Abstract
The human β globin locus spans an 80-kb chromosomal region encompassing both the five expressed globin genes and the cis-acting elements that direct their stage-specific expression during ontogeny. Sequences proximal to the genes and in the locus control region, 60 kb upstream of the adult β globin gene, are required for developmental regulation. Transgenic studies have shown that altering the structural organization of the locus disrupts the normal pattern of globin gene regulation. Procedures for introducing yeast artificial chromosomes (YACs) containing large genetic loci now make it possible to define the sequences required for stage-restricted gene expression in constructs that preserve the integrity of the β globin locus. We demonstrate that independent YAC transgenic lines exhibit remarkably similar patterns of globin gene expression during development. The switch from γ to β globin predominant expression occurs between day 11.5 and 12.5 of gestation, with no more than twofold differences in human β globin mRNA levels between lines. Human β globin mRNA levels were twofold to fourfold lower than that of mouse βmaj, revealing potentially significant differences in the regulatory sequences of the two loci. These findings provide an important basis for studying regulatory elements within the β globin locus.
THE HUMAN β GLOBIN locus has been extensively studied as a model system for understanding tissue- and developmental stage–specific expression of gene families. Generally, individual genes of the human β globin gene family are regulated appropriately by their cognate promoter elements at fetal and adult stages of development in transgenic mice. However, the levels of globin gene expression are highly variable, ranging from 0.1% to 3.0% of endogenous murine β globin synthesis.1-7 Linking sequences from the locus control region (LCR) to individual γ or β globin genes confers high-level erythroid-specific expression independent of the position of integration in the mouse genome, although stage-specific regulation of the genes may be lost.8-10 To achieve normal stage-restricted expression of the β globin gene family, transgenic constructs that more closely reproduce the organization of these genes in the native locus have been analyzed. Transgenes that include the γ and β globin genes and the sequences normally residing between them linked to the LCR have shown more precise, stage-restricted expression.10-13
These studies demonstrate that the native order and sequence context of the β globin genes are required both for appropriate timing and for levels of expression. To identify the cis-acting regulatory elements in the intact human β globin gene locus in transgenic mice, we isolated two yeast artificial chromosomes (YACs) 150 and 230 kb in length encompassing the LCR and the genes of the β globin gene family.14 We previously reported that the individual genes of the locus are expressed in a stage- and tissue-specific pattern following transfer of the intact human β globin locus in the 150-kb β globin YAC.15 However, to use the YAC transgenic model to reliably define the function of cis-acting elements, it was necessary to establish whether the murine chromatin surrounding the integrated YAC sequences influences the level and pattern of human globin gene expression.
In the present studies, we transferred the 230-kb β globin YAC and compared the expression of each of the members of the human β globin gene family in three independent transgenic lines carrying intact copies of the β globin locus and LCR. We have shown that the LCR in each of these lines directs high-level, developmental stage–specific expression of the human β globin gene family. The developmental patterns of expression are similar in all the lines, as are the levels of human β globin protein in circulating murine red blood cells. There are small but reproducible differences in the level of expression of the transgenic β globin loci in independent transgenic lines as compared with expression of the endogenous mouse βmaj globin gene.
MATERIALS AND METHODS
Isolation and purification of YAC DNA.YAC DNA was purified from total yeast chromosomal DNA of strain A85D10 as described previously.15 The DNA was concentrated and dialyzed for 48 hours against 10 mmol/L Tris, pH 7.4, and 0.16 mmol/L EDTA. Integrity of the YAC DNA was assessed by pulsed-field gel electrophoresis (PFGE) and Southern blot analysis.
Transgenic mice.Fertilized oocytes from FVB/NJ mice (Jackson Laboratories, Bar Harbor, ME) were injected with YAC DNA and transferred according to standard transgenic protocols.16 The FVB/NJ strain carries the HbbD (diffuse) globin haplotype. Founder mice were screened by polymerase chain reaction (PCR) and Southern blot analysis as previously described.15
Analysis of developmental expression of the human β globin gene family.Total RNA was extracted from yolk sacs of day 8.5 to 11.5 embryos, from livers of day 12.5 to 16.5 fetuses, or from blood or bone marrow of day 17.5 to adult animals using RNAzol (Tel-Test Inc, Friendswood, TX). RNA was annealed with primers for the mouse mɛy, βh1, and βmaj genes, as well as primers for human ɛ, γ, and β globin RNA as previously described.15
Quantitative reverse transcriptase–PCR of human β and mouse β globin RNA.Total RNA samples from at least two adult F1 animals of each of the transgenic lines were suspended in TE, and serial dilutions were made. The reverse transcriptase (RT) reaction and PCR amplification of globin cDNA was performed in the presence of α32P-dCTP as previously described.17 PCR conditions and mouse β globin primers (Bm-4 and Bm-2R) were as previously described.17 These primers amplify murine β globin genes of both the diffuse (βmaj and βmin) and single (βS and βT) alleles. The alleles can be distinguished by cleavage with Bstx 1 (present only in the diffuse alleles).17 We determined the linear range for our assays by addition of 1, 10, 100, or 1,000 ng total blood RNA to the RT reaction, followed by PCR amplification for 20 cycles using the following conditions: 94°C for 30 seconds, 62°C for 30 seconds, and 72°C for 1 minute. The human β globin primers used were as follows: HbG-3R, 5′CAGTGCAGCTCACTCAGTGT, and HbG-4R, 5′CCTGAGGAGAAGTCTGCCGT. Both the mouse and human primers flank intron sequences such that the cDNA and genomic amplified products are, respectively, 440 and 1,220 bp for mouse β globin and 296 and 450 bp for human β globin. Control samples included human and nontransgenic mouse DNA and RNA, as well as samples from each of the YAC transgenic lines. Each of these samples were incubated with mouse or human primers or both primers together in the reaction. No competition between human and mouse β globin primer sets was observed when they were combined in a single reaction. We also bred the A20.1 line onto both the HbbD (FVB/NJ) and HbbS (C57B16) backgrounds and obtained identical human β globin levels following RT-PCR of RNA samples from these animals regardless of genetic background. Amplified products were resolved on 6% acrylamide gels and quantified on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The ratio of human β to total mouse β globin RNA (combined βmaj, βmin, βS, and βT) is expressed as the percentage of mouse β globin per locus copy. For each transgenic line, we calculated the mean ± SD for seven individual PCRs, each including RNA from at least two F1 animals, at the three dilutions described. The potential statistical significance of differences between the levels of adult β globin expressed in each of the lines was determined using a two-sided Student's t-test.
Fluorescence in situ hybridization analysis.Fibroblasts were harvested from day 13.5 transgenic embryos from each of the established YAC transgenic lines and grown in culture for 4 to 5 days in DME H16/F12 medium with HEPES, penicillin/streptomycin, and 10% fetal calf serum. After splitting the fibroblasts at 1:2, the cells were processed and the slides hybridized as previously described.18,19 The probe for fluorescence in situ hybridization (FISH) was prepared from μLCRAγψβδβ cosmid DNA.11 Metaphase spreads were located and photographed using a 100X objective, with a 4-second manual exposure for DAPI and a 45- to 60-second exposure for detection of fluorescein isothiocyanate (FITC) staining, using Kodak ASA 400 Gold Print Film (Eastman Kodak, Rochester, NY). Mouse chromosomes were identified by the banding patterns with DAPI staining.
High-performance liquid chromatography and electrospray mass spectrometry analysis of globin chains.Automated analytical reversed-phase high-performance liquid chromatography (HPLC) of hemolysates was performed on a Vydac C-4 column (The Sep/a/ration Group, Hesperia, CA) (0.46 × 25 cm, 5 mm, 300 A) at a flow rate of 1 mL/min at 20°C using a Spectra Physics HPLC system (Thermal Separation Products, Fremont, CA) as previously described.20 The elution gradient was based on a previous method21 modified to allow for separation of human and mouse globins. The identity of globins eluting in discrete HPLC peaks was confirmed by molecular mass measurement. For ESMS, 5- to 10-μL aliquots of HPLC-separated fractions were injected into a stream of 50% acetonitrile and delivered into a triple-quadrupole VG BioQ electrospray ionization mass spectrometer (Micromass, Altrincham, UK) at a flow of 10 μL/min. For ESMS analysis of mixed globins, hemolysates were diluted 300-fold with 50% acetonitrile/0.2% formic acid, and 5 to 10 μL of this mixture was injected as previously described.22 The instrument was controlled and data were analyzed using MassLynx software; spectra were deconvoluted to a real molecular mass using MaxEnt software (VG Organic, Altrincham, UK) as previously described.20 23
RESULTS
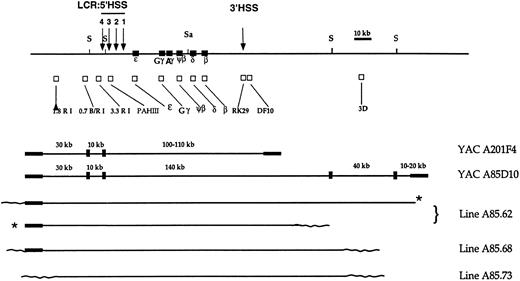
Mapping the human β globin transgenic locus.Three lines that carry all the genes of the locus and the 5′ LCR were identified following transfer of the 230-kb A85D10 YAC. The integrity of the human β globin region in these mice was examined by long-range mapping of the transgenic locus. A map of the A85D10 YAC and the transgenic human β globin locus in the lines is shown in Fig 1. Genomic DNA from transgenic mice and from the A85D10 yeast strain was digested with Sfi 1 or Sal 1 and Sfi 1 (Fig 2). To map the 5′ extent of the transgenic β globin loci in the three founder lines, blots were hybridized with the 5′ 3.3 RI probe. The correct 10-kb Sfi 1 fragment was demonstrated in all three lines (Fig 2A). Sequences immediately upstream of the most 5′ Sfi 1 site flanking the locus were also present in all of the A85D10-derived lines as identified by the 5′ 0.7 B/RI probe (data not shown). In contrast, sequences hybridizing with the 5′ 1.8 RI probe and sequences from the 5′ YAC vector arm were present in lines A85.62 and A85.68, but not in line A85.73 (data not shown), probably due to breakage of the YAC DNA during purification or injection procedures. Thus, the 5′ end of the human sequences in line A85.73 maps to a region between 16 and 28 kb upstream of HS-4 of the LCR.
Map of the human β globin locus, β globin YACs A201F4 (150 kb) and A85D10 (230 kb), and YAC transgenic lines. The top line depicts the human β globin locus and flanking regions. The positions of erythroid-specific, developmentally stable DNase I hypersensitive sites proximal to the locus are indicated by arrows and numbered (5′) 4 to 1 (3′). Restriction sites for Sal 1 (Sa) and Sfi 1 (S) appear above the linear map. The position of each member of the human β globin gene cluster is designated by a closed square; the positions of probe sequences used to map the human genomic sequences are indicated by open squares under the restriction map. Closed rectangles at either end of the A201F4 and A85D10 YAC maps designate the TRP1 and URA3 arms, respectively, of the YAC vector. Wavy lines at the extreme ends of the restriction maps for each of the transgenic lines represent the approximate position of murine genomic DNA. Asterisks indicate that the 2 copies of the YAC have integrated in tandem.
Map of the human β globin locus, β globin YACs A201F4 (150 kb) and A85D10 (230 kb), and YAC transgenic lines. The top line depicts the human β globin locus and flanking regions. The positions of erythroid-specific, developmentally stable DNase I hypersensitive sites proximal to the locus are indicated by arrows and numbered (5′) 4 to 1 (3′). Restriction sites for Sal 1 (Sa) and Sfi 1 (S) appear above the linear map. The position of each member of the human β globin gene cluster is designated by a closed square; the positions of probe sequences used to map the human genomic sequences are indicated by open squares under the restriction map. Closed rectangles at either end of the A201F4 and A85D10 YAC maps designate the TRP1 and URA3 arms, respectively, of the YAC vector. Wavy lines at the extreme ends of the restriction maps for each of the transgenic lines represent the approximate position of murine genomic DNA. Asterisks indicate that the 2 copies of the YAC have integrated in tandem.
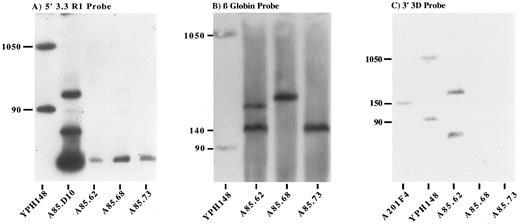
Long-range mapping of the transgenic human β globin locus by PFGE. YAC and transgenic mouse genomic DNA samples were digested with Sfi 1, resolved by PFGE, blotted, and probed with the 5′ 3.3 RI probe (A), β globin gene probe (B), and 3′ 3D probe (C). Lanes 1A and 1B and lane 2C, total chromosomal DNA from yeast strain YPH148. The 90-kb and 1,050-kb chromosomes from this strain cross-hybridize with plasmid sequences and provide useful markers. Lane 2A, partial Sfi 1 digest of the A85D10 YAC. Lane 1C, undigested total yeast DNA from YAC clone A201F4 provides a 150-kb marker. Lanes A85.62, A85.68, and A85.73 indicate Sfi 1–digested murine genomic DNA from each of the YAC transgenic lines described.
Long-range mapping of the transgenic human β globin locus by PFGE. YAC and transgenic mouse genomic DNA samples were digested with Sfi 1, resolved by PFGE, blotted, and probed with the 5′ 3.3 RI probe (A), β globin gene probe (B), and 3′ 3D probe (C). Lanes 1A and 1B and lane 2C, total chromosomal DNA from yeast strain YPH148. The 90-kb and 1,050-kb chromosomes from this strain cross-hybridize with plasmid sequences and provide useful markers. Lane 2A, partial Sfi 1 digest of the A85D10 YAC. Lane 1C, undigested total yeast DNA from YAC clone A201F4 provides a 150-kb marker. Lanes A85.62, A85.68, and A85.73 indicate Sfi 1–digested murine genomic DNA from each of the YAC transgenic lines described.
Hybridization with the ɛ, Gγ, δ IVS-2, β, and RK29 probes located on a 140-kb Sfi 1 fragment in the A85D10 YAC, revealed fragments of 140 and 180 kb in line A85.62, corresponding to two integrated copies of the YAC. Hybridization with these probes demonstrated the presence of a single integrated YAC on a 200-kb Sfi 1 fragment in line A85.68, and on a 150-kb fragment in line A85.73 (Fig 2B). Only genomic DNA from line A85.62 hybridized with the far downstream 3D probe (Fig 2C). Both the predicted 40-kb Sfi 1 fragment and a partially digested 180-kb fragment hybridized with this probe (Fig 2C). The 3′ YAC arm was deleted in all of the A85D10-derived lines. In summary, all of the lines produced by injection of the 230-kb A85D10 YAC contain the entire LCR and all the genes of the human β globin locus in an intact fragment. Lines A85.68 and A85.73 each carry a single copy of the human β globin locus in which the extreme 3′ end of the YAC has been lost, whereas line A85.62 appears to carry two tandem copies of the locus.
Chromosomal assignment for the β globin YAC transgenes.To determine the position of integration of the β globin YAC DNA in the three lines derived from the A85D10 YAC, as well as the A20.1 line described previously, we performed FISH of metaphase spreads using day 13.5 embryo fibroblasts. In these experiments, a cosmid spanning the region from the Aγ to β globin genes was used as the probe. We observed a single fluorescent locus per genome, consistent with a unique integration site of the β globin YAC sequences in each of the lines (Fig 3). The chromosomal assignment of the YAC sequences is as follows: line A20.1, chromosome 13; line A85.62, chromosome 12; line A85.68, chromosome 5; and line A85.73, chromosome 3.
FISH analysis of β globin YAC transgenic lines. Metaphase spreads from fibroblasts derived from transgenic lines A85.62 (left) and A85.68 (right). Upper panels are photomicrographs of chromosomes stained with DAPI (original magnification 1,000×). Lower panels show fields identical to those in the upper panels, with FITC labeling. β globin YAC sequences have integrated in chromosome 12 in line A85.62 and chromosome 5 in line A85.68.
FISH analysis of β globin YAC transgenic lines. Metaphase spreads from fibroblasts derived from transgenic lines A85.62 (left) and A85.68 (right). Upper panels are photomicrographs of chromosomes stained with DAPI (original magnification 1,000×). Lower panels show fields identical to those in the upper panels, with FITC labeling. β globin YAC sequences have integrated in chromosome 12 in line A85.62 and chromosome 5 in line A85.68.
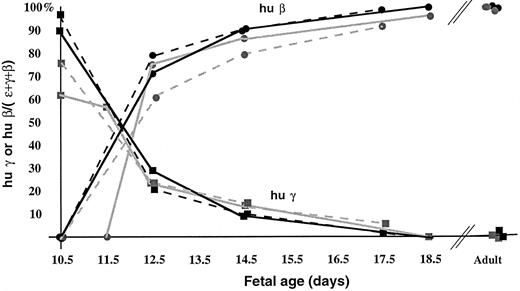
Transcriptional regulation of the human β globin gene family in lines A85.62, A85.68, and A85.73.To establish the reproducibility of human globin gene activation in our YAC transgenic mice, we compared the temporal pattern of expression. In these experiments, normalized levels of human fetal and adult mRNA were compared at embryonic, fetal, and adult stages of murine development. The human ɛ globin gene was expressed from day 9.5 of gestation until day 12.5, coincident with expression of the endogenous murine ɛy and βh1 globin genes (data not shown). We found that the switch from γ to β globin predominant gene expression occurred between day 11.5 and 12.5 of gestation in all of the A85D10 YAC-derived lines, as well as in the previously described A20.1 line (Fig 4). At day 12.5 of gestation, human β globin gene expression represents 75% to 85% of the total human globin gene expression in all of the lines. Levels of γ globin gene expression were 2% or less in perinatal mice and less than 1% in adult animals as determined by primer extension analyses. Thus, the pattern of human ɛ, γ, and β globin gene activity during ontogeny is highly reproducible among transgenic lines derived from either the A201F4 or A85D10 YACs.
Patterns of human γ and β globin gene expression in β globin YAC transgenic lines during ontogeny. Human β-like globin RNA transcripts were quantified by primer extension and PhosphorImager analysis, and the results of multiple experiments were averaged. The level of activity of human γ or β globin genes is expressed as the fraction of total human ɛ, γ, and β globin gene expression at each gestational stage, corrected for the specific activity of the primers. Solid gray line, A20.1; broken gray line, A85.68; solid black line, A85.73; broken black line, A85.62. In line A20.1, the yolk sac was harvested on day 11.5 for these experiments. The pattern of γ globin gene expression is denoted with squares, and human β globin gene expression is designated by circles.
Patterns of human γ and β globin gene expression in β globin YAC transgenic lines during ontogeny. Human β-like globin RNA transcripts were quantified by primer extension and PhosphorImager analysis, and the results of multiple experiments were averaged. The level of activity of human γ or β globin genes is expressed as the fraction of total human ɛ, γ, and β globin gene expression at each gestational stage, corrected for the specific activity of the primers. Solid gray line, A20.1; broken gray line, A85.68; solid black line, A85.73; broken black line, A85.62. In line A20.1, the yolk sac was harvested on day 11.5 for these experiments. The pattern of γ globin gene expression is denoted with squares, and human β globin gene expression is designated by circles.
The levels of human β globin gene mRNA were initially quantified using primer extension assays and then confirmed using quantitative RT-PCR studies. To assess the accuracy and reproducibility of RT-PCR assays, we first determined concentrations of RNA that were in the linear range of amplification by making 10-fold serial dilutions of RNA beginning with 1 μg to 1 ng per 10 μL RT reaction and quantifying the products of subsequent PCR amplification by PhosphorImage analysis. An example of an experimental control using human β globin primers to amplify RT products from YAC transgenic line A20.1 is shown in Fig 5A. We also confirmed that the primers for mouse β globin have equal affinity for the diffuse and single murine alleles by amplifying mouse β globin sequences from both transgenic and nontransgenic samples from C57Bl6 (HbbS) and FVB/NJ (HbbD) mice and from mice with a single and a diffuse allele (Fig 5B). There was no cross-reactivity between human and mouse β globin primers or difference in the quantity of amplified products when primer pairs were used individually or combined in the same reaction. Using serial dilutions of total blood RNA from two or more F1 animals in each line, PCR were performed in the presence of murine and human β globin primer pairs (Fig 5C). The level of human β globin gene transcripts was expressed as the percent of total mouse β globin gene expression (combined βmaj, βmin, βS, and βT) per locus and corrected for the YAC copy number. The levels of human β globin determined from these RT-PCR studies appear below the autoradiographs of reaction products from each of the lines and were as follows: line A85.62, 51.8% ± 9.3%; line A85.68, 31.9% ± 13.7%; line A85.73, 22% ± 12%; and line A20.1, 32.8% ± 6.5% (Fig 5C). The statistical significance of differences in human β globin RNA levels between the four transgenic lines was as follows: A20.1 versus A85.62, P < .0005; A85.62 versus A85.68, P < .005; and A85.62 versus A85.73, P < .0005. Human β globin mRNA levels in line A85.68 versus A20.1 or versus A85.73 were not significantly different. The level of human β globin gene expression from the transgenic locus was twofold to fourfold less than the level of murine β globin gene expression per gene copy. The lower level of transcription from the ectopic human locus suggests either that additional human sequences are required for normal levels of expression or that the different activities of the endogenous and transgenic loci are the result of divergence in regulatory sequences.
Comparison of the level of human β globin gene expression in YAC transgenic lines. (A) Autoradiograph of products of RT-PCR. Reverse transcription of 1 to 500 ng total blood RNA from line A20.1 (first 5 lanes), A20.1 transgenic RNA sample with trace genomic DNA present (F1), and RNA from a nontransgenic animal (F1-), followed by PCR amplification with human β globin primers. (B) Ethidium-stained acrylamide gel showing products of RT-PCR of total blood RNA using murine β globin primers, followed by digestion with Bstx1: line A20.1 on C57Bl6 (HbbS) genetic background (lane 1), A20.1 in mice heterozygous for the diffuse and single murine β globin alleles (lane 2), A20.1 on FVB/NJ (HbbD) background (lane 3), and nontransgenic C57Bl6 (lane 4) and FVB/NJ (lane 5) RNA. M, loaded with the φx174/HaeIII marker ladder. (C) Autoradiographs of RT-PCR products resolved by electrophoresis in 5% acrylamide gels. Samples from β globin YAC transgenic lines are indicated above each set of 4 lanes. For each line, 10-fold serial dilutions of total blood RNA were made, and 1 μg (lanes 1, 5, 9, and 13), 100 ng (lanes 2, 6, 10, and 14), 10 ng (lanes 3, 7, 11, and 15), or 1 ng (lanes 4, 8, 12, and 16) RNA was included in the RT reaction. PCR was performed in the presence of both human and mouse β globin primers. Lane F1, RT-PCR of 100 ng total blood RNA from a nontransgenic animal showing amplification of murine β globin RNA. Lane M, φx174/HaeIII marker ladder. The % human β globin/mouse β globin corrected for the number of human and mouse loci and standard deviations calculated for each line are indicated.
Comparison of the level of human β globin gene expression in YAC transgenic lines. (A) Autoradiograph of products of RT-PCR. Reverse transcription of 1 to 500 ng total blood RNA from line A20.1 (first 5 lanes), A20.1 transgenic RNA sample with trace genomic DNA present (F1), and RNA from a nontransgenic animal (F1-), followed by PCR amplification with human β globin primers. (B) Ethidium-stained acrylamide gel showing products of RT-PCR of total blood RNA using murine β globin primers, followed by digestion with Bstx1: line A20.1 on C57Bl6 (HbbS) genetic background (lane 1), A20.1 in mice heterozygous for the diffuse and single murine β globin alleles (lane 2), A20.1 on FVB/NJ (HbbD) background (lane 3), and nontransgenic C57Bl6 (lane 4) and FVB/NJ (lane 5) RNA. M, loaded with the φx174/HaeIII marker ladder. (C) Autoradiographs of RT-PCR products resolved by electrophoresis in 5% acrylamide gels. Samples from β globin YAC transgenic lines are indicated above each set of 4 lanes. For each line, 10-fold serial dilutions of total blood RNA were made, and 1 μg (lanes 1, 5, 9, and 13), 100 ng (lanes 2, 6, 10, and 14), 10 ng (lanes 3, 7, 11, and 15), or 1 ng (lanes 4, 8, 12, and 16) RNA was included in the RT reaction. PCR was performed in the presence of both human and mouse β globin primers. Lane F1, RT-PCR of 100 ng total blood RNA from a nontransgenic animal showing amplification of murine β globin RNA. Lane M, φx174/HaeIII marker ladder. The % human β globin/mouse β globin corrected for the number of human and mouse loci and standard deviations calculated for each line are indicated.
HPLC analysis of blood samples from YAC transgenic lines.The steady-state level of human β globin protein in the peripheral blood of transgenic mice was also determined. Hemolysates of peripheral blood samples from several F1 animals of each of the β globin YAC transgenic lines were analyzed by HPLC, and the level of human β globin chains was expressed as the ratio of human β globin to all mouse non–α globin (%) as follows: A85.62, 13.6 ± 1.1; A85.68, 14.2 ± 0.8; A85.73, 13 ± 0.7; and A20.1, 11.2 ± 1.6. The basis for these observations has not been proven experimentally, but our data and those of others indicate that the affinity of mouse α globin for murine β globin protein in the assembly of globin tetramers is greater than that of mouse α for human β globin chains. Analyses of mutant β globin chain assembly into hemoglobin tetramers in human samples show that electrostatic interactions and differences in surface charge affect the proportion of normal and mutant hemoglobins in heterozygous individuals.24 25 Similar interactions may contribute to different rates of assembly and steady-state levels of murine α2 human β2 as compared with murine α2β2 tetramers in mice carrying human β globin transgenes. We have demonstrated that when YAC transgenic lines are bred onto different murine thalassemic backgrounds, the steady-state level of human β globin protein in peripheral blood is inversely proportional to the level of mouse β globin chains produced (Gaensler ML, Witkowska E, unpublished results, June 1996).
DISCUSSION
We have developed a β globin YAC transgenic model to study the function of cis-acting elements in the human β globin locus in their native sequence context during ontogeny. One of the prerequisites for these studies was to determine whether the developmental timing and the level of human β globin gene expression were affected by the murine chromatin flanking different YAC integration sites. To answer this question, we generated three additional transgenic lines by microinjection of a 230-kb YAC carrying the entire human β globin locus. We showed that the YAC sequences integrated at single, unique sites in the mouse genome. We then compared the level and pattern of expression of the human β globin genes in YAC transgenic lines with the expression in the YAC transgenic line (A20.1)15 generated by microinjection of a 150-kb β globin YAC. We showed that the developmental timing of expression of human ɛ, γ, and β globin genes is specific and highly reproducible regardless of the position of integration of β globin YAC sequences. The differences in the level of human β globin gene expression may be due to the presence or absence of sequences 3′ to the locus that differ between lines, or to the effects of a second copy of the LCR on linked globin gene expression in line A85.62. Alternatively, the minor variability in the level of globin expression we observed may be attributable to the position of integration of β globin YAC sequences and the effect of adjacent murine chromatin.
We have shown that the β globin YAC transgenic lines meet important prerequisites for a model of globin gene regulation that closely approximates the expression of the human β globin locus in its endogenous context. The integrity of the locus is preserved, and human ɛ, γ, and β globin gene expression from the wild-type β globin YAC locus provides a reproducible reference for studies of the effects of targeted mutations. In addition, the high frequency of homologous recombination in yeast allows generation of specific mutations in putative regulatory sequences while avoiding artifacts associated with insertion of a selectable marker gene.
Recent analysis of a deletion of HS-2 in the murine β LCR generated by conventional homologous recombination showed that the presence of a promoter and selectable marker gene dramatically affects downstream globin gene expression.17 This mutation results in decreased expression of both embryonic and adult murine β globin genes and lethal β thalassemia in homozygotes at day 10.5 to 13.5 of gestation. Surprisingly, removal of the selectable marker gene reversed the thalassemia initially observed, and demonstrated that the HS-2 enhancer is not strictly required for globin gene transcription. Our human β globin YAC transgenic lines have been used to rescue and analyze mice with the HS-2 deletion with a selectable marker gene present (Gaensler KML, Fiering S, Groudine M, unpublished results, May 1995). The level of human β globin gene expression from the YAC locus was sufficient to rescue mice that are homozygous for the HS-2 deletion, even at embryonic and fetal stages of development when murine thalassemia is most severe.
In previous transgenic studies, plasmid constructs including individual or multiple 5′HS sites linked to globin genes usually integrated in multiple copies. Analyses of single-copy transgenic lines carrying plasmid constructs suggested that a single 5′HS-3 can direct expression of linked globin genes, but the ability of the HS-2 core enhancer to function as LCR is contingent upon integration of more than one copy.26,27 Single-copy transgenic lines carrying linked cosmids encompassing the human β globin locus with deletion of one of the four 5′HSs in the LCR show marked position-dependent differences in the level of globin gene activation.28 Thus, to reliably interpret the results of mutational analyses of cis-acting sequences within and flanking the genes of the locus, studies must be performed on constructs such as YACs that preserve the integrity of the LCR.
To date, several other groups have made transgenic lines with β globin YACs or with ligated cosmids that encompass the LCR and all the members of the human β globin gene family.12,29-32 These studies have established that (1) the order and spatial arrangement of β globin genes with respect to each other and to the LCR are important for appropriate stage-restricted expression,29 (2) the human HPFH phenotype is reproduced in β globin YAC transgenic mice following introduction of a previously defined Aγ globin promoter mutation into a modified A85D10 β globin YAC,31 and (3) the phenotypes of deletions or substitutions of hypersensitive sites-3 and -4 in human β globin YAC transgenic mice suggest that individual hypersensitive sites of the LCR are not stage-restricted in their activity.32 33 These studies provide strong evidence that transgenic studies of targeted mutations in β globin YACs will accurately predict the role of these cis-acting elements during human ontogeny.
Analysis of YAC transgenic mice will provide insights into long-range regulatory mechanisms and structural elements involved in the erythroid- and stage-specific activation of this chromatin domain in ectopic chromosomal locations. In addition, the β globin YACs will also be useful in creating murine models of human hemoglobinopathies and thalassemias. These models will be valuable for testing future strategies for gene transfer, homologous recombination, and induction of human γ globin gene expression, as all of the regulatory sequences that participate in regulation of the human β globin gene family are present in their native context.
ACKNOWLEDGMENT
The authors thank Dr Y.W. Kan for ongoing support and interest in this project, Elaine Carlson for assistance in adapting the FISH procedures, and Drs William Forrester, Steven Fiering, Elliot Epner, and Robert Debs for helpful discussions of this study.
Supported in part by National Institutes of Health Grants No. HL02711 and P60-HL20984 and the Lucille P. Markey Trust.
Address reprint requests to Karin M.L. Gaensler, MD, Department of Medicine, University of California, San Francisco, 3rd and Parnassus, San Francisco, CA 94143-0724.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal