Abstract
We have previously shown that granulocyte colony-stimulating factor (G-CSF ) delays spontaneous neutrophil apoptosis through activation of the vacuolar proton ATPase (v-ATPase). We have now examined the regulation of the v-ATPase in neutrophils exposed to G-CSF in vitro. When neutrophils were cultivated in the absence of G-CSF, the 57-kD cytosolic B subunit of the v-ATPase disappeared within 1 to 2 hours, its loss preceding the nuclear changes of apoptosis and coinciding with the onset of acidification. By contrast, in neutrophils cultured for 2 hours in the presence of G-CSF, the amount of the 57-kD subunit was similar to that in freshly isolated neutrophils. However, inhibition of protein synthesis with cycloheximide and actinomycin D led to loss of the 57-kD subunit even in the presence of G-CSF. These results indicated that ongoing protein synthesis was required to maintain the v-ATPase, and further suggested that G-CSF acted, at least in part, by maintaining synthesis of the 57-kD cytosolic subunit. G-CSF also promoted the translocation of the 57-and 33-kD cytosolic v-ATPase subunits to the membrane. Our findings suggested two coordinate mechanisms by which the activity of the v-ATPase could be increased by G-CSF: the synthesis of cytosolic v-ATPase subunits and their translocation to the membrane.
THE VACUOLAR PROTON ATPase (v-ATPase) is a multisubunit enzyme composed of V1 , a complex of 5 different cytosolic components (A to E, with molecular weights 72, 57, 40, 34, and 33 kD, respectively) that exhibit ATPase activity and V0 , a complex of membrane-associated subunits (α to γ, with molecular weights 100, 39, and 16 kD, respectively) that participate in proton translocation.1 V1 is only active when associated with V0 , the membrane complex. When the active v-ATPase is associated with the membrane of a vesicle, it pumps protons into the lumen of the vesicle, acidifying that compartment. However, the v-ATPase can also become associated with the plasma membrane, from where it exports protons out of the cell, alkalinizing the cytosol. The v-ATPase is quiescent in resting neutrophils, but can become activated when the cells are stimulated by phorbol myristate acetate (PMA).2 In this study we examine the regulation of the v-ATPase in response to granulocyte colony-stimulating factor (G-CSF ).
MATERIALS AND METHODS
Neutrophils were obtained from the peripheral blood of adult donors after obtaining informed consent. After dextran sedimentation, neutrophils were purified by centrifugation through a discontinuous Percoll gradient. Neutrophils were washed once and cultured in RPMI-1640 in 5% CO2 and 95% humidified air at 37°C with or without recombinant human G-CSF (104 U/mL), for up to 3 hours. Apoptosis was assessed by examination of nuclear morphology after staining with acridine orange (4 μg/mL).3 Cells were then treated with diiospropylfluorophosphate (DFP) 0.3 μmol/L on ice for 20 minutes, then disrupted by nitrogen cavitation. We obtained essentially identical results if the DFP treatment was done before the start of the incubation in RPMI. The nuclei were removed by centrifugation (10,000g, 30 seconds), and the postnuclear supernatant was layered over a discontinuous sucrose gradient (8.6% and 15%, wt/vol) and centrifuged 45 minutes at 100,000g in a swinging bucket rotor. Plasma membranes were recovered from the interface, pelleted, and resuspended in sample buffer. The cytosol was recovered and V1 collected by immunoprecipitation with a monoclonal antibody to the E subunit. The granule fraction (the pellet at the bottom of the sucrose gradient) was dissolved in sample buffer. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 12.5% gel), transferred to polyvinylidene difluoride (PVDF ) nylon membranes, and immunoblotted with polyclonal antibody to the 57-kD B subunit (B2 antibody4 ) and monoclonal antibody to the 33-kD E subunit (E11 antibody4 ) visualizing with a secondary antibody conjugated to alkaline phosphatase. All results presented are representative of at least three experiments.
For measurement of intracellular pH, freshly isolated neutrophils were loaded for 20 minutes with 1 μmol/L 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM) in serum-free RPMI-1640 in the incubator. Cells were washed once in sodium-free buffer (choline chloride 103 mmol/L, KCl 0.4 mmol/L, Ca gluconate 0.4 mmol/L, MgSO4 0.4 mmol/L, K2HPO4 5.6 mmol/L, HEPES 24 mmol/L, d-glucose 11.1 mmol/L, pH 7.4) and diluted in sodium-free buffer into a 96-well plate. Fluorescence was read at 530 nm with excitation of 490 nm in a plate reader (Bio-Tek, Winooski, VT). After a baseline was obtained, G-CSF (104 U/mL) and bafilomycin (100 nmol/L) were added to indicated wells and observation was begun. Measurements were made at 2-minute intervals (10-minute intervals are shown in the figures). A pH standard curve was included with each plate. All samples were prepared in triplicate.
RESULTS
In these studies, subcellular fractionation was accomplished by sonication followed by nitrogen cavitation and discontinuous sucrose density gradient centrifugation, a procedure that separates plasma membranes and secretory granules from other granule fractions. We did not detect association of the B or E subunits with the nuclei or granule fractions under any of the conditions examined (data not shown). These findings do not exclude the existence of V0 subunits associated with the granule fractions.
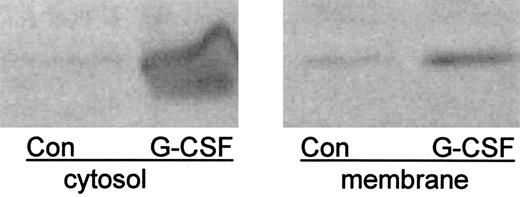
We examined the effect of G-CSF on translocation of the V1 complex (indicated by immunodetection of the B and E subunits) and observed an increase in the amount of the B subunit in both cytosol and membrane after 3 hours incubation with G-CSF compared with that of cells incubated for an equal time without G-CSF (Fig 1). We initially attributed this to increased protein synthesis in response to G-CSF. However, when we examined the content of the B subunit in freshly isolated neutrophils, we found similar amounts to that of cells incubated with G-CSF (Fig 2). Thus, it appears that cultivation of cells in the absence of G-CSF leads to progressive loss of the B subunit, suggesting that it turns over rapidly in the absence of G-CSF.
G-CSF increases the amount of B subunit in cytosol and membrane. Neutrophils were incubated for 3 hours in the presence or absence of G-CSF then disrupted by sonication. Membranes were obtained as the washed pellet of a 6-minute 100,000g centrifugation. The supernatant of the first centrifugation represented cytosol. Samples representing 2 × 107 cell equivalents were resolved by SDS-PAGE and detected with anti-B2 antibody4.
G-CSF increases the amount of B subunit in cytosol and membrane. Neutrophils were incubated for 3 hours in the presence or absence of G-CSF then disrupted by sonication. Membranes were obtained as the washed pellet of a 6-minute 100,000g centrifugation. The supernatant of the first centrifugation represented cytosol. Samples representing 2 × 107 cell equivalents were resolved by SDS-PAGE and detected with anti-B2 antibody4.
Upregulation of the B subunit depends on new protein synthesis. Neutrophils were cultivated for 2 hours in the presence (G-CSF ) or absence (Con) of G-CSF. Where noted, cycloheximide and actinomycin D (CHX/ActD) were added 30 minutes before the addition of G-CSF and included throughout the incubation. Cytosol and membrane fractions were prepared as described in the legend to Fig 1, and blots were probed with anti-B2 antibody (B) or with E-11 antibody against subunit E (E).
Upregulation of the B subunit depends on new protein synthesis. Neutrophils were cultivated for 2 hours in the presence (G-CSF ) or absence (Con) of G-CSF. Where noted, cycloheximide and actinomycin D (CHX/ActD) were added 30 minutes before the addition of G-CSF and included throughout the incubation. Cytosol and membrane fractions were prepared as described in the legend to Fig 1, and blots were probed with anti-B2 antibody (B) or with E-11 antibody against subunit E (E).
G-CSF deprivation results in loss of the B subunit. Neutrophils were disrupted by sonication either immediately (Immed) or after cultivation for 60 minutes in the presence (G-CSF ) or absence (Control) of G-CSF. Samples representing cytosol (100,000g supernatant) of 2 × 107cell equivalents were resolved by SDS-PAGE and detected with anti-B2 antibody.
G-CSF deprivation results in loss of the B subunit. Neutrophils were disrupted by sonication either immediately (Immed) or after cultivation for 60 minutes in the presence (G-CSF ) or absence (Control) of G-CSF. Samples representing cytosol (100,000g supernatant) of 2 × 107cell equivalents were resolved by SDS-PAGE and detected with anti-B2 antibody.
To determine whether the effect of G-CSF on the concentration of the B subunit caused by a G-CSF–mediated increase in the synthesis of the subunit or a decrease in its degradation, we cultivated neutrophils with G-CSF in the presence or absence of cycloheximide and actinomycin D, to inhibit new protein synthesis. We found that inhibition of protein synthesis resulted in the disappearance of the B subunit from cytosol and membrane, even in the presence of G-CSF (Fig 3). This result suggested that G-CSF promotes ongoing protein synthesis of the subunit and that in the absence of new protein synthesis, the B subunit is rapidly degraded. We also examined the effect of these interventions on the E subunit and found that G-CSF promoted translocation of the subunit to the membranes and that the amount of this subunit in the cytosolic fraction was diminished after G-CSF deprivation or inhibition of protein synthesis. However, in contrast to the B subunit, the E subunit was preserved in the membranes of G-CSF–treated neutrophils incubated with cycloheximide and actinomycin D (Fig 3). This may be consistent with a slower rate of turnover of the E subunit in the neutrophil membrane.
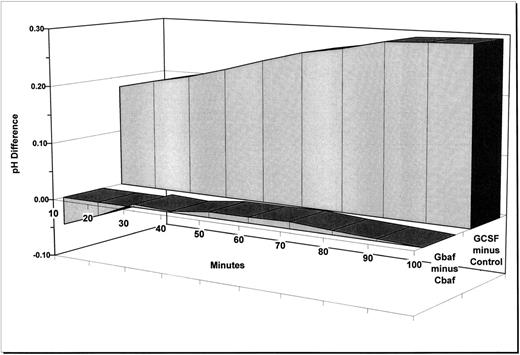
To determine if the differences in v-ATPase detected by Western blot analysis corresponded to a difference in proton export between control cells and those treated with G-CSF, we measured changes in intracellular pH in cells incubated in sodium-free media to eliminate the possible contribution of the sodium/proton antiporter. To attribute proton export to the v-ATPase, we also measured pH changes in G-CSF–treated cells and control cells incubated with bafilomycin A1 , an inhibitor of the v-ATPase. Consistent with our previously reported observations,3 we found that G-CSF attenuated cytoplasmic acidification relative to control and that the concurrent addition of bafilomycin abrogated the pH difference between the G-CSF–treated cells and control cells (Fig 4).
Effects of G-CSF and bafilomycin on pH homeostasis. Neutrophils were incubated in sodium-free media and pH-sensitive BCECF fluorescence was monitored over time. Data are presented as the average pH difference between G-CSF and controls (GCSF minus Control) and between G-CSF + bafilomycin and controls + bafilomycin (Gbaf minus Cbaf ) for six independent experiments each containing three replicates. Bars on the 100-minute time point denote standard error for six experiments. The 100-minute standard error was similar to that observed for the remainder of the time points. Areas under each of the 12 curves (6 GCSF minus control and 6 Gbaf minus Cbaf ) were calculated in arbitrary units. The difference between these two sets of areas was significant at the level of P < .02 (one-tailed Wilcoxson two-sample test).
Effects of G-CSF and bafilomycin on pH homeostasis. Neutrophils were incubated in sodium-free media and pH-sensitive BCECF fluorescence was monitored over time. Data are presented as the average pH difference between G-CSF and controls (GCSF minus Control) and between G-CSF + bafilomycin and controls + bafilomycin (Gbaf minus Cbaf ) for six independent experiments each containing three replicates. Bars on the 100-minute time point denote standard error for six experiments. The 100-minute standard error was similar to that observed for the remainder of the time points. Areas under each of the 12 curves (6 GCSF minus control and 6 Gbaf minus Cbaf ) were calculated in arbitrary units. The difference between these two sets of areas was significant at the level of P < .02 (one-tailed Wilcoxson two-sample test).
The foregoing results implied that G-CSF drives the synthesis of the cytosolic subunits of the v-ATPase and that these subunits turn over rapidly in the absence of G-CSF. To rule out the possibility that the loss of V1 subunits was caused by generalized loss of cellular protein because of late events in cell death in the G-CSF–deprived neutrophils, we scored apoptosis by morphological criteria. Fewer than 2% of the G-CSF–deprived neutrophils showed chromatin condensation or nuclear fragmentation after the 3-hour incubation, indicating that these findings could not be explained by the occurrence of apoptosis during the incubation.
DISCUSSION
These studies extend our previous observation that G-CSF delays apoptosis in neutrophils by activating the v-ATPase. The v-ATPase has been implicated as a defense against apoptosis in other systems as well; we have shown that the v-ATPase is necessary for myocardial protection against ischemia/reperfusion mediated by preconditioning5 and that inhibition of the v-ATPase induces apoptosis in WEHI cells.6 To the extent that tumorigenicity correlates with resistance to apoptosis, the v-ATPase has been shown to be important to the expression of multidrug resistance,7 and the induction of tumorigenicity in 3T3 cells.8
The observation that G-CSF causes cytoplasmic alkalinization in the first 10 minutes of observation (Fig 4) suggested that v-ATPase is rapidly activated, similar to the previously described effect of phorbol esters described by Nanda et al.2 However, our results indicated that the vATPase was regulated not only by subunit assembly and translocation but also by protein turnover. V1 translocation has been shown to occur in yeast in response to changes in the carbon source, and in Manduca sexta midgut just before eclosion.9,10 Distribution of the v-ATPase has been shown to shift from vesicular membranes to the plasma membrane in renal epithelia of acid-loaded rats,11 although it is not clear in that system whether the change is caused by membrane fusion events or assembly of new v-ATPase at the plasma membrane. Recently it has been shown that interleukin-1 causes activation of the v-ATPase in neutrophils, an event mediated by fusion of secretory granules with the plasma membrane.12 However, in that study the investigation centered on the C subunit, an element of the V1 “stalk” that enhances, but is not essential for, v-ATPase function.13 The relationship between those observations and our own will require additional study.
ACKNOWLEDGMENT
We gratefully acknowledge the generous gift of antibody to the A subunit provided by Dr Michael Forgac, Tufts University School of Medicine, Boston, MA.
Supported by USPHS Grants No. AI 01345 and AG 13501 (R.A.G.); by the Netherlands Organization for Scientific Research and the Van Helten Foundation, Royal Netherlands Academy of Arts and Sciences (H.N.); the Research Service, Deparment of Veterans Affairs (R.L.E.); and the General Clinical Research Service of The Scripps Research Institute. R.A.G. gratefully acknowledges the support of an American Society of Hematology Junior Faculty Scholar Award.
Address reprint requests to Roberta A. Gottlieb, MD, Division of Biochemistry, Department of Molecular and Experimental Medicine, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal