Abstract
The Spanish PETHEMA group designed a protocol for newly diagnosed PML/RAR-positive acute promyelocytic leukemia (APL) in which induction and consolidation followed the original AIDA regimen, except for the omission of cytarabine and etoposide from consolidation. Induction consisted of 45 mg/m2 all-trans retinoic acid (ATRA) daily until complete remission (CR) and 12 mg/m2 idarubicin on days 2, 4, 6, and 8. Patients in CR received 3 monthly chemotherapy courses: idarubicin 5 mg/m2/d × 4 (course no. 1), mitoxantrone 10 mg/m2/d × 5 (course no. 2), and idarubicin 12 mg/m2/d × 1 (course no. 3). Maintenance therapy consisted of 90 mg/m2/d mercaptopurine orally, 15 mg/m2/wk methotrexate intramuscularly, and, intermittently, 45 mg/m2/d ATRA for 15 days every 3 months. Between November 1996 and December 1998, 123 patients with newly diagnosed PML/RAR-positive APL from 39 centers were enrolled. A total of 109 patients achieved CR (89%; 95% confidence interval [CI], 83 to 95), 12 died of early complications, and the remaining 2 were resistant. Consolidation treatment was associated with very low toxicity and no deaths in remission were recorded. Molecular assessment of response by reverse transcriptase-polymerase chain reaction (RT-PCR) showed conversion to PCR-negative in 48 of 99 (51%) and 82 of 88 patients (93%) after induction and consolidation, respectively. The 2-year Kaplan-Meier estimates of overall survival and event-free survival were 82% ± 4% and 79% ± 4%, respectively. For patients who achieved CR, the 2-year disease-free survival (DFS) was 92% ± 3%. These data indicate that a significant reduction in toxicity might be obtained in APL using a less intensive consolidation without apparently compromising the antileukemic effect. These results also suggest a minor role for cytarabine and etoposide in the treatment of newly diagnosed PML/RAR-positive APL patients.
SINCE THE INTRODUCTION of all-trans retinoic acid (ATRA), several issues appear critical to the design of optimal front-line therapy in acute promyelocytic leukemia (APL). (1) Sensitivity to ATRA is genetically determined by the PML/RARα fusion that is formed as a consequence of the t(15;17).1 Demonstration of the specific chromosome abnormality or of its molecular counterpart might be considered a mandatory criterion to start tailored therapy.2 (2) The best therapy results, in terms of complete remission (CR) and disease-free survival (DFS), have been obtained using ATRA and chemotherapy for remission induction.3-9 As shown in a randomized study,9 simultaneous combination is more effective than sequential administration of ATRA and chemotherapy. (3) The additional benefit, if any, provided by cytarabine administered in induction and/or in consolidation remains unclear. In fact, although randomized trials comparing different chemotherapy induction regimens in APL have not been performed, the inclusion of cytarabine in addition to daunorubicin, with or without other chemotherapy agents (eg, thioguanine, etoposide), does not appear to significantly affect disease outcome,10 nor does it seem to provide better results than those obtained with high-dose daunorubicin11-14 or idarubicin alone.15,16 (4) Detection of minimal residual disease at relatively low sensitivity levels (10−4) during hematological CR (HCR) postconsolidation is a strong predictor of relapse,17 and the achievement of sustained molecular remission might be considered as our best therapeutic goal at present. (5) The benefits of maintenance therapy that contains ATRA have been shown in 2 large randomized studies.7 9
Keeping the above considerations in mind, in 1996 the Spanish PETHEMA group designed a protocol (LPA96) for the treatment of newly diagnosed PML/RARα-positive APL patients. This protocol included an induction phase with ATRA and idarubicin, as in the original AIDA regimen of the Italian GIMEMA group.3 In contrast with the latter treatment, however, we decided to omit cytarabine and etoposide from consolidation therapy and used an anthracycline-based consolidation with the same idarubicin and mitoxantrone dose/schedule adopted by the Italian group. Our main objectives were to evaluate the toxicity of this approach and its antileukemic efficacy in terms of molecular response and DFS.
We report here a study on 123 newly diagnosed PML/RARα positive patients enrolled in this trial. Besides the expected important reduction of treatment-related toxicity, we found that high molecular remission and DFS rates might be achieved in APL using this less intensive treatment.
MATERIALS AND METHODS
Eligibility Criteria
Eligibility criteria included: (1) confirmed genetic diagnosis by demonstration of the t(15;17) and/or PML/RARα rearrangement; (2) no prior chemotherapy; (3) normal hepatic and renal function; (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 3; (5) serum creatinine <2.5 mg/dL, serum alkaline phosphatase, bilirubin, and aspartate amino transferase (AST) <3 times the normal upper limit; (6) no cardiac contraindications to anthracycline chemotherapy; (7) negative pregnancy test; and (8) informed consent.
Protocol Design
Induction therapy consisted of oral ATRA 45 mg/m2/d, divided into 2 doses administered every 12 hours, until HCR and for a maximum of 90 days, and 4 intravenous (IV) bolus (15 to 30 minutes) of idarubicin 12 mg/m2 on days 2, 4, 6, and 8. In patients younger than 15 years, ATRA doses were adjusted to 25 mg/m2. Treatment was started as soon as a diagnosis of APL (M3 or M3 variant) was established by morphocytochemical criteria according to the French-American-British (FAB) classification. For patients in whom diagnosis was not confirmed by genetic studies, ATRA was withdrawn and alternative chemotherapy given at the physician’s discretion. Patients in HCR received 3 monthly consolidation courses consisting of idarubicin 5 mg/m2 IV daily for 4 days (course no. 1), mitoxantrone 10 mg/m2 IV daily for 5 days (course no. 2), and idarubicin 12 mg/m2 IV on only 1 day (course no. 3). After completion of consolidation, patients who tested polymerase chain reaction (PCR)-negative for the PML/RARα hybrid gene were started on maintenance therapy with mercaptopurine 90 mg/m2/day orally, methotrexate 15 mg/m2/week intramuscularly, and intermittently ATRA 45 mg/m2/d for 15 days every 3 months. Doses of mercaptopurine and methotrexate were decreased by 50% if the white blood cell (WBC) count was lower than 3.5 × 109/L and discontinued if lower than 2.5 × 109/L. Maintenance therapy was given for 2 years.
Supportive therapy.
All patients were admitted to the hospital for remission induction, while consolidation courses were always given on an outpatient basis. IV antibiotic therapy, usually consisting of β-lactamic/aminoglycoside associations, was initiated in case of fever. This standard regimen was modified thereafter according to the results of appropriate cultures. Treatment of coagulopathy during induction was based on fresh frozen plasma and/or fibrinogen transfusion, as well as on platelet support to maintain the platelet counts above 30 × 109/L until disappearance of significant coagulopathy. The use of heparin, tranexamic acid, and other measures was optional. Once the coagulopathy was under control, platelet transfusions were only used in patients with infectious or hemorrhagic manifestations or when the platelet count dropped below 20 × 109/L. At the first signs of suspected ATRA syndrome, ATRA was discontinued and patients were given 10 mg dexamethasone every 12 hours as recommended by Frankel et al.18 Prophylactic dexamethasone, using the same doses, was also administered in cases where the WBC count was greater than 5 × 109/L. The use of hydroxyurea was not recommended for patients with hyperleukocytosis.
Laboratory Studies
Bone marrow samples were collected at diagnosis, after induction, after consolidation, at 2 months during the first year, every 3 months during the second year, and every 4 to 6 months after this period. Besides morphological evaluation, samples were processed for RNA extraction and reverse transcriptase (RT)-PCR of PML/RARα. In case of doubtful or positive PCR during HCR, an extra bone marrow sample was required in 2 to 4 weeks time to confirm the result. RT-PCR tests were performed by 12 different Spanish laboratories, involved in an external quality control program, which included interlaboratory exchange of samples, as reported elsewhere.19 In addition, PCR-positivity at the end of consolidation or during clinical remission was additionally checked in a reference laboratory (P.B. and E.B., Valencia, Spain). Most laboratories followed the method of Biondi et al,20and a minor group used the method described by Borrow et al.21 Immunophenotypic and cytogenetic analyses were systematically performed at diagnosis only. Since May 1998, for rapid diagnostic refinement and patient enrollment into the protocol, we have occasionally used the immunohistochemical analysis of PML protein distribution, using monoclonal antibody PG-M3 (kindly provided by B. Falini, Institute of Hematology, University of Perugia, Perugia, Italy22).
Definitions and Study Endpoints
HCR and hematological relapse were defined according to the National Cancer Institute criteria.23 Failures were classified as resistant leukemia and early death. Early death was defined as death occurring during induction therapy or during the period of aplasia after chemotherapy. Molecular remission was defined as the disappearance on an ethidium bromide gel of the PML/RARα specific band visualized at diagnosis, using an RT-PCR assay with a sensitivity level of 10−4. Molecular relapse was defined as the reappearance of PCR-positivity in 2 consecutive bone marrow samples at any time after consolidation therapy. Chromosomal abnormalities were described according to the International System for Human Cytogenetics Nomenclature. Toxicity was graded according to the World Health Organization (WHO) grading system. According to Frankel et al,5 ATRA syndrome was defined as: definitely present in the presence of the 5 characteristic signs or symptoms of this syndrome18: fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates, and unexplained weight gain greater than 5 kg; indeterminate when 2 to 4 of the above-mentioned signs and symptoms occurred; and definitely absent in the remaining patients. Duration of neutropenia and thrombocytopenia was defined as the time, in days, from the start of chemotherapy until the day of the first measurement of absolute neutrophil count >1 × 109/L and platelets >50 × 109/L, respectively. Overall and event-free survival (OS and EFS) were calculated from the date of starting induction therapy, while relapse-free survival (RFS) and DFS were calculated from the day of HCR achievement. Failure to achieve HCR (defined above), relapse, and death in HCR were considered the “events,” whichever occurred first, to analyze, when applicable, as censored data in EFS, RFS, and DFS.
Statistical Methods
Unadjusted time-to-event analyses were performed using the Kaplan-Meier estimate,24 log-rank tests, and their generalizations.25-27 Univariate analysis for induction response was performed using Pearson’s χ2 test or, if applicable, Fisher’s exact test, and the 95% confidence intervals (CI) for difference in proportion were calculated. Variables included in the analysis are listed in Table 1.
RESULTS
Accrual and Patient Characteristics
Between November 1996 and January 1999, 136 consecutive patients from 39 Spanish institutions (see ) were registered, based on a morphocytochemical diagnosis of AML-M3 according to the FAB classification. Of these, 13 patients (10%) were subsequently excluded: 10 because of absence of the genetic hallmark upon cytogenetic and molecular analysis, 2 because of poor medical condition (ECOG grade 4 at admission due to cerebral hemorrhage, leading to death before the start of therapy), and 1 because of protocol violation. All of the remaining patients were genetically diagnosed by demonstration of the specific translocation (2), the PML/RARα hybrid gene (36) or both (85). The main clinical and biological characteristics of the 123 newly diagnosed PML/RARα-positive APL patients who were considered eligible are shown in Table 1.
Baseline Characteristics of APL Patients and Remission Induction Results
| Characteristic . | Median (range) . | N (%) . | CR (%) . | P . |
|---|---|---|---|---|
| Age | 42 (1-74) | |||
| ≤15 | 7 (6) | 5 (71) | ||
| 16-40 | 49 (40) | 45 (92) | ||
| 41-60 | 46 (37) | 44 (96) | .007 | |
| 61-70 | 13 (11) | 11 (85) | ||
| >70 | 8 (6) | 4 (50) | ||
| Gender | ||||
| Male | 71 (58) | 61 (86) | NS | |
| Female | 52 (42) | 48 (92) | ||
| Fever | 41 (33) | 33 (80) | NS | |
| Organomegaly | 9 (7) | 8 (89) | NS | |
| Coagulopathy | 77 (63) | 70 (91) | NS | |
| Hemorrhage | 92 (75) | 80 (87) | NS | |
| Cutaneous | 74 (60) | |||
| Mucosal | 62 (50) | |||
| CNS | 2 (2) | |||
| Other | 20 (16) | |||
| WBC (×109/L) | 1.9 (0.4-210) | |||
| ≤3.5 | 78 (64) | 71 (91) | ||
| 3.5-10 | 15 (12) | 13 (87) | .02 | |
| 10-50 | 20 (16) | 19 (95) | ||
| >50 | 10 (8) | 6 (60) | ||
| Hemoglobin (g/dL) | 9.3 (4.4-13.1) | |||
| ≤10 | 78 (63) | 68 (87) | NS | |
| >10 | 45 (37) | 41 (91) | ||
| Platelets (×109/L) | 21 (1-161) | |||
| ≤10 | 23 (19) | 18 (78) | ||
| 11-50 | 76 (63) | 69 (91) | NS | |
| >50 | 22 (18) | 21 (95) | ||
| FAB subtype | ||||
| Typical | 100 (81) | 90 (90) | NS | |
| Variant | 23 (19) | 19 (83) | ||
| Cytogenetics (n = 94) | ||||
| Normal | 4 (4) | 4 (100) | ||
| t(15;17) | 68 (72) | 58 (88) | NS | |
| t(15;17), +8 | 10 (11) | 10 (100) | ||
| t(15;17), plus other abnormality | 9 (10) | 9 (100) | ||
| Other | 3 (3) | 3 (100) | ||
| PML/RARα (n = 121) | ||||
| BCR1/BCR2 | 68 (56) | 60 (88) | NS | |
| BCR3 | 53 (44) | 47 (89) |
| Characteristic . | Median (range) . | N (%) . | CR (%) . | P . |
|---|---|---|---|---|
| Age | 42 (1-74) | |||
| ≤15 | 7 (6) | 5 (71) | ||
| 16-40 | 49 (40) | 45 (92) | ||
| 41-60 | 46 (37) | 44 (96) | .007 | |
| 61-70 | 13 (11) | 11 (85) | ||
| >70 | 8 (6) | 4 (50) | ||
| Gender | ||||
| Male | 71 (58) | 61 (86) | NS | |
| Female | 52 (42) | 48 (92) | ||
| Fever | 41 (33) | 33 (80) | NS | |
| Organomegaly | 9 (7) | 8 (89) | NS | |
| Coagulopathy | 77 (63) | 70 (91) | NS | |
| Hemorrhage | 92 (75) | 80 (87) | NS | |
| Cutaneous | 74 (60) | |||
| Mucosal | 62 (50) | |||
| CNS | 2 (2) | |||
| Other | 20 (16) | |||
| WBC (×109/L) | 1.9 (0.4-210) | |||
| ≤3.5 | 78 (64) | 71 (91) | ||
| 3.5-10 | 15 (12) | 13 (87) | .02 | |
| 10-50 | 20 (16) | 19 (95) | ||
| >50 | 10 (8) | 6 (60) | ||
| Hemoglobin (g/dL) | 9.3 (4.4-13.1) | |||
| ≤10 | 78 (63) | 68 (87) | NS | |
| >10 | 45 (37) | 41 (91) | ||
| Platelets (×109/L) | 21 (1-161) | |||
| ≤10 | 23 (19) | 18 (78) | ||
| 11-50 | 76 (63) | 69 (91) | NS | |
| >50 | 22 (18) | 21 (95) | ||
| FAB subtype | ||||
| Typical | 100 (81) | 90 (90) | NS | |
| Variant | 23 (19) | 19 (83) | ||
| Cytogenetics (n = 94) | ||||
| Normal | 4 (4) | 4 (100) | ||
| t(15;17) | 68 (72) | 58 (88) | NS | |
| t(15;17), +8 | 10 (11) | 10 (100) | ||
| t(15;17), plus other abnormality | 9 (10) | 9 (100) | ||
| Other | 3 (3) | 3 (100) | ||
| PML/RARα (n = 121) | ||||
| BCR1/BCR2 | 68 (56) | 60 (88) | NS | |
| BCR3 | 53 (44) | 47 (89) |
Abbreviation: NS, not significant.
Induction Therapy
Hematological and molecular response.
Of the 123 eligible patients, 109 achieved HCR (89%; 95% CI, 83 to 95), and the remaining 14 were considered as failures due to either therapy-related mortality (12) or induction resistance, which was observed in 2 patients of 41 and 71 years, respectively. Of the 12 early deaths, 3 were due to infection (occurred from day +18 through day +31), 8 to cerebral, intestinal, or pulmonary hemorrhage (days +1 through +23), and the remaining 1 to ATRA syndrome (day +28).
PML/RARα RT-PCR tests were available in 99 cases at the end of induction and/or before consolidation. Fifty-one patients (51%) tested PCR-positive and 48 (49%) PCR-negative at this time point.
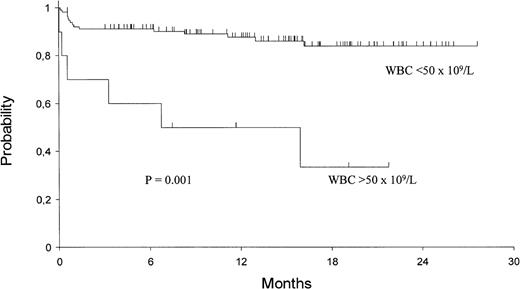
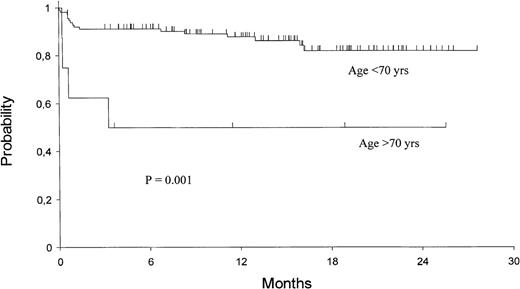
Univariate analysis demonstrated no significant relationships among clinical-biological characteristics at diagnosis and PCR status after induction therapy. However, presenting WBC count and age clearly influenced the HCR rate. A poorer response rate was, in fact, observed in older age patients and in those with higher WBC count, with the more discriminant cut-off values being 70 years and 50 × 109/L, respectively (Table 1).
Toxicity.
Apart from the above-mentioned 12 toxic deaths that occurred during induction therapy, other minor hematological and nonhematological toxicities are listed in Table 2. Due to presumed ATRA syndrome, ATRA was discontinued early for 35 patients (28%) at a median time of 9 days of treatment (range, 1 to 25). A definitely present ATRA syndrome was diagnosed in 7 patients (6%), 1 of whom died, whereas an indeterminate ATRA syndrome was reported in 25 cases (20%).
Toxic Effects During Induction Therapy
| Hematological Toxicity . | Median (range) . | N (%) . |
|---|---|---|
| Hematological recovery (days to) | ||
| PMN >1 × 109/L | 24 (5-57) | |
| Platelets >50 × 109/L | 19 (6-45) | |
| Fever | 115 (93) | |
| Not documented | 37 (32) | |
| Microbiologically documented without bacteremia | 11 (10) | |
| Bacteremia | 25 (22) | |
| Clinically documented | 42 (36) | |
| Hemorrhage | 82 (67) | |
| Pulmonary | 7 | |
| CNS | 6 | |
| Nonhematological toxicity (n = 103) Grades 3 and 4 (WHO) | ||
| Hepatic | 5 (5) | |
| Pulmonary | 17 (16) | |
| Renal | 1 (1) | |
| Cardiac | 6 (6) | |
| Neurological | 2 (2) | |
| Dermatological | 2 (2) | |
| Diarrhea | 3 (3) | |
| Oral | 15 (14) | |
| ATRA syndrome | ||
| Definitely present | 7 (6) | |
| Indeterminate | 25 (20) | |
| Definitely absent | 91 (74) |
| Hematological Toxicity . | Median (range) . | N (%) . |
|---|---|---|
| Hematological recovery (days to) | ||
| PMN >1 × 109/L | 24 (5-57) | |
| Platelets >50 × 109/L | 19 (6-45) | |
| Fever | 115 (93) | |
| Not documented | 37 (32) | |
| Microbiologically documented without bacteremia | 11 (10) | |
| Bacteremia | 25 (22) | |
| Clinically documented | 42 (36) | |
| Hemorrhage | 82 (67) | |
| Pulmonary | 7 | |
| CNS | 6 | |
| Nonhematological toxicity (n = 103) Grades 3 and 4 (WHO) | ||
| Hepatic | 5 (5) | |
| Pulmonary | 17 (16) | |
| Renal | 1 (1) | |
| Cardiac | 6 (6) | |
| Neurological | 2 (2) | |
| Dermatological | 2 (2) | |
| Diarrhea | 3 (3) | |
| Oral | 15 (14) | |
| ATRA syndrome | ||
| Definitely present | 7 (6) | |
| Indeterminate | 25 (20) | |
| Definitely absent | 91 (74) |
Supportive care and hospitalization.
The median hospital stay was 36 days (range, 5 to 70). During this period, the median time on antibiotics was 27 days (range, 5 to 67). Median red blood cell (RBC) and platelet concentrates transfused per patient were 10 U (range, 2 to 49) and 36 U (range, 3 to 138), respectively. The median time to attain 1 × 109 PMN/L and a platelet count greater than 50 × 109/L was 24 days (range, 5 to 57) and 19 days (range, 6 to 45), respectively.
Consolidation Therapy
Molecular response.
Of the 109 patients who achieved HCR, 92 have at present completed the 3 consolidation courses and 17 are still on treatment. PML/RARα RT-PCR tests were performed in 88 cases at the end of consolidation. Of these, 82 (93%) tested PCR-negative and 6 (7%) PCR-positive. All of the 40 patients found to be PCR-negative after induction and who were tested after consolidation remained in molecular remission. Of 40 patients PCR-positive after induction and who were tested after consolidation, 35 converted to PCR-negative, and 5 remained PCR-positive. Of 8 patients who were analyzed at the end of consolidation, but not after induction, 7 tested PCR-negative and 1 positive. Three of the 6 patients PCR-positive after consolidation converted to PCR-negative during maintenance. Of the remaining 3, 1 received high-dose chemotherapy and autologous stem cell transplantation (ASCT), 1 is on a waiting list for ASCT, and the confirmatory PCR is pending in the third case.
Toxicity.
All patients were able complete the 3 courses of chemotherapy as scheduled, and no deaths occurred in HCR. The incidence and type of toxicity associated with each consolidation course are reported in Table 3. A higher rate of severe and prolonged neutropenia and thrombocytopenia was observed with course no. 2, although only 63 patients had neutropenic fever during this cycle. The median number of transfused platelet and RBC units was 9 (range, 1 to 82) and 4 (range, 1 to 19), respectively.
Hematological Toxicity During Consolidation
| . | Consolidation No. 1 N = 99 . | Consolidation No. 2 N = 95 . | Consolidation No. 3 N = 92 . |
|---|---|---|---|
| Days to PMN >1 × 109/L | |||
| No neutropenia | 71 (72) | 6 (6) | 77 (83) |
| ≤15 d | 10 (10) | 5 (5) | 7 (8) |
| >15 d | 18 (18) | 84 (89) | 8 (9) |
| Days to platelets >50 × 109/L | |||
| No thrombocytopenia | 81 (82) | 18 (19) | 82 (89) |
| ≤15 d | 10 (10) | 9 (10) | 5 (6) |
| >15 d | 8 (8) | 67 (71) | 5 (5) |
| Episodes of fever | 14 (14) | 63 (66) | 7 (8) |
| . | Consolidation No. 1 N = 99 . | Consolidation No. 2 N = 95 . | Consolidation No. 3 N = 92 . |
|---|---|---|---|
| Days to PMN >1 × 109/L | |||
| No neutropenia | 71 (72) | 6 (6) | 77 (83) |
| ≤15 d | 10 (10) | 5 (5) | 7 (8) |
| >15 d | 18 (18) | 84 (89) | 8 (9) |
| Days to platelets >50 × 109/L | |||
| No thrombocytopenia | 81 (82) | 18 (19) | 82 (89) |
| ≤15 d | 10 (10) | 9 (10) | 5 (6) |
| >15 d | 8 (8) | 67 (71) | 5 (5) |
| Episodes of fever | 14 (14) | 63 (66) | 7 (8) |
Maintenance Therapy
All 92 patients who received full consolidation therapy proceeded to maintenance therapy as scheduled. Cytopenias, especially neutropenia, and slight liver function test abnormalities were commonly observed in this phase, often requiring dose reduction or temporary discontinuation of chemotherapy. In only 1 patient, who promptly developed severe acute pancreatitis associated with mercaptopurine treatment, was this drug definitely withdrawn. No deaths in CR occurred during maintenance.
Outcome.
As of February 1999, 5 patients had clinical relapse at 4 to 15 months from the achievement of HCR. Conversion to PCR-positive had been documented in 2 of them 1 and 6 months before clinical relapse, respectively. Two clinical relapses primarily occurred in the central nervous system (CNS). Of these 2 patients, 1 is alive and well, while the other 1 died of disease progression. The remaining 3 patients relapsed in the bone marrow and were treated with a simultaneous combination of ATRA, mitoxantrone, and cytarabine; 2 achieved second HCR, and 1 died during marrow aplasia. Both patients who attained second HCR underwent ASCT and died due to transplant-related toxicity and subsequent relapse, respectively.
Three additional patients relapsed at the molecular level at +5, +7, and +12 months postconsolidation. They were given intensification with ATRA plus EMA (etoposide, mitoxantrone, and cytarabine) before developing clinically overt disease. Two of these patients died early due to fungal infection, while the remaining 1 is alive and PCR-negative.
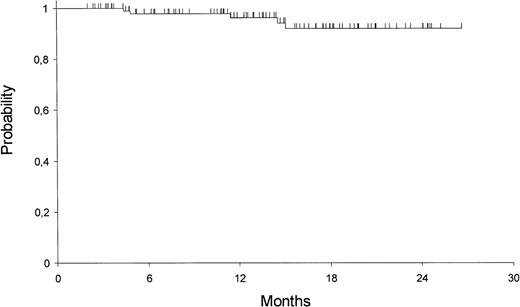
The 2-year Kaplan-Meier estimates of OS and EFS are 82% ± 4% and 79% ± 4%, respectively. For patients who achieved HCR, the 2-year DFS is 92% ± 3% (Fig 1). Univariate analysis showed that only presenting WBC count had a significant prognostic influence on both DFS and EFS (Fig 2), while age had an influence only on the EFS (Fig 3).
Kaplan-Meier product-limit estimate of overall EFS according to presenting WBC count.
Kaplan-Meier product-limit estimate of overall EFS according to presenting WBC count.
DISCUSSION
This study shows that use of an anthracycline-based regimen omitting cytarabine and etoposide for the treatment of newly diagnosed APL results in high molecular remission, DFS, and OS rates, as well as in substantially lower therapy-related toxicity.
Based on the established notion that response to ATRA correlates with PML/RARα presence in leukemic cells, we decided to enroll in this study only patients with genetically proven APL. For this purpose, the systematic use of RT-PCR in conjunction with conventional cytogenetics provided here the additional advantage of defining targets for the sensitive assessment of response at the molecular level. In line with the results of other multicenter trials in which genetic diagnosis was routinely performed,3,5 6 10% of patients initially registered in our study based on morphologic criteria subsequently tested PML/RARα-negative and were excluded from the trial.
Our protocol was designed taking into consideration the main advances derived from some recent clinical studies, especially those reported by the European APL group9 and the Italian GIMEMA group.3 These have clearly shown that the best treatment results are obtained with simultaneous ATRA treatment and chemotherapy. In addition, using idarubicin alone in combination with ATRA, the GIMEMA group reported3 impressive induction results, which are comparable to those obtained by others using induction regimens containing cytarabine.4,6,8,9 Leaving unmodified the first part of the AIDA protocol, our induction results were, as expected, similar to those reported by Mandelli et al,3 and are consequently comparable to the above-mentioned studies. In fact, all of these studies, which reported CR rates around 90%, showed that their results overlap when the confidence intervals are taken into account. Minor differences in response rates might also be explained by subtle differences in patient characteristics with potential prognostic impact (eg, morphological and/or molecular subtype, age, WBC count, etc).
The current consensus on treatment requirements of APL has led us to consider tailored therapeutic approaches differing from those used for the rest of the AMLs as regards induction and maintenance only. With very few exceptions,16 the consolidation phase has never been substantially different from that commonly used for other AMLs. In fact, even in the most recent trials,3,6-9 postremission treatment included cytarabine, usually combined with anthracyclines, and other drugs such as etoposide. All of these studies reported important toxicity during consolidation, leading to a variable, but sizable, number of patients to receive less treatment than scheduled, as well as to a death rate in remission of up to 10%, which significantly increased in elderly patients.3,8,9 Our approach to consolidation resulted in a rate of molecular remission, which is remarkably high, and is similar to that reported by the GIMEMA3 and the updated results of the Medical Research Council (MRC) trial,28 but which is associated with significantly reduced toxicity and related morbidity and mortality. In fact, all patients in the present study were able to complete the 3 consolidation courses and no deaths in remission were recorded. Combined with the preliminary findings reported by Estey et al,16 who also omitted cytarabine from postremission therapy, our study strengthens the view that this drug has little therapeutic effect in APL and suggests that patients might be spared the risks associated with intensive consolidation containing cytarabine. Although in light of the short median follow-up, we cannot establish at present the effectiveness of our treatment approach in the long-term, the molecular response rate after consolidation and the actuarial 2-year DFS rate are comparable with the best results reported thus far. Nevertheless, the low short-term relapse rate recorded in our series might also be partially due to the administration of ATRA during maintenance, which has proven to confer better DFS in two large randomized studies.7 9
Despite considerable improvement in diagnosis and management of APL, a proportion of patients who receive state-of-the-art treatment will eventually die of early complications during induction or of disease recurrence. Thus, two minor, but sizable, subsets should be considered for individualized therapeutic approaches. Identification of patients at highest risk of early death would allow prompt reinforcement of adequate supportive care and, perhaps, less intensive induction treatment. Conversely, patients at highest risk of relapse might benefit from distinct postremission intensification, such as increased anthracycline dosage and/or hematopoietic stem cell transplantation. The adjusted definition of these two risk groups is one of the major challenges for future clinical investigations in APL.
ACKNOWLEDGMENT
The authors thank Francesco Lo Coco and Guillermo F. Sanz for helpful discussion and critical reading of the manuscript. We are also grateful to Luis Benlloch for data collection and management.
The following clinical departments and personnel participated in this trial (number of patients included in parentheses): Hospital Universitario La Fe, Valencia, Sanz MA, Martı́n G (13); Hospital Central de Asturias, Oviedo, Rayón C (8); Hospital Clı́nico San Carlos, Madrid, Dı́az-Mediavilla J (6); Hospital Clı́nico Universitario, Valencia, Terol MJ (6); Hospital Insular de Las Palmas, Las Palmas, González JD (6); Hospital Clinic, Barcelona, Esteve J (6); Hospital General, Alicante, Rivas C (5); Hospital U. Germans Trias i Pujol, Badalona, Ribera JM (5); Complexo Hospitalario Xeral-Calde, Lugo, Arias J (4); Hospital Universitario, Salamanca, González M (4); Hospital de Cruces, Baracaldo, Alvarez MC (4); Complejo Hospitalario, León, Ramos F (4); Hospital Juan Canalejo, La Coruña, Debén G (4); Hospitales Ntra Sra del Pino/Sabinal, Las Palmas, Mataix R (3); Hospital Reina Sofia, Córdoba, Tabares S (3); Hospital Clı́nico Universitario, Valladolid, Fernández F (3); Hospital Universitario Vall D’Hebron, Barcelona, Bueno J (3); Hospital Son Dureta, Palma de Mallorca, Novo A (3); Hospital Xeral de Galicia, Santiago de Compostela, Pérez M (2); Hospital Ramón y Cajal, Madrid, Odriozola J (2); Hospital do Meixoeiro, Vigo, Loureiro C (2); Hospital Severo Ochoa, Leganés, Sánchez P (2); Hospital Dr. Peset, Valencia, Sayas MJ (2); Hospital 12 de Octubre, Madrid, De la Serna J (2); Hospital General de Murcia, Murcia, Moraleda JM (2); H. Universitario Virgen de la Victoria, Málaga, Pérez I (2); H.U. Puerta del Mar, Cádiz, Capote FJ (2); Hospital San Pedro de Alcántara, Cáceres, Bergua JM (2); Hospital Materno-Infantil de Las Palmas, Las Palmas, Lodos JC (1); Basurtuko Ospitalea, Basurto, Beltrán de Heredia JM (1); Hospital Rio Hortega, Valladolid, Peñarrubia MJ (1); Hospital Clı́nico Universitario Lozano Blesa, Zaragoza, Palomera L (1); Hospital General Jerez de la Frontera, Jerez de la Frontera, León A (1); Hospital General, Albacete, Romero JR (1); Hospital Xeral Cı́es, Vitoria, Poderós C (1); Hospital Txagorritxu, Vitoria, Guinea JM (1); Hospital San Pau, Barcelona, Brunet S (1); Hospital General (Oncologı́a Pediátrica), Alicante, Esquembre C (1); Hospital Rio Carrión, Palencia, Ortega F (1); Hospital U. Marqués de Valdecilla, Santander, Conde E (1); H. Universitario La Fe (Hospital Infantil), Valencia, Castell V (1).
The following laboratories and personnel participated in this trial: Hospital Universitario La Fe, Valencia, Bolufer P, Barragán E; Hospital Universitario, Salamanca, González M, Chillón C; Hospital Clinic, Barcelona, Colomer D; Hospitales Ntra Sra del Pino/Sabinal, Las Palmas, Gómez T; Hospital Reina Sofia, Córdoba, Román J; Universidad de Navarra, Pamplona, Calasanz MJ; Hospital 12 de Octubre, Madrid, Bornstein R; Hospital Clı́nico San Carlos, Madrid, Villegas A; Hospital Clı́nico Universitario, Valencia, Marugán I; Hospital Ramón y Cajal, Madrid, Ferro C; Hospital do Meixoeiro, Vigo, Loureiro C; Hospital U. Marqués de Valdecilla, Santander, Richard C.
Supported in part by Grant No. 96/1734 from the Fondo de Investigación Sanitaria (FIS), Ministerio de Sanidad of Spain, and by Grant FIJC PETH-99 from the International José Carreras Leukemia Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Miguel A. Sanz, MD, Servicio de Hematologı́a, Hospital Universitario La Fe, Av. Campanar 21, 46009 Valencia, Spain; e-mail: msanz@uv.es.