Abstract
This study details warfarin use in a large pediatric population followed in a central anticoagulation clinic. A prospective, consecutive cohort of nonselected children were studied. Patients were divided into groups by age, target international normalized ratio (INR) range, disease, medications, and vitamin K supplemented enteral nutrition use. Groups were analyzed on multiple aspects of warfarin therapy using multivariate methods. A total of 319 patients received 352 warfarin courses representing 391 treatment years. Age independently influenced all aspects of therapy. When compared with all older children, the ≤1 year of age group required increased warfarin doses, longer overlap with heparin, longer time to achieve target INR ranges, more frequent INR testing and dose adjustments, and fewer INR values in the target range. Although significantly different than children ≤1 year, children 1 to 6 years of age showed the same findings when compared with 7- to 18-year-olds. Fontan patients required 25% decreased dosage as compared with other congenital heart disease patients. Children on corticosteroids had less INRs in the target range and children on phenobarbital/carbamazepine required increased maintenance dosages of warfarin. Also, patients receiving enteral nutrition required increased dosages of warfarin. Serious bleeding occurred in 2 children (0.5% per patient year). Recurrent thromboembolic events (TEs) occurred in 8 children. Two children had recurrences while receiving warfarin (1.3% per patient year). This study outlines the profound effect of age and relative complexity of clinical management of warfarin therapy in children.
THE RELATIVELY RECENT successes in tertiary care of critically ill children have increased the frequency of long-term secondary complications. Thromboembolic events (TEs) are among the most serious of these secondary complications. The increasing risk and frequency of TEs in children has resulted in an increasing use of warfarin for both primary and secondary prophylaxis. In contrast to the adult literature, there is relatively little information on warfarin use in children.1-8 The paucity of information reflects, in part, the relatively re- cent need to use warfarin in children and lack of central- ized anticoagulation clinics that care for large numbers of children.
A pediatric thromboembolism program, which included an outpatient anticoagulation clinic, was initiated at the Hospital for Sick Children (HSC), Toronto, Canada, in July 1991. The objectives of the program were 2-fold: first, to establish consistent treatment programs using the best available data and second to develop a database of warfarin treatment in a large consecutive cohort of unselected children. Previously, we have reported on the first 115 children in this cohort.5 Because of the limited number of patients, the previous analysis was restricted, particularly in relation to the comparison of effects of other variables affecting warfarin therapy. The following study summarizes the use of warfarin in 319 children. This study expands the previous report, as large numbers of patients increased the power of the study allowing for a more in-depth statistical analysis, which includes the effect of age, target international normalized ratios (INRs), underlying disease, drug use, and diet on the clinical course of multiple parameters of warfarin therapy. In addition, the effect of a relatively uncommon clinical indication (Fontan procedure) on warfarin dosage requirements was assessed. This study details the relative complexity of warfarin management in children.
MATERIALS AND METHODS
Patient Population
Data were obtained on consecutive children referred to the pediatric thromboembolism program at the HSC between July 1, 1991 and June 30, 1996. All children had underlying disorders and were usually referred to the anticoagulation service as inpatients either through the hematology consult service or directly through the thromboembolism program. The cohort represents all children treated with warfarin at HSC. All children were followed through an outpatient anticoagulation service after discharge.
Data Collection
All data were collected prospectively. Each patient was evaluated by both a nurse practitioner and physician at the initiation and completion of therapy and a minimum of once yearly. A standardized history, physical examination, complete blood count, and prothrombin time (PT)/INR were measured at each visit. The following information was recorded: age, weight, height, thrombotic history, underlying disorder, secondary disorders, presence of central venous lines (CVLs), diet, and medications. A detailed standardized anticoagulation record was maintained for each patient and contained target INRs, dose to achieve and maintain target INRs, day of heparin therapy that warfarin was initiated, length of overlap of heparin and warfarin therapy, amount of time taken to achieve target INR range, all INR tests, and dose changes. Bleeding and TEs were recorded. All measurements were recorded in children using a whole-blood monitor to measure INRs at home.
Monitoring and Administration of Oral Anticoagulation Therapy
Monitoring.
Warfarin therapy was administered and monitored in all children using a modified standardized nomogram.9 Appropriate changes in warfarin doses were performed by the nurse coordinator with physician backup. Inpatient and outpatient laboratories measured PTs and reported both PT and corresponding INR values. A whole-blood monitor (Ciba Corning Diagnostic 512 Coagulation Monitor [CCD Monitor]) was used for 28 children.
Target INR range and duration.
The target INR range and duration of therapy with warfarin depended on the underlying disorder. Three target INR ranges were used: 1.4 to 1.8, 2.0 to 3.0, and 2.5 to 3.5. These ranges were based on international recommendations for children.9 Children received an initial loading warfarin dose of 0.2 mg/kg except in children who had undergone Fontan procedure or had liver dysfunction who received 0.1 mg/kg. Duration of therapy varied with the exception of children with mechanical heart valves (MV) who were all treated indefinitely with a target INR range of 2.5 to 3.5. Children with a first TE and no ongoing risk factor were usually treated for 3 months with a target INR range of 2.0 to 3.0. Children with a first TE and ongoing risk factors were usually treated for 3 months with a target INR range of 2.0 to 3.0 followed by a target INR range of 1.4 to 1.8 until the acquired risk factor was no longer present.
A target INR range of 1.4 to 1.8 was used for 52 children, of whom 21 had received a previous course of warfarin therapy with a target INR range of 2.0 to 3.0. The clinical reasons for selecting a target INR range of 1.4 to 1.8 were: (1) CVLs for dialysis during renal failure, total parenteral nutrition, and plasmapheresis (n = 20); (2) systemic lupus erythematosus (SLE) (n = 9); (3) congenital heart disease (CHD) (n = 6); (4) congenital antithrombin deficiency (n = 3); and (5) other indications, ie, prolonged immobilization (n = 14). A target range INR range of 2.0 to 3.0 was used for 263 children. The clinical reasons for selecting a target INR range of 2.0 to 3.0 were: (1) CHD (n = 115); (2) patients who had undergone a Fontan procedure (n = 50); (3) CVLs for dialysis during renal failure, total parenteral nutrition, and plasmapheresis (n = 21); (4) treatment of TEs in the central nervous system (CNS) (n = 19); (5) malignancy (n = 18); (6) treatment of TEs (n = 12); (7) infectious disease (n = 11); (8) SLE (n = 6); (9) idiopathic thrombosis (n = 3); and (10) other indications, ie, sickle cell disease (n = 8). A target INR range of 2.5 to 3.5 was used exclusively for 37 children with MV.
Comparison of Factors Potentially Influencing Warfarin Therapy
Age (4 groups).
The age groups were decided in advance of the analysis as clinically appropriate groupings (1) ≤1 year; (2) >1 and <6 years; (3) ≥6 and <13 years; and (4) ≥13 and ≤18 years.
Target INR (3 groups).
There were three target INR ranges: (1) 1.4 to 1.8; (2) 2.0 to 3.0; and (3) 2.5 to 3.5.
Underlying diseases (3 groups).
Three disease categories were considered: (1) children with CHD; (2) children without CHD; and (3) children who had undergone a Fontan procedure. An additional analysis of the maintenance warfarin dosage was compared between Fontan children and all other CHD children with comparable INR values.
Medication use (4 groups).
We used 4 medication usage groups based on any use of the following: (1) corticosteroids; (2) phenobarbital/carbamazepine; (3) aspirin; and (4) antibiotics.
Diet (2 groups).
Two groups were considered: (1) children using enteral nutrition and (2) children not using enteral nutrition. For the purposes of this report, the term enteral nutrition refers to both vitamin K supplemented formula and vitamin K supplemented tube feedings for infants and older children. A majority of children on enteral nutrition were on formula.
Data on children’s mean age in years, length of warfarin treatment in months, and their actual mean INR values are summarized and reported according to the potentially influencing factors listed above.
Outcomes Assessing Management of Warfarin Therapy
Treatment outcome variables.
Ten outcome variables were considered in relation to the factors listed above: (1) dose to achieve target INR; (2) dose to maintain target INR; (3) day of heparin therapy that warfarin was initiated; (4) overlap of warfarin therapy with heparin therapy; (5) amount of time taken to achieve target INR; (6) mean number of tests per month; (7) mean number of dose changes per month; (8) INR measurements within target INR range; (9) INR measurements below the target INR range; and (10) INR measurements greater than the target INR range.
Comparison of warfarin dose requirements of children who had undergone a Fontan procedure.
The maintenance dose was compared in children who had undergone a Fontan procedure as compared with all other CHD patients with comparable actual INRs. In addition, in the multivariate analysis, Fontan patients were compared with all other CHD and non-CHD for multiple parameters in all INR ranges.
Comparison of warfarin dose requirements when calculated by body weight and body surface area.
Warfarin dose requirements were calculated by both body weight (mg/kg) and by body surface area (mg/m2) in a subset of children.
Adverse Outcomes
Bleeding and TEs were considered serious adverse outcomes. Bleeding was divided into major and minor bleeding. Major bleeding was defined as clinically overt bleeding associated with a decrease of >20 g/L in hemoglobin in <24 hours and/or need for transfusion of red blood cells, or any CNS or retroperitoneal bleed. Minor bleeding was defined as all other bleeding events not meeting the criteria for a major bleed. Clinically suspected TEs were confirmed by objective radiographic tests.
Statistical Analysis
Each continuous measure is summarized by its arithmetic mean and standard deviation (SD) and reported in the tables as a mean ± 1 SD. For the sake of simplicity, interpretability, and brevity, no data transformations were used in this analysis to adjust for skewness or control variances. The multivariate nature of the relationship between each outcome measure and the 5 factors (age, target INR range, underlying disorders, medication use, and diet) was assessed using multiple regression methodology based on the generalized linear model formulation of the Minitab (release 12.21; Minitab Inc, State College, PA) general linear model (GLM) procedure. Although a full cross-classification of the factors produced 1,152 subgroups, there were children in only 81 of these cells. Again, due to the sparseness of the cross-classifications, only main effects and first-order interactions of the 5 factors (wherever possible) were considered. Because this study is essentially observational in nature and the analysis exploratory, P values <.05 were considered to be suggestive of a statistical relationship and reported in this analysis. For the factors with more than 2 levels, a posteriori pairwise comparisons between groups were conducted using Tukey’s method. For comparison of dose calculations based on body weight versus body surface area, a coefficient of variation (CV) was calculated.
RESULTS
Patient Population
Three hundred and nineteen consecutive children between the ages of 1 month and 18 years received 352 consecutive courses of warfarin between July 1, 1991 and June 30, 1996, for a total of 391 patient years. Thirty children received more than 1 course of warfarin. Three children received 3 courses and 27 children received 2 courses. There were 180 males (56%) and 139 females (44%). All children achieved their target INR range with 79% achieving it in less than 7 days. The children taking longer than 1 week to achieve their target INR were younger (4.1 ± 5.0 years v 8.1 ± 5.9 years) and a higher proportion were receiving enteral nutrition (41% v 13%) when compared with children who achieved their target INR in less than 1 week. Two hundred eight children (59%) were treated for primary prophylaxis for TEs and 144 children (41%) were receiving warfarin as secondary prophylaxis for the prevention of recurrent TEs. The latter group presented with TEs in the lower venous system (n = 48), upper venous system (n = 30), CNS (n = 27), heart (n = 10), pulmonary embolism (PE) (n = 11), and in multiple sites (n = 18). Seven of 117 children who had CVLs were diagnosed with thrombosis obstructing the vessel on the intravascular side. The mean number of days for children to achieve an INR in the target range when receiving warfarin for primary prophylaxis versus secondary prophylaxis was similar (6.0 ± 8.2 v 5.2 ± 5.1, P = .34).
CHD was the primary underlying disorder comprising 114 (36%) children. Fifty (16%) children underwent a Fontan procedure. Thirty-seven (12%) children had an MV in place. Other major disorders included renal disease (n = 23 or 7%), TEs in the CNS (n = 20 or 6%), malignancy (n = 18 or 6%), SLE (n = 11 or 3.5%), infectious diseases (n = 10 or 3%), total parental nutrition for gastrointestinal diseases (n = 8 or 2.5%), congenital prothrombotic disorders (n = 5 or 1.5%), idiopathic thrombosis (n = 3 or 1%), and others (n = 13 or 4%).
Group Characteristics
The numbers of children in each of the groups are displayed in Table 1. Although frequencies across the 4a priori determined age groups are unbalanced, sufficient numbers existed to perform the required multivariate analysis. There were no statistically significant differences in the mean ages and length of treatment. A statistically significant difference in the actual INR was seen between the 3 target INR groups with the target INR 1.4 to 1.8 group having lower INRs. In addition, the target INR range 2.0 to 3.0 group had a statistically significantly lower actual INR than the target INR range 2.5 to 3.5 group. The clinical significance of the differences in the means of the 2 groups was negligible (2.35 and 2.59, respectively).
Demographic Data of 319 Children Receiving 352 Courses of Warfarin
| Predictor . | No. of Courses of Warfarin . | Mean Age in Years . | Length of Treatment in Months . | Actual INRs . |
|---|---|---|---|---|
| INR | ||||
| 1.4-1.8 | 52 | 10.8 ± 6.3 | 20 ± 17.5 | 1.58 ± 0.10 |
| 2.0-3.0 | 263 | 7.1 ± 5.9 | 9 ± 12.5 | 2.35 ± 0.17 |
| 2.5-3.5 | 37 | 12.0 ± 5.8 | 36 ± 22.4 | 2.59 ± 0.20 |
| P value | NS | NS | <.0001 | |
| Age in years | ||||
| ≤1 | 43 | 0.7 ± 0.3 | 5 ± 7.5 | 2.3 ± 0.3 |
| >1 < 6 | 123 | 3.0 ± 1.0 | 8 ± 9.0 | 2.3 ± 0.2 |
| ≥6 < 13 | 74 | 9.3 ± 1.9 | 15 ± 17.8 | 2.2 ± 0.3 |
| ≥13 ≤ 18 | 112 | 16.0 ± 1.6 | 22 ± 21.2 | 2.2 ± 0.4 |
| P value | NS | NS | NS | |
| Disease* | ||||
| CHD (n = 114) | 121 | 6.0 ± 5.6 | 10.8 ± 13.8 | 2.3 ± 0.2 |
| Non-CHD (n = 155) | 181 | 10.8 ± 6.0 | 17.7 ± 19.7 | 2.2 ± 0.4 |
| Fontan (n = 50) | 50 | 4.0 ± 3.0 | 5.2 ± 4.1 | 2.3 ± 0.1 |
| P value | NS | NS | NS | |
| Medication | ||||
| Corticosteroids | ||||
| Yes | 38 | 11.0 ± 5.9 | 15.9 ± 16.9 | 2.1 ± 0.4 |
| No | 314 | 7.8 ± 6.1 | 13.3 ± 17.0 | 2.3 ± 0.3 |
| P value | NS | NS | .05 | |
| Phenobarbital/ Carbamazepine | ||||
| Yes | 10 | 10.0 ± 7.7 | 27.4 ± 23.7 | 2.2 ± 0.4 |
| No | 342 | 8.1 ± 6.1 | 13.1 ± 16.6 | 2.3 ± 0.3 |
| P value | NS | NS | NS | |
| Diet | ||||
| Enteral nutrition | ||||
| Yes | 82 | 2.5 ± 2.8 | 8.3 ± 10.0 | 2.3 ± 0.3 |
| No | 270 | 10.0 ± 5.9 | 15.2 ± 18.3 | 2.2 ± 0.3 |
| P value | NS | NS | NS |
| Predictor . | No. of Courses of Warfarin . | Mean Age in Years . | Length of Treatment in Months . | Actual INRs . |
|---|---|---|---|---|
| INR | ||||
| 1.4-1.8 | 52 | 10.8 ± 6.3 | 20 ± 17.5 | 1.58 ± 0.10 |
| 2.0-3.0 | 263 | 7.1 ± 5.9 | 9 ± 12.5 | 2.35 ± 0.17 |
| 2.5-3.5 | 37 | 12.0 ± 5.8 | 36 ± 22.4 | 2.59 ± 0.20 |
| P value | NS | NS | <.0001 | |
| Age in years | ||||
| ≤1 | 43 | 0.7 ± 0.3 | 5 ± 7.5 | 2.3 ± 0.3 |
| >1 < 6 | 123 | 3.0 ± 1.0 | 8 ± 9.0 | 2.3 ± 0.2 |
| ≥6 < 13 | 74 | 9.3 ± 1.9 | 15 ± 17.8 | 2.2 ± 0.3 |
| ≥13 ≤ 18 | 112 | 16.0 ± 1.6 | 22 ± 21.2 | 2.2 ± 0.4 |
| P value | NS | NS | NS | |
| Disease* | ||||
| CHD (n = 114) | 121 | 6.0 ± 5.6 | 10.8 ± 13.8 | 2.3 ± 0.2 |
| Non-CHD (n = 155) | 181 | 10.8 ± 6.0 | 17.7 ± 19.7 | 2.2 ± 0.4 |
| Fontan (n = 50) | 50 | 4.0 ± 3.0 | 5.2 ± 4.1 | 2.3 ± 0.1 |
| P value | NS | NS | NS | |
| Medication | ||||
| Corticosteroids | ||||
| Yes | 38 | 11.0 ± 5.9 | 15.9 ± 16.9 | 2.1 ± 0.4 |
| No | 314 | 7.8 ± 6.1 | 13.3 ± 17.0 | 2.3 ± 0.3 |
| P value | NS | NS | .05 | |
| Phenobarbital/ Carbamazepine | ||||
| Yes | 10 | 10.0 ± 7.7 | 27.4 ± 23.7 | 2.2 ± 0.4 |
| No | 342 | 8.1 ± 6.1 | 13.1 ± 16.6 | 2.3 ± 0.3 |
| P value | NS | NS | NS | |
| Diet | ||||
| Enteral nutrition | ||||
| Yes | 82 | 2.5 ± 2.8 | 8.3 ± 10.0 | 2.3 ± 0.3 |
| No | 270 | 10.0 ± 5.9 | 15.2 ± 18.3 | 2.2 ± 0.3 |
| P value | NS | NS | NS |
Data are reported as mean ± 1 SD.
Abbreviations: CHD, congenital heart disease; n, number; NS, not significant.
n represents number of children. Thirty children received more than 1 course of warfarin therapy.
Treatment Outcome Variables
Table 2 provides a descriptive summary of the outcome measures subdivided by the factors and reports P values for significant effects based on the regression modeling. Because no predictive ability was detected for the use of antibiotics and aspirin for any of the outcomes, they were not included. The results of the pairwise comparisons do not appear in the table, but are discussed below.
Effect of Predictors on Treatment With Warfarin
| Predictor . | Dose to Achieve Target INR (mg/kg) . | Dose to Maintain Target INR (mg/kg) . | Day of Heparin Therapy That Warfarin Was Initiated . | Overlap of Warfarin Therapy With Heparin Therapy (d) . | Amount of Time Taken to Achieve Target INR (d) . | Mean No. of Tests/ mo . | Mean No. of Dose Changes/ mo . | INR Measurements Within Target Range (%) . | INR Measure- ments < Target (%) . | INR Measure- ments > Target (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Age in years | ||||||||||
| ≤1 | 0.34 ± 0.16 | 0.33 ± 0.20 | 7.8 ± 6.5 | 6.3 ± 4.9 | 9.7 ± 8.1 | 8.1 ± 5.5 | 3.6 ± 2.0 | 37 ± 16 | 46 ± 17 | 16 ± 12 |
| >1 < 6 | 0.19 ± 0.11 | 0.15 ± 0.10 | 6.7 ± 8.2 | 4.7 ± 4.2 | 6.1 ± 9.0 | 5.7 ± 3.4 | 2.4 ± 1.6 | 45 ± 18 | 40 ± 18 | 15 ± 12 |
| ≥6 < 13 | 0.15 ± 0.07 | 0.13 ± 0.06 | 4.8 ± 3.7 | 4.4 ± 4.1 | 4.7 ± 5.6 | 4.6 ± 4.0 | 1.8 ± 2.2 | 54 ± 18 | 34 ± 18 | 13 ± 11 |
| ≥13 ≤ 18 | 0.14 ± 0.05 | 0.09 ± 0.05 | 5.0 ± 3.0 | 3.6 ± 1.8 | 3.8 ± 2.8 | 3.8 ± 3.0 | 1.5 ± 1.7 | 53 ± 19 | 32 ± 16 | 15 ± 11 |
| P value | <.0001 | <.0001 | .03 | .001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | NS |
| INR | ||||||||||
| 1.4-1.8 | 0.12 ± 0.07 | 0.11 ± 0.08 | 3.7 ± 1.9 | 2.7 ± 2.9 | 5.5 ± 5.4 | 2.7 ± 2.5 | 1.0 ± 1.3 | 49 ± 21 | 35 ± 18 | 16 ± 13 |
| 2.0-3.0 | 0.20 ± 0.12 | 0.16 ± 0.13 | 6.4 ± 6.1 | 4.6 ± 3.7 | 6.0 ± 7.6 | 5.9 ± 4.0 | 2.5 ± 1.8 | 47 ± 18 | 38 ± 17 | 15 ± 12 |
| 2.5-3.5 | 0.17 ± 0.09 | 0.14 ± 0.09 | 4.0 ± 6.0 | 5.2 ± 6.0 | 4.2 ± 4.2 | 2.7 ± 1.7 | 1.4 ± 2.2 | 61 ± 20 | 30 ± 20 | 9 ± 6 |
| P value | .02 | NS | NS | NS | NS | <.0001 | <.0001 | .011 | NS | .02 |
| Disease | ||||||||||
| CHD | 0.20 ± 0.13 | 0.17 ± 0.14 | 5.2 ± 4.5 | 4.2 ± 4.4 | 6.9 ± 8.4 | 5.4 ± 4.4 | 2.2 ± 1.8 | 46 ± 17 | 40 ± 17 | 14 ± 11 |
| Non-CHD | 0.18 ± 0.12 | 0.15 ± 0.14 | 6.1 ± 4.8 | 5.0 ± 3.6 | 4.6 ± 4.0 | 4.8 ± 3.7 | 2.0 ± 2.0 | 50 ± 21 | 35 ± 19 | 15 ± 12 |
| Fontan* | 0.18 ± 0.08 | 0.12 ± 0.07 | 8.0 ± 12.1 | 4.2 ± 4.0 | 5.9 ± 10.0 | 6.1 ± 3.5 | 2.6 ± 1.5 | 48 ± 16 | 36 ± 16 | 15 ± 10 |
| P value | .02 | .003 | NS | .03 | NS | .002 | .002 | NS | NS | NS |
| Medication | ||||||||||
| Corticosteroids | ||||||||||
| Yes | 0.18 ± 0.08 | 0.11 ± 0.08 | 4.6 ± 2.4 | 3.7 ± 2.5 | 3.7 ± 3.1 | 5.2 ± 3.6 | 2.3 ± 2.0 | 43 ± 21 | 36 ± 17 | 21 ± 11 |
| No | 0.19 ± 0.12 | 0.16 ± 0.13 | 6.3 ± 6.4 | 4.7 ± 4.0 | 5.9 ± 7.5 | 5.2 ± 4.0 | 2.1 ± 1.9 | 49 ± 19 | 37 ± 18 | 14 ± 11 |
| P value | NS | NS | NS | NS | NS | NS | NS | .02 | NS | .002 |
| Phenobarbital/ Carbamazepine | ||||||||||
| Yes | 0.24 ± 0.15 | 0.24 ± 0.17 | 7.7 ± 4.0 | 6.3 ± 4.5 | 6.6 ± 4.5 | 4.5 ± 3.5 | 2.0 ± 2.3 | 55 ± 22 | 32 ± 24 | 13 ± 8 |
| No | 0.19 ± 0.12 | 0.15 ± 0.13 | 6.0 ± 6.1 | 4.6 ± 3.9 | 5.7 ± 7.3 | 5.2 ± 4.0 | 2.2 ± 1.9 | 48 ± 19 | 37 ± 17 | 15 ± 12 |
| P value | NS | .006 | NS | NS | NS | NS | NS | NS | NS | NS |
| Diet | ||||||||||
| Enteral nutrition | ||||||||||
| Yes | 0.28 ± 0.16 | 0.26 ± 0.18 | 8.6 ± 8.9 | 6.4 ± 4.9 | 8.0 ± 10.0 | 6.8 ± 4.7 | 2.9 ± 1.8 | 41 ± 15 | 43 ± 15 | 16 ± 12 |
| No | 0.16 ± 0.07 | 0.11 ± 0.07 | 4.9 ± 3.7 | 3.9 ± 3.2 | 5.0 ± 5.7 | 4.7 ± 3.6 | 1.9 ± 1.9 | 51 ± 20 | 35 ± 18 | 14 ± 11 |
| P value | .005 | <.0001 | <.0001 | <.0001 | NS | NS | NS | NS | NS | NS |
| Predictor . | Dose to Achieve Target INR (mg/kg) . | Dose to Maintain Target INR (mg/kg) . | Day of Heparin Therapy That Warfarin Was Initiated . | Overlap of Warfarin Therapy With Heparin Therapy (d) . | Amount of Time Taken to Achieve Target INR (d) . | Mean No. of Tests/ mo . | Mean No. of Dose Changes/ mo . | INR Measurements Within Target Range (%) . | INR Measure- ments < Target (%) . | INR Measure- ments > Target (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Age in years | ||||||||||
| ≤1 | 0.34 ± 0.16 | 0.33 ± 0.20 | 7.8 ± 6.5 | 6.3 ± 4.9 | 9.7 ± 8.1 | 8.1 ± 5.5 | 3.6 ± 2.0 | 37 ± 16 | 46 ± 17 | 16 ± 12 |
| >1 < 6 | 0.19 ± 0.11 | 0.15 ± 0.10 | 6.7 ± 8.2 | 4.7 ± 4.2 | 6.1 ± 9.0 | 5.7 ± 3.4 | 2.4 ± 1.6 | 45 ± 18 | 40 ± 18 | 15 ± 12 |
| ≥6 < 13 | 0.15 ± 0.07 | 0.13 ± 0.06 | 4.8 ± 3.7 | 4.4 ± 4.1 | 4.7 ± 5.6 | 4.6 ± 4.0 | 1.8 ± 2.2 | 54 ± 18 | 34 ± 18 | 13 ± 11 |
| ≥13 ≤ 18 | 0.14 ± 0.05 | 0.09 ± 0.05 | 5.0 ± 3.0 | 3.6 ± 1.8 | 3.8 ± 2.8 | 3.8 ± 3.0 | 1.5 ± 1.7 | 53 ± 19 | 32 ± 16 | 15 ± 11 |
| P value | <.0001 | <.0001 | .03 | .001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | NS |
| INR | ||||||||||
| 1.4-1.8 | 0.12 ± 0.07 | 0.11 ± 0.08 | 3.7 ± 1.9 | 2.7 ± 2.9 | 5.5 ± 5.4 | 2.7 ± 2.5 | 1.0 ± 1.3 | 49 ± 21 | 35 ± 18 | 16 ± 13 |
| 2.0-3.0 | 0.20 ± 0.12 | 0.16 ± 0.13 | 6.4 ± 6.1 | 4.6 ± 3.7 | 6.0 ± 7.6 | 5.9 ± 4.0 | 2.5 ± 1.8 | 47 ± 18 | 38 ± 17 | 15 ± 12 |
| 2.5-3.5 | 0.17 ± 0.09 | 0.14 ± 0.09 | 4.0 ± 6.0 | 5.2 ± 6.0 | 4.2 ± 4.2 | 2.7 ± 1.7 | 1.4 ± 2.2 | 61 ± 20 | 30 ± 20 | 9 ± 6 |
| P value | .02 | NS | NS | NS | NS | <.0001 | <.0001 | .011 | NS | .02 |
| Disease | ||||||||||
| CHD | 0.20 ± 0.13 | 0.17 ± 0.14 | 5.2 ± 4.5 | 4.2 ± 4.4 | 6.9 ± 8.4 | 5.4 ± 4.4 | 2.2 ± 1.8 | 46 ± 17 | 40 ± 17 | 14 ± 11 |
| Non-CHD | 0.18 ± 0.12 | 0.15 ± 0.14 | 6.1 ± 4.8 | 5.0 ± 3.6 | 4.6 ± 4.0 | 4.8 ± 3.7 | 2.0 ± 2.0 | 50 ± 21 | 35 ± 19 | 15 ± 12 |
| Fontan* | 0.18 ± 0.08 | 0.12 ± 0.07 | 8.0 ± 12.1 | 4.2 ± 4.0 | 5.9 ± 10.0 | 6.1 ± 3.5 | 2.6 ± 1.5 | 48 ± 16 | 36 ± 16 | 15 ± 10 |
| P value | .02 | .003 | NS | .03 | NS | .002 | .002 | NS | NS | NS |
| Medication | ||||||||||
| Corticosteroids | ||||||||||
| Yes | 0.18 ± 0.08 | 0.11 ± 0.08 | 4.6 ± 2.4 | 3.7 ± 2.5 | 3.7 ± 3.1 | 5.2 ± 3.6 | 2.3 ± 2.0 | 43 ± 21 | 36 ± 17 | 21 ± 11 |
| No | 0.19 ± 0.12 | 0.16 ± 0.13 | 6.3 ± 6.4 | 4.7 ± 4.0 | 5.9 ± 7.5 | 5.2 ± 4.0 | 2.1 ± 1.9 | 49 ± 19 | 37 ± 18 | 14 ± 11 |
| P value | NS | NS | NS | NS | NS | NS | NS | .02 | NS | .002 |
| Phenobarbital/ Carbamazepine | ||||||||||
| Yes | 0.24 ± 0.15 | 0.24 ± 0.17 | 7.7 ± 4.0 | 6.3 ± 4.5 | 6.6 ± 4.5 | 4.5 ± 3.5 | 2.0 ± 2.3 | 55 ± 22 | 32 ± 24 | 13 ± 8 |
| No | 0.19 ± 0.12 | 0.15 ± 0.13 | 6.0 ± 6.1 | 4.6 ± 3.9 | 5.7 ± 7.3 | 5.2 ± 4.0 | 2.2 ± 1.9 | 48 ± 19 | 37 ± 17 | 15 ± 12 |
| P value | NS | .006 | NS | NS | NS | NS | NS | NS | NS | NS |
| Diet | ||||||||||
| Enteral nutrition | ||||||||||
| Yes | 0.28 ± 0.16 | 0.26 ± 0.18 | 8.6 ± 8.9 | 6.4 ± 4.9 | 8.0 ± 10.0 | 6.8 ± 4.7 | 2.9 ± 1.8 | 41 ± 15 | 43 ± 15 | 16 ± 12 |
| No | 0.16 ± 0.07 | 0.11 ± 0.07 | 4.9 ± 3.7 | 3.9 ± 3.2 | 5.0 ± 5.7 | 4.7 ± 3.6 | 1.9 ± 1.9 | 51 ± 20 | 35 ± 18 | 14 ± 11 |
| P value | .005 | <.0001 | <.0001 | <.0001 | NS | NS | NS | NS | NS | NS |
Data are reported as mean ± 1 SD. Each statistically significant relationship is highlighted.
Abbreviations: CHD, congenital heart disease; NS, not significant.
Fontan patients received an initial loading dose of warfarin of 0.1 mg/kg in contrast to the standard loading dose of 0.2 mg/kg.
Dose to achieve target INR.
To achieve the target INR range, children ≤1 year of age required an increased dosage of warfarin when compared with the 3 older age groups (P < .0001) (Table 2). In addition, children between >1 and <6 years of age also required an increased dosage when compared with the 2 older age groups, but required a decreased dosage than the children ≤1 year of age (P < .0001). The target INR 1.4 to 1.8 group received less warfarin than the INR 2.0 to 3.0 (P = .02), but not less than the target INR 2.5 to 3.5 group. Fontan children received less warfarin than the CHD (P = .02). Neither corticosteroids nor phenobarbital/carbamazepine influenced dose. Children receiving enteral nutrition required an increased dosage of warfarin when compared with children not on enteral nutrition (P = .005).
Dose to maintain target INR.
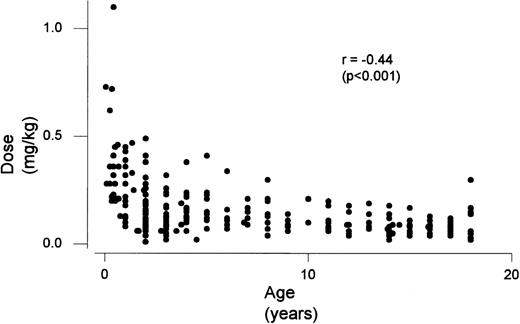
Children ≤1 year of age required an increased warfarin dosage to maintain a target INR when compared with the 3 older age groups (P < .0001) (Table 2). Children >1 and <6 years of age required an increased dosage when compared with the 2 older age groups, but less than children ≤1 year of age (P < .0001). In addition, children who were ≥6 and <13 required increased dosages when compared with the ≥13 ≤18 group (P = .04). There was a significant relationship between age and maintenance dose (P< .001) (Fig 1). Fontan children required less warfarin then the non-CHD patient group (P = .003). No effect was seen with corticosteroid use, however, children on phenobarbital/carbamazepine required an increased dosage of warfarin when compared with children not on phenobarbital/carbamazepine (P = .006). Children receiving enteral nutrition required an increased dosage of warfarin when compared with children not on enteral nutrition (P < .0001).
Day of heparin therapy that warfarin was initiated.
Children ≤1 year of age had a longer period of heparin therapy than the 2 oldest age groups (P = .001). INR group, disease group, or medication had no effect on this outcome. Children on enteral nutrition had a longer period of heparin therapy when compared with children not on enteral nutrition (P < .0001).
Overlap of warfarin therapy with heparin therapy.
Children ≤1 year of age had a longer period of overlap with heparin than the 2 oldest patient groups (P = .001). A statistically significant difference was achieved between CHD and non-CHD, however, there is no clinical significance in the difference in the mean number of days (4.2 and 5.0). There was no significant drug effect, but children on enteral nutrition required a significantly longer overlap of heparin and warfarin therapy than children not on enteral nutrition (P < .0001).
Amount of time taken to achieve target INR.
All children achieved stable INR values in their target INR range. Children ≤1 year of age required a longer period of time to achieve the therapeutic range when compared with the 3 older age groups (P < .0001). None of the other variables had an effect on the number of days taken to achieve target INR.
Mean number of INR tests per month.
Children ≤1 year of age had more tests per month than the 3 other age groups (P = .003). Children >1 and <6 years of age had more tests than the 2 oldest age groups (P = .003), but less than the children ≤1 year of age. The target INR group 2.0 to 3.0 had more INR tests per month than the target INR group 1.4 to 1.8 (P < .001) (Table 2). Children with CHD had more INR tests than non-CHD (P = .002).
Mean number of dose changes per month.
Children ≤1 year of age required more dose changes than the 3 older age groups (P < .0001) (Table 2). Children between >1 and <6 years of age required more dose changes than the oldest age group, but less than the children ≤1 year of age (P = .002). Children in the INR group 2.0 to 3.0 had significantly more dose changes than the other 2 INR groups (P < .0001). Although there was a statistically significant difference between CHD and non-CHD children (P = .002), there is no clinical significance to the difference in the means (2.2 and 2.0).
INR measurements within the target range.
Children ≤1 year and children >1 and <6 years of age had significantly fewer INR measurements within the target range when compared with the 2 oldest age groups (P = .0001 and P= .02, respectively). There were significantly more INR measurements within the target range in target INR group 2.5 to 3.5 compared with the target INR group 1.4 to 1.8 (P = .007). Children on corticosteroids had significantly fewer INR measurements within the target INR range (P = .02). No differences were seen among the disease and diet groups.
INR measurements below the target range.
Children ≤1 year of age and children >1 and <6 years of age had significantly more INRs below the target range compared with the 2 oldest age groups (P = .001 for both comparisons). No difference was observed over the target INR, disease, medication, and diet groups.
INR measurements above the target range.
The mean percentage of INRs greater than the target range was significantly less in the target INR group 2.5 to 3.5 than in the 2 other groups (P = .02) (Table 2). Children on corticosteroids had significantly more INRs greater than the target INR range (P = .002). No difference was seen over age, disease, and diet groups.
Effect of Fontan Procedure
Fontan children required significantly less warfarin (0.12 ± 0.06 mg/kg) compared with CHD children with matched current INRs (0.16 ± 0.13 mg/kg) (P = .0006). The difference in dosages was independent of age. Also, Fontan children required less warfarin than both CHD and non-CHD children independent of the target INR range (Table 2).
Comparison of Warfarin Dose Requirements When Calculated by Body Weight and Body Surface Area
We studied 121 children, 79 children above 1 m2 and 42 children below 1 m2 body surface area. These groups were compared based on the coefficient of variation (CV) of the maintenance dosages when the dose was calculated using both body weight and body surface area. Children <1 m2 body surface area were 5 ± 3 years of age and children >1 m2 body surface were 14 ± 3 years of age. For children with body surface areas less than 1 m2 the dosages were 3.9 ± 1.86 mg/m2 or 0.16 ± 0.08 mg/kg and the CVs were 47% and 50%. For children with body surfaces >1 m2 the dosages were 3.0 ± 1.41 mg/m2 or 0.09 ± 0.04 mg/kg and the CVs 47% and 44%. No substantive difference was seen between dosage variations calculated using body weight or body surface area.
Adverse Outcomes
The median follow-up was 6.0 months.
Bleeding.
Serious bleeding occurred in 2 children for an overall incidence of 0.5% per patient year. Both children were receiving warfarin for secondary prophylaxis with a target INR range 2.0 to 3.0. The first child spontaneously developed a small subdural hemorrhage. The INR value was 1.7 at the time of the event and at 6 and 3 days before the event, the INRs were 4.1 and 1.7. The second child required a blood transfusion following a soft-tissue hematoma after minor trauma with an increased PT value at the time of the event. There was evidence of minor bleeding in 9 children or 2.3% per patient year. All children recovered from their bleeding episodes.
Thrombotic events.
None of the 208 children receiving warfarin for primary prophylaxis had a TE. Eight of the 144 children receiving warfarin for secondary prophylaxis presented with recurrent deep venous thrombosis (DVT)/PE. Two patients had recurrences during warfarin therapy, which is an incidence of 1.3% per patient year. One patient suffered from thrombotic occlusion of the iliac vein and the other patient developed a PE. A further 6 children presented with recurrent TEs occurring 5 days to 8 months after warfarin therapy was discontinued. These TEs consisted of 4 local recurrences and 2 PEs.
Whole-Blood Monitor
Twenty-eight children were tested by whole-blood monitors at home for a cumulative time period of 36 patient years and an average duration of 15 ± 12 months. INR values were within the target INR range for 68% ± 17% measurements, 20% ± 15% were below the target INR range, and 13% ± 11% were greater than the target INR range. Seventy patients’ samples had parallel INR testing with the whole-blood monitor and in the clinical laboratory. Four results (5.7%) were outside the 95% confidence limit of the regression line. Data from 23 of these children has been published elsewhere.7
DISCUSSION
The purpose of this study was to evaluate warfarin therapy in children. A nonselected consecutive cohort of 319 children receiving warfarin at HSC were prospectively studied. The study database summarizes a total of 391 patient years of warfarin therapy and was analyzed for multiple parameters potentially influencing clinical management of warfarin therapy.
Uniform dosing and monitoring protocols were used in our study. There were 3 target INR ranges based on indications for warfarin therapy (INR 1.4 to 1.8, INR 2.0 to 3.0, and INR 2.5 to 3.5). The largest number of children were in the target INR range 2.0 to 3.0 group. Seventy-nine percent of children achieved an INR in their target range in less than 7 days, which is comparable to data from adult literature and 1 small pediatric case series.1 17 Children requiring more than 7 days to achieve their target INR range were younger and more frequently receiving enteral nutrition. Use of warfarin for primary as compared with secondary prophylaxis did not influence the number of days to achieve a target INR value. All children were initially treated with heparin before warfarin therapy. The duration of time that warfarin therapy overlapped heparin therapy was shorter than the amount of time taken to achieve a target INR range, indicating that not all children achieved a therapeutic INR before discontinuing heparin. In adult patients with DVT, the recommendations are that 2 consecutive results are in the target INR range before switching to warfarin. However, in primary prophylaxis, low-dose warfarin may be started without previous heparin treatment. In our study, a majority of children (59%) were receiving warfarin for primary prophylaxis. The clinical decision, therefore, was that having 2 INR results in the target range was not as important and, to expedite release from hospital, some children were not in the therapeutic range before switching to warfarin.
The influence of age on warfarin dose requirements has been suggested previously, but study sizes prevented exploration of interaction of other variables.5,8 The current study had the power to assess relationships between age, other potentially independent variables, and warfarin dosing. Children ≤ 1 year of age, as compared with all other ages, required increased warfarin doses, a longer overlap with heparin, longer periods of time to achieve target INR ranges, more frequent INR testing, more dose adjustments, fewer INR values in the target range, and more INR values below the target range. Similar findings were present for children between 1 and 6 years of age compared with the 2 older age groups. These results reflect the increased degree of difficulty in management of warfarin therapy of very young children. The profound influence of age on warfarin dose requirements overwhelmed even the patients’ target INR range, as there was no significant difference in dose requirements to maintain INR values in the different target ranges. To achieve a target INR 2.0 to 3.0, children in our study required 0.16 mg/kg of warfarin compared with 0.04 to 0.08 mg/kg in adults, suggesting the influence of age continues beyond childhood.12 18 Other variables independently influencing warfarin therapy in children included target INR range, diet, medications, and underlying disease.
Children in the target INR range of 2.0 to 3.0 required more testing and dose changes, likely related to marked heterogeneity in their underlying disorders and their shorter treatment periods. Overall, 49% to 61% of INR measurements were within the target INR range, which is consistent with 1 small pediatric study and in adults.1,19-21 The percentage of INRs in the target range reported in our study is likely related, in part, to at least two facts. First, a majority of children who received oral anticoagulants had severe underlying diseases. Second, because of the risk of bleeding with INRs above 3.5, most physicians aim for the lower end of the target INR range, which is reflected in the fact that 30% to 38% values were below the target INR range. Children with target INR range 2.5 to 3.5 were in range more frequently than the other two groups, probably because they were more homogenous. All patients in this group had MV, were relatively stable, receiving the same medications, and were all on lifelong warfarin treatment. Only a few INR values were above the target range reflecting the practice of aiming for INR values between 2.5 and 3.0. Children with a target INR range between 2.0 to 3.0 were less frequently in the target range, had more frequent testing, and dose changes when compared with the other two groups. Data from the literature suggests that more frequent INR measurements lead to an increased percentage of INR values within the target range and the use of whole-blood monitors may assist in maintaining INR values in the target ranges in children.22
The diet of children ≤1 year of age is unique and includes enteral formula. All commercially available formula are supplemented with vitamin K to prevent hemorrhagic disease of the newborn. Because warfarin is a competitive inhibitor of vitamin K, increased dietary intake of vitamin K induces warfarin-resistance.23 Our study shows that warfarin requirements in enteral nutrition-fed children were significantly greater than for children not receiving enteral nutrition. Another dietary cause of resistance to warfarin is poor absorption due to short-bowel syndrome.13 Nine children in this study required daily total parenteral nutrition. Despite removal of vitamin K supplementation from their total parenteral nutrition, these children still required an increased dosage of warfarin (0.4 mg/kg) for a target INR range of 2.0 to 3.0.
In our study, 77% of children receiving warfarin therapy also required additional medications. Interactions of drugs such as corticosteroids, carbamazepine/phenobarbital, aspirin, and antibiotics are known to influence the effect of warfarin.14 In our study, corticosteroid treatment resulted in fewer INR values in the target range and significantly more values above the target range. The mechanism responsible for the influence of corticosteroids on warfarin dosing is not clear, but may be related to regulation of hepatic production of coagulation proteins.14,24 Children treated with the anticonvulsants carbamazepine/phenobarbital required increased doses of warfarin to maintain their target INR range, which is consistent with the findings in adults.14,25 The mechanism responsible for the latter is the induced activity of hepatic oxidases, which accelerate the metabolism of anticonvulsants.25-27
Children requiring warfarin therapy have diverse underlying disorders, which may influence management. The only clinically significant differences observed were in the warfarin doses between Fontan children and CHD and non-CHD children. Children having undergone a Fontan operation are frequently treated with warfarin to prevent thrombosis in the Fontan circuit, embolization to the lung, and embolization to the CNS.28-30 Fontan children in our study had a 25% decreased dose requirement for warfarin when compared with other CHD patients. Mechanisms for the decreased requirement are not obvious, but may be related to abnormal liver function and cholestasis in children post-Fontan procedure.31
Warfarin dosages in young children may be more accurately calculated by using body surface area instead of weight.32 Our study compared warfarin dose requirements calculated by both methods and found no significant difference. The nomogram suggests that warfarin therapy begins in the first few days of heparin therapy. Children ≤1 year of age and children who were on enteral nutrition required a significantly longer time on heparin, as a majority of these children were critically ill. Doses required to maintain target INR ranges were age-dependent, with infants and small children requiring doses that were greater than the initial loading dose, which partially explains the prolonged period of time required to achieve values in the target INR range. A small pilot study was conducted in which the loading dose was increased to either 0.3 or 0.4 mg/kg. However, 15 of 33 children had INR values over 4.0 during the loading phase and the study was stopped because of the risk of bleeding. The higher loading dose was introduced without adjustment of the standard nomogram with respect to timing of dosing.33 INR measurements in the first few days of therapy are insensitive to plasma warfarin levels due to the various half-lives of the vitamin K-dependent proteins.34 A repeat loading dose on following days may increase the risk of INRs above the target range and the nomogram should be adjusted to reflect this. Carefully designed prospective clinical studies of age-adjusted nomograms are needed to resolve these issues.
Twenty-eight children in this cohort used whole-blood monitors at home; data on 23 of these children was published previously.7Management of children using the monitor was excellent with 68% of measured INR values within the target range. There has been speculation that self-testing is less valid than laboratory testing, as the responsibility for accuracy of testing is placed on parents. Data from the literature does not support this hypothesis. One randomized controlled trial and 1 prospective cohort study in adults show that self-testing patients had a greater percentage of INR values within the target range and showed excellent agreement with reference plasma PTs (83% to 96%).35 36 The former may reflect the fact that self-managed patients tend to test themselves more frequently than patients monitored by an anticoagulation clinic. These studies indicate that home monitoring is accurate and may even result in improved clinical outcome.
No child receiving primary prophylaxis had a TE. Eight children receiving secondary prophylaxis had a recurrence, 2 patients developed recurrent TEs while receiving warfarin (incidence of 1.3% per patient year). Six children developed recurrent TEs when warfarin therapy was discontinued. The overall incidence of major bleeding in our study was 0.5% per patient year. As both bleeding episodes occurred in children with target INR range of 2.0 to 3.0, the incidence of major bleeding was 1.0% per patient year in that group, which is comparable to adults (1.3% per patient year).37 One child had a CNS bleed and a second developed soft-tissue hemorrhage. No child with MV had serious bleeding. The reported risk of serious bleeding in children with MV ranges from 0% to 0.9% per patient year, while the risk in adults is 1.2% to 5.6%.38-46 Clinically insignificant minor bleeding complications occurred in 2.3% per patient year.
In summary, our study showed that age is the single most important variable influencing warfarin therapy during childhood. Our study confirms that warfarin requirements in children are highly age-dependent and extends previous work by showing that the effect of age is independent of target INR range, underlying disease, medication, and diet. Also, our study shows that younger children have a significantly more complicated clinical course. This study has shown for the first time that children who have undergone a Fontan procedure have reduced requirements for warfarin. Also, this is the first study to assess warfarin dosing based on body surface area and on weight concluding there is no difference. The extent of the clinically important effect of enteral nutrition, corticosteroids, and phenobarbital/carbamazepine on warfarin therapy in children was not previously suspected. Data from this cohort document the complicated clinical management of warfarin therapy in children. Clinical trials are required to determine the optimal intensity and duration of warfarin therapy in children.
Supported by a grant from the Heart and Stroke Foundation of Canada. L.M. is a Scholar with the Medical Research Council of Canada. M.A. is a Career Scientist with the Heart and Stroke Foundation of Canada. W.S. is the recipient of a Stipendium zur Förderung von Auslandsstudien of the University of Innsbruck, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to L. Mitchell, MSc, Hamilton Civic Hospital Research Centre, 711 Concession St, Hamilton, Ontario L9C 3G1, Canada.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal