The ribosome-inactivating protein, Shiga-like toxin-1 (SLT-1), targets cells that express the glycolipid globotriaosylceramide (CD77) on their surface. CD77 and/or SLT-1 binding was detected by flow cytometry and immunocytochemistry on lymphoma and breast cancer cells recovered from biopsies of primary human cancers as well as on B cells or plasma cells present in blood/bone marrow samples of multiple myeloma patients. Breast cancer cell lines also expressed receptors for the toxin and were sensitive to SLT-1. Treatment of primary B lymphoma, B-cell chronic lymphocytic leukemia, and myeloma B or plasma cells with SLT-1–depleted malignant B cells by 3- to 28-fold, as measured by flow cytometry. Depletion of myeloma plasma cells was confirmed using a cellular limiting dilution assay followed by reverse transcriptase-polymerase chain reaction analysis of clonotypic IgH transcripts, which showed a greater than 3 log reduction in clonotypic myeloma cells after SLT-1 treatment. Receptors for the toxin were not detected on human CD34+ hematopoietic progenitor cells (HPC). HPC were pretreated with a concentration of SLT-1 known to purge primary malignant B cells and cultured for 6 days. The number of HPC was comparable in toxin-treated and untreated cultures. HPC were functionally intact as well. Colony-forming units (CFU) were present at an identical frequency in untreated and SLT-1 pretreated cultures, confirming that CFU escape SLT-1 toxicity. The results suggest the ex vivo use of SLT-1 in purging SLT-1 receptor-expressing malignant cells from autologous stem cell grafts of breast cancer, lymphoma, and myeloma patients.
GENE MARKING STUDIES suggest that relapse observed in cancer patients treated with high-dose chemotherapy and autologous stem cell transplantation (ASCT) may reflect, at least in part, the reinfusion of contaminating tumor cells in the stem cell graft.1,2 In myeloma, recent experiments have demonstrated that malignant progenitors from granulocyte colony-stimulating factor (G-CSF)–mobilized blood transferred myeloma to immunodeficient mice as measured by the presence of lytic bone lesions and polymerase chain reaction (PCR)-detectable disease (Pilarski et al, manuscript submitted). Significant improvements in disease-free survival have been demonstrated for follicle center cell lymphoma patients transplanted with autologous stem cell grafts purged free of PCR-detectable tumor cells with anti–B-cell antibodies and rabbit complement.3 4
A limited number of strategies for purging tumor cells from stem cell grafts have been developed to date. Most approaches focus on the design of protein fusion molecules or chemically derived toxin conjugates incorporating a cytotoxic component derived from a protein toxin covalently linked to a growth factor, cytokine, or antibody domain, enabling the resulting constructs to target specific cancer cells. Such constructs were initially created to cope with the broad receptor specificity of native protein toxins. The bacterial toxin, Shiga-like toxin 1 (SLT-1), is internalized by cells that express the glycolipid receptor globotriaosylceramide (Gb3, CD77) on their surface.5,6 Our group has recently shown that SLT-1 can successfully purge a human Burkitt’s lymphoma cell line from a murine bone marrow (BM) graft ex vivo before transplantation in severe combined immunodeficiency (SCID) mice.7 The potential use of SLT-1, a ribosome-inactivating protein, is presently limited to an ex vivo application, because its toxicity includes endothelial cell damage and hemolytic uremic syndrome observed in patients after gastrointestinal infections with SLT-1–producing strains ofEscherichia coli or Shiga toxin-producing strains ofShigella dysenteriae.5,8-14 We now report the expression of SLT-1 receptors on clinical tumor specimens and cell lines of cancers that may benefit from autologous stem cell transplantation and have addressed the survival of CD34+cells after exposure to SLT-1. Our findings suggest that SLT-1 could be used as an ex vivo purging agent for autologous grafts from patients with 3 malignancies commonly treated with high-dose chemotherapy and autologous stem cell transplantation, namely breast cancer, lymphoma, and multiple myeloma.15 16
MATERIALS AND METHODS
Cell lines, tissue sources, and culture conditions.
Human breast cancer cell lines were obtained from the American Type Culture Collection (Rockville, MD) and from the laboratories of Dr Ron Buick (Ontario Cancer Institute, Toronto, Ontario, Canada) and Dr Irene Andrulis (Lunenfeld Research Institute, Toronto, Ontario, Canada). The MDA-MB series of cells were cultured at 37°C in Leibovitz L-15 medium in the absence of CO2, whereas the other breast cancer cell lines were cultured in α-minimal essential medium (α-MEM), Iscove’s modification of Dulbecco’s modified Eagle’s medium (IMDM), or RPMI 1640 in the presence of 5% CO2 with 10% fetal calf serum (FCS), except for JS-1,17 which was maintained in 20% FCS. Clinical specimens were obtained with informed consent according to protocols approved by ethics committees at Women’s College Hospital, the Toronto General Hospital, the Princess Margaret Hospital, the University of Toronto, and the Alberta Cancer Board. Lymphoma specimens (including peripheral blood, biopsies, BM, or fine-needle aspirates) were obtained from patient samples sent to the flow cytometric unit (Princess Margaret Hospital, Toronto, Ontario, Canada) for immunophenotyping. Blood and BM samples from myeloma patients were obtained from the Cross Cancer Institute (Edmonton, Alberta, Canada) or the Toronto Hospital. Blood from a B-cell chronic lymphocytic leukemia (B-CLL) patient or G-CSF–mobilized blood samples from myeloma, lymphoma, or breast cancer patients were obtained at the Cross Cancer Institute or the Red Cross Apheresis Unit (Edmonton, Alberta, Canada). Lymph node biopsies having 30% to 60% malignant B cells were obtained from 2 patients with B lymphoma (provided by Dr R.G. Coupland, Cross Cancer Institute). Lymph node cells were dispersed by passage through a sieve. Mononuclear cells (MNC) from blood, BM, or lymph nodes were purified on Ficoll Hypaque (Pharmacia, Dorval, Quebec, Canada). Breast cancer specimens were derived from surgical biopsies of primary breast cancers at Women’s College Hospital (N.M.). All functional assays were performed on freshly obtained tissues.
Monoclonal antibodies (MoAbs).
Antibodies used for immunocytochemistry were bought from the following sources: anti-CD10 (Dako, Glostrup, Denmark), biotinylated mouse antirat IgM, unconjugated rat IgM anti-CD7718 and anti–B-B4 antibodies (Serotec, Oxford, UK),19 anti-CD13 (Coulter, Burlington, Ontario, Canada), AE1/AE3 anti-cytokeratin (Zymed, South San Francisco, CA), anti-low molecular weight keratins (Cam 5.2; Becton Dickinson, Franklin Lakes, NJ). The antiepithelial cancer antibody Ber-EP420 was purchased from Dako. ONC-M38, a hybridoma producing a murine antimucin antibody,21 was from Dr Peter Linsley (Bristol-Myers Squibb, Seattle, WA). M38 antibody was purified from culture supernatants by protein G affinity chromatography. MoAb 17G10-phycoerythrin (PE) was used to detect CD45.22 Anti-CD19 MoAb (FMC63) was coupled to PE or fluorescein isothiocyanate (FITC) and used to detect B cells in lymphoma biopsies and myeloma MNC as previously described.23,24 Plasma cells in myeloma BM were identified using an anti-CD38-PE conjugate (Leu17; Becton Dickinson) and based on their light scattering properties.25 Hematopoietic progenitor cells (HPC) were detected by staining the cell population with a combination of anti-CD34-PE (8G12-PE; Becton Dickinson) and anti-CD45-Quantum red (Sigma) conjugates and gating for the CD34+45lo subset, as previously described.26
Toxin purification and labeling.
SLT-1 cytotoxicity assays for breast cancer cell lines.
The toxicity of SLT-1 towards adherent cell lines was measured using the sulforhodamine B (SRB) dye binding assay.29 Briefly, cells cultured in 96-well plates were exposed to a range of toxin concentrations for a 1-hour period in phosphate-buffered saline (PBS). The toxin-containing solution was subsequently diluted with the appropriate medium containing FCS and the treated cells were cultured for another 48 hours. The medium was removed and the remaining adherent cells were fixed with ice-cold 10% trichloroacetic acid, air-dried, and stained with 0.4% SRB (Molecular Probes, Eugene, OR) dissolved in 1% acetic acid. The excess dye was washed away, and the remaining bound SRB dye was extracted from the cells with 10 mmol/L Tris base. The absorbance of the dye was read at 540 nm using a plate reader.
Flow cytometry and immunocytochemistry.
The expression of SLT-1 receptors was detected by flow cytometry using FITC-labeled SLT-B subunit (FITC-SLT-B) and by immunocytochemistry with an unconjugated anti-CD77 MoAb. Flow cytometry of human lymphoma samples was performed as previously described.7 Flow cytometric data were acquired from human breast cancer aspirates or cells recovered from minced tumor samples stained with antibodies to surface antigens (CD77, Ber-EP4, and M38) and viability dyes. A cocktail of both Ber-EP4 and M38 antibodies was used for flow cytometry of breast cancer biopsies because of the limited recovery of cells. Cytospins of lymphoma and breast cancer cells were immunostained with anticytokeratin antibodies (cam 5.2 [Becton Dickinson] and AE1/AE3 [Zymed]). All immunocytochemistry was accomplished on paraformaldehyde-fixed samples.
Purging of ex vivo malignant B cells.
MNC derived from lymphoma biopsies or from blood/BM samples of myeloma patients were treated with 5 μg/mL SLT-1 for 60 minutes at 37°C, washed to remove unbound toxin, and cultured for 6 to 10 days in RPMI supplemented with 10% FCS. Cultured cells were harvested, and the total number of viable cells was counted and stained with FITC-labeled anti-CD19 as well as with propidium iodide (PI) to identify viable cells. The absolute number of viable CD19+ B cells remaining in the cultures was calculated as the percentage of viable CD19+ cells (CD19+PIlow) multiplied by the total number of viable cells. Plasma cells were stained with FITC-labeled anti-CD38 MoAb as well as PI.
Quantitation of clonotypic cells in myeloma.
To confirm the purging of malignant plasma cells from myeloma BM cells, a cellular limiting dilution reverse transcriptase-PCR (RT-PCR) assay24 was performed to detect the presence of clonotypic IgH VDJ rearrangements using patient-specific primers. Briefly, graded numbers of untreated or SLT-1–pretreated BM cells were deposited into PCR tubes containing lysis buffer. RNA from the lysed cells was reverse transcribed and the resulting cDNA was amplified in the same tube. The patient-specific IgH VDJ sequence was identified from single plasma cells shown to be specific for this patient and expressed by greater than 80% of individual plasma cells recovered for this patient, confirming that the myeloma-specific Ig sequence had been identified.24
Short-term culture of HPC.
Mononuclear cells from G-CSF–mobilized blood were pretreated with 5 μg/mL of SLT-1 for 60 minutes at 37°C, washed, and cultured in commercial long-term culture medium (Myelocult plus 10% Hemostim; Stem Cell Technologies, Vancouver, British Columbia, Canada) for 7 to 10 days. Harvested cells from paired untreated and SLT-1–pretreated cultures were stained with anti-CD34-PE and anti-CD45-QR conjugates. The percentage of HPC (CD34+45loScatterlow/med) was determined as previously described.26
Assay for colony-forming units (CFU).
MNC from G-CSF–mobilized blood samples were enriched for HPC by negative selection according to instructions from the manufacturer (StemSep; Stem Cell Technologies). Enriched HPC (40% to 60% HPC) were then treated or not with 5 μg/mL of SLT-1 for 60 minutes at 37°C, washed once, and cultured to monitor the number of CFU. Increasing numbers of toxin-treated or untreated cells (25 to 200 cells per well) were added to a mixture of growth factors and methyl cellulose (Methylcult; Stem Cell Technologies) according to the instructions from the manufacturer and deposited into culture wells. The number of colonies was counted under a microscope after 20 days, as recommended by the manufacturer. CFU values reported represent the average of colony numbers observed in 6 to 12 replicate wells.
RESULTS
Expression of SLT-1 receptor on breast cancer cell lines and SLT-1 cytotoxicity.
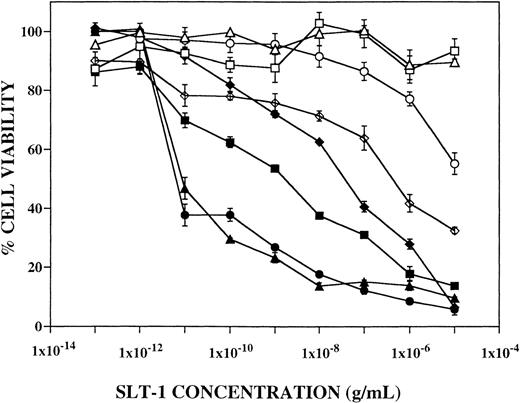
The expression of SLT-1 receptors on 18 breast cancer cell lines was analyzed by flow cytometry using FITC-SLT-B. Two antibodies were used to label breast cells: Ber-EP4, which recognizes an unknown antigen on epithelial-derived carcinomas,20 and M38,21which detects the epitope MUC1 on breast-derived mucin.30Of the 18 breast cancer cell lines studied, 5 had less than 15% positivity for FITC-SLT-B, whereas 13 (72%) expressed surface SLT-1 receptors (Table 1). Sixteen of 18 (89%) breast cancer cell lines were positive for M38 (MUC1) and 12 of 18 (67%) were positive for Ber-EP4. The results demonstrate the frequent expression of SLT-1 receptors on the surface of breast cancer cell lines. The level of expression of SLT-1 receptors on breast cancer cell lines correlates with their sensitivity to killing by SLT-1 (Fig 1 and Table 1, CD50values).
Human Breast Cancer Cell Line Surface Antigens and SLT-1 Sensitivity
| Cell Lines . | Dx . | SLT-R . | Ber-EP4 . | M38/MUC1 . | CD50 Values . |
|---|---|---|---|---|---|
| Hs578T | CAR | − | − | ++ | ND |
| MDA-MB-330 | LOB | − | − | − | ND |
| MDA-MB-468 | IDC | − | − | +++ | >10 μg/mL |
| CAMA-1 | AC | − | +++ | +++ | >10 μg/mL |
| BT-474 | IDC | − | +++ | +++ | ND |
| BT-20 | IDC | + | − | + | ND |
| DU4475 | MET (skin) | + | +++ | +++ | ND |
| MDA-MB-134 | IDC | + | +++ | ++ | ND |
| MDA-MB-435S | IDC | + | − | ++ | ND |
| ZR-75 | IDC | + | ++ | +++ | ND |
| MCF-7 | IDC | +/++ | +++ | ++/+++ | 2 ng/mL |
| MDA-MB-157 | MED | ++ | +++ | +++ | ND |
| SKBR3 | AC | ++ | +++ | ++/+++ | >10 μg/mL |
| JS-1 | MET (bone) | ++ | − | − | 40 ng/mL |
| MDA-MB-231 | AC | +++ | + | +++ | 0.01 ng/mL |
| MDA-MB-469 | ND | +++ | +++ | +++ | 0.01 ng/mL |
| T47D | IDC | +++ | +++ | +++ | 0.7 μg/mL |
| UACC-812 | IDC | +++ | +++ | +++ | ND |
| Cell Lines . | Dx . | SLT-R . | Ber-EP4 . | M38/MUC1 . | CD50 Values . |
|---|---|---|---|---|---|
| Hs578T | CAR | − | − | ++ | ND |
| MDA-MB-330 | LOB | − | − | − | ND |
| MDA-MB-468 | IDC | − | − | +++ | >10 μg/mL |
| CAMA-1 | AC | − | +++ | +++ | >10 μg/mL |
| BT-474 | IDC | − | +++ | +++ | ND |
| BT-20 | IDC | + | − | + | ND |
| DU4475 | MET (skin) | + | +++ | +++ | ND |
| MDA-MB-134 | IDC | + | +++ | ++ | ND |
| MDA-MB-435S | IDC | + | − | ++ | ND |
| ZR-75 | IDC | + | ++ | +++ | ND |
| MCF-7 | IDC | +/++ | +++ | ++/+++ | 2 ng/mL |
| MDA-MB-157 | MED | ++ | +++ | +++ | ND |
| SKBR3 | AC | ++ | +++ | ++/+++ | >10 μg/mL |
| JS-1 | MET (bone) | ++ | − | − | 40 ng/mL |
| MDA-MB-231 | AC | +++ | + | +++ | 0.01 ng/mL |
| MDA-MB-469 | ND | +++ | +++ | +++ | 0.01 ng/mL |
| T47D | IDC | +++ | +++ | +++ | 0.7 μg/mL |
| UACC-812 | IDC | +++ | +++ | +++ | ND |
Results from the flow cytometric analysis of breast cancer cell lines for surface expression of SLT-R, Ber-EP4, and M38/MUC1. Also shown are CD50 values derived from Fig 1. Expression of surface antigen in terms of percentage of cells staining positively for a marker is defined as negative or − (0% to 14%), weak or + (15% to 40%), intermediate or ++ (41% to 70%), and strong or +++ (71% to 100%).
Abbreviations: Dx, morphological diagnosis42; CAR, carcinosarcoma; LOB, lobular carcinoma; IDC, intraductal carcinoma; MET, metastatic carcinoma; MED, medullary carcinoma; AC, adenocarcinoma; CD50, dose of SLT-1 required to kill 50% of cells; ND, not determined.
Effect of SLT-1 on the viability of human breast cancer cell lines. The percentage of cell viability of 8 breast cancer cell lines based on the SRB dye binding assay was measured as a function of toxin concentration. Cell viability curves are shown for CAMA-1 (□), JS-1 (⧫), MCF-7 (▪), MDA-MB-231 (▴), MDA-MB-468 (○), MDA-MB-469 (•), SKBR3 (▵), and T47D (◊).
Effect of SLT-1 on the viability of human breast cancer cell lines. The percentage of cell viability of 8 breast cancer cell lines based on the SRB dye binding assay was measured as a function of toxin concentration. Cell viability curves are shown for CAMA-1 (□), JS-1 (⧫), MCF-7 (▪), MDA-MB-231 (▴), MDA-MB-468 (○), MDA-MB-469 (•), SKBR3 (▵), and T47D (◊).
Expression of SLT-1 receptors on breast cancer biopsies.
The expression of SLT-1 receptors on biopsies of primary human breast cancers obtained from 10 patients was analyzed by flow cytometry and immunocytochemistry. Cell suspensions were prepared from fine-needle aspirates or by mechanical disaggregation of solid tumors. Eight of 10 samples (80%) showed greater than 15% positive staining for FITC-SLT-B (Table 2) and the intensity of fluorescence staining was high (Fig2C). In contrast to the established breast cancer cell lines, Ber-EP4/M38 was expressed on 4 of 10 samples, all 4 of which were positive for SLT-1 receptors (SLT-R). Four of 8 SLT-R+samples were negative for the breast cancer markers (Ber-EP4 and M38). SLT-1 receptor-positive cells were confirmed as breast cancer cells by morphological examination and immunocytochemistry with anti-low molecular weight cytokeratin and anti-CD77 antibodies (Fig 3A and B).
Breast Cancer Biopsy Surface Antigens
| Biopsies . | Dx . | SLT-R . | Combined Ber-EP4/M38 . | LMWK . |
|---|---|---|---|---|
| BC-1 | IDC | +/++ | +/++ | ND |
| BC-2 | IDC | ++ | ++ | ND |
| BC-3 | IDC | + | − | ND |
| BC-4 | ILC | ++ | − | ND |
| BC-5 | IDC | + | − | + |
| BC-6 | ILC | + | − | + |
| BC-7 | IDC | ++ | ++ | + |
| BC-8 | IDC | − | − | ND |
| BC-9 | ILC | − | − | ND |
| BC-10 | IDC | +++ | +++ | ND |
| Biopsies . | Dx . | SLT-R . | Combined Ber-EP4/M38 . | LMWK . |
|---|---|---|---|---|
| BC-1 | IDC | +/++ | +/++ | ND |
| BC-2 | IDC | ++ | ++ | ND |
| BC-3 | IDC | + | − | ND |
| BC-4 | ILC | ++ | − | ND |
| BC-5 | IDC | + | − | + |
| BC-6 | ILC | + | − | + |
| BC-7 | IDC | ++ | ++ | + |
| BC-8 | IDC | − | − | ND |
| BC-9 | ILC | − | − | ND |
| BC-10 | IDC | +++ | +++ | ND |
Flow cytometric analysis of primary based cancer biopsy material for surface expression of SLT-R and combined Ber-EP4/M38. Also shown are results for immunohistochemical analysis of cytospins for LMWK. Expression of surface antigen in terms of percentage of cells staining positively for a marker: defined as negative or − (0% to 14%), weak or + (15% to 40%), intermediate or ++ (41% to 70%), and strong or +++ (71% to 100%).
Abbreviations: Dx, morphological diagnosis; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LMWK, low molecular weight keratins detected by immunohistochemistry, expression is defined as negative (−) or positive (+) only; ND, not determined.
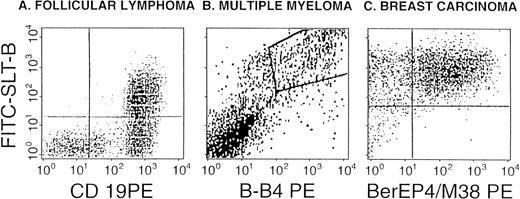
SLT-1 binding by malignant B cells, breast carcinoma cells and HPC. Representative views from the flow cytometric detection of SLT-1 receptors on follicle center lymphoma grade I (A), multiple myeloma plasma cells (B), and breast carcinoma (C). The binding of FITC-SLT-B is shown in relation to anti-CD19 labeling (a pan-B–cell marker) for the lymphocyte gate in (A) or against B-B4 (plasma cell marker) for BM cells in (B) and against the combined expression of Ber-EP4 and M38 (MUC1) for a breast cancer biopsy (C). Dual-positives are shown in the upper right quadrant (A and C) or as a boxed area (B).
SLT-1 binding by malignant B cells, breast carcinoma cells and HPC. Representative views from the flow cytometric detection of SLT-1 receptors on follicle center lymphoma grade I (A), multiple myeloma plasma cells (B), and breast carcinoma (C). The binding of FITC-SLT-B is shown in relation to anti-CD19 labeling (a pan-B–cell marker) for the lymphocyte gate in (A) or against B-B4 (plasma cell marker) for BM cells in (B) and against the combined expression of Ber-EP4 and M38 (MUC1) for a breast cancer biopsy (C). Dual-positives are shown in the upper right quadrant (A and C) or as a boxed area (B).
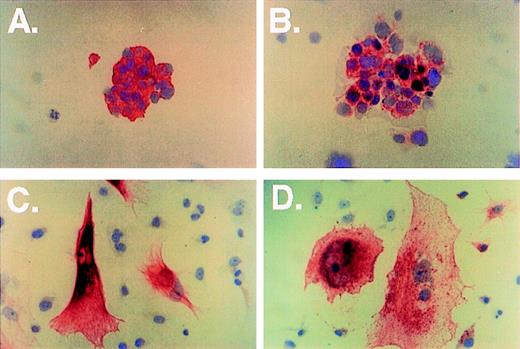
Immunocytochemical analysis of breast cancer cells (A through D, immunohistochemical stain shown in red and nuclear counterstain in blue). Cytospins of cells from a breast cancer biopsy were stained with anti-LMWK (A, original magnification × 400) and anti-CD77 (B, original magnification × 400). The metastatic breast cancer cell line JS1 was grown on plastic slides and stained with anticytokeratin AE1/AE3 (C, original magnification × 400) and anti-CD77 (D).
Immunocytochemical analysis of breast cancer cells (A through D, immunohistochemical stain shown in red and nuclear counterstain in blue). Cytospins of cells from a breast cancer biopsy were stained with anti-LMWK (A, original magnification × 400) and anti-CD77 (B, original magnification × 400). The metastatic breast cancer cell line JS1 was grown on plastic slides and stained with anticytokeratin AE1/AE3 (C, original magnification × 400) and anti-CD77 (D).
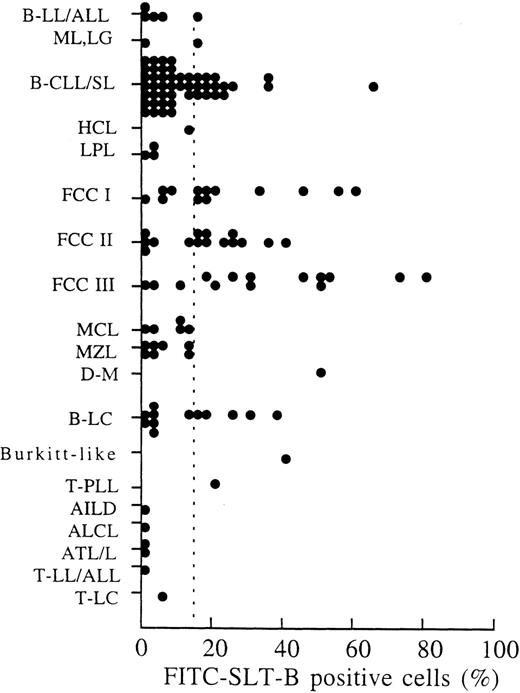
Expression of SLT-1 receptor on lymphoma and myeloma.
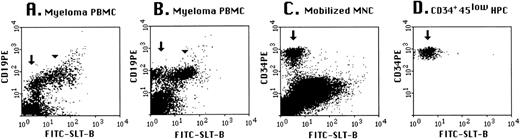
Clinical samples from patients with hematological cancers were routinely screened with FITC-SLT-B to monitor the expression of SLT-1 receptors. Flow cytometry data were collected on 134 sequential samples (Fig 4) classified according to the REAL system.31 On average, 3% cells (±4%) recovered from nonmalignant samples stained with the fluorescent probe (results not shown). Clinical samples were scored as positive when at least 15% of cells (3 SD above the mean background) bound FITC-SLT-B.7SLT-1 receptors were frequently expressed on follicle center cell lymphoma grades I, II, and III (Fig 4), with 31 of 43 (72%) patient samples positive. These results agree with the expression of CD77 on normal follicle center cells.32-37 Thirty-three percent (15 of 46 samples) of small lymphocytic lymphomas with or without chronic lymphocytic leukemia were positive for FITC-SLT-B staining, as were 42% (5 of 12 samples) of large B-cell lymphomas. FITC-SLT-B staining was not observed on mantle cell lymphomas, which are thought to arise from the normally CD77− B cells in the follicular mantle zone. Marginal zone lymphomas were also CD77−. The B-cell acute lymphocytic leukemias were essentially CD77−. Flow cytometry (2 samples) and immunohistochemistry (5 samples) were performed on BM aspirates collected from multiple myeloma patients. The marker syndecan-1, identified by the antibody B-B4, was used to detect normal plasma cells as well as multiple myeloma cells.19,38 Normal plasma cells were found to be syndecan-1+, CD77−, whereas plasma cells from multiple myeloma BM were syndecan-1+, CD77+ (results not shown). Multiple myeloma includes circulating CD19+ B-cell components of the malignant clone as well as the BM-localized plasma cells.23-25,39 On average, 66% of B cells in myeloma peripheral blood mononuclear cells (PBMC) are clonotypic24 and approximately 30% are polyclonal.25 Figure 5 shows that the set of B cells shown elsewhere to be clonotypic24express SLT-1 receptors (arrowhead), whereas the set of residual normal B cells in myeloma patients does not bind SLT-1 (arrow).
SLT-1 receptor (CD77) expression on lymphoma. The expression of SLT-1 receptors on malignant lymphoma was detected using a fluoresceinated binding subunit of SLT-1 (FITC-SLT-B). Cell suspensions recovered from patient samples (peripheral blood, fine-needle aspirates, lymph node, and BM biopsies) were sent to a flow cytometry unit (Ontario Cancer Institute) for immunophenotyping. A lymphocyte population in which 15% of the cells stained positively with FITC-SLT-B (dashed line) was defined as positive for the presence of SLT-1 receptors. This percentage value (15%) was calculated from the average percentage of FITC-SLT-B–positive cells (3% ± 4%) observed in samples of noncancerous patients, plus 3 SD. Abbreviations used: B-LL/ALL, B-cell lymphoblastic lymphoma/acute lymphoblastic leukemia; ML, LG, malignant lymphoma, low grade (nonclassifiable); B-CLL/SL, small lymphocytic lymphoma with or without chronic lymphocytic leukemia; HCL, hairy cell leukemia; LPL, lymphoplasmacytoid lymphoma; FCC I, follicle center cell lymphoma, follicular, grade I; FCC II, follicle center cell lymphoma, follicular, grade II; FCC III, follicle center cell lymphoma, follicular, grade III; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; D-M, diffuse-mixed small- and large-cell lymphoma; B-LC, diffuse large B-cell lymphoma; T-PLL, T-cell chronic lymphocytic lymphoma, prolymphocytic leukemia; AILD, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ATL/L, adult T-cell lymphoma/leukemia; T-LL/ALL, T-cell lymphoblastic lymphoma/acute lymphoblastic leukemia; T-LC, T-cell intermediate-grade large-cell lymphoma. Patients with diffuse large B-cell lymphoma who had a documented history of follicle center cell lymphoma were included in the FCC-III category.
SLT-1 receptor (CD77) expression on lymphoma. The expression of SLT-1 receptors on malignant lymphoma was detected using a fluoresceinated binding subunit of SLT-1 (FITC-SLT-B). Cell suspensions recovered from patient samples (peripheral blood, fine-needle aspirates, lymph node, and BM biopsies) were sent to a flow cytometry unit (Ontario Cancer Institute) for immunophenotyping. A lymphocyte population in which 15% of the cells stained positively with FITC-SLT-B (dashed line) was defined as positive for the presence of SLT-1 receptors. This percentage value (15%) was calculated from the average percentage of FITC-SLT-B–positive cells (3% ± 4%) observed in samples of noncancerous patients, plus 3 SD. Abbreviations used: B-LL/ALL, B-cell lymphoblastic lymphoma/acute lymphoblastic leukemia; ML, LG, malignant lymphoma, low grade (nonclassifiable); B-CLL/SL, small lymphocytic lymphoma with or without chronic lymphocytic leukemia; HCL, hairy cell leukemia; LPL, lymphoplasmacytoid lymphoma; FCC I, follicle center cell lymphoma, follicular, grade I; FCC II, follicle center cell lymphoma, follicular, grade II; FCC III, follicle center cell lymphoma, follicular, grade III; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; D-M, diffuse-mixed small- and large-cell lymphoma; B-LC, diffuse large B-cell lymphoma; T-PLL, T-cell chronic lymphocytic lymphoma, prolymphocytic leukemia; AILD, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ATL/L, adult T-cell lymphoma/leukemia; T-LL/ALL, T-cell lymphoblastic lymphoma/acute lymphoblastic leukemia; T-LC, T-cell intermediate-grade large-cell lymphoma. Patients with diffuse large B-cell lymphoma who had a documented history of follicle center cell lymphoma were included in the FCC-III category.
SLT-1 receptors are present on a B-cell subset of myeloma patients and absent on HPC. Cells from peripheral blood of myeloma patients (A and B) were stained with anti-CD19-PE and FITC-SLT-B. Mobilized blood mononuclear cells (C) were stained with anti-CD34-PE/anti-CD45-QR and FITC-SLT-B and files were gated for the HPC subset (D), as described in Materials and Methods. For (C) and (D), the arrows indicate the HPC subset. For myeloma, the peripheral blood includes both polyclonal and monoclonal B cells.24 The arrowhead indicates the monoclonal subset of B cells and the arrow highlights the polyclonal subset, presumptively normal B cells that lack SLT-1 receptors.
SLT-1 receptors are present on a B-cell subset of myeloma patients and absent on HPC. Cells from peripheral blood of myeloma patients (A and B) were stained with anti-CD19-PE and FITC-SLT-B. Mobilized blood mononuclear cells (C) were stained with anti-CD34-PE/anti-CD45-QR and FITC-SLT-B and files were gated for the HPC subset (D), as described in Materials and Methods. For (C) and (D), the arrows indicate the HPC subset. For myeloma, the peripheral blood includes both polyclonal and monoclonal B cells.24 The arrowhead indicates the monoclonal subset of B cells and the arrow highlights the polyclonal subset, presumptively normal B cells that lack SLT-1 receptors.
Hematopoietic progenitor cells do not bind SLT.
HPC are phenotypically defined as CD34+45loScatterlow/medium cells.26,40 41 Hematopoietic progenitor cells from G-CSF–mobilized blood MNC were stained in multicolor immunofluorescence to determine SLT-1 binding. A representative flow cytometry plot is shown in Fig 5 for unfractionated G-CSF–mobilized MNC (Fig 5C) and the same mobilized blood MNC gated for the HPC subset, showing that normal HPC (indicated by arrows in Fig 5C and D) do not bind SLT-1. Similar results were obtained for HPC from 4 different mobilized blood samples.
SLT-1 purging depletes malignant cells from lymphoma, B-CLL, and myeloma tissues.
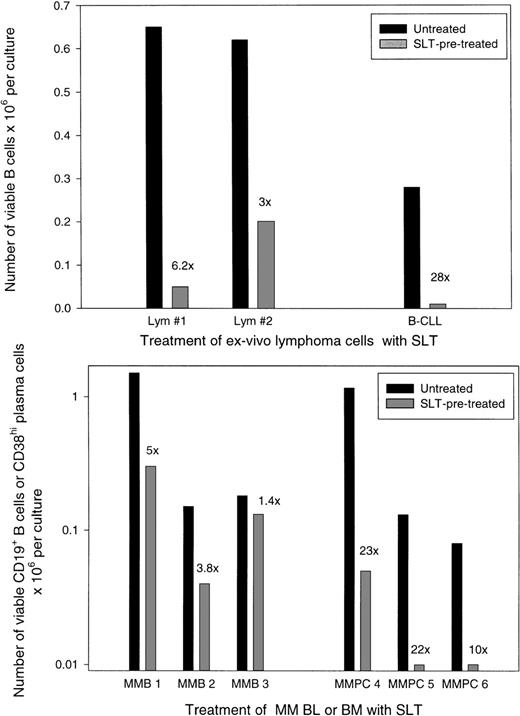
MNC from lymphoma node biopsies or blood/BM from myeloma patients were treated in vitro with SLT-1 to evaluate its ability to purge malignant cells recovered from these tissues. Unlike cell lines that, after exposure to 1 μg/mL or less of toxin, die within 2 to 3 days,7 the toxic effects of SLT-1 on freshly cultured cells required relatively high concentrations of toxin (5 μg/mL) and were detectable only after 6 to 10 days in culture (not shown). For 2 lymphoma biopsies, MNC were pretreated with 5 μg/mL SLT-1 and cultured, and the number of phenotypically defined B cells was enumerated using flow cytometry. Figure 6(top panel) shows that the absolute number of lymphoma B cells was reduced by 3- to 6-fold after pretreatment with SLT-1. For 1 patient with B-CLL, B cells were reduced 28× after pretreatment with SLT-1 (Fig 6, top panel). For B cells from myeloma blood, a similar reduction in total B cells was detected after SLT-1 pretreatment for 2 patients with the high numbers of B cells characteristic of myeloma patients (Fig 6, bottom panel). However, for 1 myeloma patient who had recovered from autologous transplant and had no detectable circulating clonotypic cells (not shown; MMB 3, Fig 6, bottom panel), there was only a marginal reduction in B cells after SLT-1 pretreatment, suggesting that only malignant B cells are targeted by this strategy. For myeloma BM, pretreatment with SLT-1 efficiently depleted the majority of plasma cells by 10- to 23-fold as measured phenotypically.
Pretreatment with SLT-1 depletes malignant B-lymphoma cells, B-CLL, and B or plasma cells from myeloma patients. MNC derived from lymphoma biopsies or PBMC from B-CLL (top panel) or from myeloma blood or BM (bottom panel) were pretreated with 5 μg/mL of SLT-1 for 60 minutes, washed, and cultured for 6 to 11 days. Viable cells were then enumerated and analyzed using anti-CD19 MoAb to detect B cells and anti-CD38 MoAb to detect plasma cells, together with PI staining to identify live versus dead cells. The absolute number of B or plasma cells remaining in cultures was calculated as the percentage of viable CD19+ or CD38+ cells times the number of viable cells per culture. The numerical values indicate the extent of B-cell or plasma cell depletion by SLT-1 as compared with the untreated control cultures. Patient MMB3 was posttransplant and had no detectable clonotypic B cells before harvest of the blood sample used here (not shown).
Pretreatment with SLT-1 depletes malignant B-lymphoma cells, B-CLL, and B or plasma cells from myeloma patients. MNC derived from lymphoma biopsies or PBMC from B-CLL (top panel) or from myeloma blood or BM (bottom panel) were pretreated with 5 μg/mL of SLT-1 for 60 minutes, washed, and cultured for 6 to 11 days. Viable cells were then enumerated and analyzed using anti-CD19 MoAb to detect B cells and anti-CD38 MoAb to detect plasma cells, together with PI staining to identify live versus dead cells. The absolute number of B or plasma cells remaining in cultures was calculated as the percentage of viable CD19+ or CD38+ cells times the number of viable cells per culture. The numerical values indicate the extent of B-cell or plasma cell depletion by SLT-1 as compared with the untreated control cultures. Patient MMB3 was posttransplant and had no detectable clonotypic B cells before harvest of the blood sample used here (not shown).
Molecular analysis confirms a greater than 3 log reduction in clonotypic myeloma cells after SLT-1 pretreatment.
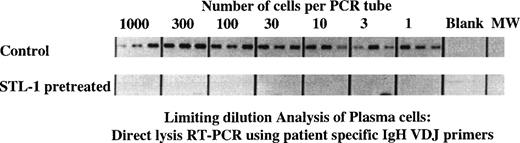
One complicating factor in evaluating the extent of purging in the experiments cited above is the presence of residual normal B lineage cells that may resist the action of the toxin. Because phenotypic analysis does not reliably distinguish between normal and malignant B or plasma cells, we evaluated the extent of purging using a novel molecular assay for clonotypic transcripts. Myeloma cells are uniquely identified by the IgH VDJ rearrangement that characterizes the malignant clone in each patient. Untreated or SLT-1 pretreated BM cells from MM patient no. 4 were deposited at limiting dilution into lysis buffer in PCR tubes, followed by RT-PCR using patient-specific primers. Figure 7 shows that, for the untreated cultures, by day 6, essentially all cells were clonotypic (3 of 3 tubes were positive at the 1 cell/tube concentration). However, for the SLT-1–treated cultures, clonotypic transcripts were undetectable even in the tubes containing 1,000 cells per tube. The frequency of clonotypic cells remaining after SLT-1 pretreatment was less than 1/3,000 cells in light of the fact that all 3 tube replicates were negative from the presence of transcripts (for a total of 3,000 cells analyzed).
SLT-1 pretreatment depletes myeloma plasma cells by more than 3 log units as measured using a cellular limiting dilution RT-PCR analysis for clonotypic transcripts. Harvested cells from sample in Fig5 were further analyzed for the presence of clonotypic cells to enumerate specifically the depletion of the malignant clone. For myeloma patient no. 4, untreated or SLT-1–pretreated cultures were harvested at day 6 and deposited at limiting dilution into PCR tubes containing lysis buffer, followed by RT-PCR analysis using patient-specific primers as described in the methods section.24 The product amplified from cells of the control cultures was a clonotypic IgH VDJ transcript of the expected size and sequence. Products amplified from cells that were pretreated with SLT-1 were of an incorrect size and did not have an Ig sequence.
SLT-1 pretreatment depletes myeloma plasma cells by more than 3 log units as measured using a cellular limiting dilution RT-PCR analysis for clonotypic transcripts. Harvested cells from sample in Fig5 were further analyzed for the presence of clonotypic cells to enumerate specifically the depletion of the malignant clone. For myeloma patient no. 4, untreated or SLT-1–pretreated cultures were harvested at day 6 and deposited at limiting dilution into PCR tubes containing lysis buffer, followed by RT-PCR analysis using patient-specific primers as described in the methods section.24 The product amplified from cells of the control cultures was a clonotypic IgH VDJ transcript of the expected size and sequence. Products amplified from cells that were pretreated with SLT-1 were of an incorrect size and did not have an Ig sequence.
HPC and CFU from G-CSF–mobilized blood escape SLT-1 toxicity.
To be effective, a purging agent must spare normal hematopoietic progenitor cells. To evaluate the effects of SLT-1 treatment on progenitor cells and their functional activity, we analyzed the survival of HPC in toxin-pretreated cultures and of cells able to give rise to hematopoietic colonies in vitro (CFU). These 2 approaches represent surrogate assays for engraftment potential. Table 3A shows that, when MNC or HPC-enriched MNC are pretreated with 5 μg/mL of SLT-1, a concentration shown above to effectively purge malignant cells, phenotypically identified HPC are present in equivalent numbers in untreated or SLT-1–treated cultures at days 6 to 7 posttreatment. For the HPC-enriched MNC population derived from mobilized blood, HPC were more frequent in SLT-1–treated than in untreated cultures (Table 3, analysis of HPC-enriched CD34+45lopopulations), probably reflecting a further enrichment of the toxin-resistant HPC as SLT-1–sensitive cells died. These experiments indicate that the HPC population as a whole survives SLT-1 pretreatment, but does not address the functional capability of these HPC.
HPC and CFU Escape SLT-1 Toxicity
| (A) Analysis of HPC . | ||
|---|---|---|
| Patient Source of Mobilized Blood . | Percentage of CD34+45lo HPC at Day 6-7 . | |
| Untreated . | SLT-1–Treated . | |
| Breast cancer | ||
| Unfractionated | 2.8% | 2.6% |
| HPC-enriched | 39.5% | 60.2% |
| Lymphoma | ||
| Unfractionated | 1.1% | 1.4% |
| HPC-enriched | 71.1% | 39.5% |
| Myeloma | ||
| HPC-enriched | 5.0% | 28.4% |
| (B) Analysis of CFC | ||
| Patient Source of Mobilized Blood | No. of Colonies/100-200 Cells Plated at Day 203-150 | |
| Untreated | SLT-1–Treated | |
| Breast cancer | ||
| HPC-enriched | 15 ± 1 | 13 ± 1 |
| Lymphoma | ||
| HPC-enriched | 17 ± 1 | 20 ± 1.5 |
| Myeloma | ||
| HPC-enriched | 17 ± 1 | 19 ± 1 |
| (A) Analysis of HPC . | ||
|---|---|---|
| Patient Source of Mobilized Blood . | Percentage of CD34+45lo HPC at Day 6-7 . | |
| Untreated . | SLT-1–Treated . | |
| Breast cancer | ||
| Unfractionated | 2.8% | 2.6% |
| HPC-enriched | 39.5% | 60.2% |
| Lymphoma | ||
| Unfractionated | 1.1% | 1.4% |
| HPC-enriched | 71.1% | 39.5% |
| Myeloma | ||
| HPC-enriched | 5.0% | 28.4% |
| (B) Analysis of CFC | ||
| Patient Source of Mobilized Blood | No. of Colonies/100-200 Cells Plated at Day 203-150 | |
| Untreated | SLT-1–Treated | |
| Breast cancer | ||
| HPC-enriched | 15 ± 1 | 13 ± 1 |
| Lymphoma | ||
| HPC-enriched | 17 ± 1 | 20 ± 1.5 |
| Myeloma | ||
| HPC-enriched | 17 ± 1 | 19 ± 1 |
Mobilized MNC were treated with 5 μg/mL SLT-1 for 60 minutes, washed, and cultured. For HPC assays, toxin-treated or untreated cells were cultured in Myelocult + 10% Hemostim culture medium for 7 days, followed by harvest of cultured cells and staining with anti-CD34-PE and anti-CD45-QR conjugates. Mobilized MNC were enriched for HPC by negative selection after antibody coating and magnetic bead depletion resulting in a cell population in which 40% to 60% of cells display the HPC phenotype.
Cells were plated in microtiter wells. Values listed represent the average number of colonies (±SE) calculated from 6 to 12 replicate wells for each condition tested.
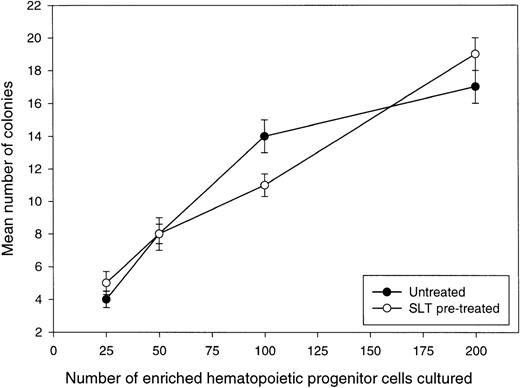
The functional potential of HPC pretreated with SLT-1 was evaluated in terms of their ability to form hematopoietic colonies (Table 3, analysis of CFU). HPC would not be expected to have sufficient generative potential to form a visible colony if their survival was compromised by their exposure to the toxin. Enriched HPC were pretreated with 5 μg/mL of SLT-1 under conditions identical to those shown to purge lymphoma or myeloma cells and assayed for their colony-forming ability. These experiments show that the number of colonies was the same for untreated or SLT-1–pretreated HPC, indicating that SLT-1 is not toxic to human CD34+hematopoietic progenitor cells. Similar results were obtained for mobilized HPC from breast cancer and lymphoma patients (Table 3). To more accurately confirm that CFU are SLT-1–resistant, a limiting dilution analysis of CFU numbers was performed in which 25 to 200 enriched HPC, pretreated or not with 5 μg/mL SLT-1, were plated and resulting colonies were counted 20 days later (Fig 8). The frequency of CFU was identical for untreated or SLT-1–treated enriched HPC (∼1 CFU per 12 enriched HPC), further confirming with a functional assay that hematopoietic progenitors escape SLT-1 toxicity.
Toxin treatment of HPC derived from mobilized blood does not affect the number of CFU observed. Mobilized HPC from a lymphoma patient were enriched using a negative selection approach described in Materials and Methods. Increasing numbers of enriched HPC (25 to 200 cells per culture) in Methylcult were treated or not with 5 μg/mL SLT-1 for 60 minutes, washed, and finally dispensed in microtiter wells to measure colony formation. At day 20, the number of colonies were counted for replicate wells at each cell concentration. Values represent the average number of colonies (±SE) calculated from counting colonies present in 8 to 12 replicate wells. HPC from this patient were also shown to be negative for binding FITC-SLT-B (not shown).
Toxin treatment of HPC derived from mobilized blood does not affect the number of CFU observed. Mobilized HPC from a lymphoma patient were enriched using a negative selection approach described in Materials and Methods. Increasing numbers of enriched HPC (25 to 200 cells per culture) in Methylcult were treated or not with 5 μg/mL SLT-1 for 60 minutes, washed, and finally dispensed in microtiter wells to measure colony formation. At day 20, the number of colonies were counted for replicate wells at each cell concentration. Values represent the average number of colonies (±SE) calculated from counting colonies present in 8 to 12 replicate wells. HPC from this patient were also shown to be negative for binding FITC-SLT-B (not shown).
DISCUSSION
Shiga-like toxin 1, a ribosomal-inactivating protein produced by pathogenic strains of E coli (eg, O157:H7), intoxicates cells expressing globotriaosylceramide (CD77, Gb3) on their surface. It has recently been demonstrated that ex vivo treatment of murine BM with SLT-1 can effectively purge SCID mice of a B-cell lymphoma xenograft while sparing normal murine hematopoietic precursor cells, suggesting its potential use as an ex vivo purging agent.7 This study demonstrates the expression of Shiga-like toxin 1 receptors on 3 of the human cancers commonly treated by autologous stem cell transplant (breast cancer, lymphoma, and multiple myeloma) and proves the absence of SLT-1 receptor on human CD34+ stem cells. Taken together, this evidence supports the clinical use of SLT-1 as an ex vivo purging agent to deplete malignant cells expressing receptors for SLT-1 from autologous stem cell grafts.
The expression of SLT-1 receptors (SLT-R) was assessed on human breast cancer cell lines and clinical biopsies of primary human breast cancer tissues in light of the growing number of breast cancer patients treated each year with stem cell transplants.16 Eighteen breast cancer cell lines were tested. Thirteen of the 18 cell lines, some derived from breast cancer metastases,42 expressed SLT-R on their surface, whereas 80% of primary breast cancer biopsies were SLT-1 receptor-positive, indicating the potentially broad expression of SLT-1 receptors on tumor cells at both primary and metastatic sites. These findings identify CD77/SLT-R as a marker on clinical specimens of breast cancer and indicate that SLT-1 could be used to purge these cells. Previous evidence for the expression of CD77 on breast tissue indicated some weak immunostaining of normal breast ductal epithelium43-46 and the presence of CD77 in human breast milk, possibly shed or secreted by ductal cells.47Our results prove conclusively that CD77 is expressed on breast carcinomas. The previous finding of globo-H glycolipid on normal mammary gland epithelia and human mammary carcinomas demonstrate that breast cells have the necessary biosynthetic enzymes to make CD77 (a precursor to globo-H) and more complex globo-series glycolipids.48 Cell lines such as CAMA-1 and SKBR3 were resistant to the toxin, whereas CD50 values observed for other human breast cancer cell lines spanned several orders of magnitude. Interestingly, these results also indicate that the expression of SLT-1 receptors on cells is related to but not linearly correlated with their sensitivity to the toxin.
Flow cytometry was performed on 134 tumor samples from patients with hematological cancers stained with FITC-SLT-B. SLT-1 receptors are predominantly expressed on follicle center cell lymphoma, diffuse large B-cell lymphoma, and small lymphocytic lymphoma with or without chronic lymphocytic leukemia. SLT-1 receptors are expressed on B-CLL/SL samples in agreement with the expression of CD77 reported by others.43,49-52 Previous work indicated the expression of CD77 on cell lines established from multiple myeloma patients.53 We detected high levels of CD77 on BM plasma cells from 5 multiple myeloma patients. Circulating clonotypic B cells, previously shown to be part of the myeloma clone,23-25 39also bind SLT-1.
To determine whether SLT-1 might be an effective purging agent in lymphoma and multiple myeloma, fresh patient cells were treated with SLT-1 and purging was assessed using flow cytometry. We found that the concentrations of SLT-1 required to mediate toxic effects on primary malignant B cells (5 μg/mL) was considerably higher than that required to kill breast cancer cell lines. For 2 lymphoma node biopsies and B-CLL cells from 1 patient, substantial B-cell cytotoxicity was detected 6 to 10 days after a pretreatment period with SLT-1. In the case of multiple myeloma, pretreatment with SLT-1 mediated cytotoxicity at days 6 to 10 for both circulating B cells and BM-localized plasma cells. The prolonged delay in observing SLT-1–initiated cell death in vitro implies that, in the case of ex vivo purging protocols, cellular events leading to purging may occur after reinfusion. A more accurate determination of the log kill of myeloma plasma cells after SLT-1 pretreatment was achieved by using a novel cellular limiting dilution analysis for clonotypic transcripts.24 This RT-PCR assay uses primers specific for the unique IgH rearrangement that characterizes the myeloma clone in each patient. In untreated control cultures of myeloma BM, essentially 100% of the cells were clonotypic. However, in the SLT-1–treated cultures at 6 days of culture, the limiting dilution analysis indicated that no clonotypic cells were detectable, indicating a frequency of less than 1/3,000. Thus, SLT-1 pretreatment depleted myeloma plasma cells by greater than 3 log units. Taken together with recent work showing that G-CSF–mobilized blood from myeloma patients includes myeloma progenitors (Pilarski et al, manuscript submitted), these data suggest that SLT-1 purging of autologous stem cell transplants for multiple myeloma patients may improve their survival.54
Hematopoietic stem cells in BM or peripheral blood represent a subpopulation of CD34+ progenitor cells capable of long-term repopulation of the immune and hematopoietic systems. The absence of CD77 on CD34+ cells, early committed progenitors, or circulating B and T cells suggests that they may resist the effects of SLT-1. The expression of CD77 on hematopoietic cell lineages has been primarily limited to a small number of activated T cells and follicle center B cells and may be also a minor determinant on monocytes and erythrocytes.55-58 Platelets may also be CD77+.11,59 Colony-forming unit–granulocyte-macrophage (CFU-GM) or CFU-C are resistant to SLT-1, but a slight decrease in burst-forming unit-erythroid (BFU-E) counts7 suggests some sensitivity to the toxin. The survival of B cells from a posttransplant myeloma patient who had no detectable circulating B cells expressing the myeloma IgH clonotype indicates that normal human B cells escape SLT-1 toxicity. Using phenotypic analysis, we show here that normal HPC lack the receptor for SLT-1. However, a key criterion for purging tumor cells from stem cell grafts is that the purging agent must not damage graft repopulating activity, making a functional assay for HPC mandatory.
To test directly the effects of SLT-1 on hematopoietic progenitor cells, we pretreated MNC from G-CSF–mobilized blood with a concentration of toxin shown to be toxic for primary malignant B cells (5 μg/mL) and analyzed the survival of HPC, as measured by phenotypic analysis. For unfractionated MNC from mobilized blood of cancer patients, the numbers of CD34+45low HPC in untreated and SLT-1–treated cultures were identical. For HPC-enriched cells, pretreatment with SLT-1 resulted in even greater enrichment of surviving HPC at day 10 of culture, indicating that HPC escape SLT-1 toxicity. However, ultimately, clinical use of SLT-1 requires a demonstration that the functional activity of treated HPC is unimpaired. To address this issue, enriched HPC from mobilized blood were pretreated with SLT-1 and the number of CFU were enumerated. Equivalent numbers of CFU were observed from either untreated or SLT-1–pretreated HPC. A limiting dilution analysis of CFU indicated that, for SLT-1–pretreated enriched HPC, the frequency of CFU was 1/12, identical to that in untreated control cultures. These findings indicate that CFU also escape any SLT-1 toxicity. Thus, ex vivo purging with SLT-1 appears to be clinically feasible. Transplantation of toxin-treated and washed murine BM cells into SCID mice did not result in toxicity to the host, suggesting that the levels of residual toxin in the graft after washing steps are low.7
In conclusion, we have demonstrated that SLT-1 receptors are found on the surface of breast cancer, B lymphoma, B-CLL, and myeloma B and plasma cells. Malignant B cells in lymphoma, CLL, and myeloma were effectively purged by a pretreatment with SLT-1. A greater than 3 log depletion of clonogenic myeloma cells could be achieved with the toxin, as monitored by RT-PCR in the case of 1 myeloma patient. Phenotypic analysis and functional CFU assays conclusively show that hematopoietic progenitors escape SLT-1 toxicity. The presence of SLT-1 receptors on breast cancer, follicle center cell lymphoma, and multiple myeloma cells, together with the demonstrated lack of SLT-1 toxicity towards CD34+ stem cells, suggests a potential for SLT-1 as an ex vivo purging agent in removing tumor cells from autologous stem cell grafts.
ACKNOWLEDGMENT
The authors thank Papar Laraya, Rose Hurren, Marijke Koekebakker, Lori-Ann Webster, James Ho, Juliet Sheldon, and Denis Bouchard for their technical help; Dr Hans Messner and Nazir Jamal for providing peripheral blood stem cells; and Drs Brent Zanke and Heather Lochnan for their helpful comments. In Edmonton, Eva Pruski provided outstanding technical assistance, Dr Robert Coupland provided biopsies of B-lymphoma nodes, and the Red Cross Apheresis Unit provided aliquots of mobilized blood. We thank Agnieszka J. Szczepek for sequencing the RT-PCR products shown in Fig 8. This work is dedicated to the memory of Dr Ron Buick (1948-1996) for all his encouragement, advice, and support over the years.
Supported by a translational research grant to J.G. from the Leukemia Society of America and by a grant from the Medical Research Council of Canada to L.M.P. and A.R.B. E.C.L. was the recipient of a fellowship from the Medical Research Council of Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jean Gariépy, PhD, Department of Medical Biophysics, Room 7-117, Ontario Cancer Institute/Princess Margaret Hospital, 610 University Ave, Toronto, ON, Canada, M5G 2M9.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal