von Willebrand factor (vWF) is synthesized exclusively by endothelial cells and megakaryocytes, and stored in the intracellular granules or constitutively secreted into plasma. ABO blood group antigens are covalently associated with asparagine-linked sugar chains of plasma vWF. The effect of ABO-mismatched bone marrow transplantation (BMT) or blood stem cell transplantation (BSCT) on the expression of ABO blood group antigens on the vWF was examined to obtain information on the origin of these antigens. In ABO-mismatched (HLA-matched) groups, 8 cases of BMT and 4 cases of BSCT were examined. In all cases, the ABO blood groups on red blood cells were gradually converted to the donor’s type within 80 to 90 days after the transplantation. The blood group antigens on the vWF were consistent with the recipient’s blood group for the period monitored by enzyme-linked immunosorbent assay (ELISA). When vWF was isolated from normal platelets and examined for the blood group antigens using ELISA or immunoblotting, it showed few antigens. However, vWF extracted from veins expressed blood group antigens. These findings indicate that platelet (megakaryocyte)-derived vWF does not contain blood group antigens and that these antigens may be specifically associated with vWF synthesized in endothelial cells and secreted into plasma. Furthermore, it is possible that the persistence of the recipient’s blood group antigens on plasma glycoproteins such as vWF, independent of the donor-derived erythrocytes, after ABO-mismatched stem cell transplantation, may influence the immunological system in the production of anti-blood group antibodies resulting in the establishment of immunological tolerance in the recipient plasma.
VON WILLEBRAND FACTOR (vWF) is a multimeric large-plasma glycoprotein that plays a key role in the initial step of hemostasis and is also a carrier of blood coagulation factor VIII (FVIII).1-3 vWF mediates the adhesion of platelets to the sites of vascular damage under high-shear stress resulting in thrombotic platelet plug formation. vWF is specifically synthesized in endothelial cells and megakaryocytes. vWF is constitutively secreted from endothelial cells into circulating plasma and subendothelial matrices or stored in endothelial Weibel-Palade bodies and platelet α granules.4-6
Human plasma vWF has ABO blood group antigens on the asparagine-linked sugar chains.7,8 It is quite unique because only limited human plasma glycoproteins (vWF, FVIII, and a part of α2-macroglobulin) covalently link these antigens.9,10 The physiological function of these antigens on plasma vWF is not clear. However, the concentration of vWF has been known to be influenced by these blood groups and is significantly lower in persons with blood group O.11-15 In addition, Shima et al16 recently reported that the concentration of plasma vWF is affected by the presence of the O gene, namely, the vWF from homotypic genotype donors (AA and BB) showed a higher concentration than those from the heterotypic genotype donors (AO and BO). There are no significant differences among the blood groups in the amount of vWF within cells,17 the specific activity, or the multimeric pattern of plasma vWF.16 These results suggest the structure and the amount of blood group sugar chains on vWF molecule, such as the terminal sialic acids,18 may determine the circulating life span of vWF.
Bone marrow transplantation (BMT) or blood stem cell transplantation (BSCT) is an established medical treatment for patients with aplastic anemia, acute leukemia, and immunodeficiency diseases. Because ABO blood group antigens are independent of HLA gene complex, HLA-matched donors may be ABO-incompatible.19-21 In such cases, the blood group antigen of the recipient on red blood cells gradually turns to the donor’s type, but there is little information about the changes in plasma components containing blood group antigens. In the present study, we examined the blood group antigens on plasma vWF after ABO-mismatched BMT and BSCT, and the origin of the vWF with blood group antigens.
MATERIALS AND METHODS
Materials.
Plasma samples were collected from normal adults, and from donors and recipients of ABO-matched (three cases) or mismatched BMT (four cases of minor, three cases of major, and one case of major-minor mismatched, but HLA-matched) at intervals before and after transplantation, and stored at −80°C after addition of 1/50 volume to each of a protease-inhibitor mixture (final 4 mmol/L EDTA, 4 mmol/LN-ethylmaleimide, 4 mmol/L benzamidine, 400 kallikrein inhibitor units/mL of aprotinin). Three cases of allogeneic peripheral blood and one case of cord blood stem cell transplantation were also examined. Standard vWF was purified from FVIII concentrates as described previously.22
Enzyme-linked immunosorbent assay (ELISA).
ELISA plate (Immuno module; Nunc Intermed, Kamstrup, Denmark) was coated with 50 or 100 μL (in each well) of anti-vWF goat immunoglobulin G (IgG) (20 μg/mL; Medical and Biological Laboratory (MBL), Nagoya, Japan) in 100 mmol/L bicarbonate buffer, pH 9.6 overnight at 4°C, and blocked with 200 μL of Tris-buffered saline (TBS; 10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl) containing 1% bovine serum albumin (BSA) overnight at 4°C. The coated plate was used within 3 weeks after preparation and washed twice with 200 μL of TBS containing 0.05% tween 20 (TwTBS) before use. Plasma samples were appropriately diluted (10 to 100-fold with TwTBS) and 50 or 100 μL of each was applied to the anti-vWF coated plate and incubated for 90 minutes at room temperature. The plate was washed five times with 200 μL of TwTBS and incubated with either 50 or 100 μL of anti-A, anti-B monoclonal antibody (MoAb) (Ortho Diagnostics Systems, Raritan, NJ) diluted with TwTBS (10-fold for anti-A, fivefold for anti-B), biotin-conjugated UEA-I lectin (5 μg/mL, EY Laboratories, San Mateo, CA) that recognizes the type-2 H structure rich in blood group O, or horseradish peroxidase (HRP)-conjugated anti-vWF rabbit IgG (1/1,000 diluted with TwTBS; Dakopatts, Glostrup, Denmark) for 60 minutes. After washing with TwTBS, the plate treated with anti-A, anti-B, and UEA-I was incubated with HRP-conjugated anti mouse IgM (1/1,000 diluted with TwTBS, Zymed Laboratories, San Francisco, CA), anti mouse IgM (1/1,000) plus anti mouse IgG (1/1,000; TAGO Immunologicals, Camarillo, CA) and streptavidin (1/1,000, Vector Laboratories, Burlingame, CA) for 45 minutes, respectively. Peroxidase reaction was performed with 100 μL of solution containing 100 mmol/L NaCl, 0.5 mg/mLo-phenylenediamine, 0.02% H2O2, and 50 mmol/L Tris-HCl, pH 7.5, for 30 to 60 minutes at room temperature in the dark. The absorbance at 490 nm was measured with a plate reader after the addition of 100 μL of 8 mol/L H2SO4. vWF concentration was measured by ELISA using the control plasma N (Dade Behring, Marburg, Germany) and expressed as U/dL. Protein concentration was determined by bicinchoninic acid protein assay kit (Pierce, Rockford, IL) using BSA as a standard.
vWF from platelets.
Platelets (three samples each for A and B) were prepared from platelet-rich plasma by centrifugation (2,800 rpm, 10 minutes at 25°C), washed with phosphate-buffered saline (PBS; 150 mmol/L NaCl, 10 mmol/L Na-phosphate, pH 7.2) containing 9 mmol/L EDTA and 15% acid-citrate-dextrose (ACD) and twice with 150 mmol/L NaCl containing 10 mmol/L HEPES buffer, pH 7.5, and stored at −80°C until use. Platelets were suspended in five volumes of TwTBS containing protease inhibitors as described above and disrupted by sonication at 0°C with a Branson Sonifier 250 (Danbury, CT). After centrifugation at 15,000 rpm at 4°C for 45 minutes, the supernatant was used for ELISA as described above.
In a separate experiment, the soluble fraction of platelets and plasma from normal adults with blood group A or B (200 μL each) were mixed with 20 μL of anti-vWF goat IgG (15 mg/mL, MBL), respectively, for 3 days at 4°C. The immunoprecipitates were collected by centrifugation (15,000 rpm, 100 minutes at 4°C) and washed with TBS.
Aliquots of the washed immunoprecipitates were resolved with 25 μL of sodium dodecyl sulfate (SDS) buffer (2% SDS, 25% glycerol, 62.5 mmol/L Tris-HCl, pH 6.5) containing 5% 2-mercaptoethanol at 95°C, subjected to SDS-polyacrylamide gel electrophoresis (PAGE)23 and transferred to a polyvinylidene difluoride (PVDF) membrane as described.24 The membrane was incubated with TwTBS containing anti-A (1/10 diluted), anti-B (1/5 diluted) MoAb, or HRP-conjugated anti-vWF antibody (1/1,000 diluted) for 90 minutes at room temperature. After washing with TwTBS, HRP-conjugated second antibodies were used for detection of anti-A and -B binding, as described above. HRP reaction was performed using 0.2 mg/mL of diaminobenzidine and 0.05% H2O2 as a substrate.
vWF from vein.
Small pieces of renal vein from postmortem individuals with type A (two samples) and O (one sample), obtained with informed consent, were carefully cleaned and canurated with PBS before freezing. The frozen veins (0.2 g, wet weight) were cut into small pieces on ice, suspended in 1 mL of TwTBS containing 10 mmol/L EDTA and protease inhibitors, and disrupted by sonication at 0°C. After centrifugation (15,000 rpm, 2 hours at 4°C), the supernatant was used for ELISA. The concentration of fibrinogen, transferrin, α2-macroglobulin and vWF in the vein extracts and normal plasma were measured by sandwich ELISA using the corresponding HRP-conjugated antibodies (MBL, Dakopatts).
Other methods.
The blood group of the red blood cells and the anti-blood group antibodies was determined by conventional hemagglutination assay using anti-A and -B MoAbs and standard human type A1 and B red blood cells (Ortho Diagnostics) according to the manufacturer’s instructions.
RESULTS
ABO blood group antigens on red blood cells.
ABO blood group antigens on the surface of red blood cells and the anti-blood group antibodies in the plasma of the recipient with acute lymphocytic leukemia in first relapse (blood group AB, male, 36 years old), who was transplanted from a sibling donor (blood group O, female, 35 years old), were monitored for 161 days at intervals before and after BMT (Fig 1A) (the recipient experienced complete remission, but relapsed 7 months after transplantation and died of fungal infection even though a second BMT was performed). The recipient’s red blood cells with type AB showed a mixed field with anti-A and -B antibodies 1 week after transplantation, but had completely lost their reactivity to the antibodies after 90 days indicating that the blood group of the recipient was gradually converted to the donor’s type by ABO-mismatched BMT. The anti-blood group antibodies assayed by standard red blood cells (type A1 and B) showed that there was no production of either of these antibodies for the duration of the monitoring period (130 days after transplantation) (Fig 1A). In all cases of ABO-mismatched BMT examined, the antibodies against the recipient’s original blood group were not found in the plasma (Table1).
Blood group antigens on plasma vWF after ABO minor mismatched BMT. (A) The recipient (patient M.N.; type AB) who suffered from acute lymphocytic leukemia was transplanted with bone marrow from an HLA-matched but ABO minor mismatched donor (sister; type O). Plasma and red blood cells (RBC) were serially collected from the recipient. The reactivity of RBC against anti-A and -B antibodies was expressed as positive (+), negative (−) or mixed field (±) observations. The anti-blood group antibody production was expressed as titers. Each plasma sample was diluted to 1:20 with TwTBS and vWF in plasma was measured by ELISA using anti-vWF antibody and the concentration of vWF (□), the binding of anti-A (•), anti-B MoAbs (○) and UEA-I (▴) were monitored. Data express the average of three measurements. Arrow indicates reactivities of the donor. (B) HLA-matched and ABO minor mismatched BMT between patient K.Y. with type A who suffered from severe aplastic anemia at the second remission and the donor with type O (father). Concentration of vWF (□) and the blood group A antigen (•) on vWF in 1:80 diluted plasma were monitored. RBC showed the mixed field to anti-A from 14 days after transplantation.
Blood group antigens on plasma vWF after ABO minor mismatched BMT. (A) The recipient (patient M.N.; type AB) who suffered from acute lymphocytic leukemia was transplanted with bone marrow from an HLA-matched but ABO minor mismatched donor (sister; type O). Plasma and red blood cells (RBC) were serially collected from the recipient. The reactivity of RBC against anti-A and -B antibodies was expressed as positive (+), negative (−) or mixed field (±) observations. The anti-blood group antibody production was expressed as titers. Each plasma sample was diluted to 1:20 with TwTBS and vWF in plasma was measured by ELISA using anti-vWF antibody and the concentration of vWF (□), the binding of anti-A (•), anti-B MoAbs (○) and UEA-I (▴) were monitored. Data express the average of three measurements. Arrow indicates reactivities of the donor. (B) HLA-matched and ABO minor mismatched BMT between patient K.Y. with type A who suffered from severe aplastic anemia at the second remission and the donor with type O (father). Concentration of vWF (□) and the blood group A antigen (•) on vWF in 1:80 diluted plasma were monitored. RBC showed the mixed field to anti-A from 14 days after transplantation.
ABO Blood Group Antigens on Plasma vWF and Red Blood Cells (RBC) of the Recipients after ABO-Matched and Mismatched BMT
| Case . | Recipient . | Blood Group . | Time after BMT (months) . | vWF Conc.(U/dL) . | Blood Group after BMT . | Anti-blood Group Antibody Production . | |||
|---|---|---|---|---|---|---|---|---|---|
| Sex (age) . | Diagnosis . | Recipient . | Donor . | vWF . | RBC . | ||||
| ABO-Mismatched BMT | |||||||||
| Major | |||||||||
| M.H. | M (39) | CML | B | AB | 11 | 171 | B | AB | none |
| S.Y. | F (9) | ALL | O | A | 7 | 169 | O | A | anti-B |
| K.K. | M (32) | AML | O | B | 5 | 192 | O | B | n.d. |
| Minor | |||||||||
| K.Y. | M (17) | SAA | A | O | 9 | 144 | A | O | anti-B |
| T.T. | M (13) | WAS | B | O | 45 | 163 | B | O | anti-A |
| M.O. | F (24) | MDS | B | O | 10 | 92 | B | O | anti-A |
| M.N. | M (36) | ALL | AB | O | 5 | 278 | AB | O | none |
| Major + Minor | |||||||||
| K.F. | F (12) | PRCA | A | B | 38 | 234 | A | B | none |
| ABO-Matched BMT | |||||||||
| Y.S. | M (44) | MDS | A | A | 18 | 189 | A | A | anti-B |
| T.H. | M (37) | ALL | B | B | 35 | 339 | B | B | anti-A |
| M.I. | F (24) | AML | AB | AB | 6 | 272 | AB | AB | none |
| Case . | Recipient . | Blood Group . | Time after BMT (months) . | vWF Conc.(U/dL) . | Blood Group after BMT . | Anti-blood Group Antibody Production . | |||
|---|---|---|---|---|---|---|---|---|---|
| Sex (age) . | Diagnosis . | Recipient . | Donor . | vWF . | RBC . | ||||
| ABO-Mismatched BMT | |||||||||
| Major | |||||||||
| M.H. | M (39) | CML | B | AB | 11 | 171 | B | AB | none |
| S.Y. | F (9) | ALL | O | A | 7 | 169 | O | A | anti-B |
| K.K. | M (32) | AML | O | B | 5 | 192 | O | B | n.d. |
| Minor | |||||||||
| K.Y. | M (17) | SAA | A | O | 9 | 144 | A | O | anti-B |
| T.T. | M (13) | WAS | B | O | 45 | 163 | B | O | anti-A |
| M.O. | F (24) | MDS | B | O | 10 | 92 | B | O | anti-A |
| M.N. | M (36) | ALL | AB | O | 5 | 278 | AB | O | none |
| Major + Minor | |||||||||
| K.F. | F (12) | PRCA | A | B | 38 | 234 | A | B | none |
| ABO-Matched BMT | |||||||||
| Y.S. | M (44) | MDS | A | A | 18 | 189 | A | A | anti-B |
| T.H. | M (37) | ALL | B | B | 35 | 339 | B | B | anti-A |
| M.I. | F (24) | AML | AB | AB | 6 | 272 | AB | AB | none |
CML: chronic myeloid leukemia; ALL: acute lymphocytic leukemia; AML: acute myelogenous leukemia; SAA: severe aplastic anemia; WAS: Wiskott-Aldrich syndrome; MDS: myelodysplastic syndrome; PRCA: pure red cell aplasia; n.d.: not determined.
ABO blood group antigens on plasma vWF.
vWF in plasma was monitored using ELISA. Concentration of the vWF in the patients receiving a BMT was significantly higher (204 ± 42 U/dL, n = 6, P < .001) when compared with the average concentration in normal adults (91 ± 22 U/dL, n = 9) regardless of ABO-matched or mismatched BMT (Table 1). Plasma vWF, especially, was transiently increased after transplantation and gradually decreased as reported25,26 (Fig 1A and 1B). The level of ABO blood group antigens on the vWF also varied with the concentration of vWF, but it never converted to the donor’s type after ABO-mismatched BMT (Fig 1A and 1B). UEA-I lectin reacts with vWF from blood group O because it has more H-substance than the other groups.9 Reactivity of the recipient’s plasma vWF against UEA-I was less than that of the donor’s vWF except for a short period after BMT (Fig 1A).
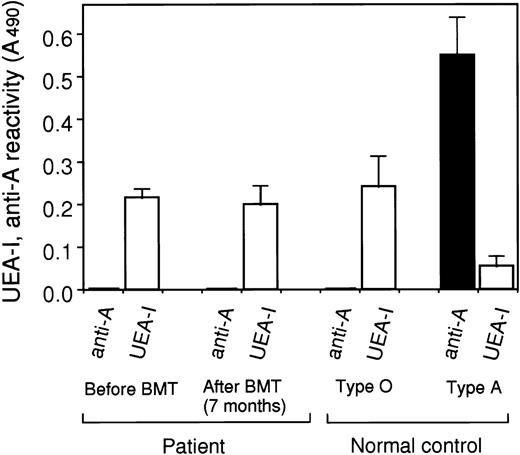
In the case of major mismatched BMT from a type A donor to a type O recipient (Fig 2), plasma vWF of the recipient at 7 months after transplantation still showed UEA-I binding activity similar to the level before transplantation. No reactivity was shown to anti-A antibody, indicating neither a significant reduction of the vWF with blood group O, nor the production of vWF with blood group A antigen.
Blood group antigens on plasma vWF after ABO major mismatched BMT. The reactivities of the recipient’s plasma vWF before and after (7 months) major mismatched BMT (patient S.Y. with type O and the donor with type A) against UEA-I (white) and anti-A MoAb (black) were measured using ELISA and normalized as the reactivity (A490) of 6 U/dL (1 μg/mL) of vWF solution. The reactivities of plasma vWF from normal subjects with blood group O and A against UEA-I and anti-A antibody were also measured as a control. Data express the means ± SE (n = 3) for a patient and the means ± SD (n = 6) for a control.
Blood group antigens on plasma vWF after ABO major mismatched BMT. The reactivities of the recipient’s plasma vWF before and after (7 months) major mismatched BMT (patient S.Y. with type O and the donor with type A) against UEA-I (white) and anti-A MoAb (black) were measured using ELISA and normalized as the reactivity (A490) of 6 U/dL (1 μg/mL) of vWF solution. The reactivities of plasma vWF from normal subjects with blood group O and A against UEA-I and anti-A antibody were also measured as a control. Data express the means ± SE (n = 3) for a patient and the means ± SD (n = 6) for a control.
In the eight cases of ABO-mismatched BMT examined, there was no change in the blood group of plasma vWF (Table 1). Examination 3 years, 9 months after transplantation showed that the vWF still continued to express the recipient’s blood group antigens but not the donor’s type.
In addition to bone marrow, BSCT was also examined. Three cases of allogeneic peripheral and one case of cord ABO-mismatched BSCT showed the same results as BMT (Table 2). Plasma vWF expressed the original blood groups in contrast to red blood cells, which were converted to express the donor’s type.
Blood Group Antigens on Plasma vWF and RBC of the Recipients after ABO-Mismatched Allogeneic-Peripheral Blood Stem Cell (PBSCT) and Cord Blood Stem Cell Transplantation (CBT)
| Case . | Recipient . | Blood Group . | Time after Transplantation (months) . | vWF Conc.(U/dL) . | Blood Group after Transplantation . | Anti-blood Group Antibody Production . | |||
|---|---|---|---|---|---|---|---|---|---|
| Sex (age) . | Diagnosis . | Recipient . | Donor . | vWF . | RBC . | ||||
| PBSCT | |||||||||
| N.T. | M (16) | CML | A | O | 7 | 247 | A | O | anti-B |
| A.T. | M (3) | JMML | A | O | 21 | 169 | A | O | anti-B |
| M.I. | F (8) | ALL | O | A | 15 | 117 | O | A | anti-B |
| CBT | |||||||||
| S.Y. | F (3) | FHL | B | O | 6 | 202 | B | O | anti-A |
| Case . | Recipient . | Blood Group . | Time after Transplantation (months) . | vWF Conc.(U/dL) . | Blood Group after Transplantation . | Anti-blood Group Antibody Production . | |||
|---|---|---|---|---|---|---|---|---|---|
| Sex (age) . | Diagnosis . | Recipient . | Donor . | vWF . | RBC . | ||||
| PBSCT | |||||||||
| N.T. | M (16) | CML | A | O | 7 | 247 | A | O | anti-B |
| A.T. | M (3) | JMML | A | O | 21 | 169 | A | O | anti-B |
| M.I. | F (8) | ALL | O | A | 15 | 117 | O | A | anti-B |
| CBT | |||||||||
| S.Y. | F (3) | FHL | B | O | 6 | 202 | B | O | anti-A |
JMML: juvenile myelomonocytic leukemia; FHL: familial hemophagocytic lymphohistiocytosis.
Blood group antigens on vWF from platelets and vein.
To elucidate the origin of blood group antigens on plasma vWF, we examined vWF extracted from both platelets and the renal vein. vWF immunoprecipitated from platelets and plasma both showed the 270 kD subunit band with some minor degraded bands by immunoblotting with anti-vWF antibody (Fig 3). Plasma vWF showed the corresponding blood group, whereas platelet vWF had no or only a faint blood group antigenicity. The latter showed two bands at about 110 and 130 kD that were reactive to the corresponding anti-blood group antibody, but did not react with anti-vWF antibody. Also using ELISA, platelet vWF showed no significant binding to the anti-blood group antibody (Fig 4).
Blood group antigens on vWF from plasma and platelets. vWF was immunoprecipitated from plasma (Pls) and the platelets (Plt) extracts from normal subjects with blood groups A and B. Aliquots of each immunoprecipitates and the standard vWF (vWF, 0.5 μg) were solubilized in SDS-buffer and subjected to SDS-PAGE under reducing conditions. Proteins were transferred to a PVDF membrane followed by immunoblotting with anti-vWF antibody, anti-A and B MoAbs. vWF showed the subunit band at about 270 kD. Blood group antigens were detected on the vWF band including minor degraded band at about 140 kD prepared from plasma and the purified vWF. vWF from platelets showed no or a very faint reactivity against blood group antibody, but platelets contained smaller bands at about 110 and 130 kD that weakly reacted with anti-blood group antibodies but not with anti-vWF antibody. Numbers on the left indicate the positions of molecular mass standard (kDa). The same results were obtained when using the other two platelet specimens from blood groups A and B.
Blood group antigens on vWF from plasma and platelets. vWF was immunoprecipitated from plasma (Pls) and the platelets (Plt) extracts from normal subjects with blood groups A and B. Aliquots of each immunoprecipitates and the standard vWF (vWF, 0.5 μg) were solubilized in SDS-buffer and subjected to SDS-PAGE under reducing conditions. Proteins were transferred to a PVDF membrane followed by immunoblotting with anti-vWF antibody, anti-A and B MoAbs. vWF showed the subunit band at about 270 kD. Blood group antigens were detected on the vWF band including minor degraded band at about 140 kD prepared from plasma and the purified vWF. vWF from platelets showed no or a very faint reactivity against blood group antibody, but platelets contained smaller bands at about 110 and 130 kD that weakly reacted with anti-blood group antibodies but not with anti-vWF antibody. Numbers on the left indicate the positions of molecular mass standard (kDa). The same results were obtained when using the other two platelet specimens from blood groups A and B.
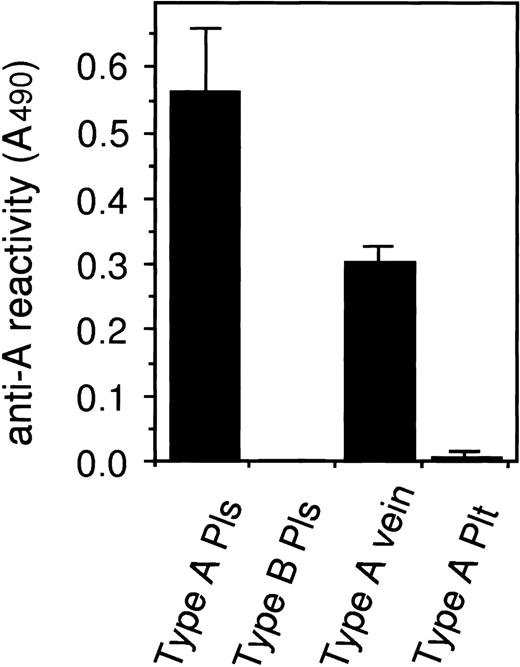
Blood group antigens on vWF extracted from renal veins and platelets. vWFs in the normal plasma (Pls) and in the extracts of veins and platelets (Plt) from the subjects with type A were captured with anti-vWF antibody on an ELISA plate. The concentration of vWF and the reactivity against anti-A MoAb was measured and normalized as the anti-A reactivity (A490) of 6 U/dL (1 μg/mL) of vWF solution. Each value indicates means ± SD (n = 3 except for type A vein, n = 2).
Blood group antigens on vWF extracted from renal veins and platelets. vWFs in the normal plasma (Pls) and in the extracts of veins and platelets (Plt) from the subjects with type A were captured with anti-vWF antibody on an ELISA plate. The concentration of vWF and the reactivity against anti-A MoAb was measured and normalized as the anti-A reactivity (A490) of 6 U/dL (1 μg/mL) of vWF solution. Each value indicates means ± SD (n = 3 except for type A vein, n = 2).
vWF in the renal vein extracts was examined using ELISA. Among the samples from two subjects with type A and one subject with type O, vWF from the type A subjects clearly showed blood group A antigenicity, although the reactivity was about half that of plasma vWF from normal blood type A subjects (Fig 4). Vein vWF from the type O subject showed only UEA-I reactivity (data not shown). To address the question of whether vWF in the vein extracts used was mostly derived from contaminated plasma, we measured the contents of several plasma proteins in the extracts and compared them with those in normal plasma. Fibrinogen, transferrin, and α2-macroglobulin in the vein extracts used were estimated to be 0.4 ± 0.1, 0.4 ± 0.1, and 0.6 ± 0.3 U/dL, respectively (n = 3), whereas the vWF in the extracts was 12.2 ± 2.2 U/dL. These findings suggest that the vein extracts contained a greater amount of vWF compared with other plasma proteins and that vWF derived from plasma appeared to be less than 1%.
DISCUSSION
HLA-matched but ABO-mismatched BMT has no influence on marrow engraftment, graft rejection, and graft-versus-host disease if appropriate care, such as plasma exchange and antibody absorption, is performed to avoid acute hemolysis.19-21 However, delayed hemolysis, retarded growth of erythroblast, or undergrown erythrocytes have often been observed as complications.27-29 Production of anti-blood group antibody must be controlled by the remaining host antigens and the donor-derived lymphocytes. It has been reported that blood group antigens are covalently linked to vWF, FVIII, and α2-macroglobulin in plasma.7-10 In the present study, the blood group antigens on erythrocytes gradually converted to the donor’s type, but the blood group on plasma vWF did not change after ABO-incompatible BMT (and BSCT). No antiblood group antibody against the recipient’s original blood group was detected after the transplantation. Wernet and Mayer30 reported that isoagglutinins against the recipient’s original red blood cell type are produced only during the early days after transplantation even though the patient has converted to the donor’s red blood cell type after ABO-mismatched BMT. Although it is probable that the immunosuppressing treatment might interfere with the production of antibodies in the recipient, the observed pattern of antibody production (Table 1) suggests that the remaining blood group antigens on plasma glycoproteins such as vWF might contribute to the establishment of immunological tolerance.
The finding that plasma vWF continued to express the recipient’s blood group after ABO-mismatched BMT suggested two possibilities for the origin of these antigens on the vWF. One was that vWF produced in megakaryocytes, differentiated from bone marrow stem cells, would not be secreted but stored in platelets. The other was that vWF in platelets originally had no blood group antigens. Almost all plasma vWF has been shown to be supplied from endothelial cells rather than platelets by crossed BMT of pigs,31 suggesting that the secretion of vWF from platelets is limited to the local area at thrombosis. Platelets have been known to have both covalently and noncovalently bound blood group antigens.32,33 Recently, the covalently bound antigens have been found in platelet membrane glycoproteins (GP) such as GPIa, Ib, IIa, IIb, IIIa,34,35IV, and V,36 suggesting that platelets (megakaryocytes) have a machinery to assemble the blood group antigens. We have prepared platelet vWF, but it has no or only a faint blood group antigenicity. The 110 and 130 kD proteins observed in the immunoprecipitated platelet vWF (Fig 3) seem to be GPs coprecipitated with vWF. The absence of blood group antigens in platelet vWF has also been recently reported by two groups.37 38 The very faint blood group reactivity observed in the platelet vWF (Fig 3 and 4) might be a contamination from the plasma vWF adsorbed onto the platelets.
Another vWF producing site is endothelial cells. We found that the renal vein extracts contained vWF with blood group antigens. Expression of the blood group antigens by the vWF molecule was about half that of the plasma vWF, suggesting that vWF molecules with no or a small amount of blood group antigens also exist. It is possible that these vWFs are incompletely glycosylated. Alternatively, the glycosylation may be different between vWF that is constitutively secreted and that stored in the regulated Weibel-Palade body pathway. Recently, Yamamoto et al39 reported that the synthesis of vWF in endothelial cells varied among the organs in mice. Glycosylation is regulated by glycosyltransferases and trimming glycosidases in cells, suggesting that the blood group antigen production might also be altered by each organ. When we analyzed vWF extracted from cultured human umbilical vein endothelial cells, no significant blood group antigens were observed and neonatal plasma vWF showed a lower expression of these antigens (Matsui, unpublished observations), suggesting that the blood group antigens on plasma glycoproteins may also be developmentally regulated like embryonic antigens.40
Our present findings, together with the recent findings of Brown et al37 showing that plasma vWF with blood group antigens was rapidly increased after administration of DDAVP to a type 1 von Willebrand disease patient, strongly suggest that vWF with blood group antigens is specifically glycosylated in endothelial cells but not in megakaryocytes. It is also possible that the blood group antigens are attached to vWF extracellularly by plasma glycosyltransferases after secretion. A or B transferases are still present in plasma in accordance with the recipient’s type even after ABO-mismatched BMT.41 However, transplanted O-type erythrocytes to type-A recipients did not show A antigens even though the plasma contained A-type vWF (Table 1) and A transferase (Sako, unpublished observation). Furthermore, it is not likely that the plasma contains enough sugar nucleotide donors such as UDP-N-acetylgalactosamine and UDP-galactose for A and B transferase, respectively.
Although the biological function of the blood group antigens on vWF is still not clear, the different glycosylation between platelet and endothelial vWF might influence the function of each pool of vWF in hemostasis or in its association with FVIII. Recently, Sarode et al38 reported that the blood group sugar chains on vWF influenced the ristocetin-induced platelet agglutinating activity. Further studies on the relationships between thrombotic complications followed by ABO-mismatched BMT or BSCT and the presence of blood group antigens on vWF may contribute to more successful transplantation.
ACKNOWLEDGMENT
We thank S. Nishida and S. Ishihara for their technical assistance. We also thank Dr D. Mrozek for editing the manuscript.
Supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (to T.M. and K.T.) and Fujita Health University (to K.T. and J.H.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal