We have recently shown that, in human neutrophils, interleukin-10 (IL-10) fails to induce specific DNA-binding activities to the gamma-interferon response region (GRR), a regulatory element located in the FcγRI gene promoter, which is required for transcriptional activation by IL-10 and interferon γ (IFNγ) in monocytic cells. In this study, we report that IL-10 is also unable to induce the binding of STAT1 or STAT3 to the serum-inducible element (hSIE/m67), despite the fact that both proteins are expressed in neutrophils. Whereas IFNγ and granulocyte colony-stimulating factor (G-CSF) are efficient inducers of STAT1 and STAT3 tyrosine phosphorylation in polymorphonuclear neutrophils (PMN), IL-10 fails to trigger STAT1 and STAT3 tyrosine and serine phosphorylation, therefore explaining its inability to induce the FcγRI expression in these cells. By contrast, we demonstrate that IL-10 alone represents an efficient stimulus of CIS3/SOCS3 mRNA expression in neutrophils. CIS3/SOCS3 belongs to the recently cloned cytokine-inducible SH2-containing protein (CIS) gene family (which also includes CIS1, CIS2, CIS4, CIS5, and JAB) that is believed to be, at least in part, under the control of STAT transcription factors and whose products are potential modulators of cytokine signaling. Moreover, IL-10 synergizes with lipopolysaccharide (LPS) in upregulating CIS3/SOCS3 mRNA expression in PMN through a mechanism that involves mRNA stabilization. In contrast to CIS3/SOCS3, mRNA transcripts encoding other family members are unaffected by IL-10 in neutrophils. Finally, transfection of CIS3/SOCS3 in murine M1 myeloid cells suppresses LPS-induced growth arrest, macrophage-like differentiation, and nitric oxide synthesis, but not IL-6 mRNA expression. Collectively, our data suggest that, in neutrophils, the activation of STAT1 and STAT3 phosphorylation is neither required for CIS3/SOCS3 induction by IL-10 nor involved in the regulatory effects of IL-10 on cytokine production.
INTERLEUKIN-10 (IL-10) is an 18-kD nonglycosylated protein that is mainly synthesized by helper T cells, B cells, activated monocytes, macrophages, thymocytes, and keratinocytes and has the capacity to attenuate a wide range of inflammatory and immune responses.1 Although IL-10 downregulates specific effector functions in monocytes, natural killer (NK) cells, B cells, and Th1 cells,1,2 recent studies performed in our laboratory, as well as by other groups, have shown that a number of polymorphonuclear neutrophil (PMN) functional responses are also regulated by IL-10.3 Among the latter, the modulation of cytokine and chemokine production by IL-10 has been the focus of intensive investigation.4-9 However, more recently, we10 and others11,12 found that IL-10 fails to induce the surface expression of the high-affinity receptor for IgG (FcγRI/CD64) in PMN. The inability of IL-10 to induce FcγRI in neutrophils was in a sense surprising, in view of earlier studies performed in human monocytes and murine macrophages, which had demonstrated that FcγRI gene and surface expression are upregulated by IL-10, both in vitro and in vivo.13-15 A likely explanation for this cell-type–specific action of IL-10 is related to the fact that the FcγRI promoter contains a 39-bp region that is necessary and sufficient for the induction of gene expression by interferon γ (IFNγ) or IL-10.14,16-21 Consistent with this interpretation, we10 and others14,20-22reported that gamma-interferon response region (GRR)-binding complexes containing STAT1 and STAT3 are effectively induced in monocytes and peripheral blood mononuclear cells (PBMC) in response to IL-10, whereas we found that, in neutrophils, no GRR-binding activities are inducible by IL-10.10
In this study, we further investigated the inability of neutrophils to activate GRR-binding complexes in response to IL-10. In agreement with previous studies,20,22,23 we observed that the IL-10–elicited induction of GRR-binding activities is accompanied by the tyrosine phosphorylation of STAT1 and STAT3 in PBMC. However, in neutrophils, neither STAT1 nor STAT3 becomes tyrosine or serine phosphorylated in response to IL-10. To gain further insight into IL-10 signaling, we also investigated whether the expression of cytokine-induced Src homology 2-containing (CIS) proteins might be modulated by IL-10 in neutrophils. Members of this novel family of cytokine-inducible genes are currently generating much interest, because they have the potential to negatively regulate the JAK-STAT signaling pathway.24-31 We now report that IL-10 directly stimulates the expression of CIS3 mRNA, both in human neutrophils and PBMC, and that stable transfection of CIS3 into a murine myeloid cell line suppresses macro- phage differentiation and nitric oxide synthesis induced by lipopolysaccharide (LPS). The implications of our findings and the possible role of CIS3 in the context of IL-10 signaling are discussed.
MATERIALS AND METHODS
Cell purification and culture.
Highly purified granulocytes (>99.5%) and PBMC were isolated under LPS-free conditions from buffy coats of healthy donors by centrifugation on a Ficoll-Hypaque gradient, as previously described.32 Monocytes were isolated from PBMC after centrifugation over Percoll gradients, as described earlier.33 After purification, leukocytes were suspended in RPMI 1640 supplemented with 10% low endotoxin fetal calf serum (FCS; <0.006 ng/mL; Hyclone Laboratories Inc, Logan, UT) and treated with either 100 U/mL IFNγ or up to 2,000 U/mL IL-10 (kindly provided by Dr K. Moore, DNAX and Schering-Plough Corp, Palo Alto, CA).10Optimal biological effects were obtained using either 100 U/mL IL-10 from DNAX4,5 or 20 ng/mL IL-10 purchased from Peprotech Inc (Rocky Hill, NJ). In selected experiments, PMN were also treated with 1,000 U/mL granulocyte colony-stimulating factor (G-CSF; Granulokine; Hoffmann-LaRoche, Basel, Switzerland), 100 ng/mL phorbol myristate acetate (PMA; purchased from Sigma, St Louis, MO), 100 ng/mL LPS (fromEscherichia coli, serotype 026:B6; Sigma), or 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Genetics Institute, Boston, MA). Leukocytes thus treated were cultured in polystyrene flasks or polypropylene tubes (Greiner, Nürtingen, Germany) at 37°C under a 5% CO2 humidified atmosphere or (for electrophoretic mobility shift assays [EMSA] studies) at room temperature.10 Murine M1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% horse serum, as previously described.26,27 34 All reagents were of the highest available grade and all buffers were prepared using pyrogen-free water for clinical use.
Cellular extracts.
After stimulation for the indicated times, neutrophils (1 to 2 × 108/condition) or monocytes/PBMC (0.3 to 1 × 108/condition) were diluted in ice-cold phosphate-buffered saline (PBS) and centrifuged twice at 500g for 5 minutes at 4°C. The cells were then suspended in relaxation buffer and disrupted in a nitrogen bomb (Parr Instruments, Mobile, IL), and extracts were prepared exactly as described.35Alternatively, cells were pelleted, washed in ice-cold PBS, and resuspended in lysis buffer (20 mmol/L HEPES-KOH, pH 7.5, 350 mmol/L KCl, 1 mmol/L MgCl2, 0.5 mmol/L EDTA, 0.1 mmol/L EGTA, 20% [vol/vol] glycerol, 1% [vol/vol] Nonidet P-40, and 5 mmol/L dithiothreitol [DTT]) containing protease and phosphatase inhibitors (1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 10 μg/mL pepstatin A, 5 mg/mL α1-antitrypsin, 1 mmol/L Na3VO4, and 10 mmol/L NaF).36 After 15 minutes of incubation on ice, cell debris were spun down (12,000g for 20 minutes at 4°C), and the supernatants were frozen and stored at −80°C. Small aliquots of the various extracts were routinely processed for protein content determination by using a protein assay kit (Bio-Rad, Hercules, CA).
EMSA.
Protein-DNA complexes were detected by EMSA analysis of the various extracts as previously described,10,35 with the following modifications: 4 to 40 μg of cytoplasmic or 5 to 20 μg of nuclear extracts was usually incubated for 10 minutes at room temperature in a buffer containing 10 mmol/L Tris, pH 7.5, 100 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L DTT, 100 μg/mL poly (dI-dC).poly (dI-dC), 50 μg/mL salmon sperm, and 10% glycerol, followed by the addition of a 32P-labeled double-stranded oligonucleotide probe corresponding to the GRR element located within the promoter of the FcγRI gene16 (5′ CTT TTC TGG GAA ATA CAT CTC AAA TCC TTG AAA CAT GCT 3′) or the high-affinity synthetic derivative of the c-sis-inducible element (SIE), hSIE/m67 (5′ gtc gaC ATT TCC CGT AAA TCg 3′) for 15 minutes.36 Supershift experiments were performed by incubating the proteins with 0.5 μg of the various anti-STAT antibodies for 30 minutes at room temperature, before adding the labeled probe. Anti-STAT1 (E-23, raised against amino acids 688-710), anti-STAT3 (C20, raised against amino acids 750-769), and anti-STAT5 (C17, raised against amino acids 711-727 of STAT5b p80 of mouse origin, and specific for STAT5a and STAT5b) antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA).
Immunoprecipitations and immunoblots.
For the immunoprecipitation experiments, nuclear plus cytoplasmic fractions from cavitated neutrophils (2 mg) or PBMC (0.85 mg) were incubated for 2 hours at 4°C on a rotating wheel with 20 μL of a 50% slurry of protein A agarose (Boehringer Mannheim, Mannheim, Germany) in the presence of a 1:300 dilution of anti-STAT3 antibody (C-20) or anti-STAT1 antiserum (a generous gift from Dr K. Ozato, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD).37 The immunoprecipitates were washed 5 times with a rinsing buffer (40 mmol/L Tris, pH 8, 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L Na3VO4, and 50 mmol/L NaF) and once with TBS (20 mmol/L Tris, pH 7.6, 137 mmol/L NaCl) before elecrophoretic separation on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent transfer to nitrocellulose by electroblotting. Nitrocellulose membranes were incubated overnight at 4°C in blocking buffer (TBS, 0.2% Tween-20, 7.5% bovine serum albumin [BSA]), before 2 hours of incubation at room temperature with the primary antibodies at the following dilutions: antiphosphotyrosine antibody 4G10 (Upstate Biotechnology Inc, Lake Placid, NY), 1:1,000; anti-STAT3 (K-15; Santa Cruz), 1:2,000; and anti-STAT1 (C-111; Santa Cruz), 1:1,500.
For the direct detection of tyrosine-phosphorylated STAT1 and STAT3 and of serine-phosphorylated STAT1 and STAT3, detergent lysates prepared from PMN and PBMC according to the method described by Vollebregt et al38 were electrophoresed and electroblotted as described above. Membranes were first blocked for 1 hour at room temperature in TBS/T (20 mmol/L Tris-HCl, pH 7.6, 137 mmol/L NaCl, 0.1% Tween 20) containing 5% BSA and then incubated overnight at 4°C in the presence of the phospho-specific primary antibodies. The latter were phospho-specific STAT1 antibody (Tyr701; 9171S; New England Biolabs, Beverly, MA) diluted 1:500 in blocking buffer, phospho-specific STAT3 antibody (Tyr705; 9131S; New England Biolabs) diluted at 1:1,000, phospho-specific STAT1 antibody (Ser727; 06-802; Upstate Biotechnology) diluted 1:1,000 in blocking buffer, and phospho-specific STAT3 antibody (Ser727; 06-803 [Upstate Biotechnology] or 9134S [New England Biolabs]) diluted at 1:1,000. Membranes were then probed with anti-STAT1 (E-23; Santa Cruz) or anti-STAT3 (C-20; Santa Cruz) diluted 1:2,000 in blocking buffer. Antibody binding was detected by using horseradish peroxidase-conjugated antimouse or antirabbit IgG and shown using the chemiluminescence system (ECL; Amersham, Arlington Heights, IL) according to the manufacturer’s instructions.
Northern blot analyses.
Total RNA was extracted from PMN, PBMC, or M1 cells by the guanidinium isothiocyanate method and processed for Northern blot analysis, as already described.32 Individual mRNA species in human cells were detected by autoradiography after hybridization of nylon filters with cDNA probes labeled with 32P using Ready-to-go kits (Pharmacia, Uppsala, Sweden). The probes used consisted of full-length cDNA fragments encoding CIS,27 IL-8, tumor necrosis factor α (TNFα), IL-1 receptor antagonist,4,5 and actin (kindly provided by Dr G. Trinchieri, Wistar Institute, Philadelphia, PA). The extent of hybridization was quantitatively analyzed in an InstantImager (Packard Instruments, Meriden, CT) and plotted after actin normalization. For mRNA half-life experiments, data were plotted on semilogarithmic graphs as the percentage of remaining mRNA versus time decays in minutes, and the resulting values were plotted against time. Half-lives were calculated by regression analysis. For M1 cells, total RNA was extracted after stimulation with 100 ng/mL LPS, hybridized with digoxigenin (DIG)-labeled riboprobes, and then visualized using alkaline-phosphatase–labeled anti-DIG antibodies according to the manufacturer’s instructions (Boehringer Mannheim). cDNA for murine IL-6 and G3PDH have been previously described.27
LPS-induced differentiation of M1 cells.
M1 stable transfectants were obtained by electroporation with pcDNA carrying Myc-tagged full-length CIS3 and selected with 0.8 mg/mL G418, as previously described.27 Two to 4 independent clones were tested for LPS-induced differentiation and growth arrest. Briefly, 105 parental M1 cells and CIS3-expressing clones were cultured in medium containing 10% horse serum supplemented with 100 ng/mL LPS for 3 days and then subjected to May-Grunwald-Giemsa staining.
Assay for nitric oxide (NO) synthesis by M1 cells.
Parental M1 cells and CIS3 transfectants were cultured in 24-well tissue culture plates at a density of 106 cells/well at 37°C. After 1 and 3 days of culture in the presence or absence of LPS, the NO content of culture supernatants was measured using the Griess reagents. Nitrite concentrations were calculated from a standard curve derived from the reaction of NaNO2 in the assay.
RESULTS
Lack of induction of STAT-binding complexes by IL-10 in neutrophils.
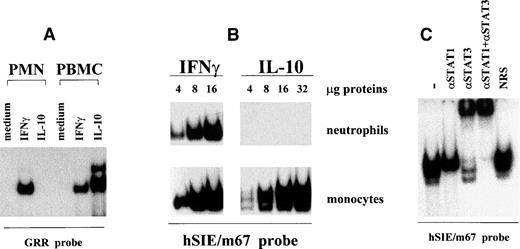
Figure 1A shows that no GRR-binding activities are detectable in cytoplasmic (or nuclear) extracts of IL-10–treated PMN. In sharp contrast, GRR-binding activities are consistently detectable in preparations from IL-10–stimulated PBMC or from IFNγ-stimulated cells (Fig 1A). Identical results were obtained when increasing amounts of neutrophil and monocyte extracts were analyzed by EMSA using a serum-inducible element (hSIE) probe (Fig 1B) that binds STAT protein complexes with a higher affinity than the GRR.39,40 Figure 1C shows that the hSIE-binding complexes induced in monocytes by IL-10 contain both STAT1 and STAT3, but not STAT5 (data not shown), in keeping with previous observations made using a GRR probe.10 By comparison, the hSIE/m67-binding activities induced by IFNγ in PMN contain only STAT1 (not shown).
Lack of induction of DNA-binding complexes by IL-10 in neutrophils. (A) PMN and autologous PBMC were incubated for 20 minutes in the presence or absence of 100 U/mL IL-10 or IFNγ. Cytoplasmic extracts were then prepared by nitrogen cavitation and analyzed in EMSA, using a 32P-labeled GRR oligonucleotide. For PMN extracts, 40 μg of protein was used in the binding reactions, whereas 10 μg of protein was used for PBMC extracts. This experiment is representative of at least 10. (B) PMN and autologous monocytes were incubated for 15 minutes with 100 U/mL IL-10 or IFNγ, and the resulting whole-cell extracts were analyzed in EMSA, using a32P-labeled hSIE/m67 oligonucleotide. Amounts of extract used are indicated. This experiment is representative of 2. (C) Characterization of the hSIE/m67-binding complexes induced in IL-10–treated monocytes. Purified monocytes were treated for 20 minutes with 100 U/mL IL-10, and whole-cell extracts were analyzed in EMSA. Binding reactions were performed in the presence or absence of specific anti-STAT antibodies as indicated, before the addition of the hSIE/m67 probe. This experiment is representative of 4.
Lack of induction of DNA-binding complexes by IL-10 in neutrophils. (A) PMN and autologous PBMC were incubated for 20 minutes in the presence or absence of 100 U/mL IL-10 or IFNγ. Cytoplasmic extracts were then prepared by nitrogen cavitation and analyzed in EMSA, using a 32P-labeled GRR oligonucleotide. For PMN extracts, 40 μg of protein was used in the binding reactions, whereas 10 μg of protein was used for PBMC extracts. This experiment is representative of at least 10. (B) PMN and autologous monocytes were incubated for 15 minutes with 100 U/mL IL-10 or IFNγ, and the resulting whole-cell extracts were analyzed in EMSA, using a32P-labeled hSIE/m67 oligonucleotide. Amounts of extract used are indicated. This experiment is representative of 2. (C) Characterization of the hSIE/m67-binding complexes induced in IL-10–treated monocytes. Purified monocytes were treated for 20 minutes with 100 U/mL IL-10, and whole-cell extracts were analyzed in EMSA. Binding reactions were performed in the presence or absence of specific anti-STAT antibodies as indicated, before the addition of the hSIE/m67 probe. This experiment is representative of 4.
STAT1 and STAT3 are not phosphorylated after IL-10 stimulation in neutrophils.
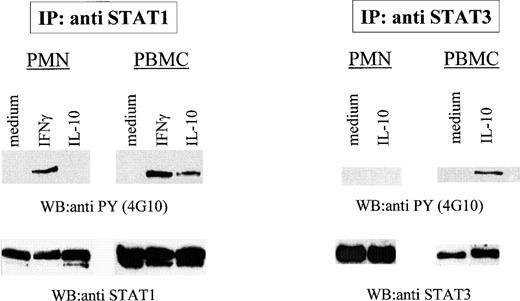
We previously showed that homodimers and heterodimers of these proteins can be induced to bind STAT-binding probes (GRR and hSIE/m67) in response to G-CSF stimulation.41 Thus, the lack of responsiveness of PMN to IL-10 in terms of GRR- or hSIE/m67-binding activities cannot be attributed to a general inability of these cells to activate STAT1 and STAT3. Because tyrosine phosphorylation of STAT proteins is required for DNA binding,42,43 we initially investigated whether STAT1 and STAT3 become tyrosine phosphorylated after IL-10 stimulation of PMN, as previously shown for monocytes and lymphocytes.20,22 23 For this purpose, neutrophils and autologous PBMC were stimulated for 15 minutes with either IL-10 (up to 1,000 U/mL) or IFNγ, before disruption of the cells by nitrogen cavitation. The resulting cytoplasmic preparations were immunoprecipitated with anti-STAT3 or anti-STAT1 antibodies and analyzed by immunoblot using an anti-phosphotyrosine (4G10) antibody. As shown in Fig 2, IFNγ and (to a lesser extent) IL-10 both induced the tyrosine phosphorylation of STAT1 in PBMC, whereas, in neutrophils, STAT1 became tyrosine phosphorylated only in response to IFNγ. Similarly, IL-10 promoted the phosphorylation of STAT3 on tyrosine residues in PBMC, but failed to do so in PMN (Fig 2). Stripping and reprobing the nitrocellulose membranes with anti-STAT1 or anti-STAT3 antibodies ascertained that comparable amounts of proteins had been immunoprecipitated in these experiments. Identical results were obtained using neutrophil or PBMC whole-cell extracts (data not shown).
Defective activation of STAT1 and STAT3 tyrosine phosphorylation in IL-10–treated PMN. PMN and autologous PBMC were incubated in the presence or absence of 100 U/mL IL-10 or IFNγ for 15 minutes before lysis. STAT1 and STAT3 were immunoprecipitated and the membranes were blotted with antiphosphotyrosine antibodies (4G10). Similar amounts of immunoprecipitated material were loaded in each lane, as judged from subsequent reblotting with appropriate antibodies. The data shown are representative of 4 independent experiments.
Defective activation of STAT1 and STAT3 tyrosine phosphorylation in IL-10–treated PMN. PMN and autologous PBMC were incubated in the presence or absence of 100 U/mL IL-10 or IFNγ for 15 minutes before lysis. STAT1 and STAT3 were immunoprecipitated and the membranes were blotted with antiphosphotyrosine antibodies (4G10). Similar amounts of immunoprecipitated material were loaded in each lane, as judged from subsequent reblotting with appropriate antibodies. The data shown are representative of 4 independent experiments.
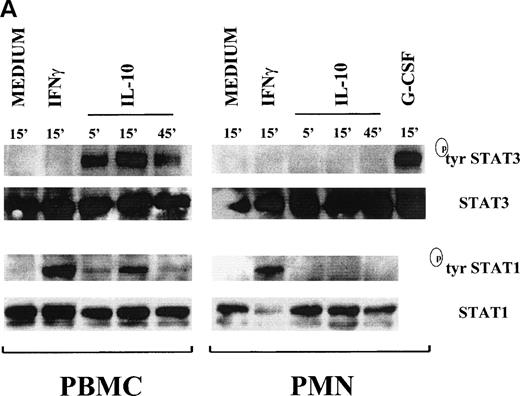
Time course experiments on whole-cell extracts prepared by detergent lysis and analyzed with antibodies that specifically recognize the phosphotyrosine forms of STAT1 and STAT3 confirmed that STAT1 and STAT3 are phosphorylated on tyrosine residues after treatment of neutrophils with IFNγ and G-CSF, respectively, but not with IL-10 (Fig 3A). In PBMC, a strong tyrosine phosphorylation of STAT3 in response to IL-10 was already evident by 5 minutes, reached a maximum at 15 minutes, and was still detectable at 45 minutes; STAT1 tyrosine phosphorylation followed an analogous time course, albeit with a less intense signal (Fig 3A).
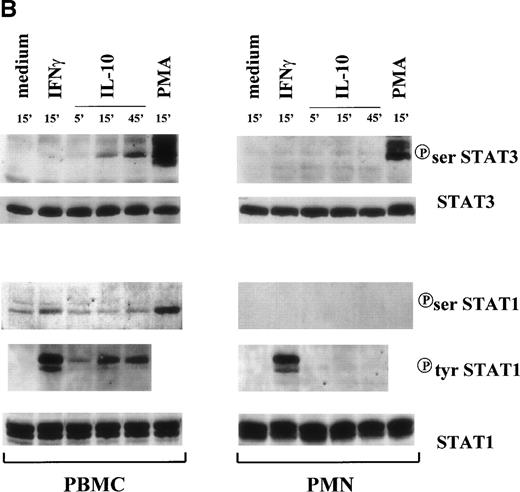
Time course of STAT1 and STAT3 phosphorylation in IL-10–treated PMN and PBMC. Cells were treated with 100 U/mL IL-10, 100 U/mL IFNγ, and 1,000 U/mL G-CSF (A) or 20 ng/mL IL-10 (Peprotech), 100 U/mL IFNγ, and 100 ng/mL PMA (B) before lysis under denaturating conditions as described in Materials and Methods. One hundred fifty micrograms of PMN lysate and 80 μg of PBMC lysate were loaded on the gels; and immunoblots were performed using antibodies specific for tyrosine or serine phosphorylated forms of STAT1 and STAT3. Subsequent reblotting with the indicated anti-STAT1 or anti-STAT3 antibodies was performed to ensure that similar amounts of material were deposited in each lane. The data for each panel are representative of 4 independent experiments.
Time course of STAT1 and STAT3 phosphorylation in IL-10–treated PMN and PBMC. Cells were treated with 100 U/mL IL-10, 100 U/mL IFNγ, and 1,000 U/mL G-CSF (A) or 20 ng/mL IL-10 (Peprotech), 100 U/mL IFNγ, and 100 ng/mL PMA (B) before lysis under denaturating conditions as described in Materials and Methods. One hundred fifty micrograms of PMN lysate and 80 μg of PBMC lysate were loaded on the gels; and immunoblots were performed using antibodies specific for tyrosine or serine phosphorylated forms of STAT1 and STAT3. Subsequent reblotting with the indicated anti-STAT1 or anti-STAT3 antibodies was performed to ensure that similar amounts of material were deposited in each lane. The data for each panel are representative of 4 independent experiments.
Under the same conditions, we also assessed the ability of IL-10 to induce serine phosphorylation of STAT1 and STAT3. Serine phosphorylation appears to increase the magnitude of gene transcription induced by tyrosine phosphorylation, even though it is not sufficient per se to activate STAT proteins.44 Western blot analyses showed that, whereas STAT3 was unphosphorylated on ser-727 in resting neutrophils and PBMC, IL-10 treament only led to a time-dependent serine phosphorylation of STAT3 in PBMC (Fig 3B). Despite this, G-CSF (not shown) and PMA were effective inducers of STAT3 serine phosphorylation in neutrophils (Fig 3B). With respect to STAT1, low constitutive levels of serine phosphorylated protein were present in PBMC, which remained unchanged after IL-10 treatment. In contrast, PMA (and to lesser extent) IFNγ both augmented STAT1 serine phosphorylation in PBMC (Fig 3B). No STAT1 serine phosphorylation was detected in neutrophils after any treatment (Fig 3B).
Expression of CIS family members in neutrophils and their induction by IL-10.
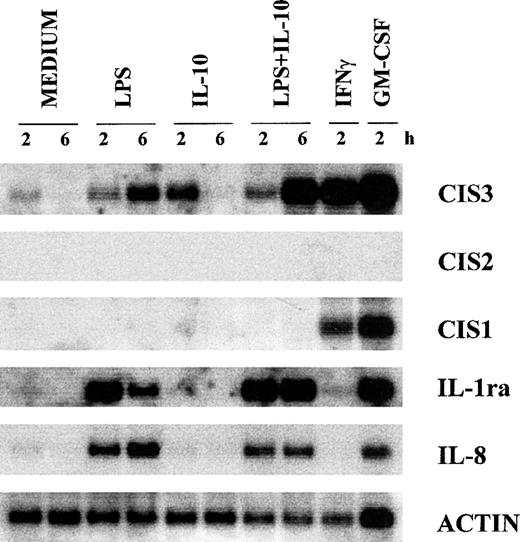
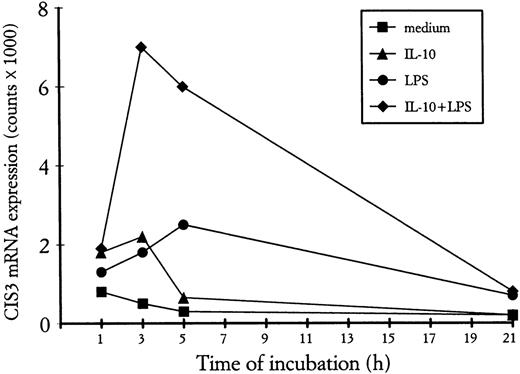
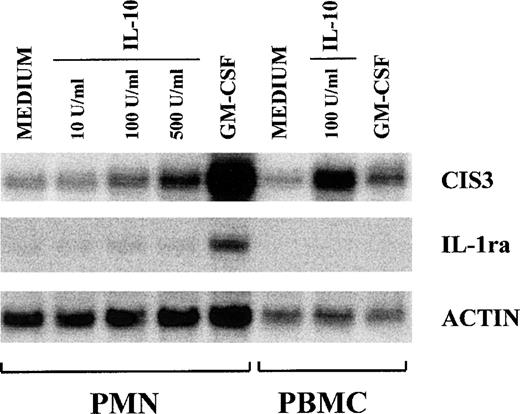
A new family of STAT-regulated genes, the CIS family, has recently been the focus of much attention, because its members are potential negative regulators of cytokine signaling.24-31 In view of their potential involvement in regulating IL-10 signaling, we examined whether the gene expression of CIS proteins might be influenced, in turn, by IL-10 in human neutrophils. For this purpose, we performed Northern blot analyses on total RNA isolated from PMN incubated with IL-10 (100 U/mL) and/or LPS (100 ng/mL) for 2 and 6 hours. Because of the well-established capacity of both GM-CSF and IFNγ to upregulate CIS mRNA expression in myeloid cells,27,45 total RNA was also extracted from neutrophils incubated for 2 hours with either agent for comparison purposes. Figure 4 shows that CIS3 mRNA (but not CIS1 or CIS2 mRNA) is constitutively expressed in unstimulated neutrophils. Whereas the gene expression of CIS1, CIS3, and JAB (not shown) was upregulated by both GM-CSF and IFNγ, IL-10 and LPS selectively enhanced the expression of CIS3 mRNA (Fig 4). Neither IL-10 nor LPS had any effect on the gene expression of either JAB or CIS5 (data not shown). In the same experiments (Fig 4), the LPS-induced accumulation of IL-8 mRNA was inhibited by IL-10 (by ∼50% at 6 hours), whereas that of IL-1ra mRNA was greatly enhanced, in agreement with previous studies.4-7 As shown in Fig 4, and better explored in the experiment depicted in Fig 5, both the IL-10–mediated and LPS-mediated increase in CIS3 mRNA steady-state levels was time-dependent, peaking at 2 to 3 hours in the case of IL-10 stimulation (Figs 4 and 5) and gradually decreasing thereafter. Remarkably, coincubation of PMN with IL-10 and LPS yielded a synergistic augmentation of CIS3 gene expression; this synergism was already evident at 3 hours and persisted even when, in IL-10–treated neutrophils, CIS3 mRNA levels had returned to baseline levels (Fig 5). Further investigation showed that the IL-10–induced accumulation of CIS3 mRNA occurred in a dose-dependent manner and that the pattern of CIS3 and IL-1ra mRNA inducibility markedly differed between neutrophils and autologous PBMC (Fig 6). Importantly, the latter observation also provides convincing evidence that the findings that we report here for CIS family members in neutrophils are unlikely to result from the small proportion of contaminating PBMC that are present in our neutrophil preparations.
Effect of IL-10 and LPS on the gene expression of various CIS family members, IL-8, and IL-1ra in neutrophils. PMN were preincubated with or without 100 U/mL IL-10 for 15 minutes before the addition of LPS, IFNγ, or GM-CSF for the times indicated. Total RNA was extracted and analyzed by Northern blotting. This experiment is representative of 4.
Effect of IL-10 and LPS on the gene expression of various CIS family members, IL-8, and IL-1ra in neutrophils. PMN were preincubated with or without 100 U/mL IL-10 for 15 minutes before the addition of LPS, IFNγ, or GM-CSF for the times indicated. Total RNA was extracted and analyzed by Northern blotting. This experiment is representative of 4.
Time course of CIS3 mRNA expression in PMN treated with IL-10 and/or LPS. PMN were incubated for the indicated times with 500 U/mL IL-10 in the presence or absence of LPS, before total RNA extraction and Northern blot analysis for CIS3 mRNA expression. The extent of hybridization was quantitatively analyzed in an InstantImager (Packard Instruments) and plotted after actin normalization. This experiment is representative of 3.
Time course of CIS3 mRNA expression in PMN treated with IL-10 and/or LPS. PMN were incubated for the indicated times with 500 U/mL IL-10 in the presence or absence of LPS, before total RNA extraction and Northern blot analysis for CIS3 mRNA expression. The extent of hybridization was quantitatively analyzed in an InstantImager (Packard Instruments) and plotted after actin normalization. This experiment is representative of 3.
Dose-dependent effect of IL-10 on CIS3 mRNA expression in neutrophils. PMN were incubated in the presence or absence of increasing concentrations of IL-10 for 2 hours before total RNA extraction. For comparative purposes, RNA was also extracted from PMN stimulated with GM-CSF and from autologous PBMC. CIS3 and IL-1ra gene expression was analyzed by Northern blot. This experiment is representative of 2.
Dose-dependent effect of IL-10 on CIS3 mRNA expression in neutrophils. PMN were incubated in the presence or absence of increasing concentrations of IL-10 for 2 hours before total RNA extraction. For comparative purposes, RNA was also extracted from PMN stimulated with GM-CSF and from autologous PBMC. CIS3 and IL-1ra gene expression was analyzed by Northern blot. This experiment is representative of 2.
Effect of IL-10 on CIS3 mRNA stability.
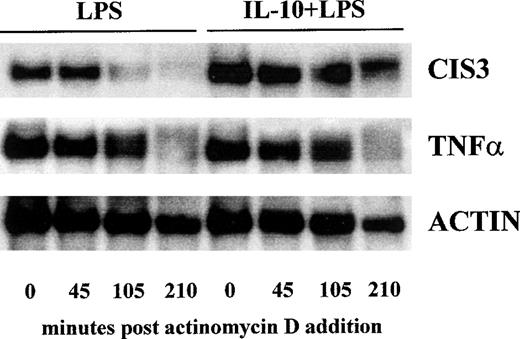
In an attempt to elucidate the mechanisms whereby IL-10 modulates the accumulation of CIS3 transcripts in LPS-treated PMN, we examined the influence of IL-10 on CIS3 mRNA stability. PMN were stimulated with LPS for 3.5 hours in the presence or absence of IL-10 and then treated with actinomycin D to block the formation of additional transcripts. At increasing intervals thereafter, the cultures were processed for Northern blot analysis, and changes in the amount of cytokine mRNA were quantitated by InstantImager scanning. Whereas our preliminary experiments established that neither IL-10 nor LPS stimulation significantly alters CIS3 mRNA stability (not shown), Fig 7 shows that IL-10 treatment markedly prolonged CIS3 mRNA half-life in LPS-stimulated cells (65 v 175 minutes, respectively). Under the latter conditions, IL-10 did not significantly affect the stability of TNFα and actin mRNA isolated from LPS-treated PMN (Fig 7). A similar effect of IL-10 on the turnover rate of CIS3 transcripts was exerted in cells stimulated with LPS for 5 hours, a time point at which the differences in CIS3 mRNA levels were still evident (not shown).
Effect of IL-10 on the turnover rate of CIS3 mRNA in LPS-stimulated PMN. PMN were cultured with 1 μg/mL LPS in the presence or absence of 100 U/mL IL-10. After 3.5 hours, actinomycin D (5 μg/mL) was added and the cells were further cultured for the indicated times. Total RNA was prepared and analyzed by Northern blotting with CIS3, TNF, and actin cDNA probes. This experiment is representative of 3.
Effect of IL-10 on the turnover rate of CIS3 mRNA in LPS-stimulated PMN. PMN were cultured with 1 μg/mL LPS in the presence or absence of 100 U/mL IL-10. After 3.5 hours, actinomycin D (5 μg/mL) was added and the cells were further cultured for the indicated times. Total RNA was prepared and analyzed by Northern blotting with CIS3, TNF, and actin cDNA probes. This experiment is representative of 3.
Negative effect of CIS3 on LPS signaling.
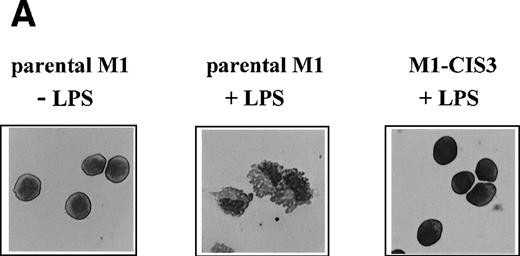
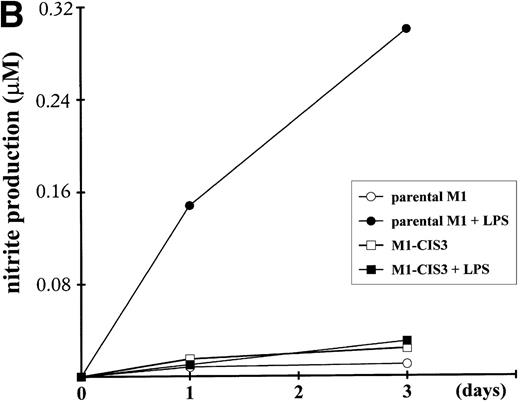
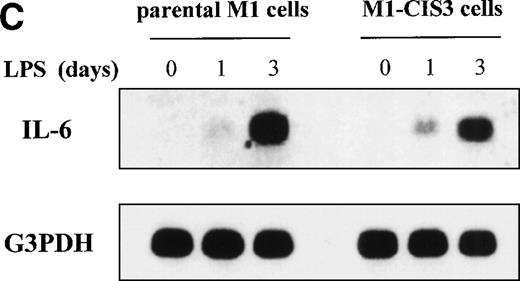
To address the potential function of CIS3, we stably transfected CIS3 in the murine M1 myeloid cell line and examined the effects thereof on LPS-induced differentiation, NO synthesis, and cytokine mRNA expression. This leukemic cell line does not constitutively express CIS3 and has been widely used as a model system not only for the study of LPS-induced macrophage-like differentiation,26,46,47 but also for the JAK/STAT signaling pathway.34 48 As shown in Fig 8A, stable transfectants expressing CIS3 were almost completely resistant to LPS-induced differentiation, whereas LPS-treated parental M1 cells exhibited typical morphological changes such as vacuolation and chromatin condensation. Further incubation of M1 cells in LPS resulted in apoptotic cell death, whereas CIS3 transfectants grew normally (data not shown). In addition, LPS induced a large accumulation of nitrites into the culture medium of parental M1 cells, whereas no LPS-dependent NO synthesis was seen in CIS3 transfected cells (Fig 8B). In contrast, LPS-induced expression of IL-6 mRNA, or of TNFα production as well (A. Yoshimura, personal communication, April 1999), were not affected in CIS3-transfected cells (Fig 8C).
Negative effects of stable CIS3 transfection on LPS-signaling in murine M1 cells. (A) Suppression of LPS-induced differentiation in M1 cells. Parental M1 cells (M1-P) and stable transfectants expressing CIS3 (M1-CIS3) were incubated without or with 100 ng/mL LPS. After 72 hours of culture, cells were spun down on slide glass using a cytospin and examined after May-Grunwald Giemsa staining. (B) Inhibition of NO synthesis of M1 cells by CIS3. Parental M1 cells (5 × 105; parent) and stable transfectants expressing CIS3 were cultured with or without 100 ng/mL LPS for the days indicated. No synthesis was determined by the Griess method. Similar results were obtained with at least 2 independent clones of each transfectants. (C) Expression of IL-6 mRNA in M1 cells and transformants. Parental M1 cells (parent) and stable transfectants expressing CIS3 were stimulated with 100 ng/mL LPS for the days indicated. Total RNA was extracted and expression of IL-6 and G3PDH was analyzed by Northern blotting.
Negative effects of stable CIS3 transfection on LPS-signaling in murine M1 cells. (A) Suppression of LPS-induced differentiation in M1 cells. Parental M1 cells (M1-P) and stable transfectants expressing CIS3 (M1-CIS3) were incubated without or with 100 ng/mL LPS. After 72 hours of culture, cells were spun down on slide glass using a cytospin and examined after May-Grunwald Giemsa staining. (B) Inhibition of NO synthesis of M1 cells by CIS3. Parental M1 cells (5 × 105; parent) and stable transfectants expressing CIS3 were cultured with or without 100 ng/mL LPS for the days indicated. No synthesis was determined by the Griess method. Similar results were obtained with at least 2 independent clones of each transfectants. (C) Expression of IL-6 mRNA in M1 cells and transformants. Parental M1 cells (parent) and stable transfectants expressing CIS3 were stimulated with 100 ng/mL LPS for the days indicated. Total RNA was extracted and expression of IL-6 and G3PDH was analyzed by Northern blotting.
DISCUSSION
Several studies have established that, in monocytic cells, IL-10 has the ability to induce the gene and surface expression of the high-affinity IgG receptor, FcγRI.10,13-15 In marked contrast, we and others have reported that, in neutrophils, IL-10 fails to exert a similar effect.10-12 In this regard, we recently demonstrated that, whereas IL-10 induces the binding of multimeric complexes containing both STAT1 and STAT3 to the GRR sequence of the FcγRI gene promoter, no GRR-binding complexes were induced by IL-10 in autologous neutrophils.10 In the current report, we confirmed these observations by showing that, in neutrophils, IL-10 fails to elicit any detectable DNA-binding activities to an oligonucleotide probe, hSIE/m67, which binds STAT protein complexes with a higher affinity than the GRR.39,40 We also extended our previous findings by showing that neither STAT1 nor STAT3 is phosphorylated on serine or tyrosine residues in response to IL-10 stimulation of neutrophils, in keeping with the fact that the DNA-binding activity of STAT proteins is critically dependent on these phosphorylations.42,43 It is noteworthy that both STAT1 and STAT3 can nevertheless undergo tyrosine phosphorylation in neutrophils in response to stimuli other than IL-10, as shown here in the case of IFNγ and G-CSF or in other works performed with GM-CSF–stimulated neutrophils.49,50 By comparison, we found that IL-10 rapidly triggers the tyrosine phosphorylation of both STAT1 and STAT3 in autologous PBMC, as previously reported.20,22,23,40 In addition, we show for the first time that stimulation of PBMC with IL-10 also results in the phosphorylation of STAT3 on serine residues. Collectively, our data suggest that the inability of IL-10 to induce the FcγRI gene in neutrophils involves a defective postreceptor event that is upstream from STAT1 and STAT3 phosphorylation. However, the nature of this upstream event remains unclear. Although it can be envisaged that IL-10 somehow triggers an inhibitory signal in neutrophils, we tend to exclude this possibility, because costimulation of neutrophils with IL-10 plus either IFNγ or G-CSF did not influence the IFNγ-elicited or G-CSF–elicited GRR-binding activities or FcγRI gene expression.10 Another explanation could be that, in PMN, the IL-10 receptor (IL-10R) complex differs from that present in monocytes. A functional IL-10R is in fact a multicomponent structure composed of the IL-10R151 and of the recently identified CRFB4/CRF-2 molecule,51-54 in which IL-10R1 associates with JAK1 and recruits STAT340 and CRFB4/CRF-2 associates with TYK2.55 We previously assessed the presence of IL-10R on the neutrophil surface by flow cytometry using biotinylated IL-10 and showed that freshly isolated neutrophils clearly possess IL-10 binding sites, albeit to a lesser extent than peripheral blood monocytes or lymphocytes.10 More recently, we have also investigated by Northern blot analysis the mRNA expression of IL-10R1 and CRFB4/CRF-2 and found that the constitutive expression of IL-10R1 transcripts is higher in PBMC than in neutrophils (our unpublished observations). However, whether this differential mRNA expression in neutrophils and PBMC has a functional consequence remains an open question. For instance, the IL-10 receptor in neutrophils might lack the intracellular distal portion containing the docking sites for the recruitment of STAT3.40 In this regard, it is noteworthy that transfection of the murine IL-10R1 chain into L929 fibroblasts conferred to these cells the ability to bind murine IL-10; however, despite this, IL-10 failed to induce STAT activation in these cells.56 Finally, an obvious reason for the lack of inducible STAT tyrosine phosphorylation in IL-10–treated PMN might be that IL-10 fails to activate JAK1 and/or TYK2, the 2 tyrosine kinases previously shown to catalyze the phosphorylation of STAT1 and STAT3 after cell treatment with IL-10.22,23Whereas JAK1 and TYK2, as well as JAK2 and JAK3, can all be detected at low levels in neutrophils by immunoblot,50,57JAK2 is the only member of the JAK family that to date has been reported to be activated in response to GM-CSF stimulation of neutrophils.49 50
In the present work, we also demonstrate that IL-10 selectively regulates CIS3 mRNA steady-state levels in both neutrophils and PBMC, in that no effects of IL-10 on CIS1, CIS2, CIS5, and JAB mRNA expression were observed. In IL-10–treated neutrophils, CIS3 mRNA accumulation was also found to be transient, peaking after 2 to 3 hours of incubation. To our knowledge, this constitutes the first demonstration of the ability of IL-10 to modulate the gene expression of a CIS family member in neutrophils. In agreement with our data, IL-10 was shown to upregulate CIS3 mRNA expression in monocytes while the present manuscript was under revision.58 CIS protein expression is known to be induced via the JAK-STAT pathway in response to a wide range of cytokines, including GM-CSF, G-CSF, macrophage colony-stimulating factor (M-CSF), IL-1, IL-2, IL-3, IL-6, leukemia inhibitory factor, and IFNγ,24-31,45 and as such, is thought to negatively regulate signal transduction. For instance, expression of CIS1 appears to reduce the proliferative response of hemopoietic cells to erythropoietin and IL-3,24 whereas JAB and CIS3 appear to be potent inhibitors of M1 cell differentiation in response to a number of cytokines.27 Likewise, overexpression of JAB and CIS3, but not of CIS1 or CIS2, inhibits the ability of growth hormone to regulate gene expression in adypocites.59 Interestingly, we show here that, similar to IL-10, LPS also upregulates the expression of CIS3 mRNA in neutrophils and that it synergizes with IL-10, resulting in a sustained (as opposed to transient) upregulation of CIS3 mRNA levels. Under the latter conditions, IL-8 and IL-1ra mRNA expression was modulated exactly as reported previously,4-7 indicating that IL-10 was acting as an anti-inflammatory agent. Further investigation showed that IL-10 substantially increased the half-life of CIS3 transcripts in LPS-treated cells. Consistent with this finding is the finding that a similar posttranscriptional action of IL-10 has also been reported in the case of IL-1ra.5 Although other effects of IL-10 at the level of gene transcription cannot be excluded, it should be interesting to know in this context whether CIS3 contains the AU-rich sequences in its 3′-untranslated regions that are believed to be involved in the regulation of mRNA stability.60
The function of CIS3 was examined using the murine M1 myeloid cell line stably expressing CIS3. Our data showed that CIS3 transfection in M1 monocytic cells suppresses LPS-induced growth arrest, macrophage-like differentiation, and NO synthesis, but not IL-6 mRNA expression or TNFα production. Because LPS induces IL-6 in M1 cells, it is highly probable that an autocrine/paracrine activation of the JAK/STAT pathway plays an essential role in the LPS-induced differentiation.26,27,46 At the light of the established ability of CIS3 to bind JAKs and inhibit their protein kinase activity,46 it is tempting to speculate that the mechanism whereby CIS3 transfection blocks LPS-induced differentiation and NO synthesis occurs at the JAK level. The idea is consistent with a previous report showing that tyrosine kinase inhibitors suppress LPS-induced NO synthesis in macrophages,61 but it is in contrast with a more recent study showing that CIS3 was unable to inhibit JAK kinase activity in vitro.48 Although M1 cells are not responsive to IL-10 (A. Yoshimura, personal communication, April 1999), our data suggest that, at least in this cellular experimental model, CIS3 negatively modulates specific LPS-elicited responses. Although the data raise the possibility that CIS3 may act as an anti-inflammatory mediator induced by IL-10, the question of whether CIS3 induction negatively affects the signaling pathways mobilized by IL-10 or whether CIS3 might mediate the modulation by IL-10 of LPS-elicited responses in neutrophils remains to be elucidated. However, whatever the case may be, the fact that CIS3 transfection does not influence IL-6 mRNA expression or TNFα production in LPS-treated cells might indicate that CIS3 is not involved in the mechanisms by which IL-10 modulates cytokine expression.
Our finding that IL-10 enhances CIS3 mRNA expression represents the first molecular demonstration of a direct effect of IL-10 towards neutrophils. Importantly, our study also makes it clear that the activation of STAT1 and STAT3 via tyrosine or serine phosphorylation is not required for CIS3 induction by IL-10 in neutrophils. By inference, our data raise the possibility that the modulation by IL-10 of cytokine production in human neutrophils3,62 and, similarly, in other cell types,1,2 might occur independently of STAT protein activation. In support of this notion, it has been shown that, in STAT1-deficient mice, IL-10 retains the ability to inhibit TNFα production in LPS-stimulated macrophages and to induce the formation of DNA-binding complexes consisting of STAT3 homodimers.63Furthermore, transfection studies using a truncated STAT3 acting as a dominant negative mutant showed that the inhibition of monokine production by IL-10 is similarly independent of STAT3 activation.64 However, it should be pointed out that 2 studies performed in the mouse instead support a critical role of the JAK1-STAT3 pathway in the IL-10–mediated deactivation of macrophages and neutrophils. In one of them, macrophages derived from JAK1-deficient mice were shown to be unresponsive to IL-10 in terms of inhibition of LPS-induced TNFα production,65 thereby highlighting an obligatory role for JAK1 in mediating this action of IL-10. In the other one,66 macrophages and neutrophils derived from mice engineered to express a genetic STAT3 deficiency in the myeloid cell compartment failed to respond to IL-10 and secrete high levels of TNFα upon stimulation with IL-10 plus LPS. The discrepancies between human and murine neutrophils might be partially explained by our preliminary experiments, in which IL-10 induced STAT3 tyrosine phosphorylation in murine neutrophils (Gasperini et al, unpublished data, May 1999). These considerations indicate that human neutrophils could represent an interesting cellular model for the study of the IL-10R signaling occurring independently from STAT activation.
ACKNOWLEDGMENT
The authors thank Dr P.P. McDonald for his invaluable criticisms and suggestions.
Supported by grants from MURST (40%, 60% funds, and “cofinanziamento MURST-Universita”), AIRC, and “Progetto Sanità, Fondazione CARI-VR-VI-BL-AN.”
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marco A. Cassatella, MD, Department of Pathology, Strada Le Grazie 4, I-37134 Verona, Italy; e-mail: MCNCSS@borgoroma.univr.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal