Abstract
Stem cell factor (SCF) exerts its biological effects by binding to a specific receptor, the tyrosine kinase c-Kit, which is expressed on the cell surface. Although normal cellular trafficking of growth factor receptors may play a critical role in the modulation of receptor function, the mechanisms that regulate the distribution of c-Kit on the cell surface and the internalization of c-Kit have not been fully defined. We investigated whether signal transduction via Src family kinases is required for normal c-Kit trafficking. Treatment of the SCF-responsive human hematopoietic cell line MO7e with the inhibitor of Src family kinases PP1 blocked SCF-induced capping of c-Kit and internalization of c-Kit. c-Kit was able to associate with clathrin in the presence of PP1, suggesting that entry of c-Kit into clathrin-coated pits occurs independently of Src family kinases. SCF-induced internalization of c-Kit was also diminished in the D33-3 lymphoid cell line in which expression of Lyn kinase was disrupted by homologous recombination. These results indicate that Src family kinases play a role in ligand-induced trafficking of c-Kit.
SIGNAL TRANSDUCTION BY the receptor encoded by c-kit plays a critical role in hematopoiesis, in mast cell production and function, and in the development of germ cells and melanocytes (reviewed in Broudy1). Binding of stem cell factor (SCF) to c-Kit induces homodimerization and intermolecular tyrosine phosphorylation of the receptor, creating docking sites for a number of SH2-containing signal transduction molecules.2These include phosphatidylinositol 3′-kinase (PI 3K) and phospholipase Cγ-1,3-5 Stat1,6 the Src family kinases Src, Lyn, and Fyn,7-9 and the GTPase activating protein GAP.10 Binding of SCF to c-Kit results in activation of Protein Kinase C,5,11 Rac1,9JNK,9 Raf-1,12 tyrosine phosphorylation of JAK2,13 and association of the adaptor protein CRKL and of p120cbl.14 An SH2 domain-containing tyrosine phosphatase, SHP-1, associates with a tyrosine in the juxtamembrane region of c-Kit, and downmodulates c-Kit signal transduction.15 16
Like other cell surface hematopoietic growth factor receptors, c-Kit is rapidly internalized after ligand binding.17-20Internalization of the cytokine-receptor complex may be a mechanism to attenuate cellular response to the ligand.21,22Additionally, internalization and normal cellular trafficking of the receptor may be necessary for activation of the full spectrum of signal transduction pathways associated with the receptor.23 For these reasons, the mechanisms that regulate endocytosis of cell surface receptors have been intensely studied.24 25
Clathrin-coated pits constitute a major mechanism of endocytosis of cell surface proteins. Specific sequences in the cytoplasmic tail of cell surface proteins mediate association with the adaptor complex AP-2, which targets the cell surface protein to clathrin-coated pits.26 The GTPase dynamin assembles into high molecular weight multimers at the neck of the clathrin-coated pit, 27and mediates scission of the clathrin-coated pit from the plasma membrane.28 29 The contents of the vesicle can then be transported to the lysosome for degradation, or recycled to the cell surface.
Prior studies of c-Kit internalization have shown the requirement for c-Kit kinase activity, PI 3K activation, and calcium influx for rapid receptor internalization. Introduction of a point mutation at aspartic acid 790 in the kinase domain of c-Kit ablated kinase activity, and diminished the rate of SCF-stimulated receptor internalization.18 In contrast, introduction of a point mutation at tyrosine 719 in the kinase insert region, the binding site of the p85 subunit of PI 3K, did not impair c-Kit internalization,18,19 but disrupted normal intracellular trafficking of the receptor. However, when both calcium influx and PI 3K activation were prevented, the cells failed to internalize the receptor.19 Mutation of a c-Kit autophosphorylation site at tyrosine 821 did not ablate ligand-induced receptor internalization.18 These studies did not examine the role of Src family kinases in c-Kit internalization.
Because Src family kinases (reviewed in Corey and Anderson30) associate with c-Kit in normal hematopoietic cells and in hematopoietic cell lines,8,9 and because overexpression of Src in fibroblasts increases the rate of internalization of the ligand-occupied epidermal growth factor receptor,31 we investigated whether signal transduction by Src family members is necessary for normal cellular trafficking of c-Kit. We focused on the ability of c-Kit to cap, to enter clathrin-coated pits, and to be internalized. The results show that lack of Src family kinase activity inhibits SCF-induced c-Kit trafficking.
MATERIALS AND METHODS
Cells and reagents.
The SCF-responsive human leukemic cell line MO7e was maintained in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT) plus 3 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (obtained from Dr Kenneth Kaushansky, University of Washington, Seattle, WA). The DT40 chicken B-cell line and the D33-3 Lyn-deficient cell line (a subclone of DT40 generated by targeted disruption of Lyn)32 were maintained in RPMI 1640 supplemented with 2 mmol/L glutamine, 50 μmol/L β-mercaptoethanol, 10% FCS, and 1% chicken serum (Sigma Chemical Co, St Louis, MO). Recombinant human SCF1–165, expressed in Escherichia coli, was obtained from Amgen, Inc (Thousand Oaks, CA). The inhibitor of Src family kinases PP133 was obtained from Calbiochem (La Jolla, CA). The non-neutralizing anti–c-Kit monoclonal antibody (MoAb) 104D234 was a gift from Dr Hans-Jörg Bühring (University of Tübingen, Tübingen, Germany). The SR-1 anti–c-Kit antibody was generated in our laboratory.35 The anticlathrin heavy chain MoAb and the antidynamin II MoAb were obtained from Transduction Labs (Lexington, KY). Polyclonal antibody used to immunoprecipitate Lyn was kindly provided by Dr Joe Bolen (DNAX, Palo Alto, CA) and has previously been described.36 MoAb used to identify Lyn by immunoblotting was purchased from Transduction Labs.
Flow cytometric analysis of c-Kit internalization.
The MO7e cells were incubated in the absence or presence of PP1 (10 μmol/L) for 30 minutes at 37°C, before the addition of SCF (200 ng/mL). Aliquots of cells were removed at various time points (0 to 60 minutes), fixed in 0.5% paraformaldehyde, labeled with the 104D2 antibody (2 μg/mL) followed by goat antimouse immunoglobulin G (IgG)-PE (Jackson ImmunoResearch, West Grove, PA), and analyzed in a Coulter Epics Elite flow cytometer (Coulter, Miami, FL) to detect cell surface c-Kit.
Expression and internalization of c-Kit in the DT40 cell line and in the lyn-deficient D33-3 cell line.
The DT40 cell line and the D33-3 Lyn-deficient cell line were electroporated with the pTracer/CMV2 vector (Invitrogen, Carlsbad, CA) containing the full-length human c-kit complementary DNA (cDNA) using techniques previously described.37 Stable transfectants were selected by culture in zeocin (200 μg/mL; Invitrogen). For some experiments, the cells were labeled with the SR-1 anti–c-Kit MoAb, followed by goat antimouse IgG-PE, and the 2% of cells expressing the highest quantity of cell surface c-Kit were selected by cell sorting in a Coulter Epics Elite flow cytometer, and subsequently maintained in RPMI 1640 in the presence of 2 mmol/L glutamine, 50 μmol/L β-mercaptoethanol, 10% FCS, 1% chicken serum, and 200 μg/mL zeocin. The cells were incubated without or with SCF (200 ng/mL) for 60 minutes at 37°C, then analyzed for c-Kit internalization by flow cytometry as described above, with the following modification. Aliquots of the DT40-c-Kit cells and the D33-3-c-Kit cells were labeled with purified mouse IgG1 (Southern Biotechnology Associates, Birmingham, AL) in parallel to labeling with the 104D2 antibody, and the mean fluorescence intensity of cells labeled with the control mouse IgG1 was subtracted from the mean fluorescence intensity of cells labeled with the 104D2 antibody. Because sorted and unsorted cells gave similar results, all of the experiments were analyzed together.
Immunoprecipitation, electrophoresis, and immunoblotting.
Immunoprecipitations were performed with the antibodies indicated. The immune complexes were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon as previously described.8 In brief, the blots were blocked and incubated with the unconjugated primary antibody indicated. After rigorous washing, the blots were incubated first with biotinylated secondary antibody (rabbit or mouse, as appropriate), then with peroxidase-conjugated streptavidin. Proteins were visualized using the Renaissance Reagent for enhanced chemiluminescence (New England Nuclear, Boston MA).
Immune complex kinase assay.
The cells were washed twice in RPMI 1640 and lysed in 1 mL of buffer containing 1% Triton X-100, 150 mmol/L sodium chloride, 20 mmol/L Tris, 10 mmol/L EDTA, 100 μmol/L sodium fluoride, 1 mmol/L MgCl2, 2 mmol/L sodium orthovanadate, 10% glycerol, 200 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μmol/L pepstatin, and 1 mmol/L PMSF. Lyn kinase assays were performed as follows. Clarified cell lysates were immunoprecipitated with anti-Lyn polyclonal antibodies, washed six times with lysis buffer, then incubated 15 minutes at 30°C in kinase buffer (25 mmol/L Hepes and 10 mmol/L MnCl2, pH 7.5) containing 100 μCi/mL [γ-32P]ATP. Samples were then washed and eluted from the protein A sepharose with SDS sample buffer. c-Kit kinase assays were performed as described above except that a MoAb specific for c-Kit (SR-1) was used for the immunoprecipitations. Radiolabeled proteins were resolved with SDS-PAGE, transferred to Immobilon, and visualized with autoradiography. Protein loading was assessed by immunoblotting with the indicated antibodies as described above.
Localization of c-Kit by immunofluorescence microscopy.
Fluorescence microscopy was used to examine the cell surface distribution of c-Kit, and to provide an alternative way to evaluate cell surface versus internalized receptor. The MO7e cells were incubated overnight in 1% FCS and 1 ng/mL GM-CSF to minimize exposure to SCF present in serum,38 then incubated without or with PP1 (10 μmol/L) for 30 minutes at 37°C. The cells were labeled with the 104D2 antibody (5 μg/mL) for 30 minutes at 4°C, then SCF (200 ng/mL) or no SCF was added, the cells were incubated at 37°C, and aliquots of cells were removed at time 0, 3 minutes, or 30 minutes for analysis. The cells were fixed in 0.5% paraformaldehyde and one half of each aliquot of cells was permeabilized with 0.05% Triton X-100 to permit detection of intracellular c-Kit.39 All of the samples were incubated with goat antimouse IgG-fluorescein isocyanate (FITC), and with propidium iodide (0.5 μg/mL; Molecular Probes, Inc, Eugene, OR) to stain the nuclei. The cells were analyzed and photographed in a Nikon Eclipse E800 Fluorescent Microscope (Tokyo, Japan), using a 60× 0.95 Nikon dry objective lens and filters to detect FITC fluorescence at 515 to 555 nm, and propidium iodide fluorescence above 590 nm. Caps were defined as an intensely fluorescent area of the cell surface encompassing less than 1/4 of the cell circumference.
Analysis of MO7e migration.
SCF-induced chemotaxis of MO7e cells was assessed by a fluorescence-based assay using 96-well chemotaxis chambers containing polycarbonate filters with 8 μm pores (ChemoTx, Neuro Probe Inc, Gaithersburg, MD). MO7e cells (5 × 106/mL) in RPMI 1640 containing 10% FCS were incubated for 45 minutes at 37°C with 5 μg/mL calcein AM (Molecular Probes), then washed twice with RPMI 1640 containing 0.1% human serum albumin (Sigma, St. Louis, MO) (RPMI-HSA) and resuspended at 4 × 106 cells/mL in RPMI-HSA for the assay. The chamber wells were filled with 29 μL of varying concentrations of SCF (1 ng/mL to 1 μg/mL) diluted in RPMI-HSA, or with RPMI-HSA alone as a negative control. The filter was applied, and MO7e cells (25 μL containing 1 × 105cells) were placed directly onto the filters. All conditions were performed in quadruplicate in each experiment. The chambers were incubated for 4 hours at 37°C in the SCF dose-response studies, and for up to 6 hours in the time-course experiments. To assess the potential role of Src family kinases in SCF-mediated chemotaxis, MO7e cells were preincubated for 30 minutes at 37°C with PP1 (10 μmol/L). At the end of the incubation period, nonmigrating cells on the origin (top) side of the filter were removed by washing and aspirating with excess RPMI-HSA and gentle wiping with a tissue. To determine the percentage of cells that migrated into the bottom chamber during the course of the experiment, the chemotaxis chamber was placed in a multiwell fluorescent plate reader (Cyto Fluor II, PerSeptive Biosystems, Framingham, MA) and fluorescence was determined in the bottom-read position (excitation 485 nm, emission 530 nm). The data are reported as the percentage of MO7e cells (mean ± SD) that migrated into the bottom chamber during the course of the experiment.
RESULTS
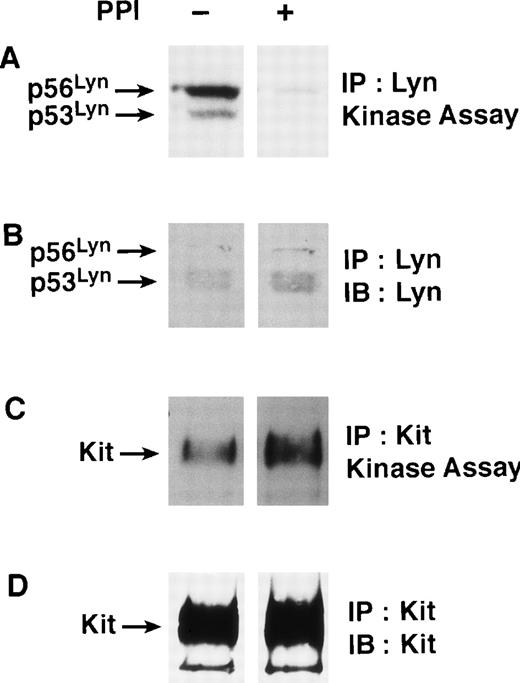
To examine the role of Src family members in the early events in SCF-induced c-Kit internalization we used PP1, a Src family inhibitor.33 Because Lyn is the most highly expressed Src family member in MO7e cells and is also associated with c-Kit, we chose to examine the effects of PP1 on Lyn kinase activity.8Figure 1A shows that PP1 dramatically inhibited Lyn autophosphorylation activity, whereas no differences in the expression of the Lyn protein were found (Fig 1B). Importantly, PP1 has no effect on the catalytic activity (Fig 1C) or the protein expression (Fig 1D) of c-Kit.
PP1 inhibits Lyn kinase activity but not c-Kit kinase activity in MO7e cells. MO7e cells were treated for 30 minutes at 37°C in the presence or absence of PP1 (10 μmol/L). The cells were then lysed and assessed for either Lyn or c-Kit activity as described in the Materials and Methods section. (A) Lyn kinase activity is inhibited after treatment with PP1. Immune complex assays were performed on Lyn immunoprecipitated from cell lysates, resolved with SDS-PAGE, transferred to Immobilon, and radiolabeled Lyn was visualized with autoradiography. (B) Equivalent amounts of Lyn protein are present in the immune complex kinase assays. Immunoblotting with Lyn-specific antibody was performed on the immunoprecipitates shown in Panel A. (C) PP1 does not impair c-Kit kinase activity. Immune complex assays were performed on c-Kit immunoprecipitated from cell lysates, resolved with SDS-PAGE, transferred to Immobilon, and radiolabeled c-Kit was visualized with autoradiography. (D) c-Kit immunoblot of Panel C.
PP1 inhibits Lyn kinase activity but not c-Kit kinase activity in MO7e cells. MO7e cells were treated for 30 minutes at 37°C in the presence or absence of PP1 (10 μmol/L). The cells were then lysed and assessed for either Lyn or c-Kit activity as described in the Materials and Methods section. (A) Lyn kinase activity is inhibited after treatment with PP1. Immune complex assays were performed on Lyn immunoprecipitated from cell lysates, resolved with SDS-PAGE, transferred to Immobilon, and radiolabeled Lyn was visualized with autoradiography. (B) Equivalent amounts of Lyn protein are present in the immune complex kinase assays. Immunoblotting with Lyn-specific antibody was performed on the immunoprecipitates shown in Panel A. (C) PP1 does not impair c-Kit kinase activity. Immune complex assays were performed on c-Kit immunoprecipitated from cell lysates, resolved with SDS-PAGE, transferred to Immobilon, and radiolabeled c-Kit was visualized with autoradiography. (D) c-Kit immunoblot of Panel C.
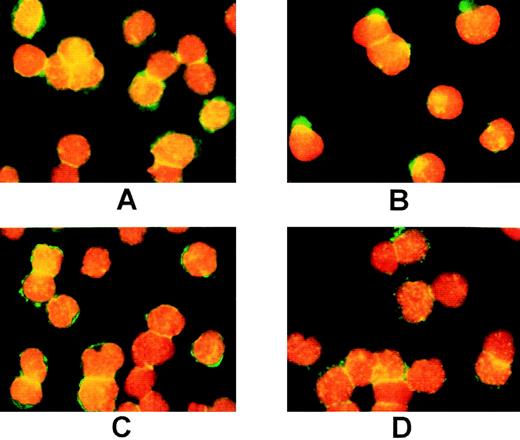
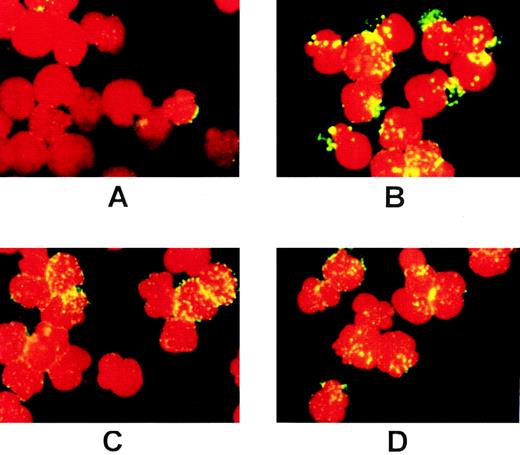
Previous studies from our laboratory have shown that treatment with SCF induces rapid capping of c-Kit before internalization.39 We therefore examined the effect of PP1 on SCF-induced capping of c-Kit using immunofluorescence microscopy. In the absence of PP1, a 3-minute exposure to SCF resulted in dramatic changes in the distribution of the receptor on the cell surface. The diffuse pattern of cell surface c-Kit display (Fig 2A) changed to a large cap in one region of the cell, plus multiple punctate clusters (Fig 2B, Table1). In MO7e cells pretreated with PP1 and then exposed to SCF for 3 minutes, the caps were not observed, and the location of c-Kit in the multiple punctate clusters was more pronounced (Table 1, Fig 2D). These data show that PP1 impairs SCF-induced capping of c-Kit and suggest a role for Src family activity in redistribution of c-Kit on the cell surface. In addition, one explanation for the punctate staining pattern of c-Kit in Fig 2 is association with clathrin-coated pits.
Treatment with PP1 blocks capping of c-Kit. MO7e cells were preincubated without PP1 (A,B) or with PP1 (C,D) as described in Fig 1, then labeled with the 104D2 antibody. Before exposure to SCF (A,C), or after a 3-minute exposure to SCF (200 ng/mL; B,D), the cells were fixed and labeled with goat antimouse IgG-FITC plus propidium iodide, and photographed at a magnification of 60×.
Treatment with PP1 blocks capping of c-Kit. MO7e cells were preincubated without PP1 (A,B) or with PP1 (C,D) as described in Fig 1, then labeled with the 104D2 antibody. Before exposure to SCF (A,C), or after a 3-minute exposure to SCF (200 ng/mL; B,D), the cells were fixed and labeled with goat antimouse IgG-FITC plus propidium iodide, and photographed at a magnification of 60×.
PP1 Treatment Inhibits Capping of c-Kit
| PP1 . | Time in SCF (minutes) . | % of Cells With a Cap . |
|---|---|---|
| 0 | 0 | 3.6 |
| 0 | 1 | 3.0 |
| 0 | 3 | 76.5 |
| 0 | 5 | 68.1 |
| + | 0 | 4.3 |
| + | 1 | 3.3 |
| + | 3 | 0.0 |
| + | 5 | 3.5 |
| PP1 . | Time in SCF (minutes) . | % of Cells With a Cap . |
|---|---|---|
| 0 | 0 | 3.6 |
| 0 | 1 | 3.0 |
| 0 | 3 | 76.5 |
| 0 | 5 | 68.1 |
| + | 0 | 4.3 |
| + | 1 | 3.3 |
| + | 3 | 0.0 |
| + | 5 | 3.5 |
MO7e cells were cultured in IMDM supplemented with 1% FCS and 0.5% GM-CSF for 16 hours, treated without or with PP1 (10 μmol/L) for 30 minutes at 37°C, labeled with the 104D2 MoAb (5 μm/mL) for 45 minutes at 4°C, treated with SCF (200 ng/mL) for 0, 1, 3, or 5 minutes at 37°C, fixed, and labeled with goat antimouse IgG-FITC plus propidium iodide. The proportion of cells exhibiting a fluorescent cap in one region of the cell was determined using a Zeiss fluorescent microscope. For each data point, 50 to 90 cells were analyzed for the presence of caps.
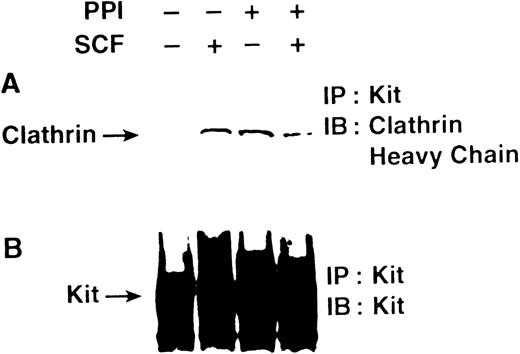
To determine if Src family kinase activity is necessary for incorporation of c-Kit into clathrin-coated pits, we examined the effect of PP1 on the capacity of the receptor to coimmunoprecipitate with clathrin after stimulation with SCF (Fig3). These experiments show that a 5-minute treatment with SCF induced association of c-Kit and clathrin. In contrast, c-Kit and clathrin were associated in MO7e cells treated with PP1 both before and after stimulation with SCF. These data show that Src family kinase activity is not necessary for entry of c-Kit into clathrin-coated pits. The constitutive association of c-Kit and clathrin after PP1 treatment suggests that there is normally a basal level of c-Kit internalization via clathrin-coated pits, and that when internalization is blocked, c-Kit accumulates in the clathrin-coated pits.
PP1 does not impair SCF-induced association of c-Kit with clathrin. MO7e cells were incubated without or with PP1 as described in Fig 1, then treated without or with SCF for 5 minutes. The cells were lysed and immunoprecipitated with the SR-1 anti–c-Kit antibody, and analyzed by SDS-PAGE followed by immunoblotting with a polyclonal antibody specific for the clathrin heavy chain (A), or immunoblotting with a polyclonal antibody specific for c-Kit (B).
PP1 does not impair SCF-induced association of c-Kit with clathrin. MO7e cells were incubated without or with PP1 as described in Fig 1, then treated without or with SCF for 5 minutes. The cells were lysed and immunoprecipitated with the SR-1 anti–c-Kit antibody, and analyzed by SDS-PAGE followed by immunoblotting with a polyclonal antibody specific for the clathrin heavy chain (A), or immunoblotting with a polyclonal antibody specific for c-Kit (B).
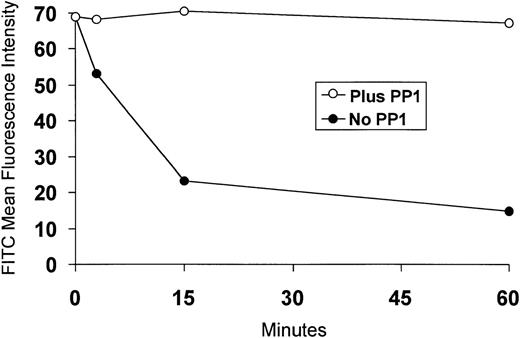
Assembly of receptors in clathrin-coated pits is rapidly followed by scission from the cell surface and trafficking through endocytic organelles. Consistent with prior reports,17-20 39 we found that SCF induces internalization of c-Kit (Fig4). However, SCF-induced internalization of c-Kit is blocked in MO7e cells pretreated with PP1 (Fig 4).
PP1 inhibits SCF-induced internalization of c-Kit. MO7e cells were preincubated without PP1 (◍) or with PP1 (○) for 30 minutes at 37°C, then SCF (200 ng/mL) was added. Aliquots of cells were removed at time 0, 3, 15, and 60 minutes after the addition of SCF, fixed in 0.5% paraformaldehyde, labeled with the anti–c-Kit MoAb 104D2, followed by PE-conjugated goat antimouse IgG, and analyzed by flow cytometry. The MO7e cells incubated in the absence of PP1 exhibited a PE mean fluorescence intensity at time 0 identical to that of the MO7e cells incubated in the presence of PP1. One additional experiment gave similar results.
PP1 inhibits SCF-induced internalization of c-Kit. MO7e cells were preincubated without PP1 (◍) or with PP1 (○) for 30 minutes at 37°C, then SCF (200 ng/mL) was added. Aliquots of cells were removed at time 0, 3, 15, and 60 minutes after the addition of SCF, fixed in 0.5% paraformaldehyde, labeled with the anti–c-Kit MoAb 104D2, followed by PE-conjugated goat antimouse IgG, and analyzed by flow cytometry. The MO7e cells incubated in the absence of PP1 exhibited a PE mean fluorescence intensity at time 0 identical to that of the MO7e cells incubated in the presence of PP1. One additional experiment gave similar results.
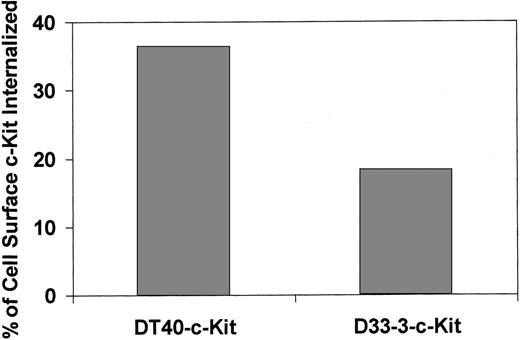
To investigate whether Lyn kinase has a role in SCF-induced c-Kit internalization, we introduced c-Kit into the DT40 lymphoid cell line that expresses Lyn kinase, and into the D33-3 subclone that lacks Lyn kinase due to targeted disruption of Lyn.32 The DT40-c-Kit cells and the D33-3-c-Kit cells were incubated in the presence of SCF for 60 minutes, and cell surface display of c-Kit was assessed by flow cytometry (Fig 5). In a total of six experiments, the Lyn-deficient D33-3 cells internalized 51.2 ± 9.8% as much cell surface c-Kit as the parental DT40 cell line (mean ± SEM of 6 experiments). In addition, we expressed a kinase-inactive form of Lyn (Lyn K275R)37 in MO7e cells. Expression of Lyn K275R resulted in reduction of SCF-induced internalization of c-Kit, in comparison with MO7e cells transfected with the vector alone (data not shown).
Internalization of c-Kit is impaired in Lyn-deficient cells. The DT40-c-Kit and the Lyn-deficient D33-3-c-Kit cells were incubated in SCF (200 ng/mL) for 1 hour at 37°C, and analyzed for c-Kit internalization by flow cytometry as described in Fig 4. The results of one of six similar experiments is shown.
Internalization of c-Kit is impaired in Lyn-deficient cells. The DT40-c-Kit and the Lyn-deficient D33-3-c-Kit cells were incubated in SCF (200 ng/mL) for 1 hour at 37°C, and analyzed for c-Kit internalization by flow cytometry as described in Fig 4. The results of one of six similar experiments is shown.
We used immunofluorescence microscopy to examine the cellular localization of the receptor after treatment of MO7e cells with SCF. In the absence of PP1, treatment with SCF for 30 minutes resulted in loss of c-Kit expression on the surface of MO7e cells (Fig6A), and appearance of c-Kit in the intracellular compartment (Fig 6B). In contrast, 30 minutes after the addition of SCF to PP1-treated MO7e cells, c-Kit remains distributed in a punctate pattern on the cell surface (Fig 6C), consistent with imprisonment in clathrin-coated pits. Permeabilization of the PP1-treated MO7e cells to permit detection of cell surface plus cytoplasmic c-Kit (Fig 6D) was similar to results shown in Fig 6C, indicating that no additional c-Kit is detected intracellularly in the PP1-treated cells. In total, these data show that lack of Src family kinase activity impairs internalization of c-Kit in response to SCF, and suggest a role for Src family members in these events.
c-Kit remains on the surface of PP1-treated cells. MO7e cells were preincubated without PP1 (A,B) or with PP1 (C,D) as described in Fig 1, then labeled with the 104D2 antibody. After a 30-minute incubation in SCF (200 ng/mL), the cells were fixed and labeled with goat antimouse IgG-FITC under conditions to detect cell surface c-Kit (A,C), or cell surface plus cytoplasmic c-Kit (B,D), as described in Materials and Methods.
c-Kit remains on the surface of PP1-treated cells. MO7e cells were preincubated without PP1 (A,B) or with PP1 (C,D) as described in Fig 1, then labeled with the 104D2 antibody. After a 30-minute incubation in SCF (200 ng/mL), the cells were fixed and labeled with goat antimouse IgG-FITC under conditions to detect cell surface c-Kit (A,C), or cell surface plus cytoplasmic c-Kit (B,D), as described in Materials and Methods.
Because the formation of caps in one region of the cell may be involved in cell locomotion,40 and because SCF is a potent chemotactic agent for hematopoietic cells and for mast cells,41-43 we investigated whether Src family kinase activity is required for SCF-induced migration of MO7e cells. The ability of SCF to induce chemotaxis of MO7e cells was assessed in a fluorescence-based assay using 96-well chemotaxis chamber plates (Fig7). SCF-induced migration of MO7e cells was evident within 30 minutes and maximal by 4 hours, at which time 21.5% ± 2.0% (mean ± standard deviation) of the MO7e population was observed to migrate (Fig 7A). Optimal MO7e migration occurred at 100 ng/mL SCF, and preincubation with PP1 (10 μmol/L) reduced migration induced by SCF (100 ng/mL) by greater than 80% (Fig 7B).
SCF induces chemotaxis of MO7e cells via a Src family kinase-mediated mechanism. SCF-induced chemotaxis was measured by a fluorescence-based assay using 96-well chemotaxis chamber plates (see Materials and Methods). Panel A depicts time-course analysis of SCF-induced (100 ng/mL) migration of MO7e cells. Panel B shows the concentration dependence of SCF-induced chemotaxis and PP1 (10 μmol/L) inhibition of SCF-induced chemotaxis of MO7e cells. The data are reported as the percentage of MO7e cells migrating into the lower chamber (mean ± standard deviation). The results are representative of three independent experiments that yielded similar results. The percentage of cells migrating in the presence of SCF plus PP1 was not different than the percentage of cells migrating in the control (P > .05, Student’s t-test).
SCF induces chemotaxis of MO7e cells via a Src family kinase-mediated mechanism. SCF-induced chemotaxis was measured by a fluorescence-based assay using 96-well chemotaxis chamber plates (see Materials and Methods). Panel A depicts time-course analysis of SCF-induced (100 ng/mL) migration of MO7e cells. Panel B shows the concentration dependence of SCF-induced chemotaxis and PP1 (10 μmol/L) inhibition of SCF-induced chemotaxis of MO7e cells. The data are reported as the percentage of MO7e cells migrating into the lower chamber (mean ± standard deviation). The results are representative of three independent experiments that yielded similar results. The percentage of cells migrating in the presence of SCF plus PP1 was not different than the percentage of cells migrating in the control (P > .05, Student’s t-test).
DISCUSSION
One of the major mechanisms of endocytosis of cell surface receptors is via clathrin-coated pits, although alternative mechanisms of endocytosis have also been described.24,44 Recruitment of receptors into clathrin-coated pits depends on association of specific sequences in the cytoplasmic tail of the receptor with the AP-2 complex, which is followed by association with clathrin. Coimmunoprecipitation of c-Kit and clathrin has been shown (Gommerman et al19 and the present report), implying that c-Kit can be internalized via the clathrin-coated pit mechanism. We found that signal transduction by Src family kinases is not required for entry of c-Kit into clathrin-coated pits. However, internalization of c-Kit was impaired in the PP1-treated MO7e cells and in the Lyn-deficient D33-3 cells, suggesting that Src family kinases are involved in this process.
Clathrin-coated pits have been estimated to comprise 2% of the cell surface area, and to have a half-life on the cell surface of 1 to 2 minutes.45 One question is whether activated signaling receptors enter preexisting clathrin-coated pits, or initiate assembly of new clathrin-coated pits that then internalize the ligand-receptor complex. The high-affinity Fc receptor for IgE can be sequestered in preexisting clathrin-coated pits; activation of this receptor did not induce new clathrin-coated pit formation.46 However, epidermal growth factor (EGF) binding to the EGF receptor promotes clathrin redistribution to the cell periphery, and this process is mediated at least in part by phosphorylation of clathrin heavy chain by Src.47 The latter data clearly show a role for Src family kinases in the regulation of clathrin localization and function. Of note, although phosphorylation of clathrin occured within 2 minutes of EGF binding, recruitment of clathrin to the cell periphery was detected 10 to 20 minutes after EGF binding.47 Coimmunoprecipitation of c-Kit and clathrin can be detected within 2 to 5 minutes after exposure of cells to SCF (Gommerman et al19 and the present report), and the association of c-Kit and clathrin does not require Src family kinase activity. These results suggest that although Src can directly phosphorylate clathrin,47 neither Src family kinase-induced phosphorylation of clathrin nor clathrin redistribution to the cell periphery is necessary for initial entry of ligand-activated c-Kit into clathrin-coated pits.
Scission of clathrin-coated pits from the cell surface requires the participation of the GTPase dynamin. Dynamin contains an aminoterminal GTP binding domain, a pleckstrin homology motif, and a carboxy-terminal proline-rich region, which contains binding sites for SH3-containing proteins. Dynamin can bind members of the Src kinase family,48-50 including Lyn,51 and Src family members stimulate the GTPase activity of dynamin in vitro.48 Deletion mutagenesis of SH3 binding sites in the proline-rich region of dynamin impairs dynamin colocalization with clathrin in the plasma membrane, self assembly, and GTPase activity.51-53 Furthermore, ligand-induced activation of the β2-adrenergic receptor induces Src to associate with dynamin and to phosphorylate dynamin,50 which is essential for its function in the internalization of the β2-adrenergic receptor. Together, these data implicate Src family kinases in the regulation of dynamin function. The diminished ability of the PP1-treated MO7e cells or the Lyn-deficient D33-3 cells to internalize c-Kit is consistent with these results,50 and may be due to a requirement for Src family kinase activity for normal dynamin function.
Capping of receptors in one region of the cell surface may serve as a mechanism to facilitate the association of the receptor and critical signal transduction molecules in a macromolecular complex.54 Clustering and lateral signaling among receptors may amplify a ligand binding signal in prokaryotic cells,55and possibly in eukaryotic cells as well.56 Previous work has shown that expression of a mutant c-Kit lacking a Src family binding site,9 or treatment of cells with PP1,8 9 inhibits SCF-induced proliferation. It is possible that inhibition of c-Kit capping or other receptor trafficking events may play a role in the ability of PP1 to impair SCF-induced proliferation.
Cap formation is found in motile cells, and may be required for cell migration.40 Because treatment with PP1 inhibited SCF-induced capping of c-Kit, we investigated whether SCF-induced migration of the cells would also be impaired. These experiments showed that inhibition of Src family kinase activity diminished SCF-induced chemotaxis of MO7e cells (Fig 7). In contrast to the present results with c-Kit, phosphorylation of the PDGF β-receptor by Src negatively regulates PDGF BB-induced mobility of endothelial cells.57Inhibition of Src family kinase activity by overexpression of low molecular weight phosphotyrosine-protein phosphatase enhanced PDGF BB-stimulated chemotaxis of NIH 3T3 cells.58 However, activation of Src family kinases is not required for PDGF BB-induced endothelial cell chemotaxis mediated by the PDGF α-receptor.59 Thus Src family kinases may play distinct roles in cell migration mediated by different members of the Type-III tyrosine kinase receptor family.
ACKNOWLEDGMENT
The authors thank Dr Hans-Jörg Bühring for providing the 104D2 MoAb, and Dr Thalia Papayannopoulou for the use of the Nikon Eclipse Fluorescent Microscope.
Supported by National Institutes of Health Grants No. DK44194, DK43719, CA31615, HL53515, and AI07763, and by the National Cancer Institute. This project was funded in part with the federal funds under the National Cancer Institute, National Institutes of Health under contract No. N01-CO-56000.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Virginia C. Broudy, MD, Division of Hematology, University of Washington, Box 357710, Seattle, WA 98195-77105; e-mail:vcbroudy@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal