Abstract
Cellular immunophenotypic studies were performed on a cohort of randomly selected IgM+ B-chronic lymphocytic leukemia (B-CLL) cases for which Ig VH and VL gene sequences were available. The cases were categorized based on V gene mutation status and CD38 expression and analyzed for treatment history and survival. The B-CLL cases could be divided into 2 groups. Those patients with unmutated V genes displayed higher percentages of CD38+ B-CLL cells (≥30%) than those with mutated V genes that had lower percentages of CD38+ cells (<30%). Patients in both the unmutated and the ≥30% CD38+ groups responded poorly to continuous multiregimen chemotherapy (including fludarabine) and had shorter survival. In contrast, the mutated and the <30% CD38+ groups required minimal or no chemotherapy and had prolonged survival. These observations were true also for those patients who stratified to the Rai intermediate risk category. In the mutated and the <30% CD38+ groups, males and females were virtually equally distributed, whereas in the unmutated and the ≥30% CD38+ groups, a marked male predominance was found. Thus, Ig V gene mutation status and the percentages of CD38+B-CLL cells appear to be accurate predictors of clinical outcome in B-CLL patients. These parameters, especially CD38 expression that can be analyzed conveniently in most clinical laboratories, should be valuable adjuncts to the present staging systems for predicting the clinical course in individual B-CLL cases. Future evaluations of new therapeutic strategies and drugs should take into account the different natural histories of patients categorized in these manners.
B CELL CHRONIC lymphocytic leukemia (B-CLL) is the most common leukemia in the Western world1; ∼7,500 individuals develop and ≈ 5,000 die of this disease each year.2 Age is an important factor, as the incidence of B-CLL increases linearly with each decade above the age of 40.3,4 In addition, gender is relevant because men outnumber women by ∼ 2:1 ratio and may have a worse clinical outcome.5 6
Patients with B-CLL follow heterogeneous clinical courses. Some survive for prolonged periods without requiring definitive therapy, while others die rapidly despite aggressive treatment.1,7 Two major staging systems have been developed to address this clinical heterogeneity.8-10 Although these systems have provided valuable information regarding survival, they have been unable to accurately predict which early stage or intermediate risk patients will experience an indolent or an aggressive course. Therefore, novel identifiers of the clinical subsets with favorable versus poor prognoses would be very helpful for patient management. For this reason, several parameters such as lymphocyte doubling time,11 circulating levels of β2microglobulin12,13 and soluble CD23,14 serum thymidine kinase levels,15,16bone marrow histology,17 and cytogenetic abnormalities18 are currently being evaluated as adjuncts to these staging systems.
B-CLL is characterized by the clonal accumulation of CD5+ B cells.19 Although these cells originally were considered antigen-inexperienced “virgin” lymphocytes, recent data indicate that at least half of these cases represent expansions of previously-triggered, postgerminal center (GC) “memory” B cells.20,21 This conclusion is based on the presence of significant numbers of somatic mutations in the Ig heavy (H) chain variable region (V) genes. Indeed, in our previous study of 83 (64 IgM+ and 19 non-IgM+) B-CLL cases,21 we found significant numbers of VHgene mutations in ∼50% of the IgM+ and ∼75% of the non-IgM+ (IgG and IgA) cases. We recently extended these studies to the VL genes of these cases. These newer analyses indicate that ∼10% of B-CLL patients have mutations in their VL genes only (Ghiotto et al, in preparation). Taken together, these VH and VL sequencing data suggest that ∼60% of B-CLL cases can be considered post-GC memory B cells. Thus, B-CLL cases can be divided into 2 categories according to Ig V gene mutation status.
The expression of specific cell surface markers distinguishes subsets of normal human B cells that differ in differentiation and activation stages and in biologic properties.22 Analyses of CD38 and IgD expression have been especially useful in distinguishing B cells at various stages of differentiation from naı̈ve through memory cells.23 Therefore, in this study, we investigated whether the mutated and unmutated B-CLL cases could also be distinguished in this way. In addition, we asked whether distinctions based on surface membrane phenotype or Ig V gene mutation status might predict different clinical courses and outcomes.
Our data indicate that both the percentages of CD38+ B-CLL cells and the presence or absence of Ig V gene mutations (VH + VL) can be used as prognostic indicators in this disease. Because the percentage of CD38+B-CLL cells inversely correlates, in a statistically significant manner, with the presence or absence of Ig V gene mutations in B-CLL, CD38 may be the preferred adjunct to the current staging systems because of its relative ease of ascertainment.
MATERIALS AND METHODS
Patients.
The Institutional Review Boards of North Shore University Hospital, Manhasset, NY, and Long Island Jewish Medical Center, New Hyde Park, NY, approved these studies. The patients in this study are a subset (n = 47) of the well-defined cohort (n = 64) of randomly chosen, typical IgM+ B-CLL patients described previously.21Patients were selected for the present study based on the availability of detailed clinical histories (Don Monti Division of Medical Oncology, North Shore University Hospital, and the Hematology/Oncology Division, Long Island Jewish Medical Center) and the availability of DNA sequences for both the Ig VH21 24 and VL (Ghiotto et al, in preparation) genes in each case. The clinical courses of the patients that were analyzed in this study were not significantly different from those that could not be studied because of lack of sample or follow-up. There were 34 males and 13 females in this group, with a mean age of 63.4 years (range, 38 to 80). The mean ages of the unmutated (mean, 61.3; range, 38 to 79) and mutated (mean, 65.5; range, 47 to 80) cases or of the ≥30% CD38+ (mean, 63.5; range, 38 to 79) and <30% CD38+ (mean, 63.6; range, 44 to 79) cases were similar.
Fresh or cryopreserved B-CLL cells were available for surface marker analyses on 37 patients (20 unmutated and 17 mutated). These samples had been obtained at various points in the clinical follow-up of these patients. There were no differences in the timing of sample acquisition between the unmutated and mutated groups.
Cellular immunophenotypic analysis.
The following antibody conjugates were used: anti–CD23-fluorescein isothiocyanate (FITC; Immunotech, Inc, Westbrook, ME), goat antihuman IgD-FITC (Southern Biotechnology Associates, Birmingham, AL), and anti–CD5-FITC, anti–CD5-phycoerythrin (PE), anti–CD38-PE, anti–CD19-allophycocyanin (APC), anti–CD45-FITC, and anti–CD14-PE (Simultest LeucoGATE; all from Becton Dickinson Immunocytometry Systems, San Jose, CA).
Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood by density gradient centrifugation using Ficoll-Paque (Pharmacia LKB Biotechnology, Piscataway, NJ) and used either immediately or after thawing samples that had been cryopreserved with a programmable cell freezing machine (CryoMed, Mt. Clemens, MI). PBMC were analyzed for surface expression of CD19/CD5/CD38 and CD19/IgD/CD38 and CD19/CD5/CD23 by triple color immunofluorescence.25 For the detection of CD19/CD5/CD38-expressing cells, monoclonal antibody (MoAb) labeled with the following fluorochromes were used: anti–CD19-APC, anti–CD5-FITC, and anti–CD38-PE; for the detection of CD19/IgD/CD38-expressing cells, anti–CD19-APC, anti–IgD-FITC, and anti–CD38-PE MoAb were used; for the detection of CD19/CD5/CD23-expressing cells, anti–CD19-APC, anti–CD23-FITC, and anti–CD5-PE MoAb were used. Isotype-matched negative controls were used in all assays to determine positive from negative results. Flow cytometric analyses were performed on a Becton-Dickinson FACS Calibur flow cytometer equipped with argon and red diode lasers. Measurements of forward and side scatter were combined with CD45 and CD14 determinations to identify lymphocytes and exclude monocytes. The CellQuest software system (Becton Dickinson Immunocytometry Systems) was used to acquire and analyze data.
Statistical analyses.
The percentages of CD5+/CD19+ B cells that coexpressed CD38 or IgD or CD23 were determined for each patient and statistical differences between the unmutated and mutated groups analyzed using the Mann-Whitney test. Patients were also classified according to the percentage of B-CLL cells expressing CD38 into ≥30% CD38+ and <30% CD38+ groups.
To determine the degree of association between the individual patients based on the actual percentages of CD38-expressing cells and on the V gene mutation status or on the percentages of CD23-expressing cells, the Spearman coefficient was calculated. To determine the degree of association between the patients classified into 2 groups based on percentages of CD38-expressing cells and on the V gene mutation status, the Kappa coefficient was used. Standard methods for estimating proportions and associated exact confidence intervals (CI) were used for estimating sensitivity of high numbers of CD38+ B-CLL cells (≥30%) as a marker for “unmutated” V genes. In standard epidemiological terminology, the unmutated gene corresponded to the “disease” state and accordingly sensitivity was computed using the number of patients with unmutated genes as the denominator. Similarly, specificity of low numbers of CD38+ B-CLL cells (<30%) was computed using the patients with mutated genes as the denominator. Accuracy was defined as the percentage of patients who were classified correctly as unmutated or mutated using the CD38 criteria. Positive and negative predictive values were computed using Bayes’ Rule.
Comparisons of V gene mutation status and CD38 expression with clinical course were made “blindly”. The investigator (T.W.) who reviewed the clinical histories of these patients was unaware of the laboratory data during the retrospective chart review. The two-tailed Fisher’s Exact test was used to determine whether chemotherapy requirements, Rai stage at diagnosis, or gender were significantly associated with V gene mutation status or with CD38 percentage. Survival analyses were performed using the Kaplan-Meier product-limit method and the log-rank test.
RESULTS
Percentages of CD38+ B-CLL cells among the unmutated and mutated cases.
The DNA sequences of the Ig VH and VL genes expressed by the leukemic cells of the 47 typical IgM+B-CLL cases included in this study were determined previously. Based on the numbers of somatic mutations detected in these genes, the cases were divided into 2 categories: “unmutated” or “mutated”. As per current convention, “unmutated” cases were defined as those with <2% differences from the most similar germline gene in both the expressed VH and VL genes; “mutated” cases were defined as those in which the B-CLL cells displayed ≥2% differences in either the expressed VH or VL gene.
To determine whether these genetic differences reflected cellular phenotypic differences, we analyzed the expression of CD5, CD23, CD38, and IgD on the B-CLL cells of the 37 patients in whom PBMC were available (20 unmutated and 17 mutated). Analyses of CD38 and IgD expression were chosen for these studies because they distinguish B cells at various stages of differentiation.22 23
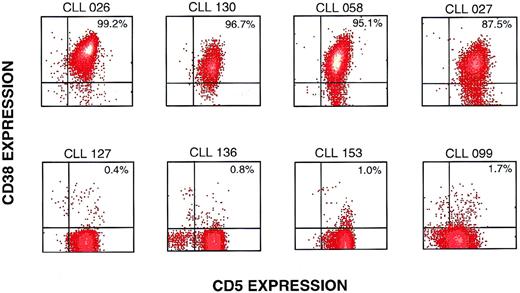
The unmutated and mutated B-CLL cases were similar in CD19+B cells coexpressing CD5, CD23, and IgD, both in the percentages of positive cells and in mean fluorescence intensity (data not shown). However, the percentage of CD38+ cells was dramatically different between the unmutated and mutated cases. Figure 1 illustrates 8 representative B-CLL cases analyzed for CD19/CD5/CD38-expressing cells. The VHand VL genes of the 4 cases listed in the upper panel were not mutated, whereas the VH and/or VL genes in the lower panel were mutated. Note that the unmutated cases have a much higher percentage of CD38+ cells among the CD5+/CD19+ cells than the mutated cases in the lower panel.
Representative flow cytometric profiles of CD38 expression on mutated and unmutated CD5+/CD19+ B-CLL cases. B-CLL cases were analyzed by flow cytometry after exposure to anti–CD19-APC, anti–CD5-FITC, and anti–CD38-PE. The events illustrated were obtained by gating on cells expressing CD19. Density plots of CD38 and CD5 expression are shown for 8 representative B-CLL cases. The upper 4 cases had less than 2% mutations in either the VH or VL genes, whereas the lower 4 cases had mutations in the VH and/or VL genes.
Representative flow cytometric profiles of CD38 expression on mutated and unmutated CD5+/CD19+ B-CLL cases. B-CLL cases were analyzed by flow cytometry after exposure to anti–CD19-APC, anti–CD5-FITC, and anti–CD38-PE. The events illustrated were obtained by gating on cells expressing CD19. Density plots of CD38 and CD5 expression are shown for 8 representative B-CLL cases. The upper 4 cases had less than 2% mutations in either the VH or VL genes, whereas the lower 4 cases had mutations in the VH and/or VL genes.
When the percentages of CD38+ B-CLL cells in the unmutated and mutated groups were compared statistically, very significant differences were found (means, 63.9% v 7.3%, respectively;P = .00001). The Spearman correlation between the individual percentages of CD38+ B-CLL cells in each case versus the actual percentages of V gene mutations was r = -0.75 (P < .001), indicating a relatively strong inverse relationship. A low to moderate direct correlation existed between the percentages of CD23+ B-CLL cells and the percentage of V gene mutation (r = 0.42; P = .01). There was no correlation between CD38 expression and CD23 expression (data not shown).
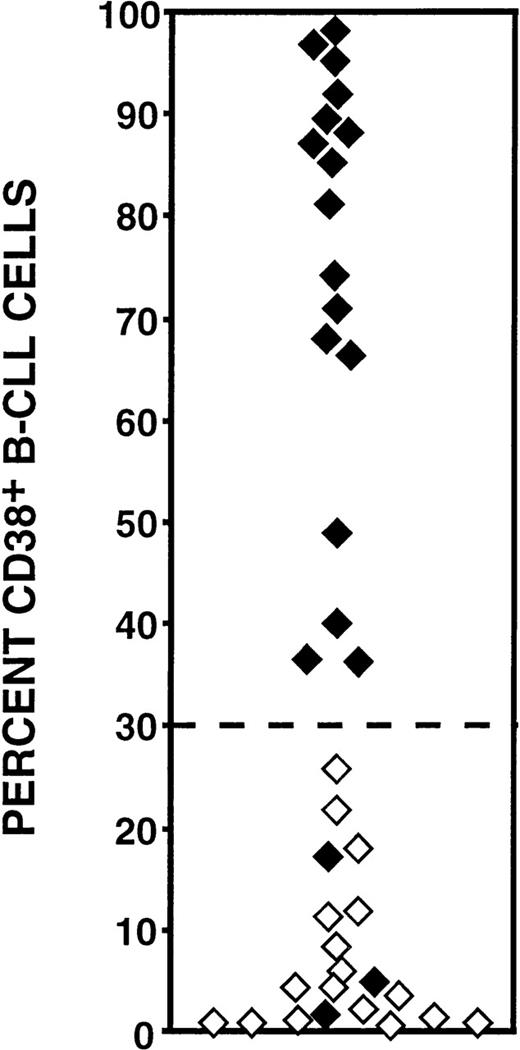
When the results on percentages of CD38-expressing cells were plotted individually ( Fig 2), the cases could be segregated into 2 distinct sets, 1 with ≥30% CD38+ cells and the other with <30% CD38+ cells. The 30% cut off value was chosen empirically based on the observed distributions on the plot. Furthermore, an inverse relationship existed between CD38 expression and V gene mutation status. The set with the higher percentages of CD38+ B-CLL cells was comprised solely of unmutated cases, whereas the set with the lower percentages of CD38+ cells contained all of the mutated cases and 3 of the unmutated cases. The Kappa coefficient calculated for association between these 2 sets of CD38+ B-CLL cases versus the unmutated and mutated groups was -0.84, indicating a strong inverse relationship.
Percentages of CD38+/CD5+/CD19+ cells among mutated and unmutated B-CLL cases. The percentages of CD38-expressing B-CLL cells among patients (n = 37) whose Ig VH and VL genes had been sequenced. Unmutated cases (⧫) display <2.0% differences from the most similar germline gene; mutated samples (⋄) display ≥2% differences. Note that all of the cases (17 of 17) that have ≥30% CD38+ B-CLL cells were unmutated, whereas only 3 unmutated cases expressed low numbers (<30%) of CD38+ B-CLL cells. These comparisons are statistically significant (P = .00001; Mann-Whitney test).
Percentages of CD38+/CD5+/CD19+ cells among mutated and unmutated B-CLL cases. The percentages of CD38-expressing B-CLL cells among patients (n = 37) whose Ig VH and VL genes had been sequenced. Unmutated cases (⧫) display <2.0% differences from the most similar germline gene; mutated samples (⋄) display ≥2% differences. Note that all of the cases (17 of 17) that have ≥30% CD38+ B-CLL cells were unmutated, whereas only 3 unmutated cases expressed low numbers (<30%) of CD38+ B-CLL cells. These comparisons are statistically significant (P = .00001; Mann-Whitney test).
Furthermore, high percentages of CD38+ B cells (≥30%) indicated the presence of <2% mutations with 100% specificity (95% CI: 84% to 100%). Because 3 patients with unmutated V genes were found to have <30% CD38+ B-CLL cells (Fig 2), the sensitivity of using ≥30% CD38+ B-CLL cells as a marker for significant percentages of VH or VL gene mutations was 85% (95% CI: 62% to 97%). Based on this specificity and sensitivity and on a prevalence of 60% for ≥2% mutations in either VH or VL, the positive predictive value of ≥30% CD38+ B cells indicating the “unmutated” genotype was 100%. Conversely, the predictive value of <30% CD38+ B cells indicating the “mutated” genotype was 82%. These CD38 criteria indicate V gene mutation status with 92% accuracy.
The differences in CD38 expression were stable over time and were not influenced by chemotherapy. Sixteen patients (7 with CD38 values ≥30% and 9 with <30%) were studied at 2 or more time points, separated by as much as 6 years. Indeed, the percentages of CD38+ B-CLL cells detected never differed by more than 10% in any instance. One patient with 95% circulating CD38+ B-CLL cells was studied on 5 occasions over a 24-month period and the percentages of CD38+ cells detected in each sample were very similar (<5% difference).
Clinical course and outcome of the unmutated versus mutated B-CLL cases.
The treatment histories of the patients with unmutated and mutated Ig V region genes were very different (Table 1). Eighteen of the 23 mutated cases (78.3%) required either no chemotherapy (52.2%) or minimal treatment (26.1%), while only 20.8% of the unmutated cases required no (16.6%) or minimal therapy (4.2%). These differences were highly significant (P = .0001). Furthermore, 79.2% (19 of 24) of the unmutated cases required continuous chemotherapy or chemotherapy using 2 or more agents or regimens. Although 18 of these 19 patients (94.7%) received fludarabine, only 2 achieved a durable clinical response.
Comparison of Treatment Histories Based on Either Ig V Gene Mutation Status or the Percentages of CD38+ B-CLL Cells
| . | Unmutated . | Mutated . |
|---|---|---|
| Patients requiring no or minimal*treatment | 20.8% (5/24) | 78.3% (18/23) |
| Patients requiring continuous chemotherapy or chemotherapy with 2 or more agents or regimens | 79.2% (19/24) | 21.7% (5/23) |
| P = .0001† | ||
| ≥30% CD38+ B-CLL Cells | <30% CD38+ B-CLL Cells | |
| Patients requiring no or minimal* treatment | 23.5% (4/17) | 73.7% (14/19) |
| Patients requiring continuous chemotherapy or chemotherapy with 2 or mor eagents or regimens | 76.5% (13/17) | 26.3% (5/19) |
| P = .0067† | ||
| . | Unmutated . | Mutated . |
|---|---|---|
| Patients requiring no or minimal*treatment | 20.8% (5/24) | 78.3% (18/23) |
| Patients requiring continuous chemotherapy or chemotherapy with 2 or more agents or regimens | 79.2% (19/24) | 21.7% (5/23) |
| P = .0001† | ||
| ≥30% CD38+ B-CLL Cells | <30% CD38+ B-CLL Cells | |
| Patients requiring no or minimal* treatment | 23.5% (4/17) | 73.7% (14/19) |
| Patients requiring continuous chemotherapy or chemotherapy with 2 or mor eagents or regimens | 76.5% (13/17) | 26.3% (5/19) |
| P = .0067† | ||
Minimal treatment is defined as less than 6 months of therapy in the years of follow-up.
Statistical analyses performed using the 2-tailed Fisher’s exact test.
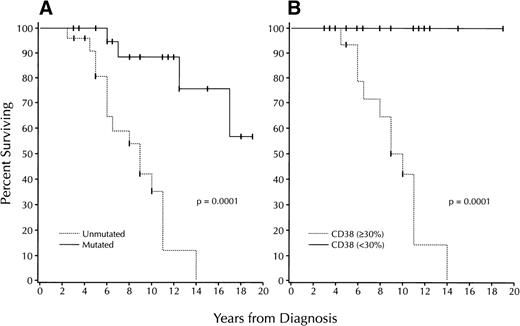
These significant differences in chemotherapy requirements were reflected in significant differences in survival (Fig 3A). The median survival of the patients in the unmutated group was 9 years, whereas median survival for the mutated group was not reached for the duration of follow-up (P = .0001).
Survival based on V gene mutation status and CD38 expression. (A) Kaplan-Meier plot comparing survival based on the absence (“unmutated”: . . . . . . ) or presence (“mutated”: ____) of significant numbers (≥2%) of V gene mutations in 47 B-CLL cases (unmutated: 24 cases; mutated: 23). Median survival of unmutated group: 9 years; median survival of mutated group not reached; P = .0001; log-rank test). (B) Kaplan-Meier plot comparing survival based on the detection of ≥30% (. . . . . . ) or <30% CD38+ B-CLL cells ( ≥30%: 17 cases; <30%: 19). Median survival of the ≥30% CD38+ group: 10 years; median survival of the <30% CD38+ group: not reached (P = .0001; log-rank test).
Survival based on V gene mutation status and CD38 expression. (A) Kaplan-Meier plot comparing survival based on the absence (“unmutated”: . . . . . . ) or presence (“mutated”: ____) of significant numbers (≥2%) of V gene mutations in 47 B-CLL cases (unmutated: 24 cases; mutated: 23). Median survival of unmutated group: 9 years; median survival of mutated group not reached; P = .0001; log-rank test). (B) Kaplan-Meier plot comparing survival based on the detection of ≥30% (. . . . . . ) or <30% CD38+ B-CLL cells ( ≥30%: 17 cases; <30%: 19). Median survival of the ≥30% CD38+ group: 10 years; median survival of the <30% CD38+ group: not reached (P = .0001; log-rank test).
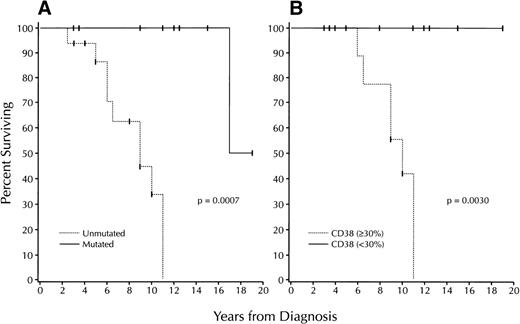
Finally, we compared V gene mutation status with the clinical stage at the time of diagnosis using the modified Rai system. Our patients stratified to all Rai modified clinical stages at the time of diagnosis (Table 2). Patients who stratify to the Rai intermediate risk group are the most heterogeneous in treatment requirements and survival and represent those in whom outcome is the most difficult to predict.1 7 Therefore, we analyzed the survival of the 25 patients in this group (9 mutated cases v 16 unmutated cases; Fig 4A). The median survival of the unmutated cases was 9 years, compared with 17 years for the mutated cases (P = .0007).
Comparison of Modified Rai Stage at Diagnosis With Ig V Gene Mutation Status and the Percentages of CD38+ B-CLL Cells
| Stage . | Unmutated . | Mutated . |
|---|---|---|
| Low* | 22.7% (5/22) | 52.4% (11/21) |
| Intermediate* | 72.7% (16/22) | 42.9% (9/21) |
| High | 4.6% (1/22) | 4.7% (1/21) |
| P = .123 | ||
| ≥30% CD38+ B-CLL Cells | <30% CD38+ B-CLL Cells | |
| Low† | 20.0% (3/15) | 50.0% (9/18) |
| Intermediate† | 73.3% (11/15) | 50.0% (9/18) |
| High | 6.7% (1/15) | 0.0% (0/18) |
| P = .138 | ||
| Stage . | Unmutated . | Mutated . |
|---|---|---|
| Low* | 22.7% (5/22) | 52.4% (11/21) |
| Intermediate* | 72.7% (16/22) | 42.9% (9/21) |
| High | 4.6% (1/22) | 4.7% (1/21) |
| P = .123 | ||
| ≥30% CD38+ B-CLL Cells | <30% CD38+ B-CLL Cells | |
| Low† | 20.0% (3/15) | 50.0% (9/18) |
| Intermediate† | 73.3% (11/15) | 50.0% (9/18) |
| High | 6.7% (1/15) | 0.0% (0/18) |
| P = .138 | ||
Comparison of V gene mutation status among patients in the low and intermediate risk categories (P = .058; 2-tailed Fisher’s Exact test).
Comparison of CD38 expression among patients in the low and intermediate risk categories (P = .147; 2-tailed Fisher’s Exact test).
Survival based on V gene mutation status and CD38 expression among B-CLL patients who stratify to the Rai intermediate risk category. (A) Kaplan-Meier plot comparing V gene mutation status with survival among the cases within the Rai intermediate risk category (unmutated: 16 cases; mutated: 9). Median survival of the mutated group: 9 years; median survival of the unmutated group: 17 years (P = .0007; log-rank test). (B) Kaplan-Meier plot comparing numbers of CD38+ B-CLL cells with survival among the cases within the Rai intermediate risk category (≥30%: 11 cases; <30%: 9). Median survival of the 30% CD38+ group: 10 years; median survival of the <30% CD38+ group: not reached (P = .0030; log-rank test). None of the patients in the <30% CD38+ group died during the follow-up period.
Survival based on V gene mutation status and CD38 expression among B-CLL patients who stratify to the Rai intermediate risk category. (A) Kaplan-Meier plot comparing V gene mutation status with survival among the cases within the Rai intermediate risk category (unmutated: 16 cases; mutated: 9). Median survival of the mutated group: 9 years; median survival of the unmutated group: 17 years (P = .0007; log-rank test). (B) Kaplan-Meier plot comparing numbers of CD38+ B-CLL cells with survival among the cases within the Rai intermediate risk category (≥30%: 11 cases; <30%: 9). Median survival of the 30% CD38+ group: 10 years; median survival of the <30% CD38+ group: not reached (P = .0030; log-rank test). None of the patients in the <30% CD38+ group died during the follow-up period.
Clinical course and outcome of B-CLL cases with ≥30% or <30% CD38+ cells.
Because there was a significant correlation between V gene mutation and CD38 expression by the B-CLL cells, we also compared chemotherapy requirements and survival as a function of the percentages of CD38+ leukemic cells. Significant differences were found for both. Seventy-three percent (14 of 19) of the <30% CD38+ cases required either no or minimal chemotherapy, compared with 23.5% (4 of 17) of the ≥30% CD38+ cases (P = .0067; Table 1). Conversely, 76.5% of the ≥30% CD38+ cases required either continuous chemotherapy or chemotherapy with 2 or more agents or regimens.
Median survival for the patients in the ≥30% CD38+ group was 10 years (Fig 3B). In contrast, this value could not be determined for the patients in the <30% CD38+ group, as all patients in this group were alive for the duration of follow-up (P = .0001). Highly significant differences in survival also were found among the patients in the Rai intermediate risk group (Fig 4B). Median survival for the ≥30% CD38+patients was reached in 10 years, whereas all patients in the <30% CD38+ group remained alive throughout the years of follow-up (P = .003).
Studies of IgG+ and IgA+ B-CLL cases.
The preceding observations were also true for a cohort of non-IgM producing (IgG or IgA) B-CLL patients (n = 16), whose V gene sequence analyses were published previously.21 24 The median survival of the unmutated non-IgM cases was only 3 years, whereas it was not reached for the mutated cases at 15 years (P = .004, log-rank test; data not shown). Similar data were obtained when the cases were compared based on CD38 expression, although the small numbers of available samples (n = 8) precluded accurate statistical analysis. When these non-IgM+ cases were pooled with the IgM+ cases described above (bringing the total number of patients studied to 63), the median survival for the unmutated group (n = 29) was 8 years and for the mutated group (n = 34) was not reached for the duration of follow-up (P = .0001). Similar data were obtained for the CD38 groups: median survival for the ≥30% CD38+ (n = 19) was 9 years, whereas median survival for the <30% CD38+ group (n = 25) was not reached (P = .0001).
Gender of the B-CLL cases based on either V gene mutation or CD38 expression.
The cohort of IgM+ B-CLL patients in this study consisted of 34 males and 13 females (M:F = 2.6:1). However, the M:F ratio of the patients stratified by either V gene mutation status or CD38 expression was very different (Table 3). In the mutated group, males and females were virtually equally distributed, whereas in the unmutated group, a marked male predominance was found (M:F = 11:1; P = .003). A similar disparity in gender distribution was seen when the patients were compared based on the percentages of CD38+ B-CLL cells. The numbers of males and females among the <30% CD38+ group were almost equal (M:F = 1.1:1), whereas males outnumbered females in the ≥30% CD38+ group (M:F = 7.5:1; P = .031).
Gender Differences Based on Either Ig V Gene Mutation Status or the Percentages of CD38+ B-CLL Cells
| . | Unmutated . | Mutated . |
|---|---|---|
| Male3-150 | 91.7% (22/24) | 52.2% (12/23) |
| Female3-150 | 8.3% (2/24) | 47.8% (11/23) |
| P = .0033-150 | ||
| Male:female ratio | 11.0:1 | 1.1:1 |
| ≥30% CD38+ B-CLL Cells | <30% CD38+ B-CLL Cells | |
| Male3-150 | 88.2% (15/17) | 52.6% (10/19) |
| Female3-150 | 11.8% (2/17) | 47.4% (9/19) |
| P = .0313-150 | ||
| Male:female ratio | 7.5:1 | 1.1:1 |
| . | Unmutated . | Mutated . |
|---|---|---|
| Male3-150 | 91.7% (22/24) | 52.2% (12/23) |
| Female3-150 | 8.3% (2/24) | 47.8% (11/23) |
| P = .0033-150 | ||
| Male:female ratio | 11.0:1 | 1.1:1 |
| ≥30% CD38+ B-CLL Cells | <30% CD38+ B-CLL Cells | |
| Male3-150 | 88.2% (15/17) | 52.6% (10/19) |
| Female3-150 | 11.8% (2/17) | 47.4% (9/19) |
| P = .0313-150 | ||
| Male:female ratio | 7.5:1 | 1.1:1 |
Statistically significant difference in gender distribution between the unmutated and mutated groups and between the ≥30% CD38+ and <30% CD38+ groups (2-tailed Fisher’s Exact test).
DISCUSSION
The preceding data indicate that Ig V gene mutation status and CD38 expression are distinct and reliable prognostic indicators of clinical course and outcome in B-CLL. Indeed, those patients in either the unmutated or ≥30% CD38+ groups experienced a worse clinical course than those patients in the mutated or <30% CD38+ groups. This was true for both chemotherapy requirements (Table 1) and survival (Fig 3).
Possibly our most clinically relevant correlation was found among those patients who presented initially in the Rai intermediate risk category (Fig 4). These patients are frustratingly difficult for clinicians to treat because they can have either an indolent course requiring no or minimal therapy or a rapid downhill course despite aggressive treatment. Both CD38 expression and V gene mutation status were able to segregate those Rai intermediate risk patients who followed an indolent course from those whose course was much more aggressive (Fig 4).
Relevant to our observations on Ig V gene mutations and survival is the study of Oscier et al26 indicating that B-CLL cells with unmutated VH genes frequently contain 3 copies of chromosome 12, a cytogenetic marker that is associated with poor clinical outcome. This study was recently extended by Hamblin et al26a to a larger cohort of patients. These new data are consistent with our observations and show clearly that unmutated VH genes are associated with a more aggressive form of B-CLL.
When our patients were stratified according to V gene mutation status or CD38 expression (Table 3), a clear preponderance of males was noted in the unmutated and ≥30% CD38+ (poor outcome) groups (M:F: 11:1 and 7.5:1, respectively). These ratios are much higher than those reported previously.5 Our data, however, agree with the studies indicating that women with B-CLL have a more favorable clinical course than men. Although women comprised only ∼10% of the unmutated and ≥30% CD38+ (poor outcome) groups, they constituted ∼50% of the mutated and <30% CD38+ (good outcome) groups (Table 3). These data support a role for gender indirectly influencing clinical outcome and possibly B-cell maturation and differentiation. The mechanism(s) responsible for these differences are obscure at this point.
The 2 sets of B-CLL cases characterized in this study appear to represent B cells transformed at different stages of B-cell differentiation and/or activation. Thus, those B-CLL cases with mutated V genes and low numbers of CD38+ B-CLL cells are characteristic of post-GC, memory B cells.22,23 Some of these B-CLL cells may be derived from the small subset of IgM+/IgD+ memory cells found in the blood27 or bone marrow28 or from cells similar to the IgD+ memory B cells identified in tonsils.29 Although CD27 is another marker that distinguishes pre-GC from post-GC B cells,27,30,31 we did not find differences in CD27 expression among our CD5+B-CLL cases, either in density per cell or in cell number (data not shown). These data are in agreement with those of others.32 33
In contrast, those B-CLL cases with unmutated V genes and high numbers of CD38+ B-CLL cells display surface markers characteristic of B cells that have not entered a GC. Because CD38, as detected by MoAb conjugated with PE, is expressed on most blood B cells,34 the ≥30% CD38+/unmutated B-CLL cells could be derived from either naı̈ve B cells or activated B cells that have not entered a GC and have not generated Ig V gene mutations. Based on analyses of the HCDR3 characteristics of unmutated B-CLL cases,21 35 we favor the hypothesis that some of these unmutated B-CLL cells have been activated and selected by foreign or autoantigen.
The physiological significance of CD38 expression primarily by the unmutated cases and its potential function in cell survival and proliferation is presently unknown. However, previous studies suggest that CD38 expression identifies those B-CLL clones that are capable of transducing signals through their B-cell antigen receptors that may increase or decrease their chance for survival.36,37 In this regard, Zupo et al38 have reported that CD38+ B-CLL cells can be induced to undergo apoptosis in vitro after exposure to anti-Ig antibodies, whereas CD38− B-CLL cells are resistant to these effects. Although these data appear to be at variance with our clinical observations that those B-CLL cases with higher percentages of CD38+ B cells have a worse clinical outcome, the quality of the antigen receptor stimulus and the presence of associated stimuli may lead to diverse endpoints (apoptosis v survival). Similarly, triggering through the CD38 molecule can have different effects on the survival of B cells depending on the state of maturation/activation of the cell. Whereas anti-CD38–mediated signaling results in the death of immature B cells,34mature B cells can be rescued from apoptosis by CD38 triggering.36 37 Further studies will be necessary to determine how these in vitro data correlate with our clinical observations.
In conclusion, our studies identify CD38 expression and V gene mutation status as novel prognostic indicators that appear to identify mutually overlapping groups of B-CLL patients (Figs 1 and 2 and Results). However, because CD38 expression can be determined more conveniently and rapidly than Ig V gene mutations, this parameter may be the preferred adjunct to the current staging systems. Indeed, the results of this simple test should enable physicians to predict with considerable accuracy whether a patient is likely to have a favorable or unfavorable clinical course. Furthermore, because the leukemic cells appear to be fixed in their level of expression of this marker, determining the percentage of CD38+ B-CLL cells may be useful at any point in the clinical course of the individual B-CLL patient. However, we cannot exclude the possibility that CD38 expression might change with the alterations in chromosomal structure and gene expression that occur in Richter’s transformation.39-41
It will be important to confirm our findings and to compare more extensively the value of CD38 expression versus V gene mutation status as prognostic indicators. In addition, these 2 indicators need to be compared and correlated with other prognostic markers such as lymphocyte doubling time,11 circulating levels of β2microglobulin12,13 and soluble CD23,14 serum thymidine kinase levels,15,16bone marrow histology,17 and cytogenetic abnormalities.18
ACKNOWLEDGMENT
We thank Cathy Rapelje and Grace Lee for performing flow cytometric analyses, Drs Qiuhu Shi, Cristina Sison, and Martin L. Lesser for performing statistical calculations, Gurmeet Sahansra for helping with chart review, and Drs Charles C. Chu, Thomas J. Degnan, and Peter K. Gregersen for critically reviewing the manuscript.
R.N.D. and T.W. contributed equally to this study.
Supported in part by US Public Health Service (USPHS) Grant No. AI 10811 from the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID), the Joseph Eletto Leukemia Research Fund, the Jean Walton Fund for Lymphoma and Myeloma Research, the Sass Foundation for Medical Research, and the Richard and Nancy Leeds Fund of the Department of Medicine of North Shore University Hospital.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Nicholas Chiorazzi, MD, North Shore University Hospital, 350 Community Dr, Manhasset, NY 11030; e-mail:nchizzi@nshs.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal