Abstract
The ETV6 gene (also known as TEL) is the main target of chromosomal translocations affecting chromosome band 12p13. The rearrangements fuse ETV6 to a wide variety of partner genes in both myeloid and lymphoid malignancies. We report here 4 new cases of acute myeloid leukemia (AML) with very immature myeloblasts (French-American-British [FAB]-M0) and with a t(4;12)(q11-q12;p13). In all cases, ETV6 was found recombined to a new gene, homologous to the mouse Brx gene. The gene was named BTL (Brx-likeTranslocated in Leukemia). Reverse transcriptase-polymerase chain reaction (RT-PCR) experiments indicate that the expression of the BTL-ETV6 transcript, but not of the reciprocal ETV6-BTL transcript, is a common finding in these leukemias. In contrast to the majority of other ETV6 fusions, both the complete helix-loop-helix (HLH) and ETS DNA binding domains of ETV6 are present in the predicted BTL-ETV6 fusion protein, and the chimeric gene is transcribed from theBTL promoter.
ETV6 (ALSO KNOWN AS TEL) belongs to the ETS family of transcription factors characterized by 2 important domains: the HLH (helix-loop-helix or pointed) domain that mediates protein–protein interactions and the ETS DNA binding domain. The gene was originally discovered as the 12p gene involved in the t(5;12)(q33;p13) in chronic myelomonocytic leukemia.1 It is now clear that ETV6 is the major target of translocations involving 12p13 in hematologic malignancies.2 In the fusion protein resulting from these rearrangements, the HLH-domain of ETV6 activates the kinase domain of respectively PDGFRB1, ABL3, JAK24 or modifies the properties of the transcription factor CBFA2.5 A different type of fusion was described for the t(3;12) in myeloid disease whereETV6 drives the expression ofMDS1/EVI1.6 From a mechanistic point of view, this might be similar to a t(12;13) case where the 5′ part of ETV6 drives the expression of the complete homeobox geneCDX2.7 Still another type of fusion was characterized from a t(12;22), which results in the fusion of MN1 to ETV6 generating a chimeric transcription factor with the ETV6 DNA-binding domain.8
The t(4;12)(q11-q13;p12-p13) was previously described as a recurrent translocation. Twelve cases of acute leukemia were reviewed by Harada et al9: 3 acute lymphocytic leukemia (ALL), 8 acute myeloid leukemia (AML), and 1 acute undifferentiated leukemia (AUL). The AML cases occurred predominantly in adults, and the ALL cases were found exclusively in children. We have collected 4 new cases (1 myeloid/natural killer [NK] cell leukemia, and 3 AML-M0), with a t(4;12)(q11-q12;p13) and characterized molecularly the genes affected by the rearrangement.
MATERIALS AND METHODS
Case reports.
Four patients (3 men, 1 woman), aged 54 to 81, presented with acute leukemia at 4 different hospitals. Clinical and cytogenetic data are summarized in Table 1. The patients had no particular antecedents, except for case 2, who was treated previously for chronic lymphocytic leukemia (CLL). The onset of the diseases was acute. Case 1 had extensive lymph node enlargement and was diagnosed as myeloid/NK cell leukemia,10 while the 3 other cases were classified as AML-M0. None presented with hepatosplenomegaly. The bone marrow showed dysplasia and blast cells of an undifferentiated phenotype. Remarkably, all cases showed the same very immature myeloid immunophenotype: CD7+, CD13+, CD33+. Response to intensive chemotherapeutic regimen for myeloid leukemia was obtained in 2 cases.
Clinical and Cytogenetic Data at Presentation and Relapse (Case 4)
| Case . | Sex/ Age . | Blood . | BM . | Immunophenotype . | Diagnosis (FAB) . | Treatment . | Response and Survival (mo) . | Cytogenetics . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) . | Pt (×109/L) . | Blasts (%) . | Cytology . | Blasts (%) . | Tissue . | Karyotype . | ||||||
| 1 | F/76 | 5.0 | 150 | 0 | Hypercellular, dysgranulopoiesis, dysmegakaryopoiesis | 24 | CD2+, CD5+, CD7+, CD13+, CD56+, CD33+, cyMPO+ | Myeloid/ NK cell leukemia | Intensive chemotherapy | CR1 (8+) | LN BM | 46, XX, t(4;12) (q12;p13) [20] 46, XX, t(4;12) (q12;p13) [9] / 46, XX [1] |
| 2 | M/70 | 36.0 | NA | 80 | Dry tap | NA | CD7+, CD13+, CD34+, HLA-DR+ | AML-M0 | Chemotherapy | Death at induction | BM | 46, XY, t(4;12) (q11;p13) [13] / 47, XY, t(4;12) (q11;p13) del(1) (p11p35) [18] |
| 3 | M/81 | 23.3 | 117 | 49 | Hypercellular, dysgranulopoiesis, dyserythropoiesis | 70 | CD4+, CD7+, CD13+, CD33+, CD34+, CD56+, HLA-DR+ | AML-M0 | Mild chemotherapy (supportive) | NR (3+) | BM | 46, XY, t(4;12) (q12;p13) [7] / 46, XY, t(4;12) (q11;p13), del(5) (q13q33), add(11)(p15) [13] |
| 4 | M/54 | 5.8 | 7 | 57 | Normocellular, dysgranulopoiesis, dysmegakaryopoiesis | 60 | CD7+, CD13+, CD33+, CD34+ | AML-M0 | Intensive chemotherapy | CR1 (26) | BM | 46, XY, t(4;12) (q11;p13) |
| NA | Relapse | 20 | Relapse | Chemotherapy and BM transplantation | CR2 (24+) | BM | 46, XY, t(4;12) (q11;p13) [3] / 46, XY [12] | |||||
| Case . | Sex/ Age . | Blood . | BM . | Immunophenotype . | Diagnosis (FAB) . | Treatment . | Response and Survival (mo) . | Cytogenetics . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) . | Pt (×109/L) . | Blasts (%) . | Cytology . | Blasts (%) . | Tissue . | Karyotype . | ||||||
| 1 | F/76 | 5.0 | 150 | 0 | Hypercellular, dysgranulopoiesis, dysmegakaryopoiesis | 24 | CD2+, CD5+, CD7+, CD13+, CD56+, CD33+, cyMPO+ | Myeloid/ NK cell leukemia | Intensive chemotherapy | CR1 (8+) | LN BM | 46, XX, t(4;12) (q12;p13) [20] 46, XX, t(4;12) (q12;p13) [9] / 46, XX [1] |
| 2 | M/70 | 36.0 | NA | 80 | Dry tap | NA | CD7+, CD13+, CD34+, HLA-DR+ | AML-M0 | Chemotherapy | Death at induction | BM | 46, XY, t(4;12) (q11;p13) [13] / 47, XY, t(4;12) (q11;p13) del(1) (p11p35) [18] |
| 3 | M/81 | 23.3 | 117 | 49 | Hypercellular, dysgranulopoiesis, dyserythropoiesis | 70 | CD4+, CD7+, CD13+, CD33+, CD34+, CD56+, HLA-DR+ | AML-M0 | Mild chemotherapy (supportive) | NR (3+) | BM | 46, XY, t(4;12) (q12;p13) [7] / 46, XY, t(4;12) (q11;p13), del(5) (q13q33), add(11)(p15) [13] |
| 4 | M/54 | 5.8 | 7 | 57 | Normocellular, dysgranulopoiesis, dysmegakaryopoiesis | 60 | CD7+, CD13+, CD33+, CD34+ | AML-M0 | Intensive chemotherapy | CR1 (26) | BM | 46, XY, t(4;12) (q11;p13) |
| NA | Relapse | 20 | Relapse | Chemotherapy and BM transplantation | CR2 (24+) | BM | 46, XY, t(4;12) (q11;p13) [3] / 46, XY [12] | |||||
Abbreviations: Pt, platelet; WBC, white blood cell count; BM, bone marrow; LN, lymph node; NA, not available; CR1/CR2, first (second) complete remission; NR, no remission.
Cloning of the t(4;12) fusion.
Fluorescent in situ hybridization (FISH) was performed as described previously.11 First-strand cDNA for anchored polymerase chain reaction (PCR) (rapid amplification of cDNA ends [RACE]) was synthesized from 1 μg of total RNA using MuMLV-reverse transcriptase (GIBCO-BRL, Gaithersburg, MD) using, respectively, the oligonucleotide ETV6R3a, derived from exon 3 of ETV6 for the 5′-RACE experiments and the oligonucleotide 465 for the 3′-RACE experiments (the sequence of all PCR primers is given at the end of this section). For the 5′-RACE experiments, the first-strand cDNA was tailed with deoxyadenosine triphosphate (dATP). Second-strand synthesis was performed using Klenow DNA polymerase (GIBCO-BRL) and the primers 466 (5′-RACE) and ETV6F1a (3′-RACE). Anchored PCR was performed for 35 cycles with primers ETV6R3b-467 (5′-RACE) and ETV6F1a-467 (3′-RACE). Nested PCR was performed with the primers ETV6R2-468 (5′-RACE) and ETV6F1b-468 (3′-RACE). PCR products were cloned into pGEM-T Easy (Promega, Madison, WI) and sequenced.
Reverse transcriptase (RT)-PCR.
RT-PCR experiments on cases 1 and 2 were performed on 1 μg of total RNA using the Titan RT-PCR system from Boehringer (Mannheim, Germany). The BTL-ETV6 transcript was detected using the primers BTLF1 – ETV6R3b for the first round PCR and primers BTLF2 – ETV6R2 for the nested PCR. For detection of the ETV6-BTLtranscript, the primers ETV6F1a – BTLR6a were used, followed by the nested primers ETV6F1b – BTLR6b. For cases 3 and 4, first-strand cDNA was generated as described above using the primer 465. On 10% of this cDNA, PCR was performed with the primers BTLF2 – ETV6R3b followed by the nested primers BTLF3 – ETV6R2 to detect theBTL-ETV6 transcript and with the primers ETV6F1a – BTLR6b and the nested primers ETV6F1b – BTLR4 to detect theETV6-BTL transcript.
Cloning of BTL.
A 500-bp BTL probe was generated from cDNA obtained from the K562 cell line by PCR using the primers BTLF2 and BTLR6a. A human fetal kidney cDNA library cloned into the λgt10 vector (Clontech, Palo Alto, CA) was screened using a standard protocol. Genomic PAC clones containing BTL were isolated by screening high-density filters carrying the RPCI1 and RPCI5 PAC libraries (Roswell Park Cancer Institute, Buffalo, NY).
Oligonucleotides.
(Oligonucleotides derived from ETV6 and BTL are indicated in Fig 1A).
ETV6F1a: 5′ TGAGACATGTCTGAGACTCCTGCT 3′; ETV6F1b: 5′ ACTCCTGCTCAGTGTAGCATTAAG 3′; ETV6R3a: 5′ GAATGAGGAGATCGATAGCG 3′; ETV6R3b: 5′ TCCCTGCTCCAGTAAATTGGCTGCAAG 3′; ETV6R2: 5′ ACTGGAACATGAAGTGGCGT 3′; BTLF1: 5′ CAGGATGGCGGATTTCGACG 3′; BTLF2: 5′ GGTCACGTCACCGTATTTGGA 3′; BTLF3: 5′ CGTTGGCTACTTTGTGGCTG 3′; BTLR6a: 5′ CCACACGATTCTGTAGAACC 3′; BTLR6b: 5′ AATCTGGTCGAAAAATCGGTG 3′; BTLR4: 5′ TCCCATTCTAATAACTTCTC 3′; 465: 5′ CCAGTGAGCAGAGTGACGAGGACTCGAGCTCAAGCNNNNNN 3′; 466: 5′ CCAGTGAGCAGAGTGACGAGGACTCGAGCTCAAGCTTTTTTTT 3′; 467: 5′ CCAGTGAGCAGAGTGACG 3′; 468: 5′ GAGGACTCGAGCTCAAGC 3′.
RESULTS AND DISCUSSION
Cloning of the t(4;12).
Metaphases from case 1, a patient with myeloid/NK cell leukemia characterized by a t(4;12)(q11-q12;p13), were analyzed by FISH using probes covering, respectively, the 5′- and the 3′-end ofETV6.12 13 Cosmid 50F4 (intron 1-exon 2 ofETV6) showed split FISH signals (results not shown), indicating that the breakpoint occurred in intron 1 of ETV6. To identify the fusion partner of ETV6, 5′- and 3′-RACE experiments were performed on reverse transcribed RNA of the tumor cells. Nested oligonucleotides located, respectively, in exons 3 and 2 of ETV6 were used for 5′-RACE and oligonucleotides located in exon 1 were designed for the 3′-RACE experiments. Both experiments showed new sequences fused in frame to, respectively, exon 2 (5′-RACE) and exon 1 (3′-RACE) of ETV6. These sequences showed high similarity to the murine Brx gene (Brain specific X-linked gene, Accession no.Y11896). Therefore, the gene was named BTL (forBRX-like Translocated inLeukemia). In addition, 2 Caenorhabditis elegans predicted proteins from the cosmid W06E11 (accession no. U20862) were detected in the databases.
Cloning of the BTL gene.
Oligonucleotides were designed to amplify a 500-bp fragment of theBTL cDNA. A human fetal kidney cDNA phage library was screened with this probe and 4 positive plaques were analyzed. A 1-kb consensus cDNA sequence, containing an open reading frame of 495 bp, was constructed and submitted to GenBank (accession no. AF159423). On a Northern blot carrying total RNA of K562 and U937 cells, 1 single transcript of approximately 1.4 kb was detected in both cell lines (results not shown), indicating that our 1-kb cDNA sequence is almost full-length.
The open reading frame of BTL predicts a protein of 165 amino acids that shows 49% similarity to the murine Brx protein. No similarity was found with any known functional protein domain, nor is there any information available about the function of Brx. Analysis of the amino acid sequence of BTL showed the presence of a hydrophobic stretch of 23 amino acids, which could represent a transmembrane domain.
The 500-bp BTL cDNA probe was also used to screen a genomic PAC library. Ten PAC clones were identified. The exact content of each PAC was investigated by hybridization with oligonucleotides derived from exons of the BTL gene (Fig 1A). Only PAC 238H24 contained the complete gene, whereas PAC 200D9 and PAC 1146G14 contained the 5′ end and the 3′ end, respectively, ofBTL. Sequence analysis of EcoRI and HindIII subclones of these PACs showed the presence of 6 different exons. The exon sequences confirmed the consensus cDNA sequence. The length of introns 1 and 5 was measured by long range PCR. Intron 3 could not be amplified by PCR, most probably because it is more than 25 kb long. A map of the genomic structure of BTL is shown in Fig 1A.
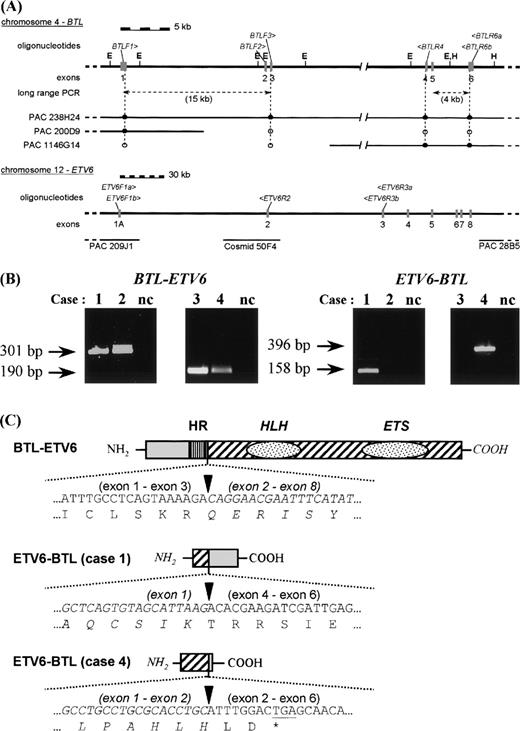
RT-PCR and schematic representation of the results. (A) Genomic structure of BTL and ETV6. The position of the PAC clones covering BTL was determined by Southern hybridization using oligonucleotides derived from the exon sequences (•, positive by hybridization; ○, negative by hybridization). The genomic structure of ETV6 was taken from Baens et al.12 The different primers used for PCR are shown in italics above the respective genomic structures. Exons are represented by gray boxes. E, EcoRI; H, HindIII. (B) Detection of the BTL-ETV6 and ETV6-BTL transcripts in the 4 cases by RT-PCR. Only the results of the second-round PCR are shown. nc, negative control. (C) Schematic representation of the predicted fusion proteins. Sequences derived from ETV6 are drawn in italics. Arrowheads mark the bounderies between the ETV6 and BTL parts. HR, hydrophobic region of BTL; HLH, helix-loop-helix domain of ETV6; ETS, DNA binding domain of ETV6.
RT-PCR and schematic representation of the results. (A) Genomic structure of BTL and ETV6. The position of the PAC clones covering BTL was determined by Southern hybridization using oligonucleotides derived from the exon sequences (•, positive by hybridization; ○, negative by hybridization). The genomic structure of ETV6 was taken from Baens et al.12 The different primers used for PCR are shown in italics above the respective genomic structures. Exons are represented by gray boxes. E, EcoRI; H, HindIII. (B) Detection of the BTL-ETV6 and ETV6-BTL transcripts in the 4 cases by RT-PCR. Only the results of the second-round PCR are shown. nc, negative control. (C) Schematic representation of the predicted fusion proteins. Sequences derived from ETV6 are drawn in italics. Arrowheads mark the bounderies between the ETV6 and BTL parts. HR, hydrophobic region of BTL; HLH, helix-loop-helix domain of ETV6; ETS, DNA binding domain of ETV6.
Molecular characterization of new t(4;12) cases.
In addition to the case described above, 3 other cases with a t(4;12)(q11-q12;p13) were collected. These 3 cases showed a very immature myeloid immunophenotype and were diagnosed as AML-M0. First, the rearrangements were analyzed by FISH (results not shown). PAC 1146G14 (3′-BTL) was found to span the breakpoint at 4q in the first 3 cases, clearly indicating a break within the BTLgene. For case 4, only interphase nuclei were available and the colocalization of PAC 200D9 (5′-BTL) and PAC 28B5 (3′-ETV6) was observed (for localization of these probes, see Fig 1A).
Second, RT-PCR experiments were performed as described in Materials and Methods. All PCR products were cloned and sequenced to confirm their identity. In all 4 cases, the same BTL-ETV6 transcript (exon 1-3 of BTL fused to exon 2-8 of ETV6) was detected (Fig 1B). The reciprocal ETV6-BTL (exon 1-exon 4) transcript was detected only in case 1 and absent in cases 2 and 3. In case 4, however, a different ETV6-BTL transcript was detected with a fusion of ETV6 exon 2 to BTL exon 2 (Fig 1B) that cannot be the reciprocal of the BTL exon 3-ETV6 exon 2 fusion detected in this case. Experiments aiming at the detection of a BTL-ETV6 (exon 1-exon 3) fusion transcript were consistently negative. All experiments were repeated including the negative controls to exclude contamination and showed the same results. This suggests that case 4 involved a more complicated rearrangement with duplication of regions from 4q and/or 12p. Unfortunately, this could not be confirmed by FISH or genomic analyses, as no metaphases or genomic DNA were available.
Taken together, these data indicate that in all 4 cases a transcript is present predicting a protein with the amino terminal 110 amino acids of BTL (exon 1-3) fused to the carboxyterminal 441 amino acids of ETV6 (exons 2-8) (Fig 1C).
The presence of the BTL-ETV6 transcript in 4 acute leukemia cases strongly suggests that it is involved in the leukemogenic process. The fusion protein contains both the HLH oligomerization domain and the ETS DNA binding domain of ETV6, which is most similar to the MN1-ETV6 fusion.8 No specific domains are present in BTL, which would shed light on its possible function. A striking feature of BTL is the presence of a hydrophobic stretch of 23 amino acids, which is also present in the BTL-ETV6 fusion protein. It remains to be investigated whether BTL and BTL-ETV6 are indeed membrane bound proteins and whether this is related to the potential oncogenic properties of this fusion protein.
At least 15 more translocations with a cytogenetically similar t(4;12)(q11-q13;p13) have been reported in the literature.9,14,15 Interestingly, 4 of the AML cases reported by Harada et al9 show a similar immature immunophenotype (CD7+, CD13+, CD33+/CD34+) as our cases and for 2 of these cases, evidence was collected that ETV6 is involved. The PAC clones isolated by us for BTL should allow us to investigate whether the 4q breakpoint is homogeneous at the molecular level.
ACKNOWLEDGMENT
We thank Dr P. Van den Berghe and Dr G. Verhoef (UZ Leuven, Leuven, Belgium) for providing us patient material and clinical data.
Supported by Grants No. G.0153.96 and G.0377.97 from the Fonds voor Wetenschappelyk Onderroek, Vlaanderen (F.W.O.) (to P.M. and A.H.). This report represents results of the Belgian Program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister’s Office, Science Policy Programming. P.M. is an Onderzoeksdirecteur and J.C. an Aspirant of the Fonds voor Wetenschappelijk Onderzoek, Vlaanderen.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter Marynen, PhD, Center for Human Genetics, Flanders Interuniversity Institute for Biotechnology, University of Leuven, Campus Gasthuisberg, Herestraat 49, B-3000 Leuven, Belgium; e-mail: Peter.Marynen@med.KULeuven.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal