Abstract
Autoimmune neutropenia (AIN) in children can be divided into 2 forms. In primary AIN, neutropenia is the sole abnormality, and although neutrophil counts are generally below 500 μL−1, mild bacterial infections occur. Primary AIN is mostly seen in young children and shows a self-limited course. AIN occurring in association with autoimmune diseases (secondary AIN) often shows more severe infectious complications. We analyzed clinical and serological data from 28 pediatric patients with AIN to evaluate whether there is a possible relationship between specificity of the neutrophil autoantibodies and the clinical course of the disease. Specificity of the circulating antibodies was determined with the indirect granulocyte immunofluorescence test (GIFT) and a panel of phenotyped donor neutrophils. The samples were further analyzed in the monoclonal antibody immobilization of granulocyte antigens assay (MAIGA) for neutrophil antigen (NA)1, NA2, CD11a, and CD11b specificity. With the indirect GIFT, an antibody specificity was deduced in 26 of the 28 analyzed samples. In all but 3 sera from patients with primary AIN, NA1-(76%) or NA2-(10%) specific antibodies were detected with the indirect GIFT. In 2 samples, the reactivity in the indirect GIFT was too weak to draw conclusions, but the MAIGA showed NA1 and/or NA2 specificity of the antibodies. One serum, from a patient with primary AIN with a persistent neutropenia for more than 6 years, contained NA1, possibly pan-FcγRIIIb, and CD11a antibodies. In 4 sera from patients with primary AIN, weak antibodies with CD11a or CD11b specificity were detected with the MAIGA. Sera from 7 patients with secondary AIN contained in all cases antibodies with pan-FcγRIIIb specificity, as deduced from the indirect GIFT results and absorbance/elution experiments performed with 2 sera. The MAIGA confirmed this for only 1 of the 5 tested sera. Furthermore, CD11a antibodies were detected in 1 of the 5 tested sera. In conclusion, our results indicate that primary AIN is usually associated with NA-specific antibodies, whereas secondary AIN seems to be associated with pan-FcγRIIIb antibodies. Thus, characterization of the antibodies in sera from children with AIN discriminates patients with primary AIN from those with secondary AIN.
CHRONIC NEUTROPENIA is defined by an absolute neutrophil count (ANC) below 1,500 cells per μL of blood, lasting for at least 6 months. In 1975, Lalezari et al1demonstrated that chronic neutropenia can be caused by autoantibodies. Autoimmune neutropenia (AIN) is divided into primary and secondary forms.2 In primary AIN, neutropenia is the sole hematologic abnormality. Primary AIN is the most common form of neutropenia in young children, generally diagnosed at the age of about 8 months and disappearing around the age of 3 years. In case of secondary AIN, neutropenia is present in association with another autoimmune disorder or a malignant lymphoproliferative disease. Secondary AIN can occur at any age and the clinical course is variable. Previous studies showed that in patients with primary AIN, the neutrophil autoantibodies are frequently directed against 1 of the alloforms of the neutrophil antigen (NA) system and, in particular, against the NA1 alloform.1-5 The specificity of autoantibodies in children with secondary AIN has not yet been studied in detail in a large number of patients.6
The NA antigens are located on the IgG-Fc receptor type IIIb (FcγRIIIb; CD16), which is exclusively expressed by neutrophils.7 The NA phenotype frequency in the white population is as follows: 11% of the population is NA(1+, 2−), 45% is NA(1−, 2+), and 43% is NA(1+, 2+).7 Recently, a new polymorphism of theNA system was found to encode the so-called SH antigen, a genetic variant involved in neonatal alloimmune neutropenia, which is expressed by approximately 4% of the white population.8 9
We investigated the neutrophil-autoantibody specificity in 21 children with primary AIN and in 7 children with secondary AIN. In patients with self-limited primary AIN, the autoantibodies were in all cases directed against 1 of the NA alloforms, whereas in patients with secondary AIN, we found only antibodies with pan-FcγRIIIb specificity.
MATERIALS AND METHODS
Patients.
Serum and/or EDTA-anticoagulated blood was sent to our laboratory for diagnostic evaluation of neutropenia. Informed consent and clinical information were obtained via the attending pediatrician. Chronic neutropenia was defined as an absolute neutrophil count (ANC) below 1,500 neutrophils/μL of blood present for at least 6 months.
In 1992, a diagnostic procedure based on the diagnostic procedure of the International Severe Chronic Neutropenia Registry was introduced in The Netherlands for pediatric patients with chronic neutropenia. This procedure starts with a 6-week period in which peripheral blood leukocyte and neutrophil counts are measured twice weekly. When persistent neutropenia is diagnosed and no spontaneous recovery occurs within 3 months, a more extensive procedure is advised, including immunologic tests, eg, immunoglobulin profile and screening for autoantibodies, screening for metabolic diseases and viral infections, bone marrow aspiration, neutrophil mobilization tests, and measurement of leukocytes and ANC in the blood from the parents of the patients. In patients with severe bacterial infections and/or clinical features of a certain syndrome, the diagnostic procedure is accelerated and extended to avoid delay in appropriate treatment.
The pediatric patients (n = 43) for whom the blood was sent to our laboratory for detection of autoantibodies against neutrophils formed the basis of the study, and only patients with positive neutrophil-autoantibody tests were included (n = 28).
Monoclonal antibodies (MoAabs) and human antisera.
Anti-pan–FcγRIIIb MoAbs (CD16) used were: CLBFcRgran1, 3G8 (Medarex, West Lebanon, NH), BW209/2, and MEM154. BW209/2 was a generous gift from Dr R. Kurrle (Behring Werke, Marburg, Germany) and MEM154 was a kind gift from Dr V. Horejsi (Prague, Czech Republic). CLBFcRgran1 (CD16), TB133 (CD11a), W6/32 (anti-HLA class I), and irrelevant murine MoAbs were obtained from our institute, the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB), Amsterdam, The Netherlands. S131 (CD11b), IB4 (CD18), and J3D3 (CD35) were obtained via the Fourth and Fifth Workshop on Human Leukocyte Differentiation Antigens.
NA1-, NA2-, NB1-, CD11a-, CD11b- and I-reactive human antisera were obtained either from women immunized during pregnancies or from patients immunized by blood transfusions. Sera from healthy volunteers (AB positive) were used as controls. Fluorescein isothiocyanate (FITC)-labeled F(ab′)2 fragments of goat antimurine Ig and FITC-labeled goat anti-human Ig were from the CLB.
Isolation of the cells.
Fresh anticoagulated blood from volunteers or patients was centrifuged over a Ficoll-Hypaque layer (Pharmacia Fine Chemicals AB, Uppsala, Sweden) with a specific gravity of 1.076 g/mL. Mononuclear cells were harvested from the interphase, and the pellet was treated with ice-cold NH4Cl solution (155 mmol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L EDTA, pH 7.4) to lyse the erythrocytes. The remaining cells were more than 95% neutrophils.
Detection of neutrophil antibodies in the direct and indirect granulocyte immunofluorescence test (GIFT).
The isolated neutrophils were fixed with 1% (wt/vol) paraformaldehyde (PFA). In the direct GIFT, the patients’ neutrophils were incubated with FITC-labeled F(ab′) fragments of goat anti-human Ig to detect neutrophil-bound antibodies. In all cases, the patients’ sera was tested with the indirect GIFT for circulating neutrophil antibodies with a panel of phenotyped donor neutrophils (Table 1).10 11 In the indirect GIFT, donor neutrophils were incubated with serum for 30 minutes at room temperature (RT). After washing with phosphate-buffered saline (PBS) containing 0.2% (wt/vol), bovine serum albumin (BSA), the neutrophils were stained with FITC-labeled F(ab′)2 fragments of goat anti-human Ig. Binding of the antibodies was measured with a FACScan (Becton Dickinson, San Jose, CA).
Clinical Characteristics of Patients
| Patient no. | 21 | 7 |
| Age at onset of neutropenia | <1 yr | 0.5-15 yr |
| ANC at diagnosis | <500/μL | 0-1,200/μL |
| Infections | Mild | Mild-moderate |
| Associated disease | None | Evans syndrome (n = 3) |
| AIHA, ITP, IDDM (n = 1) | ||
| AIHA and CID (n = 1) | ||
| ITP (n = 1) | ||
| AI-thyroiditis (n = 1) | ||
| Spontaneous recovery ANC | Yes (n = 18) Follow-up <2 yr (n = 2) No recovery after 6 years (n = 1) | No (n = 7) |
| Diagnosis | Primary AIN | Secondary AIN |
| Patient no. | 21 | 7 |
| Age at onset of neutropenia | <1 yr | 0.5-15 yr |
| ANC at diagnosis | <500/μL | 0-1,200/μL |
| Infections | Mild | Mild-moderate |
| Associated disease | None | Evans syndrome (n = 3) |
| AIHA, ITP, IDDM (n = 1) | ||
| AIHA and CID (n = 1) | ||
| ITP (n = 1) | ||
| AI-thyroiditis (n = 1) | ||
| Spontaneous recovery ANC | Yes (n = 18) Follow-up <2 yr (n = 2) No recovery after 6 years (n = 1) | No (n = 7) |
| Diagnosis | Primary AIN | Secondary AIN |
Abbreviations: AIHA, autoimmune hemolytic anemia; ITP, immune thrombocytopenic purpura; IDDM, insulin-dependent diabetes mellitus; CID, combined immune deficiency; AI, autoimmune.
Detection of neutrophil antibodies with the MoAb-specific immobilization of granulocyte antigens (MAIGA).
Sera were tested in the MAIGA as described previously.11Briefly, 1 × 106 neutrophils expressing either NA1 or NA2, or FcγRIIIb-negative neutrophils were incubated with 50 μL of serum for 30 minutes at 37°C. The cells were washed with PBS/BSA, 10 μL of diluted MoAb was added, and the incubation was continued for 30 minutes at 37°C. MoAb 3G8 and BW209/2 were used if NA1-positive neutrophils were tested and MoAbs 3G8, MEM154, and BW209/2 were used in assays with NA2-positive neutrophils. TB133 (CD11a), S131 (CD11b), IB4 (CD18), and J3D3 (CD35) were only used in assays with FcγRIIIb-negative neutrophils. The neutrophils were washed 3 times with PBS/BSA and were lysed with 1% (vol/vol) Triton-X100–containing buffer in the presence of protease inhibitors EDTA (5 mmol/L), phenylmethylsulphonyl fluoride (PMSF, 2 mmol/L), and soybean-trypsin inhibitor (200 ng/L) for 30 minutes at 4°C. After centrifugation of the lysate for 30 minutes at 15,000g, 70 μL of the lysate was diluted with 180 μL of Tris-buffered NaCl (0.9%, wt/vol) containing 1% Triton-X 100 (vol/vol), 0.05% (vol/vol) Tween-20, and 0.5 mmol/L CaCl2. A total of 100 μL of the diluted lysate was added to the wells of an enzyme-linked immunosorbent assay (ELISA) plate (Maxisorp, Nunc, Roskilde, Denmark), coated with goat anti-mouse IgG (3 μg/mL). Incubation was performed overnight at 4°C. The plates were washed, 100 μL of horseradish peroxidase-labeled goat anti-human IgG was added, and the incubation was continued for 2 hours. After washing, a substrate was added to measure the amount of bound antibody. All tests were performed in duplicate. In each assay, incubation of neutrophils with a serum containing HLA antibodies followed by incubation with MoAb W6/32 served as a control for the whole procedure. Sera with known NA1 and NA2 specificity and serum from an AB-positive control served as positive and negative controls, respectively.
Evaluation of FcγRIIIb or NA allospecificity.
To evaluate the presence of NA1-, NA2-, or pan-FcγRIIIb-specific antibodies in the sera, 150 μL of serum was incubated twice with 7.5 × 106 neutrophils from either NA1 or NA2 homozygous positive donors for 45 minutes at RT. Absorbed antibodies were eluted from the cells by incubation with 1 mol/L isotonic citrate buffer (pH 2.8) for 10 minutes at RT, and the pH of the harvested eluates was neutralized by adding an equal volume of 1 mol/L isotonic Tris-HCl (pH 10.6).12 13 The reactivity of the absorbed sera and eluates was tested with NA1- and NA2-positive neutrophils. NA1- and NA2-specific antisera and sera from AB-positive donors served as controls. The elution procedure did not alter the specificity of the control sera, indicating that no artificial immune complexes were formed that could bind to the neutrophil FcγRs.
Immunoprecipitation.
A total of 2 × 107 neutrophils of an NA(1+, 2+) genotyped donor was radiolabeled with 37 MBq 125iodide by the Iodogen method according to the manufacturer’s instructions (Pierce, Rockford, IL). Labeled cells (2 × 106) were incubated with either 1 of the patient sera, control human serum, or CLBFcRgran1 MoAb for 1 hour at RT. The cells were washed and lysed in 0.5% (wt/vol) Nonidet P-40 in the presence of protease inhibitors EDTA (5 mmol/L), PMSF (2 mmol/L), and soybean-trypsin inhibitor (200 ng/mL) for 30 minutes at 4°C. The lysed samples were centrifuged for 30 minutes at 12,000g. Subsequently, precipitation was performed with protein-G–coated sepharose beads (Pharmacia). The immune precipitate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and to autoradiography.
RESULTS
Patient groups.
Blood samples from 43 pediatric patients were analyzed. These were samples from patients with a chronic neutropenia, who were screened according to a standard diagnostic procedure, as described in Materials and Methods. Fifteen patients were excluded from our study. The sera of these patients contained no detectable antibodies against neutrophils. Four of these patients had neutropenia in association with infections, lasting only 3 to 5 months. In 8 other patients, the following diagnoses were made: Kostmann’s disease (n = 1), familial neutropenia (n = 1), cyclic neutropenia (n = 1), glycogen storage disease (n = 1), lazy leukocyte syndrome (n = 1), combined immune deficiency (n = 2), idiopathic neutropenia with limb malformations (n = 1). One patient developed acute lymphoblastic leukemia after a 4-month period of neutropenia. In 2 patients, no classifying diagnosis was made. The antibody tests, performed with both GIFT and MAIGA, were negative. One of these last 2 patients has a neutropenia persisting for more than 5 years now. In the other patient, neutropenia spontaneously resolved after 13 months. Although this last patient was likely to have had AIN, as a result of failure to detect antibodies, this patient was not included in the study.
Twenty-eight patients were included in the study. Their clinical characteristics are shown in Table 2. Based on the clinical data, the patients were divided into 2 groups. The first group consisted of 21 patients with the typical features of primary AIN: neutropenia was the only clinical problem and was diagnosed before the age of 1 year. Bacterial infections were mild and usually affected the skin, the middle ear, the oropharynx, and the upper respiratory tract. In 18 cases, the neutropenia resolved spontaneously within 30 months (range, 16 to 52 months; mean, 28 months). Three patients in the primary AIN group showed persistent neutropenia during the study period (Table 2). However, 2 of these patients were diagnosed less than 2 years ago. One patient, who is now 6 years old, has a consistent neutrophil count of less than 500/μL without any clinical problems.
Results of Antibody Screening With the Indirect GIFT Test and a Panel of Donor Granulocytes
| . | Primary AIN n = 21 . | Secondary AIN n = 7 . |
|---|---|---|
| Antibody specificity* | ||
| NA1 | 16 | 0 |
| NA2 | 2 | 0 |
| FcγRIIIb | 1† | 7 |
| Inconclusive | 2‡ | 0 |
| . | Primary AIN n = 21 . | Secondary AIN n = 7 . |
|---|---|---|
| Antibody specificity* | ||
| NA1 | 16 | 0 |
| NA2 | 2 | 0 |
| FcγRIIIb | 1† | 7 |
| Inconclusive | 2‡ | 0 |
The observed difference in antibody specificity between children with primary AIN (NA-specific) or secondary AIN (pan-FcγRIIIb) was highly significant. Fisher’s exact test: P < .01.
Further analysis showed the presence of NA1, FcγRIIIb and CD11a antibodies.
The MAIGA showed the presence of NA1 antibodies in 1 of these sera and NA1 and NA2 reactivity in the other serum.
The second group consisted of 7 patients who all suffered from secondary AIN (Table 2). The age of onset of the neutropenia was variable, ranging from 6 months to 14 years. In 2 patients, neutropenia was the presenting problem. One of these patients showed neutropenia at the age of 6 months. Ten months later, immune-mediated thrombocytopenia was diagnosed. In the other patient, neutropenia was diagnosed at the age of 13 months followed by autoimmune hemolytic anemia 1 month afterwards. The duration of neutropenia in these patients ranged from 1 to 6 years. Infections in this group were severe in 3 cases, and 1 patient also with combined immune deficiency died. Two patients, who both suffered from severe skin and respiratory tract infections, were treated with recombinant human granulocyte colony-stimulating factor (rh-G–CSF) for more than 2 years. Subsequently, the ANC normalized and no more bacterial infections occurred. In 3 other patients, immune-modulating therapy, eg, corticosteroids or high-dose intravenous immunoglobulin administration, were temporarily beneficial with respect to the ANC. Withdrawal of therapy gave a relapse of the neutropenia in 2 of these 3 patients, whereas 1 patient showed a normal ANC during 1 year and was subsequently lost for follow-up. Finally, 1 of the patients, suffering from mild autoimmune hemolytic anemia and immune thrombocytopenic purpura (ITP) (Evans syndrome), received prophylaxis with antibiotics (cotrimoxazole) and was clinically well.
Specificity of the neutrophil antibodies as determined with the indirect GIFT.
Because of the neutropenia, the direct GIFT could only be performed in 4 patients, with a positive result in all cases. The presence of circulating neutrophil antibodies was one of the inclusion criteria of this study. The presence of HLA antibodies was excluded in all cases by simultaneously testing the donor lymphocytes (data not shown). Table 2shows the results of the antibody screening with a panel of phenotyped donor granulocytes. In patients with primary AIN (n = 21), we found NA1 specificity in 16 (76%) and NA2 specificity in only 2 patients (10%). In 3 cases, no specificity could be determined based on the results with the panel, but they were directed against FcγRIIIb epitopes because the neutrophils of a FcγRIIIb-negative donor were not stained by these sera. The antibodies from 1 of these patients seemed to show pan-FcγRIIIb specificity in this test, although weaker binding to homozygous NA2-positive neutrophils compared with homozygous NA1-positive neutrophils was noted. In the other 2 patients, the reactivity of the serum was too weak to draw conclusions. In 5 patients, serial antibody tests were performed. The antibody tests in these patients were negative after 6 to 15 months of follow-up. In all of the patients, disappearance of the antibodies preceded the recovery of the neutrophil counts by several months.
The sera from the patients with secondary AIN showed all pan-FcγRIIIb specificity. These sera were positive with the neutrophils from all NA-positive donors and negative with the cells of the FcγRIIIb-negative donor.
Reactivity of patients sera in the MAIGA with CD16, CD11a, and CD11b MoAbs.
To confirm our results obtained with the screening in the indirect GIFT, we performed the MAIGA. All available sera (n = 23) were tested in duplicate with homozygous NA1-positive and homozygous NA2-positive neutrophils (Table 3). Thirteen sera with NA1 antibodies in the GIFT were tested. Confirmation of NA1 specificity was obtained in 3 cases, whereas 6 sera showed negative results (Table3). Four sera showed variable reactions, dependent on the MoAb used in the MAIGA (see Table 3). The 2 sera with NA2-reactive antibodies in the GIFT showed positive results in the MAIGA with homozygous NA2-positive neutrophils (Table 3). The 2 sera with too weak reactivity in the indirect GIFT to draw conclusions on antibody specificity reacted positively in the NA1-MAIGA, whereas 1 of these sera was also weakly positive in the NA2-MAIGA (Table 3).
Antibody Specificity and Comparison of the Results Obtained With Indirect GIFT and MAIGA
| Patients with primary AIN | ||
| Indirect GIFT | MAIGA | |
| NA1 (n = 13)3-150 | NA1 | (n = 3) |
| NA2 | (n = 2)3-151 | |
| NA1 and NA2 | (n = 2)3-152 | |
| Negative | (n = 6) | |
| NA2 (n = 2)3-153 | NA2 | (n = 2) |
| pan FcγRIIIb (n = 1) | NA1 and NA2 | (n = 1) |
| inconclusive (n = 2) | NA1 | (n = 1) |
| NA1 + NA23-155 | (n = 1) | |
| Patients with secondary AIN | ||
| Pan FcγRIIIb (n = 5)3-154 | NA1 and NA2# | (n = 1) |
| NA1 | (n = 1) | |
| Negative | (n = 3) |
| Patients with primary AIN | ||
| Indirect GIFT | MAIGA | |
| NA1 (n = 13)3-150 | NA1 | (n = 3) |
| NA2 | (n = 2)3-151 | |
| NA1 and NA2 | (n = 2)3-152 | |
| Negative | (n = 6) | |
| NA2 (n = 2)3-153 | NA2 | (n = 2) |
| pan FcγRIIIb (n = 1) | NA1 and NA2 | (n = 1) |
| inconclusive (n = 2) | NA1 | (n = 1) |
| NA1 + NA23-155 | (n = 1) | |
| Patients with secondary AIN | ||
| Pan FcγRIIIb (n = 5)3-154 | NA1 and NA2# | (n = 1) |
| NA1 | (n = 1) | |
| Negative | (n = 3) |
Three sera were not available for testing.
One serum showed only positive results with MEM154 and not with 3G8.
One serum showed negative results with 3G8 and only positive results with BW209/2, both with homozygous NA1- and NA2-positive neutrophils.
For 1 patient, no material was available to perform both the NA1- and the NA2-MAIGA.
In the MAIGA with NA2-positive neutrophils, a positive result was obtained with MEM154, but not with 3G8.
Two sera were not available for testing.
#Positive results were obtained with BW209/2, but not with 3G8, both in the NA1- and in the NA2-MAIGA.
All of these sera were also tested in the MAIGA with CD11a and CD11b MoAbs. Two sera containing NA1 antibodies showed weak reactivity in the MAIGA with the CD11a MoAb, and 1 serum containing NA2 antibodies showed weak reactivity in the MAIGA with the CD11a MoAb and strong reactivity in that with the CD11b MoAb (data not shown).
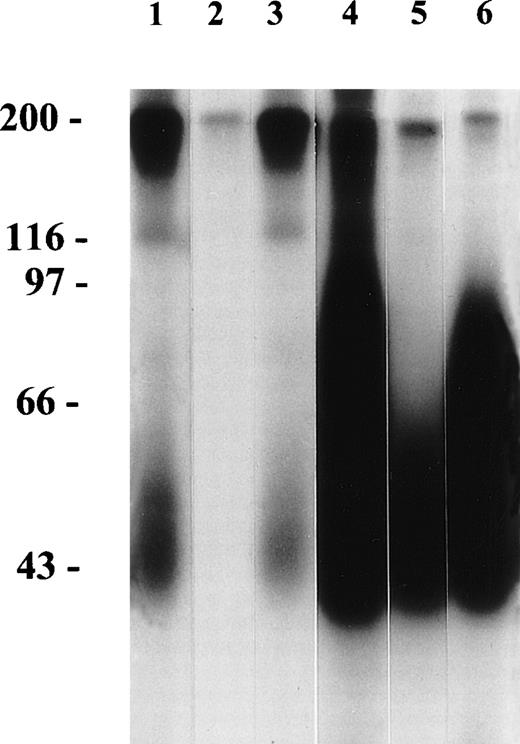
With the MAIGA, the presence of pan-FcγRIIIb–specific antibodies in 5 tested sera from patients with secondary AIN was confirmed in 1 case, whereas 3 sera showed negative results. One serum showed only a positive result in the MAIGA with BW209/2 and homozygous NA1-positive cells; with 3G8 and NA2-positive cells, all results were negative. A weak reaction in the MAIGA with the CD11a MoAb was obtained with the serum in which pan-FcγRIIIb antibodies were confirmed with the MAIGA. One patient, diagnosed as having primary AIN, but with persistent neutropenia after 6 years, showed in her serum pan-FcγRIIIb specificity of the antibodies concluded from the indirect GIFT. The serum of this patient showed the presence of both NA1 and NA2 antibodies in the MAIGA (Table 3). Furthermore, very strong reactivity in the MAIGA with the CD11a MoAb and weak reactivity in the CD11b MAIGA were observed. To further characterize the neutrophil antibodies present in this serum, immune precipitation was performed with NA(1+, 2+) phenotyped neutrophils; only NA1-FcγRIIIb was precipitated (Fig 1). As shown in Fig 1, lane 3, NA1-Fcγ RIIIb was precipitated by another serum from a patient with primary AIN and NA1 antibodies, and in 4 of the 9 additionally tested cases, NA1-FcγRIIIb was precipitated (data not shown). All immune precipitation experiments with sera from patients with secondary AIN, containing antibodies with pan-FcγRIIIb specificity, showed negative results (Fig 1, lane 2, in total 5 sera were tested, data not shown).
Immune precipitation from 125I-labelled NA(1+,2+)-phenotyped neutrophils by neutrophil antisera and CD16 MoAbs. Lane 1, serum from the patient with the clinical pattern of primary AIN, but with chronic neutropenia. This serum reacted positively in the indirect GIFT and MAIGA with both NA(1+, 2−) and NA(1−, 2+)-phenotyped neutrophils. Lane 2, serum from a patient with secondary AIN with pan-FcγRIIIb antibodies as shown by the indirect GIFT. Lane 3, serum from a patient with primary AIN and NA1 antibodies, shown in the GIFT and the MAIGA. Lane 4, control serum containing isoantibodies with pan-FcγRIIIb specificity. Lane 5, MoAb CLB-gran11 (NA1-FcγRIIIb). Lane 6, MoAb CLB-FcRgran1 (pan-FcγRIIIb).
Immune precipitation from 125I-labelled NA(1+,2+)-phenotyped neutrophils by neutrophil antisera and CD16 MoAbs. Lane 1, serum from the patient with the clinical pattern of primary AIN, but with chronic neutropenia. This serum reacted positively in the indirect GIFT and MAIGA with both NA(1+, 2−) and NA(1−, 2+)-phenotyped neutrophils. Lane 2, serum from a patient with secondary AIN with pan-FcγRIIIb antibodies as shown by the indirect GIFT. Lane 3, serum from a patient with primary AIN and NA1 antibodies, shown in the GIFT and the MAIGA. Lane 4, control serum containing isoantibodies with pan-FcγRIIIb specificity. Lane 5, MoAb CLB-gran11 (NA1-FcγRIIIb). Lane 6, MoAb CLB-FcRgran1 (pan-FcγRIIIb).
Confirmation of pan-FcγRIIIb specificity by absorption/elution tests.
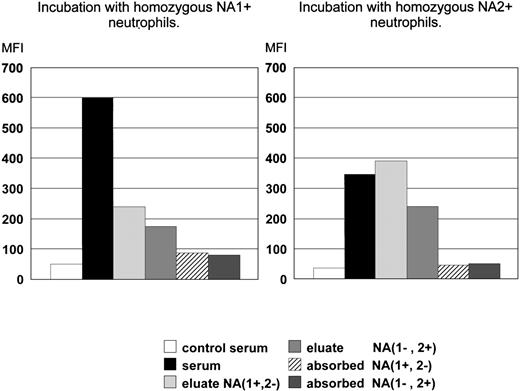
To prove that the sera from patients with secondary AIN contained antibodies with pan-FcγRIIIb specificity, absorbance and elution experiments were performed with 2 of these sera. We observed that both homozygous NA1-positive and homozygous NA2-positive neutrophils absorbed all reactivity: the twice incubated sera no longer reacted with neutrophils of the same or the opposite NA phenotype (Fig 2). Eluates prepared from serum-incubated NA1-positive neutrophils reacted positively with fresh homozygous NA1-positive and with homozygous NA2-positive neutrophils and vice versa (Fig 2). The staining of homozygous NA1-positive neutrophils was brighter compared with that of homozygous NA2-positive neutrophils. This explains the relatively higher binding to NA2-positive neutrophils of the eluate prepared from NA1-positive neutrophils.
Analysis of the presence of allospecific antibodies in FcγRIIIb-antisera from patients with secondary AIN. The left panel shows the reactivity of the sera or eluates with homozygous NA1-positive cells and the right panel with homozygous NA2-positive cells. The mean of experiments with 2 different sera is depicted.
Analysis of the presence of allospecific antibodies in FcγRIIIb-antisera from patients with secondary AIN. The left panel shows the reactivity of the sera or eluates with homozygous NA1-positive cells and the right panel with homozygous NA2-positive cells. The mean of experiments with 2 different sera is depicted.
DISCUSSION
In this study, we analyzed the neutrophil antibody specificity in sera from 2 pediatric patient groups with autoimmune-mediated neutropenia. The patients were selected by the criteria of a positive neutrophil antibody test. We used the indirect GIFT with a panel of donor granulocytes, including a FcγRIIIb-negative donor for detection of neutrophil antibodies. In the report of the Second International Granulocyte Serology Workshop, Bux et al14 concluded that a combination of the GIFT and GAT (granulocyte agglutination test) is the best means of antibody detection. From the results of the Workshop and from our own experience, it follows that the GAT is especially necessary for detection of antibodies with 9a and 5b specificity. In pediatric patients and especially in patients with AIN, 5b and 9a antibodies have never been described. Therefore, we did not use the GAT as a second test for screening antibodies in this patient group. The sera of the pediatric patients with neutropenia of unknown origin were all tested in the GIFT and in the MAIGA. From the literature, it is known that children with primary AIN have, in up to 95% of the tested cases, circulating antibodies directed against neutrophils. Specificity of these antibodies could be detected in about one third of the cases. In the majority of these cases, antibodies showed NA specificity. The specificity of the neutrophil antibodies in children with secondary AIN has not previously been studied extensively. We detected a difference in antibody specificity between patients with primary AIN and secondary AIN. In serum from all children with secondary AIN (n = 7), antibodies with pan-FcγRIIIb specificity were detected, whereas in all sera from patients with primary AIN (n = 21), antibodies with NA specificity were found. The serum of 1 patient with the clinical picture of primary AIN, but with chronic neutropenia by now lasting for 6 years, seemed to contain both antibodies with NA1 specificity and with pan-FcγRIIIb specificity, as concluded from the screening with the indirect GIFT, the MAIGA, and immune precipitation results. In addition to these antibodies, in the MAIGA performed with neutrophils from a FcγRIIIb-negative donor, strongly reactive CD11a-specific antibodies and weakly reactive CD11b antibodies were detected in this serum. Until now, no other autoimmune or hematological problems have evolved in this patient. The persistence of the neutropenia for more than 6 years, as well as the different specificity of antibodies in the serum compared with the sera of the other 20 patients with primary AIN, suggests a different type of neutropenia than the classical primary AIN.
Overall, weak CD11a- or CD11b-specific antibodies were detected in 3 of the 13 tested sera from other patients with primary AIN and in 1 of the 4 tested sera from patients with secondary AIN. In the 3 patients with primary AIN, whose sera showed both NA-specific and CD11a or CD11b antibodies, the neutropenia resolved. Bux et al found 21% of the samples positive in a MAIGA with CD11b and CD18 MoAbs, which is similar to the 28% (4 of 14 tested samples) we found. Hartman and Wright15 described the presence of antibodies with CD11b/CD18 specificity in 14% of 50 adult patients with immune neutropenia. These investigators used an immunobead assay for detection of antibodies. Six patients with CD11b/CD18 antibodies in this study had multiple autoimmune problems, and 5 of the 7 patients had recurrent infections. Thus, CD11b/CD18 antibodies are detectable both in adult and childhood populations with immune-mediated neutropenia.
In all but 3 cases of primary AIN (86%), we defined the NA specificity of the neutrophil antibodies by screening of the sera with a panel of phenotyped donor granulocytes. In a recent report on analysis of antibody specificity in 240 cases of AIN, Bux et al5 found with the same test NA specificity in only 34% of the cases, in all other tested cases the serum reacted both with NA1-and with NA2-positive neutrophils. Furthermore, these investigators observed that NA1- and NA2-reactive antibodies were detected in the MAIGA in sera from patients with primary AIN.5 We observed that not in all cases all tested MoAbs showed a positive result. In general, the positive signals obtained in the MAIGA with sera from patients with AIN were much lower compared with those obtained with sera containing allo- or isoantibodies (data not shown). In our opinion, screening of patients’ sera with a well-defined donor granulocyte panel is to be preferred over testing of the sera in the MAIGA, although this latter test can be helpful to further analyze antibody specificities present in indirect-GIFT–negative samples.
The etiology of AIN is not clear. In primary AIN, the very early onset of the disease, together with the self-limited course, is remarkable. In secondary AIN, the existence of other autoimmune diseases is of special interest. In their review, Shastri and Logue16discussed the pathophysiology of immune neutropenia. Their discussion is based on the 2 major pathways for immune dysregulation. In the first pathway, antibodies to foreign antigens, eg, viruses or bacteria, cross-react with self-antigens. The antibodies found in this situation are supposed to be polyclonal, and in the case of a normally developed immune system, this type of immune reaction is self-limited. Hence, primary AIN may be explained by a mechanism of immunity due to cross-reactivity. There are, however, questions that remain. The antibodies are present during a relatively long period (months or years). A possible explanation for this phenomenon, suggested by other investigators,17 is that full development of the suppressor T-cell function occurs during the first years of life. Apparently, the NA1 antigen forms a risk factor contracting primary AIN. Parvovirus B19 has been believed to play a role in the etiology,18 but this was not confirmed.5 The second mechanism in the pathway of immune dysregulation involves loss of suppression of a clone of cells that are able to react with self-antigens. Little is known about the clonality of the antibodies in AIN in children. In secondary AIN, the development of other autoimmune diseases suggests an etiology in which disturbance of the self-tolerance plays an important, but until now, incompletely understood role.
In conclusion, our data show that children with immune-mediated chronic neutropenia and NA-specific antibodies in their sera suffer from primary AIN and therefore have a benign, and in most cases, self-limited disease. On the contrary, children with chronic neutropenia and antibodies with pan-FcγRIIIb specificity have a risk of developing other autoimmune diseases and therefore should be followed in a different way . Especially in young children with AIN, characterization of the antibodies can be helpful in differentiating between primary and secondary AIN.
ACKNOWLEDGMENT
The authors acknowledge all pediatricians who sent material and data for this study. We thank the Department of Leukocyte and Thrombocyte Serology of the CLB for serologic screening and for their help with the MAIGA.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Masja de Haas, MD, PhD, Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Plesmanlaan 125, 1066 CX Amsterdam, PO Box 9190, 1006 AD Amsterdam, The Netherlands; e-mail: m_de_haas@clb.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal