Abstract
Human immunodeficiency virus-1 (HIV-1)-Tat, the transactivating gene product of HIV-1, has been shown to interact with different cell types, inducing gene expression, altering their growth and migratory behavior. In this study we examined whether Tat might affect functions of acquired immunodeficiency syndrome (AIDS)-related non-Hodgkin’s lymphoma (NHL), relevant to the in vivo dissemination. Our results show that Tat significantly augmented the motility of the two AIDS-related Burkitt’s lymphoma cell lines (AS283 and PA682PB) and AIDS-primary effusion lymphoma cell line (HBL-6-AIDS-PEL). Mutations in RGD or basic domain of Tat (KGE-MBP and LxI-MBP, respectively) sharply reduced migration compared with wild type, suggesting that both domains are required for migration. In contrast, a Tat protein mutation outside the active domains (NH2-TAT-GST) did not reduce lymphoma cell migration. The treatment of lymphoma cells with Tat did not influence their adhesion to matrix proteins or to human vascular endothelial cells, but endothelial cells treated with Tat became more adhesive to lymphoma cells. Flow cytometric analysis showed that treatment of endothelial cells with Tat induced the cell surface expression of the adhesion molecules vascular cell adhesion molecule-1 (VCAM-1) and E-selectin and increased the expression of intercellular adhesion molecule-1 (ICAM-1). Only antibodies against VCAM-1 on endothelial cells or against the VLA-4 integrin expressed on AS283 cells inhibited the increment of adhesion, indicating the relevance of this pathway in the adhesion of lymphoma cells to vascular endothelium. In our work, we show for the first time that Tat can enhance the migration of lymphoma cells and their adhesion to endothelial cells, two processes that may contribute to the malignant behavior of NHL in patients with AIDS.
NON-HODGKIN’S LYMPHOMA (NHL) represents one of the most frequent complications in acquired immunodeficiency syndrome (AIDS).1,2 The majority of AIDS-related NHLs are diffused aggressive B-cell lymphomas with extranodal involvement of the central nervous system in particular.1,3 Their histology includes four categories, namely small noncleaved-cell (including Burkitt’s lymphoma), large-cell immunoblastic plasmocytoid, anaplastic-large cell, and AIDS-related body-cavity–based lymphoma.1,4 Small noncleaved-cell lymphomas are characterized by activating mutations ofc-myc, frequent alterations of p53, and occasional Epstein-Barr virus infection.5,6 Large cell subtype lymphomas are strictly associated with Epstein-Barr virus infection and with rearrangements of c-myc and BCL-6.5,7Finally, AIDS-related body-cavity–based lymphoma is consistently associated with the coinfection of human herpesvirus type-8 and Epstein-Barr virus,8 whereas the anaplastic subtype has not been studied extensively. A feature common to the four AIDS-NHL subtypes is the presence of mutations in the noncoding region ofBCL-6 in the proximity of the gene promoter.9
The mechanism by which AIDS-NHL cells home into the central nervous system is largely unknown and could involve signals from endothelial cells of blood-brain barrier infected by human immunodeficiency virus-1 (HIV-1)10,11 or specific chemoattractants for lymphoma cells. Microvascular endothelial cells originated from the brain or the bone marrow and infected by HIV-1 support the growth and the adhesion of neoplastic B cells through the surface expression of CD40 that binds CD40 ligand present on lymphoma cells.12 Tat is a potent inducer of the expression of endothelial cell adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1)13that is involved in the migration of B cells to and within the germinal center of lymphonodes.14
Tat is a viral protein that transactivates viral genes and increases the replication rate of virion.15 Tat is also able to regulate the expression of host genes, including tumor necrosis factors α and β, interleukin-2 (IL-2), IL-6, major histocompatibility complex of class I, IL-2 receptor, p53 tumor suppressor gene,c-fos, and superoxide dismutase in T cells.16-22Tat can also be released by infected cells, regardless of whether these cells are alive or not,23,24 and extracellular Tat acts as a cytokine-like molecule with a broad range of activities on mesenchymal cells.13,25-33 One of the most striking effects of Tat on vascular endothelial cells is the induction of functions related to angiogenesis and inflammation, including migration, proliferation, and expression of adhesion molecules.13,31 34-36
MATERIALS AND METHODS
Cells.
The AS283 and PA682PB AIDS-related Burkitt’s lymphoma and KD488 Burkitt’s lymphoma cell lines were obtained through the courtesy of Dr I.T. Magrath (National Cancer Institute, Bethesda, MD) and are described by Kiwanuka et al.37 The Namalwa and BJAB Burkitt’s lymphoma, the diffuse histocytic lymphoma-4 (DHL-4) follicular lymphoma, and the Capo Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines were all obtained from Dr J. Golay (Mario Negri Institute, Milan, Italy). We also used the HBL-6-AIDS-primary effusion lymphoma cell line (HBL-6-AIDS-PEL) obtained from Dr G. Gaidano (University of Piemonte Orientale “A. Avogadro”, Turin, Italy). All these lines were maintained in suspension in RPMI 1640 supplemented with 2 mmol/L glutamine and 10% fetal calf serum (FCS) 3T3-NIH mouse fibroblasts (American Type Cell Collection, Bethesda, MD) and human vascular smooth muscle cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mmol/L glutamine and 10% FCS.
Endothelial cells (EC) were isolated from human umbilical vein as previously described38 and maintained in medium 199 supplemented with 10% FCS, 10% newborn calf serum, 50 μg/mL endothelial growth supplement (crude extract from bovine brain), 100 μg/mL heparin, and 20 mmol/L HEPES. All culture reagents were from GIBCO (Paisley, Scotland).
Preparation and biological activity of HIV-1-Tat molecules.
The 358-bp Pst I-BamHI fragment of Tat gene containing exon I and II was kindly donated by Dr A. Caputo (Institute of Microbiology, University of Ferrara, Ferrara, Italy) and has been subcloned in the pMAL-c2 vector (New England Biolabs, Beverly, MA) and then expressed in Escherichia coli according to the manufacturer’s instructions. Tat fused to the maltose binding protein (Tat-MBP) was purified by affinity chromatography on amylose resin and used as fusion proteins. The purified protein gave a unique band detected by silver staining (Gelcode; Pierce Chemical Co, Rockford, IL) after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE); 10%) and was able to induce transcriptional activation of HIV-1 long terminal repeat (LTR) in HL3T1 cells that contain the bacterial gene of chloramphenicol acetyltransferase (CAT) directed by HIV-1 LTR.39 Tat-MBP dose dependently stimulated CAT expression. Briefly, in prewarmed phosphate-buffered saline (PBS), our Tat-MBP preparation was added to confluent HL3T1 cells in a 100-mm diameter dish. Cells were immediately scraped from the plastic surface, then resuspended in fresh medium and centrifuged. The cells were again plated in CO2 incubator, and a CAT assay was performed after 6 hours according to Ausubel et al.40 In our experimental condition, just scraping of the cells in absence of Tat-MBP had no effect on LTR-directed CAT gene expression (125 ± 56 [3H]acetylated chloramphenicol, n = 3). However, CAT gene expression was markedly elevated in cells that received Tat-MBP (0.5 μg/mL: 423 ± 86 [3H]acetylated chloramphenicol; 1 μg/mL: 2,345 ± 167 [3H]acetylated chloramphenicol; 5 μg/mL: 6,543 ± 567 [3H]acetylated chloramphenicol).
Tat-MBP was also active in terms of the migration of Kaposi’s sarcoma cells, human vascular endothelial cells, and human monocytes.27,31,41 For mutagenesis, cDNA Tat was subcloned in the pALTER-Ex1 vector (Promega, Madison, WI). Side-direct mutagenesis was performed with the altered sites mutagenesis kit (Promega) by using mutant oligonucleotides (synthesized by TibMol-Biol, Genova, Italy) to introduce specific mutations (KGE-MBP [Tat-R78K/D80E]: 5′ CCC GAG GGG AAC CGA CAG GCC 3′ and 5′ ACC TCC CAA TCC AAA GGG GAA CCG AC 3′; LXI-MBP [Tat-G49/I50]: 5′ CTC CTA TGG CGG GAT CAA GCG GAG ACA 3′ and 5′ CGG GAT CAA GCT AAT ACA GCG ACG AAG 3′). Specific mutations induced in the cDNA were verified by DNA sequencing by using Sequenase method (Amersham, Buckinghamshire, UK). The mutated cDNAs (356-bp salI-BamHI fragments), subcloned into the pMAL-c2 vector, expressed in E coli as fused protein and purified as described above. NH2-TAT-GST, lacking the first 21 NH2 amino acids, and GST protein were prepared as described.42
Wild-type Tat-MBP, the mutated proteins, and MBP were lipolysaccharide-free, assessed by Limulus assay (Sigma Chemical Co, St Louis, MO). Proteins were stored at −80°C in the dark, in buffered-phospate saline containing 0.1 mmol/L ZnCl2, 0.1% human serum albumin (Farma Biagini, Lucca, Italy), and 1 mmol/L dithiothreitol.
Reagents.
Human recombinant IL-1β (IL-1; specific activity, 107U/mg) was obtained from BASF, Bioresearch Corp (Cambridge, MA). Monoclonal antibodies to VCAM-1 (CD106; clone 4B2), E-selectin (CD62A; clone 13D5), intercellular adhesion molecule-1 (ICAM-1) (CD54; clone 11C8/1), and α4β1 (VLA-4;CD49d/CD29; clone 2B4) were obtained from British Biotech Pharmaceuticals Ltd (Oxford, UK). Anti-α5β1 (CD49e/CD29; clone SAM-1) was obtained from Immunotech S.A. (Marseille, France), and monoclonal antibodies to αvβ3 (CD51/CD61; clone LM609) and αvβ5 (CD51/CD-; clone P1F6) were purchased from Chemicon (Temecula, CA). Monoclonal antibodies to αLβ2 (lymphocyte function-associated antigen-1 [LFA-1]; CD11a/CD18) were a kind gift from Dr A. Rambaldi (Ospedali Riuniti, Bergamo, Italy). Recombinant soluble VCAM-1 and E-selectin were obtained from Dr J. Clements (British Biotech Pharmaceuticals Ltd). Heparin was purchased from Sigma. Monoclonal antibody to Tat (clone 15.1) was from Intracel (London, UK).
Chemotaxis.
Chemotaxis was conducted in the Boyden chamber, as described previously.43 Different dilutions of Tat-MBP, KGE-MBP, LxI-MBP, MBP, NH2-Tat-GST, and GST with 0.5 μg/mL heparin were added to the lower compartment of the chamber. To block the chemotactic effect of Tat, Tat proteins were mixed with monoclonal antibodies to Tat (dilution 1:50), or Tat proteins were boiled for 10 minutes and then added to the lower compartment of the chamber. Eight-micrometer pore size polyvinylpyrrolidone (PVP)-free polycarbonate Nucleopore filters (Costar, New York, NY) were coated with gelatin by immersing them overnight in a solution of 100 μg/mL gelatin in 0.1% acetic acid and then air dried. The filter separated the attractants from the upper part of the chamber in which AS283 cells were added. After 4 hours of incubation at 37°C, filters were stained with Diff-Quick (Merz-Dade, Dudingen, Switzerland), and the migrated cells in 20 high-power fields were counted. Checkerboard analysis of the chemotactic response was performed by varying the concentrations of Tat-MBP in the upper and lower compartment of the Boyden chamber, as described previously.43
Adhesion assay.
EC grown to confluent monolayers in 96-well plates were activated for 4 hours with the indicated concentrations of Tat-MBP, KGE-MBP, LxI-MBP, or MBP diluted in culture medium. IL-1 at 2 ng/mL was used as a reference control to activate EC.44 After incubation, monolayers were washed twice, and wells were refilled with 50 μL medium 199 containing 1% bovine serum albumin (BSA; test medium). AS283 cells were radiolabeled with [125I]-Iododeoxyuridine (Amersham), as described previously.45 Fifty microliters of the cell suspension (3 × 104 cells) was added to each well and incubated at 37°C for 30 minutes. After incubation, nonadherent cells were removed by careful aspiration and three washes with test medium. Wells were incubated for 10 minutes with 100 μL of 0.1 mol/L NaOH, and the lysate was counted in a gamma counter.
To test the adhesion of AS283 cells on purified VCAM-1, 96-well plates (Falcon Pro-bind; Becton Dickinson Labware, Franklin Lakes, NJ) were coated with soluble VCAM-1 by adding 50 μL of protein (5 μg/mL) and incubating them overnight at 4°C. After two washes in PBS, the coated surfaces were incubated for 1 hour with PBS containing 1% BSA and then used for the adhesion assay according to the procedure described above.
To test the involvement of adhesion molecules expressed on EC and AS283 cells in the adhesion assays, activated EC were preincubated with test medium alone or containing 20 μg/mL monoclonal antibody (MoAb) anti–VCAM-1, anti–E-selectin, or anti–ICAM-1. Lymphoma cells were preincubated with test medium with or without 20 μg/mL MoAb anti–VLA-4 or anti–LFA-1 for 30 minutes at 37°C before the adhesion assay.
Adhesion was expressed as the percentage of attached tumor cells relative to the total added cells.
Flow cytometric analysis.
EC and AS283 cells were treated for 4 hours with Tat-MBP (20 ng/mL), equimolar concentrations of MBP or IL-1 (2 ng/mL). The expression of adhesion molecules on EC and AS283 cells was measured by indirect immunofluorescence using a FACSort (Becton Dickinson). After incubation with the appropriate primary antibody (1:50 dilution) in PBS with 2.5% FCS for 30 minutes at 4°C, cells were washed and incubated with an affinity-purified fluorescein isothiocyanate (FITC)–labeled goat antimouse Ig antiserum for 30 minutes at 4°C (Tecno Genetics, Italy). Cells were washed again and fixed with PBS containing 1% formalin. Results were expressed as percentage positive cells and the mean of the channel fluorescence intensity.
Statistics.
The Student’s t-test was used to determine statistical differences. Statistical significance was set at P < .05.
RESULTS
Induction of AS283 lymphoma cell migration by Tat-MBP.
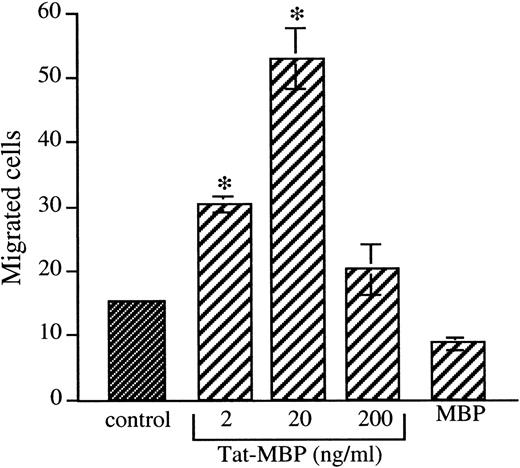
Tat-MBP (2 to 200 ng/mL) used as chemoattractant stimulate the motility of AS283 cells in a concentration-dependent manner (Fig1). At the optimal concentration of 20 ng/mL Tat-MBP, the number of migrated AS283 cells augmented from 14.5 ± 0.7 in the control wells to 50.5 ± 4.5 in wells with Tat-MBP (3.5-fold increase). The fusion protein MBP alone did not affect migration (Fig 1), nor did heparin alone (data not shown). Similar results were obtained with the AIDS-related lymphoma lines PA682PB and HBL-6-AIDS-PEL, in which 20 ng/mL Tat-MBP induced a 3.1-fold and a 2-fold increase in migration, respectively (Table1). In contrast, the migration of the Burkitt’s lymphomas (KD488, Namalwa, and BJAB), the follicular lymphoma (DHL-4), and the EBV-transformed lymphoblastoid cell line (Capo) was not increased by Tat-MBP (Table 1). Fibroblasts and vascular smooth muscle cells used as a normal cell control did not respond to Tat-MBP (Table 1).
Induction of AS283 lymphoma cell motility by Tat-MBP. Motility response of AS283 lymphoma cells to increasing amounts of Tat-MBP (2 to 200 ng/mL). Control medium, Tat-MBP, and MBP were used as attractants in the presence of heparin (0.5 μg/mL). Data are expressed as the number of migrated cells in 20 high-power fields. Columns, mean of three replicates and representative of 1 out of 3 experiments; bars, SD. *P < .001 compared with control.
Induction of AS283 lymphoma cell motility by Tat-MBP. Motility response of AS283 lymphoma cells to increasing amounts of Tat-MBP (2 to 200 ng/mL). Control medium, Tat-MBP, and MBP were used as attractants in the presence of heparin (0.5 μg/mL). Data are expressed as the number of migrated cells in 20 high-power fields. Columns, mean of three replicates and representative of 1 out of 3 experiments; bars, SD. *P < .001 compared with control.
Induction of Lymphoma Cell Migration by Tat-MBP
| Cell Type . | Cell Line . | Chemoattractant . | ||
|---|---|---|---|---|
| None . | Tat-MBP . | Positive Control . | ||
| AIDS-Burkitt’s lymphoma | AS283 | 14 ± 2 | 50 ± 4* | 216 ± 41-a |
| PA682PB | 11 ± 1 | 35 ± 6* | 29 ± 71-a | |
| Burkitt’s lymphoma | KD488 | 35 ± 6 | 34 ± 4 | 72 ± 81-a |
| Namalwa | 6 ± 2 | 2 ± 1 | 235 ± 31-a | |
| BJAB | 7 ± 2 | 1 ± 1 | 14 ± 61-b | |
| AIDS-primary effusion lymphoma | HBL-6-AIDS-PEL | 15 ± 8 | 30 ± 4* | 56 ± 401-b |
| Follicular lymphoma | DHL-4 | 8 ± 1 | 8 ± 1 | 110 ± 261-a |
| EBV-transformed lymphoblastoid | Capo | 3 ± 0 | 5 ± 2 | 107 ± 261-a |
| Fibroblasts | 3T3-NIH | 8 ± 4 | 11 ± 5 | 55 ± 121-c |
| Vascular smooth muscle cells | VSMC | 16 ± 5 | 12 ± 3 | 85 ± 121-c |
| Cell Type . | Cell Line . | Chemoattractant . | ||
|---|---|---|---|---|
| None . | Tat-MBP . | Positive Control . | ||
| AIDS-Burkitt’s lymphoma | AS283 | 14 ± 2 | 50 ± 4* | 216 ± 41-a |
| PA682PB | 11 ± 1 | 35 ± 6* | 29 ± 71-a | |
| Burkitt’s lymphoma | KD488 | 35 ± 6 | 34 ± 4 | 72 ± 81-a |
| Namalwa | 6 ± 2 | 2 ± 1 | 235 ± 31-a | |
| BJAB | 7 ± 2 | 1 ± 1 | 14 ± 61-b | |
| AIDS-primary effusion lymphoma | HBL-6-AIDS-PEL | 15 ± 8 | 30 ± 4* | 56 ± 401-b |
| Follicular lymphoma | DHL-4 | 8 ± 1 | 8 ± 1 | 110 ± 261-a |
| EBV-transformed lymphoblastoid | Capo | 3 ± 0 | 5 ± 2 | 107 ± 261-a |
| Fibroblasts | 3T3-NIH | 8 ± 4 | 11 ± 5 | 55 ± 121-c |
| Vascular smooth muscle cells | VSMC | 16 ± 5 | 12 ± 3 | 85 ± 121-c |
Motility response of lymphoma cells and non-neoplastic cells to Tat-MBP. Control medium and 20 ng/mL Tat-MBP were used as attractants in the presence of 0.5 μg/mL heparin. Appropriate positive control chemoattractants were used for each single cell line:
25 μg/mL fibronectin,
conditioned medium of 3T3-NIH cells,
5 ng/mL platelet-derived growth factor (PDGF). Data are expressed as the number of migrated cells ± SD in 20 high-power fields. Data are the mean of three replicates and representative of 1 of 2 experiments minimum.
P < .001.
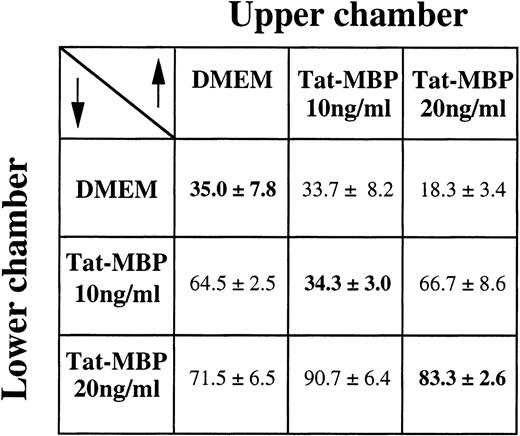
Checkerboard analysis showed that Tat-MBP mainly induced a directional motility response of AS283 lymphoma cells, although a chemokinetic component was also present (Fig 2).
Checkerboard analysis of AS283 motility response to Tat-MBP. Different gradient conditions were obtained by adding the indicated concentrations of Tat-MBP to the upper and lower compartments of the Boyden chamber. Cells, in the upper compartment, were exposed to conditions of null gradient (same concentration of Tat-MBP in the upper and lower compartments, diagonal), positive gradients (higher concentrations of Tat-MBP in the lower compartment, below the diagonal), or negative gradients (higher concentrations of Tat-MBP in the upper compartment, above the diagonal). Data are expressed as the number of migrated cells in 20 high-power fields (mean and SD of triplicates).
Checkerboard analysis of AS283 motility response to Tat-MBP. Different gradient conditions were obtained by adding the indicated concentrations of Tat-MBP to the upper and lower compartments of the Boyden chamber. Cells, in the upper compartment, were exposed to conditions of null gradient (same concentration of Tat-MBP in the upper and lower compartments, diagonal), positive gradients (higher concentrations of Tat-MBP in the lower compartment, below the diagonal), or negative gradients (higher concentrations of Tat-MBP in the upper compartment, above the diagonal). Data are expressed as the number of migrated cells in 20 high-power fields (mean and SD of triplicates).
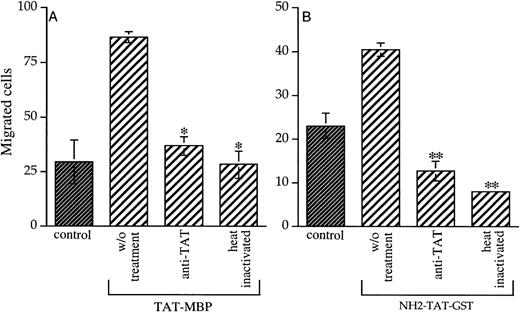
To identify the molecular domain of Tat responsible for the stimulation of AS283 motility, we tested two mutated Tat-MBP proteins, one mutated in the RGD domain (KGE-MBP) and one in the basic domain (LxI-MBP). Figure 3A shows that KGE-MBP and LxI-MBP have drastically lost the ability to induce the migration of AS283 cells. Even higher concentrations of the two mutated Tat-MBP proteins did not influence the migration of lymphoma cells (data not shown). These data indicate that the chemotactic properties of Tat-MBP might be dependent on a collaboration between the RGD and the basic domains. A Tat protein mutated in the apparently noninvolved N-terminal domain was also used (NH2-Tat-GST). NH2-Tat-GST showed similar activity on AS283 cell migration as did Tat-MBP (Fig 3B). The fusion protein GST alone had no effect at all. The specificity of Tat-MBP has been confirmed by the finding that neutralizing antibodies against Tat and heat inactivation blocked its activity on AS283 cell motility (Fig 4A). Similar results were obtained using NH2-Tat-GST (Fig 4B).
Chemotactic activity of mutated Tat proteins. (A) Inhibition of AS283 lymphoma cell motility by mutated Tat-MBP proteins. KGE-MBP and LxI-MBP proteins are mutated, respectively, in the RGD and basic domain of Tat. (B) AS283 lymphoma cell motility induced by a Tat protein mutated in the N-terminal domain. Each protein was tested at the concentration of 20 ng/mL. MBP or GST alone were used at the equimolar concentration. Data are expressed as the number of migrated cells in 20 high-power fields. Columns, mean of three replicates and representative of 1 out of 3 experiments; bars, SD. *P < .05 compared with corresponding control.
Chemotactic activity of mutated Tat proteins. (A) Inhibition of AS283 lymphoma cell motility by mutated Tat-MBP proteins. KGE-MBP and LxI-MBP proteins are mutated, respectively, in the RGD and basic domain of Tat. (B) AS283 lymphoma cell motility induced by a Tat protein mutated in the N-terminal domain. Each protein was tested at the concentration of 20 ng/mL. MBP or GST alone were used at the equimolar concentration. Data are expressed as the number of migrated cells in 20 high-power fields. Columns, mean of three replicates and representative of 1 out of 3 experiments; bars, SD. *P < .05 compared with corresponding control.
Inactivation of chemotactic activity of Tat. Monoclonal antibodies against Tat (dilution 1:50) were mixed with Tat proteins, or Tat proteins were boiled for 10 minutes and then added to the lower compartment of the chamber. Tat-MBP and NH2-Tat-GST were used in (A) and (B), respectively. Each protein was tested at the concentration of 20 ng/mL. Data are expressed as the number of migrated cells in 20 high-power fields. Columns, mean of 3 replicates (and representative of 1 out of 2 experiments done); bars, SD. *P< .001 compared with Tat-MBP without treatment, **P < .05 compared with NH2-Tat-GST without treatment.
Inactivation of chemotactic activity of Tat. Monoclonal antibodies against Tat (dilution 1:50) were mixed with Tat proteins, or Tat proteins were boiled for 10 minutes and then added to the lower compartment of the chamber. Tat-MBP and NH2-Tat-GST were used in (A) and (B), respectively. Each protein was tested at the concentration of 20 ng/mL. Data are expressed as the number of migrated cells in 20 high-power fields. Columns, mean of 3 replicates (and representative of 1 out of 2 experiments done); bars, SD. *P< .001 compared with Tat-MBP without treatment, **P < .05 compared with NH2-Tat-GST without treatment.
Induction of EC adhesiveness by Tat-MBP.
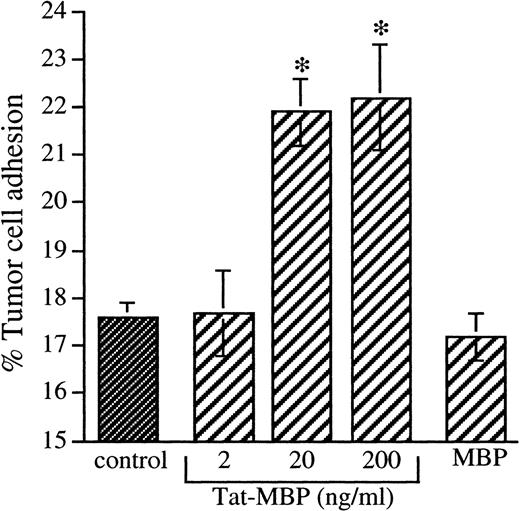
The adhesion of AS283 lymphoma cells was significantly increased on EC activated with Tat-MBP for 4 hours with maximal stimulation at 20 ng/mL Tat-MBP (Fig 5). Tat-MBP induced approximately a 1.6-fold increase of adhesion, reproducible in all the experiments performed. MBP alone had no effect.
Adhesion of AS283 lymphoma cells to endothelial cells activated with Tat-MBP. AS283 lymphoma cell adhesion was tested on EC activated with different concentrations of Tat-MBP. EC were treated with medium alone (control), Tat-MBP (20 to 200 ng/mL), or equimolar concentration of MBP. EC were activated for 4 hours, and adhesion of AS283 lymphoma cells was evaluated after 30 minutes. Results show attached cells as a percentage of total added cells. Columns, mean of three replicates and representative of 1 of 3 experiments; bars, SD. *P < .01 compared with control.
Adhesion of AS283 lymphoma cells to endothelial cells activated with Tat-MBP. AS283 lymphoma cell adhesion was tested on EC activated with different concentrations of Tat-MBP. EC were treated with medium alone (control), Tat-MBP (20 to 200 ng/mL), or equimolar concentration of MBP. EC were activated for 4 hours, and adhesion of AS283 lymphoma cells was evaluated after 30 minutes. Results show attached cells as a percentage of total added cells. Columns, mean of three replicates and representative of 1 of 3 experiments; bars, SD. *P < .01 compared with control.
We therefore investigated by fluorescence-activated cell sorter (FACS) analysis whether the increased adhesiveness was sustained by an increment of adhesion molecules. Twenty nanograms/milliliter Tat-MBP induced the expression of E-selectin and VCAM-1 and increased the expression of ICAM-1 after 4 hours of activation (Table2). MBP alone had no significant effect on the expression of adhesion molecules. The increment of lymphoma cell adhesion to EC treated with Tat-MBP was lower compared with IL-1–activated EC, used as a positive control (3.1-fold increase of adhesion). The treatment of EC together with Tat-MBP did not have any additional effect with regards to enhancing AS283 cell adhesion (data not shown). Accordingly, Tat-MBP was less potent than IL-1 in augmenting the expression of adhesion molecules on EC (Table 2). Increasing the concentration of Tat-MBP had no further effect on the expression of these adhesion molecules. Treatment of AS283 cells with Tat-MBP did not increase their adhesion to EC (data not shown). Indeed, adhesion molecules such as ICAM-1, LFA-1, and VLA-4 expressed on AS283 cells did not change after Tat-MBP (20 ng/mL) treatment (data not shown). Similar to that of the AS283 cells, the adhesion of PA682PB cells was also augmented on EC activated by Tat-MBP (data not shown).
Induction of Adhesion Molecules on EC by Tat-MBP
| Antigen . | Endothelial-Cell Treatment* . | |||
|---|---|---|---|---|
| Control . | IL-1 . | Tat-MBP . | MBP . | |
| ICAM-1 | 69 (65)† | 99 (347) | 78 (128) | 79 (84) |
| E-selectin | 4 (55) | 91 (209) | 66 (102) | 7 (56) |
| VCAM-1 | 1 (23) | 45 (26) | 13 (33) | 3 (17) |
| Antigen . | Endothelial-Cell Treatment* . | |||
|---|---|---|---|---|
| Control . | IL-1 . | Tat-MBP . | MBP . | |
| ICAM-1 | 69 (65)† | 99 (347) | 78 (128) | 79 (84) |
| E-selectin | 4 (55) | 91 (209) | 66 (102) | 7 (56) |
| VCAM-1 | 1 (23) | 45 (26) | 13 (33) | 3 (17) |
Results represent one typical experiment out of three performed.
EC were treated for 4 hours with medium (control), IL-1 (2 ng/mL), Tat-MBP (20 ng/mL), or equimolar concentrations of MBP.
Results (determined by FACS analysis) are expressed as the percentage of positive cells and the mean channel of fluorescence intensity.
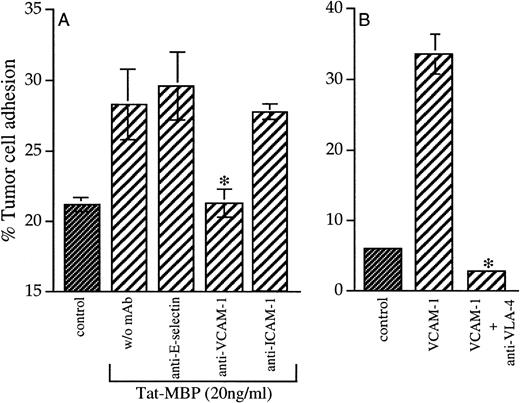
To identify the adhesion molecules involved in AS283/EC adhesion, EC were treated with antibodies against VCAM-1, E-selectin, and ICAM-1. Only antibodies against VCAM-1 blocked the increased adhesion of AS283 cells to EC (Fig 6A). Antibodies against E-selectin and ICAM-1 had no effect on the adhesion to EC (Fig 6A). To confirm that AS283 cell adhesion on Tat-MBP–activated EC depended on the VCAM-1/VLA-4 interaction, we found that AS283 cells selectively attached themselves to VCAM-1–coated plastic, and this adhesion could be blocked by antibodies against VLA-4 to tumor cells (Fig 6B).
(A) Inhibition of AS283 lymphoma cell adhesion to Tat-activated EC. EC were treated for 4 hours with medium alone (control) or Tat-MBP (20 ng/mL) and thereafter incubated with antibodies against the adhesion molecules E-selectin, VCAM-1, and ICAM-1. Adhesion of AS283 lymphoma cells was evaluated after 30 minutes. (B) AS283 lymphoma cell adhesion to VCAM-1. Wells were coated with 5 μg/mL VCAM-1 or buffer only (control). AS283 cells treated or not with antibodies against VLA-4 were incubated on soluble VCAM-1–coated plastic for 30 minutes. Results show the attached cells as a percentage of total cells added. Columns, mean of three replicates and representative of 1 of 3 experiments; bars, SD. *P< .05 compared with EC treated with Tat-MBP without MoAb (A) or to VCAM-1 (B).
(A) Inhibition of AS283 lymphoma cell adhesion to Tat-activated EC. EC were treated for 4 hours with medium alone (control) or Tat-MBP (20 ng/mL) and thereafter incubated with antibodies against the adhesion molecules E-selectin, VCAM-1, and ICAM-1. Adhesion of AS283 lymphoma cells was evaluated after 30 minutes. (B) AS283 lymphoma cell adhesion to VCAM-1. Wells were coated with 5 μg/mL VCAM-1 or buffer only (control). AS283 cells treated or not with antibodies against VLA-4 were incubated on soluble VCAM-1–coated plastic for 30 minutes. Results show the attached cells as a percentage of total cells added. Columns, mean of three replicates and representative of 1 of 3 experiments; bars, SD. *P< .05 compared with EC treated with Tat-MBP without MoAb (A) or to VCAM-1 (B).
DISCUSSION
It has been found that biological functions of mesenchymal cells, like monocytes and endothelial cells, can be modulated by the HIV-1-Tat protein.20,29,31,34,36 Recently, it has been described that Tat can upregulate Fas expression in B lymphocytes,32suggesting that Tat may contribute to B-cell hyperactivation during HIV infection. Polyclonal hyperactivation and proliferation of B cells has been associated with the development of NHL in AIDS patients.46 In this study, we show that Tat induces the migration of two AIDS-related Burkitt’s lymphoma cell lines (AS283 and PA682PB)37 and increases their adhesiveness to human endothelium, two processes that are required for the spreading of highly malignant AIDS-NHL cells. We also tested other kinds of lymphoma cells and found that only the HBL-6-AIDS-PEL lymphoma cell line migrated against Tat-MBP (Table 1), thus suggesting a selective role for the Tat protein in the migration of lymphomas derived from HIV-infected patients. An extensive study on primary lymphoma cells from patients with AIDS is likely to be necessary to address this hypothesis.
Tat protein has been found extracellularly, and induction of lymphoma cell migration was observed at concentrations that have been detected in AIDS patients.26 Similar concentrations of Tat have been reported to induce monocyte29 and EC31 motility and also to upregulate FAS expression in B cells.32 We therefore propose that secreted Tat can promote lymphoma migration and extravasation into tissues. Tat concentrations detected in the serum of AIDS patients are approximately 1 to 2 ng/mL.31 In our experiments, we observed that concentrations between 2 and 20 ng/mL are able to significantly stimulate cell motility. It is possible that the phenomenon of lymphoma cell recruitment by Tat becomes relevant at sites of Tat production and release, where concentrations are presumably higher than those in the circulation.
Extracellular activities of Tat can be mediated through the RGD-containing region or through the basic domain.31,47 To investigate the role of Tat domains in the induction of lymphoma cell motility, two mutated Tat-MBP proteins were used: the KGE-MBP protein that is mutated in the RGD domain, and the LxI-MBP protein that is mutated in the basic domain. We found that neither the RGD nor the basic domain-mutated Tat protein had any effect on lymphoma cell migration. A third Tat protein, NH2-Tat-GST, which is deleted of the first 21 amino acids and does not contain the cysteine-rich domain, had similar activity on lymphoma migration as Tat-MBP, and the data obtained exclude a role for this domain in Tat-induced lymphomas. These data indicate that the chemotactic properties of Tat for AIDS-related lymphoma cells might require both the RGD and the basic domain of Tat. In contrast, the cysteine-rich domain, which has been recently implicated in monocyte chemotaxis induced by Tat,48 seems to be irrelevant for the motility of lymphoma cells. At variance with our findings, it has been shown that the motility of EC was equally stimulated by synthetic peptides containing both the basic or the RGD domain of Tat,47though angiogenesis in vivo was only induced by the basic peptide.31 The fact that AS283 cells did not express the integrin receptors αvβ3 and α5β1 that recognize the RGD sequence of Tat,35 nor the integrin αvβ5 that binds the basic domain of the Tat protein49 (data not shown), suggests that other ligands are involved in the interaction of Tat with AS283 cells. Altogether, these data indicate that different cell types respond to different domains of Tat. Furthermore, it is also possible that unique Tat domains are responsible for different physiological functions, such as migration in vitro and angiogenesis in vivo on the same cell type.31 47
We have recently shown that Tat-induced migration of endothelial cells and monocytes occurs through the activation of the high affinity tyrosin kinase receptors for vascular endothelial growth factor (VEGF), VEGFR-2/KDR/Flk-1, and VEGFR-1/Flt-1, respectively.27,31Here we found that VEGF (20 ng/mL) induced AS283 cell migration and that pretreatment of AS283 cells with VEGF resulted in an inhibition of a subsequent response to Tat (data not shown). This result is reminiscent of the observation that VEGF is an autocrine/paracrine growth factor for acute myeloid cells.50 Although these findings indicate that AS283 cell migration induced by Tat and VEGF depended on the same receptor, VEGFR-1/Flt-1 and VEGFR-2/Flk-1/KDR receptors were not expressed on AS283 cells as analyzed by Northern blot analysis (data not shown). This may indicate the presence of another receptor on AS283 cells. Recently, a third VEGF coreceptor, identical to human neuropilin-1, has been described on endothelial cells and different types of tumor cells.51 Whether this or another receptor is responsible for Tat-induced lymphoma cell motility needs further investigation.
Another important event in the lymphoma dissemination is the interaction between tumor cells and vascular endothelial cells. We show that the treatment of EC with Tat enhances their adhesiveness to lymphoma cells. The upregulation of ICAM-1, VCAM-1, and E-selectin on EC after Tat treatment has been previously shown.13 The role of VCAM-1 and ICAM-1 in the adhesion of B lymphocytes has been shown.52,53 Here we show that the augmented adhesion of AS283 lymphoma cells to Tat-activated EC is mainly associated with an increased expression of VCAM-1, and this is abolished only by antibodies against anti–VCAM-1, but not against ICAM-1. Accordingly, two different reports have shown that lymphocyte adhesion (including Burkitt’s lymphoma cell) to cytokine-activated EC is preferentially mediated by the upregulation of VCAM-1.54,55 The lack of adhesion of Burkitt’s lymphoma to ICAM-1 expressed on endothelial cells is explained by Patarroyo et al.56 They compared a panel of 10 lymphoblastoid cell lines with 10 Burkitt’s lymphoma for the expression of LFA-1 and found that Burkitt’s lymphoma expresses only low levels of this integrin, whereas lymphoblastoid cell lines, representing various stages of B-lymphocyte development, express considerable levels of LFA-1. Although we found that by FACS analysis our AS283 cell line expressed LFA-1, its adhesion to Tat-activated EC was not abolished by antibodies against LFA-1, supporting further the preferential use of the VCAM-1/VLA-4 pathway in the adhesion to Tat-activated EC. The VCAM-1/VLA-4–dependent adhesion of AS283 has been further shown by completely blocking its adhesion to soluble VCAM-1–coated plastic and by treating AS283 cells with antibodies against VLA-4.
It has been shown that anti-CD40 stimulates the heterotypic adhesion of normal and transformed B cells to EC, and this is mainly mediated by VCAM-1/VLA-4 pathway in alternative to ICAM-1/LFA-1.53Furthermore, it has been found that HIV-infected microvascular EC promotes the attachment and growth of malignant B-cell lymphoma.12 It is worth noting that HIV infection of microvascular EC stimulates the expression of CD40, resulting in preferential induction of VCAM-1 after CD40 triggering,12once again showing the importance of the VCAM-1/VLA-4 pathway. However, we did not find CD40 expression on endothelial cells treated with Tat (data not shown), suggesting that this pathway is not involved in our experimental model.
In conclusion, AIDS-related lymphomas frequently involve extranodal sites, particularly in the gastrointestinal tract and the central nervous system, which represent the primary sites of involvement in a significant fraction of the cases.1-3 5 We showed in this work that HIV-1-Tat can enhance the migration of AIDS-related lymphoma cells and their adhesion to endothelial cells. In AIDS patients, this may contribute to the homing and growth of malignant lymphomas at these extranodal sites.
ACKNOWLEDGMENT
The authors thank Dr L.T. Magrath (National Cancer Institute, Bethesda, MD), Dr G. Golay (M. Negri Institute for Pharmacological Research, Milan, Italy), and Dr G. Gaidano (University of Piemonte Orientale “A. Avogadro”, Turin, Italy) for providing some of the lymphoma cell lines used in this study.
Supported by grants from the Istituto Superiore di Sanità (AIDS Project and Program on Tumor Therapy), and Associazione Italiana per la Ricerca sul Cancro (AIRC).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Raffaella Giavazzi, PhD, Mario Negri Institute for Pharmacological Research, Via Gavazzeni 11, 24125 Bergamo, Italy; e-mail: giavazzi@irfmn.mnegri.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal