Abstract
The recombinant fragment of von Willebrand factor (vWF) spanning Ala444 to Asp730 and containing an Arg545Cys mutation (denoted AR545C) has antithrombotic properties that are principally a consequence of its ability to inhibit platelet adhesion to subendothelial matrix. Endothelial-derived nitric oxide (NO) can also inhibit platelet function, both as a consequence of inhibiting adhesion as well as activation and aggregation. Nitric oxide can react with thiol functional groups in the presence of oxygen to form S-nitrosothiols, which are naturally occurring NO derivatives that prolong the biological actions of NO. Because AR545C has a single free cysteine (Cys545), we attempted to synthesize the S-nitroso-derivative of AR545C and to characterize its antiplatelet effects. We successfully synthesized S-nitroso-AR545C and found that it contained 0.96 mol S-NO per mole peptide. S-nitroso-AR545C was approximately 5-fold more potent at inhibiting platelet agglutination than was the unmodified peptide (IC50 = 0.02 ± 0.006 μmol/L v 0.1 ± 0.03 μmol/L, P = .001). In addition and by contrast, S-nitroso-AR545C was a powerful inhibitor of adenosine diphosphate–induced platelet aggregation (IC50 = 0.018 ± 0.002 μmol/L), while AR545C had no effect on aggregation. These effects were confirmed in studies of adhesion to and aggregation on extracellular matrix under conditions of shear stress in a cone-plate viscometer, where 1.5 μmol/L S-nitroso-AR545C inhibited platelet adhesion by 83% and essentially completely inhibited aggregate formation, while the same concentration of AR545C inhibited platelet adhesion by 74% and had significantly lesser effect on aggregate formation on matrix (P ≤ .004 for each parameter by ANOVA). In an ex vivo rabbit model, we also found that S-nitroso-AR545C had a more marked and more durable inhibitory effect on botrocetin-induced platelet aggregation than did AR545C, and these differences were also reflected in the extent and duration of effect on the prolongation of the bleeding time in these animals. These data show that S-nitroso-AR545C has significant and unique antiplatelet effects, inhibiting both adhesion and aggregation, by blocking platelet GPIb receptor through the AR545C moiety and elevating platelet cyclic 3′,5′-guanosine monophosphate through the -SNO moiety. These observations suggest that this NO-modified fragment of vWF may have potential therapeutic benefits as a unique antithrombotic agent.
VON WILLEBRAND FACTOR (vWF) is a multimeric glycoprotein synthesized by megakaryocytes and endothelial cells, and is released both into the circulation and the subendothelial space.1 At sites of vascular injury, platelet activation is initiated by interactions of vWF and a specific receptor on platelets, glycoprotein Ib (GPIb). The interaction of vWF with GPIb is critical for the initiation of platelet deposition, both during normal hemostasis2 and in the setting of arterial thrombosis.3-5 This initial hemostatic effect is triggered by vWF associated with subendothelial matrix, and is modulated by shear stress evoked by flowing blood in the vasculature. Platelet aggregation is then further promoted by activation of another platelet receptor complex, glycoprotein IIb/IIIa (GPIIb/IIIa), leading to the binding of fibrinogen or vWF to the platelet surface.6
The A1 domain of the vWF molecule is known to contain the GPIb-binding site, first assigned to a tryptic fragment spanning amino acids 449 to 728 that contains a large intrachain disulfide-linked (Cys509-Cys695) loop.7,8 The pharmacological inhibition of the high shear stress-induced platelet adhesion to vWF with monoclonal anti-vWF antibodies,5,9 aurin tricarboxylic acid,10,11or the recombinant A1 domain fragment VCL12 reduces thrombus formation in various animal models, emphasizing the crucial role vWF plays in arterial thrombogenesis.
We recently reported that the recombinant vWF fragment spanning Ala444 to Asp730 and containing an Arg545Cys mutation, denoted AR545C, has antithrombotic properties in vitro and in vivo.13 R545C is a gain of function mutation that results in an increased and also spontaneous binding of the fragment to platelet GPIb,13thereby blocking the initial interaction between native vWF and platelet GPIb, preventing any further process of platelet activation. Indeed, the mutated AR545C fragment inhibited ristocetin- and botrocetin-induced platelet agglutination of human and rabbit platelets, respectively, and enhanced the thrombolytic effect of recombinant tissue-type plasminogen activator in a rabbit thrombosis model.13
Endothelium-derived nitric oxide (NO) inhibits platelet aggregation14,15 and prevents adhesion of platelets to the subendothelium,16 and does so in association with elevating intracellular cyclic 3′,5′-guanosine monophosphate (cGMP). NO is stabilized by reacting with sulfhydryl groups in the presence of oxygen to form S-nitrosothiols, thereby prolonging its half-life and preserving its biological activity.17 The AR545C molecule contains 3 cysteine residues involved in interchain bonds (residues 459, 461, and 464), 2 pairs of intrachain disulfide bonds (residues 471-474 and 509-695), and 1 apparently free cysteine (residue 545).18 S-nitrosation of AR545C (S-nitroso-AR545C) at Cys545 should, therefore, endow the molecule with potent and long-lasting NO-like effects. This compound may be of potential clinical interest because 2 independent antiplatelet activities are combined in the same molecule, viz, antiadhesive and antiaggregatory effects. The aim of the present study is to synthesize and characterize the antiplatelet effects of S-nitroso-AR545C in vitro and ex vivo, and to compare these effects to the parent peptide AR545C.
MATERIALS AND METHODS
Construction, synthesis, and purification of the peptide AR545C.
The sequence encoding alanine 444-aspargine 730 and containing the arginine-to-cysteine substitution at amino acid residue 545 (AR545C) was derived from a full-length cDNA for human vWF.13 The coding sequence was inserted into a pZEM229 expression vector and expressed in a thymidine kinase–deficient BHK cell line, BHK-570, using methotrexate for growth selection as previously described.13 The AR545C peptide was purified from the BHK media by heparin-Sepharose CL-6B affinity chromatography (Pharmacia Biotech, Piscataway, NJ), yielding essentially pure material. The purity of the peptide was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing or reducing conditions, as well as by reverse-phase high-performance liquid chromatography with a Vydac C8 column (Vydac, Hesperia, CA).13 The amount of the peptide was quantified by a sandwich enzyme-linked immunosorbent assay (ELISA) using 1:100 rabbit anti-human vWF (Dakopatts A082; Dako, Glostrup, Denmark) as the coating antibody and 1:1,000 peroxidase-conjugated anti-vWF antibody (Dakopatts P226) as the detecting antibody. The standard was a human pool of platelet-poor plasma (30 volunteers) that was assumed to contain 10 μg/mL of vWF. ELISAs were developed with o-phenylenediamine as the colorimetric substrate and quantified at A490 on an ELISA reader (Molecular Devices, USA), as described previously.13
Synthesis of S-nitroso-AR545C peptide.
S-nitrosation of AR545C was performed by 2 methods: (1) direct nitrosation by acidified NaNO2,17 and (2) transnitrosation by the NO congener S-nitrosoglutathione (SNO-Glu).19,20 SNO-Glu was prepared within 5 minutes of use, kept at 4°C, and incubated with the purified AR545C fragment for 1 hour at an approximate 28:1 molar ratio (SNO-Glu:AR545C). The acidic pH of the mixture was then neutralized to pH 7.4 with NaOH and the unbound SNO-Glu was separated from the nitrosated AR545C (S-nitroso-AR545C) fragment by Sephadex G-25M (Pharmacia Biotech) column chromatography. The protein concentration of S-nitroso-AR545C was determined by the Coomassie Plus Protein Assay reagent (Pierce, Rockford, IL). The formation of the S-nitroso-AR545C peptide was confirmed by the Saville method21 and UV-visible spectroscopy.19 In the Saville method, NO is displaced from the S-nitrosothiol group with Hg2+ and assayed by diazotization of sulfanilamide with subsequent coupling to the chromophore N-(1-naph)-ethylenediamine; absorbance at 540 nm is measured to determine S-NO concentration.21 In this method, the sample containing S-nitroso-AR545C was first mixed with 0.5% ammonium sulfamate in 0.4 N HCl for 1 minute to remove free NO2− or HNO2 from the sample. The S-nitroso content was calculated according to a standard curve constructed with 2.5 to 20 μmol/L NaNO2.
UV-visible absorbance spectroscopy.
Spectra were recorded at room temperature on a Cary 4E UV Visible spectrophotometer (Varian, Inc, Australia Pty, Ltd). Recorded spectra represent the absorbance of S-nitrosated AR545C compared with nonnitrosated AR545C.
Preparation of platelet-rich plasma (PRP).
After obtaining informed consent, 9 vol of peripheral blood from healthy adult volunteers was drawn into 1 vol of 0.129 mol/L trisodium citrate. After centrifugation (150g, 15 minutes, 22°C), the supernatant PRP was separated by removing the top two thirds of the plasma layer. Platelet-poor plasma (PPP) was prepared by centrifugation of PRP at 1,200g for 10 minutes and used immediately.
Preparation of gel-filtered platelets (GFP).
GFP were obtained by passing PRP over a Sepharose-2B column (Pharmacia Biotech) in Tyrode’s-HEPES-buffered saline as previously described.22 Platelet counts were determined using a Coulter Counter, model Technicon H2 (Bayer Diagnostics, Tarrytown, NY).
Cyclic nucleotide assay.
Measurements of cGMP were performed using an ELISA methodology using anti-cGMP antiserum (Amersham Pharmacia Biotech, Uppsala, Sweden). cGMP was extracted from platelets as previously described.23Briefly, 10% trichloroacetic acid (TCA) was added to PRP before (negative control) and 5 minutes after the addition of SNO-Glu (positive control), AR545C, or S-nitroso-AR545C. Samples were vortexed, placed on ice, and centrifuged (8,000g, 4 minutes) at room temperature. The supernatant was extracted 4 times with diethyl ether and assayed for cGMP as above. Acetylation of samples with acetic anhydride was used to increase the sensitivity of the cGMP assay.
Effect of AR545C or S-nitroso-AR545C on ristocetin-induced platelet agglutination.
Ristocetin-induced platelet agglutination was performed using lyophilized, formalin-fixed platelets (Helena Hemostasis, Beaumont, TX) as described previously.13 Various concentrations of either AR545C or S-nitroso-AR545C were incubated with the platelets (2 × 108 platelets/mL) for 10 minutes in a platelet PACK-4 aggregometer (Helena Laboratories, Beaumont, TX) at 37°C before the addition of PPP as a source of vWF and 1.5 mg/mL of ristocetin (Sigma Chemical Co, St Louis, MO). The extent of agglutination was monitored to quantify the agglutination response.
Effect of AR545C or S-nitroso-AR545C on platelet aggregation.
Platelet aggregation experiments were conducted using human PRP or GFP. Various concentrations of AR545C or S-nitroso-AR545C were incubated for 10 minutes with stirring at 37°C with PRP or GFP, and aggregation was induced with 5 μmol/L adenosine diphosphate (ADP). In some of the experiments, methylene blue (final concentration, 5 μmol/L) was added to the reactions containing S-nitroso-AR545C. Extent of aggregation was recorded in a PACK-4 aggregometer (Helena Laboratories).
Effect of AR545C or S-nitroso-AR545C on platelet interaction with extracellular matrix (ECM).
Platelet adhesion and aggregation on ECM was tested in the cone-plate viscometer analysis system (Galai, Beit Ha’emek, Israel), as described previously.24 In brief, 0.23 mL of citrated whole blood was placed on an ECM-covered plate under a shear rate of 1,300 s−1 for 2 minutes. The sample was then washed and stained with May-Grünwald-Giemsa stain. Platelet adhesion and aggregation were determined using an image analysis system. The degree of adhesion was assessed by calculating the percentage of total area covered by platelets, and quantified as the percentage of surface coverage (SC); the normal value of SC is 19% ± 5.9% at this shear rate. The extent of platelet aggregation on the surface was estimated by measuring the frequency distribution of platelet aggregates of different size and the average size of ECM-bound platelet aggregates; the latter parameter is expressed as average size (AS) of the aggregates with the normal value of AS being 47.5 ± 15.2 μm2 at this shear rate. To evaluate the effect of AR545C or S-nitroso-AR545C on the above-described parameters, the blood samples were preincubated at room temperature for 10 minutes with various concentrations of each of the peptides and the extent of adhesion and aggregation was recorded.
Effect of AR545C or S-nitroso-AR545C on botrocetin-induced aggregation of rabbit platelets ex vivo.
All animals used in this study were approved by the Institutional Animal Care and Use Committee at the Neufeld Cardiac Research Institute (Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel). New Zealand white female rabbits, each weighing 2.5 to 3.0 kg, were anesthetized with intravenous sodium pentobarbital (Nembutal, 30 mg/kg, followed by 10 mg at 30- to 60-minute intervals) administered through the marginal ear vein. The auricular artery was cannulated for blood sampling. The rabbits were injected intravenously with 1 mg/kg AR545C (n = 3) or 0.5 mg/kg S-nitroso-AR545C (n = 3). In each animal, 3 mL blood was drawn before, and at various time intervals after, administration of the peptides. Eight parts of rabbit blood were drawn into 1 part of 0.129 mol/L trisodium citrate and rabbit PRP prepared. The effect of bolus injection of either AR545C or S-nitroso-AR545C on rabbit botrocetin-induced platelet aggregation was evaluated in an aggregometer (Helena Laboratories) as in the ristocetin-induced agglutination assay: 1 μg/mL botrocetin (Sigma Chemical Co) was added to rabbit PRP containing 2 × 108 platelets/mL and 5 mmol/L EDTA at 37°C with stirring as described previously,13 and the extent of aggregation was then recorded. ADP-induced platelet aggregation experiments were performed using rabbit PRP and 5 μmol/L ADP, and the extent of aggregation was recorded in a PACK-4 aggregometer (Helena Laboratories) as described above.
Coagulation tests and bleeding time.
Prothrombin time (PT) and activated partial thromboplastin time (PTT) assays of rabbit plasma were performed using standard techniques. Innovin reagent (Dade, Miami, FL) was used for PT and Thrombosil I (Hemoliance Ortho Diagnostic Systems Inc, Raritan, NJ) or Actin FS (Dade) reagents were used for PTT. Bleeding time was measured as described previously.25 Briefly, a shaved rabbit ear was placed into a 37°C saline bath for 5 minutes. A full-thickness standardized incision was made with a Surgicutt pediatric device (International Technique Corp, Edison, NJ). The ear was then returned to the saline bath, observed until all blood flow ceased, and the time recorded.
Statistical analysis.
Statistical comparisons were performed using the 2-tailed Student’st-test for means and 2-way analysis of variance for dose-response. P values <.05 were considered significant. In the experiments with ECM comparison between AR545C and nitroso-AR535C, fragments for each dose were evaluated by 1-way analysis of variance (ANOVA). Ex vivo dose effect of the 2 fragments was evaluated by 2-way analysis of variance.
The synergism of the 2 agents was tested using isobole method for mutually exclusive and nonexclusive compounds as described previously.26 For mutually exclusive compounds the isobole (I) representing the effect of the combination is calculated using the following formula: I = (A/Ae) + (B/Be) = 1, whereAe and Be are the corresponding doses of the individual compounds producing the same quantitative effect, and A and B are the doses of the compounds used in combination showing the same effect. For mutually nonexclusive drugs, the equation is changed to: I = (A/Am) + (B/Bm) + (A/Am) (B/Bm) = 1, whereAm and Bm represent the concentrations yielding the median effect. I is less than 1 when the compounds interact to produce synergistic effect.
RESULTS
Chemical analysis of S-nitroso-AR545C.
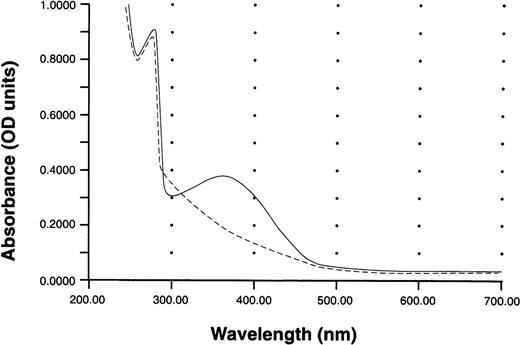
Analysis of the formation of S-nitroso-AR535C performed by the method of Saville21 showed that the S-nitrosothiol content was 0.96 mol of S-NO/1 mol of AR545C. UV-visible spectroscopy of S-nitroso-AR545C compared with AR545C is presented in Fig 1. The characteristic 350-nm absorption peak of the S-nitrosothiol group of nitrosated AR545C is illustrated, confirming that the peptide was nitrosated. The additional absorption peak at 280 nm represents the protein content of the S-nitroso-AR545C. Absorption spectra of AR545C shows only the 280-nm peak characteristic for the peptide absorbance.
UV-Vis absorption spectra of S-nitroso-AR545C and AR545C. Spectra were recorded against a blank containing phosphate-buffered saline. Solid line indicates S-nitroso-AR545C; dashed line indicates AR545C.
UV-Vis absorption spectra of S-nitroso-AR545C and AR545C. Spectra were recorded against a blank containing phosphate-buffered saline. Solid line indicates S-nitroso-AR545C; dashed line indicates AR545C.
The putative structure of the S-nitrosated AR545C monomer is presented in Fig 2. It seems reasonable that the apparently free cysteine at residue 545 will be nitrosated; however, additional cysteines of the molecule may be involved in the nitrosation process as well.
Structure of S-nitroso-AR545C. Note that the first 3 cysteines are depicted as disulfide-linked to their corresponding cysteines in a second monomer, only the partial structure of which is shown, as S-nitrosation was performed with the intact dimer.
Structure of S-nitroso-AR545C. Note that the first 3 cysteines are depicted as disulfide-linked to their corresponding cysteines in a second monomer, only the partial structure of which is shown, as S-nitrosation was performed with the intact dimer.
Effect of AR545C or S-nitroso-AR545C on ristocetin-induced platelet agglutination.
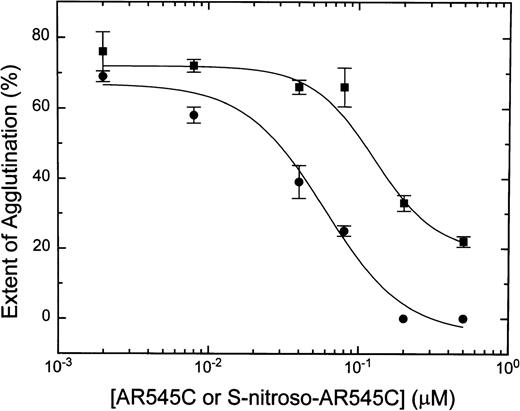
Preincubation of human platelets with either AR545C or S-nitroso-AR545C resulted in inhibition of ristocetin-induced platelet agglutination in a dose-dependent manner. As shown in Fig3, whereas 0.2 μmol/L AR545C decreased agglutination by 40%, the same concentration of S-nitroso-AR545C completely abolished it. The concentration of AR545C required to inhibit ristocetin-induced agglutination by 50% (IC50) was 0.1 ± 0.03 μmol/L, whereas the IC50 for S-nitroso-AR545C was 0.02 ± 0.006 μmol/L (P = .001). Thus, S-nitrosation of AR545C inhibited platelet agglutination approximately 5-fold.
Dose-dependent ristocetin-induced inhibition of human platelet agglutination by S-nitroso-AR545C and AR545C. Formalin-fixed platelets (2 × 108/mL) were incubated with either AR545C (▪) or S-nitroso-AR545C (•) for 10 minutes at various concentrations followed by addition of normal PPP and 1.5 mg/mL ristocetin.
Dose-dependent ristocetin-induced inhibition of human platelet agglutination by S-nitroso-AR545C and AR545C. Formalin-fixed platelets (2 × 108/mL) were incubated with either AR545C (▪) or S-nitroso-AR545C (•) for 10 minutes at various concentrations followed by addition of normal PPP and 1.5 mg/mL ristocetin.
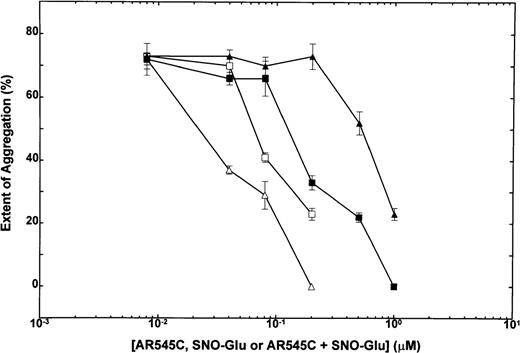
To test the effect of synergistic interactions between NO and AR545C, subthreshold concentrations of NO congener (SNO-Glu) and AR545C used alone or in combination with one another were added to PRP, and ristocetin-induced platelet aggregation was recorded in aggregometer. As shown in Fig 4, subthreshold concentrations of SNO-Glu or AR545C alone did not inhibit platelet aggregation. However, when the subthreshold concentrations of these agents were used in combination, inhibition of aggregation was obtained. Using an isobole method to establish synergy,26the isobole index for mutually exclusive and nonexclusive compounds was calculated for each combination. When combination of 0.08 μmol/L AR545C with 0.2 μmol/L or 0.5 μmol/L of SNO-Glu was analyzed, isobole indices of 0.57 and 0.81, respectively, were obtained. Similarly, combination of 0.2 μmol/L AR545C with 0.2 μmol/L or 0.5 μmol/L SNO-Glu revealed isobole indices of 0.41 and 0.53, respectively. Thus, the result of this analysis showed that all the isobole indices were below 1, indicating synergy.
Inhibition of human ristocetin-induced platelet aggregation: Dose-response to combination of SNO-Glu and AR545C. Human PRP (2.5 × 108/mL) was incubated with increasing concentrations of AR545C followed by addition of 1.5 mg/mL ristocetin in the absence (▪) or presence of 2 concentrations of SNO-Glu: (□), 0.2 μmol/L SNO-Glu; (▵), 0.5 μmol/L SNO-Glu. (▴), Increasing concentrations of SNO-Glu in the absence of AR545C. Each point represents mean ± SEM of 3 experiments.
Inhibition of human ristocetin-induced platelet aggregation: Dose-response to combination of SNO-Glu and AR545C. Human PRP (2.5 × 108/mL) was incubated with increasing concentrations of AR545C followed by addition of 1.5 mg/mL ristocetin in the absence (▪) or presence of 2 concentrations of SNO-Glu: (□), 0.2 μmol/L SNO-Glu; (▵), 0.5 μmol/L SNO-Glu. (▴), Increasing concentrations of SNO-Glu in the absence of AR545C. Each point represents mean ± SEM of 3 experiments.
Effect of AR545C or S-nitroso-AR545C on ADP-induced platelet aggregation.
The effects of AR545C or S-nitroso-AR545C were first studied in gel-filtered platelets and confirmed in PRP experiments. Results of aggregation experiments are provided in Fig5. AR545C did not affect platelet aggregation. In contrast, dose-dependent inhibition of ADP-induced platelet aggregation was observed with S-nitroso-AR545C, with an IC50 = 0.018 ± 0.002 μmol/L. Platelet aggregation was completely inhibited by S-nitroso-AR545C at concentrations above 0.5 μmol/L. This inhibition was reversed by addition of 5 μmol/L methylene blue, cGMP inhibitor (Fig 5, ▵). NO congener SNO-Glu inhibited ADP-induced platelet aggregation only at concentrations above 1 μmol/L (Fig 5, ▴).
Dose-dependent inhibition of human ADP-induced platelet aggregation by S-nitroso-AR545C. Human PRP (2.5 × 108/mL) were incubated with increasing concentrations of S-nitroso-AR545C (•), AR545C (▪), or SNO-Glu (▴), and aggregation was induced with 5 μmol/L ADP. (▵), Aggregations obtained after addition of methylene blue (final concentration, 5 μmol/L) to the mixture of PRP and S-nitroso-AR545C.
Dose-dependent inhibition of human ADP-induced platelet aggregation by S-nitroso-AR545C. Human PRP (2.5 × 108/mL) were incubated with increasing concentrations of S-nitroso-AR545C (•), AR545C (▪), or SNO-Glu (▴), and aggregation was induced with 5 μmol/L ADP. (▵), Aggregations obtained after addition of methylene blue (final concentration, 5 μmol/L) to the mixture of PRP and S-nitroso-AR545C.
Effect of AR545C or S-nitroso-AR545C on platelet cGMP.
Normal PRP was incubated with 1.5 μmol/L SNO-Glu (positive control), 0.5 μmol/L AR545C, or 0.5 μmol/L S-nitroso-AR545C, the platelet proteins were precipitated with TCA, and the protein-free supernatant was assayed for cGMP content as described in Materials and Methods. Compared with PRP alone (1.7 ± 0.4 pmol/109 platelets, negative control), addition of AR545C did not significantly alter basal cGMP level (2.4 ± 0.3 pmol/109 platelets) (P = .19) (Table 1). In contrast, there was a significant increase in cGMP levels with the addition of SNO-Glu or S-nitroso-AR545C (P < .0001 for each compound compared to AR545C) (Table 1). Thus, the increases in platelet cGMP levels after platelet exposure to SNO-Glu or S-nitroso-AR545C correlate with the inhibition of platelet aggregation by the effect of NO molecules provided by these compounds.
Platelet cGMP Content
| . | Control . | SNO-Glu (1.5 μmol/L) . | AR545C (0.5 μmol/L) . | S-nitroso-AR545C (0.5 μmol/L) . |
|---|---|---|---|---|
| pmol/109 platelets | 1.7 ± 0.4 | 16 ± 0.5 | 2.4 ± 0.2 | 8.3 ± 0.2*,† |
| . | Control . | SNO-Glu (1.5 μmol/L) . | AR545C (0.5 μmol/L) . | S-nitroso-AR545C (0.5 μmol/L) . |
|---|---|---|---|---|
| pmol/109 platelets | 1.7 ± 0.4 | 16 ± 0.5 | 2.4 ± 0.2 | 8.3 ± 0.2*,† |
Values represent mean ± SEM of 3 determinations, each performed in duplicate.
P < .0001 compared with control.
P < .0001 compared with AR545C.
Effect of AR545C or S-nitroso-AR545C on platelet interaction with ECM.
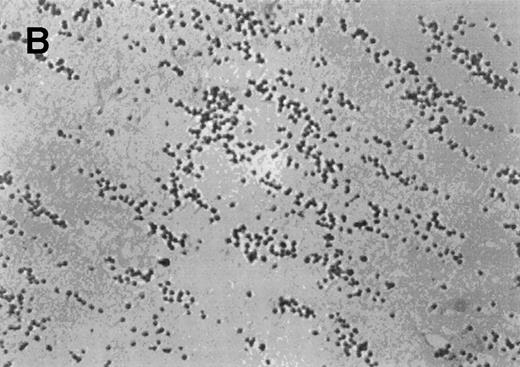
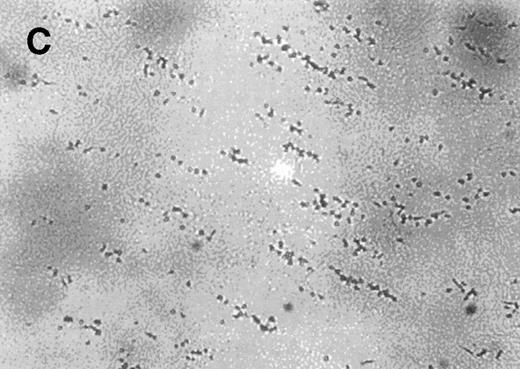
Normal whole blood tested in the cone-plate viscometer analysis system exhibited a typical adhesion and aggregation pattern with surface coverage of 28.4% ± 5.9% and an average size of the aggregates of 47.2 ± 15.2 μm2 (Table2). The normal blood sample was then preincubated for 10 minutes at room temperature with increasing concentrations of AR545C or S-nitroso-AR545C. As shown in Table 2, a dose-dependent inhibition of adhesion (represented by surface coverage) and aggregation (represented by average size of the aggregates) was observed with either fragment compared to control. However, the inhibitory effect of S-nitroso-AR545C was significantly more pronounced at concentration above 1.0 μmol/L. A representative picture shown in Fig 6 demonstrates that incubation of normal blood (Fig 6A) with 1.5 μmol/L AR545C resulted in a 74% decrease in adhesion and 64% inhibition in aggregate formation (Fig6B). Similar concentration of S-nitroso-AR545C resulted in complete inhibition of both adhesion and aggregate formation (Fig 6C). Analysis of the frequency distribution of aggregate sizes shows that S-nitroso-AR545C significantly shifted the size distribution leftward compared with control blood and with AR545C (Fig 6D through F). Thus, a more marked antiplatelet effect of S-nitroso-AR545C compared with AR545C was observed in these experiments, both with respect to inhibition of adhesion and aggregation under conditions of high shear.
Effect of AR545C or S-nitroso-AR545 on Platelet Interaction With ECM Under Flow Conditions
| . | Control . | AR545C . | S-nitroso-AR545C . | ||||
|---|---|---|---|---|---|---|---|
| 0.5 μmol/L . | 1.0 μmol/L . | 1.5 μmol/L . | 0.5 μmol/L . | 1.0 μmol/L . | 1.5 μmol/L . | ||
| Surface coverage (%) | 28.4 ± 5.9 | 12.9 ± 1.1 | 10.0 ± 1.4 | 7.5 ± 1.8 | 10.2 ± 0.9 | 5.5 ± 1.3* | 4.8 ± 1.1* |
| P = .09† | P = .05† | P = .004† | |||||
| Average size of aggregates (μm2) | 47.2 ± 15.2 | 36.2 ± 8.1 | 21.4 ± 5.7 | 17.1 ± 1.1 | 18.7 ± 2.2 | 13.0 ± 3.1* | 11.5 ± 0.7* |
| P = .07† | P = .2† | P = .003† | |||||
| . | Control . | AR545C . | S-nitroso-AR545C . | ||||
|---|---|---|---|---|---|---|---|
| 0.5 μmol/L . | 1.0 μmol/L . | 1.5 μmol/L . | 0.5 μmol/L . | 1.0 μmol/L . | 1.5 μmol/L . | ||
| Surface coverage (%) | 28.4 ± 5.9 | 12.9 ± 1.1 | 10.0 ± 1.4 | 7.5 ± 1.8 | 10.2 ± 0.9 | 5.5 ± 1.3* | 4.8 ± 1.1* |
| P = .09† | P = .05† | P = .004† | |||||
| Average size of aggregates (μm2) | 47.2 ± 15.2 | 36.2 ± 8.1 | 21.4 ± 5.7 | 17.1 ± 1.1 | 18.7 ± 2.2 | 13.0 ± 3.1* | 11.5 ± 0.7* |
| P = .07† | P = .2† | P = .003† | |||||
Results are expressed as mean ± SEM of 3 determinations.
P < .05 compared with control.
Compared with the same concentration of AR545C.
The effect of the S-nitroso-AR545C or AR545C on platelet interaction with ECM under flow conditions (shear rate of 1,300 s−1). Citrated whole blood (0.25 mL) was tested in the cone-plate viscometer analysis system after a 10-minute preincubation with either control buffer, 1.5 μmol/L AR545C, or 1.5 μmol/L S-nitroso-AR545C, and shown in representative field of the video screen (A, B, and C, respectively). The corresponding frequency histograms (D, E, and F, respectively) for the size distribution of the adhered platelets and platelet aggregates are also depicted.
The effect of the S-nitroso-AR545C or AR545C on platelet interaction with ECM under flow conditions (shear rate of 1,300 s−1). Citrated whole blood (0.25 mL) was tested in the cone-plate viscometer analysis system after a 10-minute preincubation with either control buffer, 1.5 μmol/L AR545C, or 1.5 μmol/L S-nitroso-AR545C, and shown in representative field of the video screen (A, B, and C, respectively). The corresponding frequency histograms (D, E, and F, respectively) for the size distribution of the adhered platelets and platelet aggregates are also depicted.
Effect of AR545C or S-nitroso-AR545C on botrocetin or ADP-induced aggregation of rabbit platelets ex vivo.
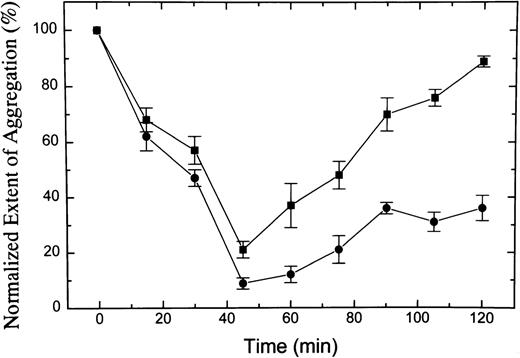
Rabbits were injected with 1 mg/kg AR545C or 0.5 mg/kg S-nitroso-AR545C (3 in each group), and the effect on ex vivo botrocetin or ADP-induced rabbit platelet aggregation was monitored, as shown in Figs 7 and 8. In animals injected with AR545C, the aggregation induced by botrocetin was significantly inhibited in a time-dependent manner, reaching a maximal effect 45 minutes after injection. The inhibitory effect was completely reversed by 2 hours after the injection. By contrast, in animals injected with S-nitroso-AR545C at one half the dose of AR545C, botrocetin-induced aggregation was completely abolished 45 minutes after the injection and the inhibitory effect persisted, showing 60% inhibition 2 hours after injection (Fig 7) (P < .0001 by 2-way ANOVA). AR545C had no effect on ADP-induced platelet aggregation (data not shown), but S-nitroso-AR545C exhibited time-dependent inhibition of platelet aggregation, reaching a maximal effect (almost 60% inhibition) at 1 hour after the injection and persisting for 1 additional hour (Fig 8).
Effect of S-nitroso-AR545C or AR545C on rabbit platelet aggregation ex vivo. Female rabbits were injected with 0.5 mg/kg of S-nitroso-AR545C or 1 mg/kg AR545C intravenously (3 in each group). Blood samples were drawn before and at different time intervals after the injection. Platelet aggregation was induced ex vivo with 1 μg/mL botrocetin added to PRP prepared from animals treated with AR545C (▪) or S-nitroso-AR545C (•) and plotted relative to the pretreatment values. Results are expressed as mean ± SEM values for n = 3 animals in each group.
Effect of S-nitroso-AR545C or AR545C on rabbit platelet aggregation ex vivo. Female rabbits were injected with 0.5 mg/kg of S-nitroso-AR545C or 1 mg/kg AR545C intravenously (3 in each group). Blood samples were drawn before and at different time intervals after the injection. Platelet aggregation was induced ex vivo with 1 μg/mL botrocetin added to PRP prepared from animals treated with AR545C (▪) or S-nitroso-AR545C (•) and plotted relative to the pretreatment values. Results are expressed as mean ± SEM values for n = 3 animals in each group.
Effect of S-nitroso-AR545C on rabbit platelet aggregation ex vivo. Female rabbits were injected with 0.5 mg/kg S-nitroso-AR545C and blood samples were collected before and at different time intervals after the injection. Platelet aggregation was induced in PRP with 5 μmol/L ADP. Results are expressed as mean ± SEM for n = 3 animals.
Effect of S-nitroso-AR545C on rabbit platelet aggregation ex vivo. Female rabbits were injected with 0.5 mg/kg S-nitroso-AR545C and blood samples were collected before and at different time intervals after the injection. Platelet aggregation was induced in PRP with 5 μmol/L ADP. Results are expressed as mean ± SEM for n = 3 animals.
Effect of AR545C or S-nitroso-AR545C on hemostatic parameters in rabbits.
The hemostatic parameters measured before and at different time intervals after the injections of AR545C or S-nitroso-AR545C are presented in Table 3. No change in the platelet count, PT, or PTT values was observed. However, prolongation of the bleeding time was noted in both groups. Twofold prolongation of bleeding time was observed 1 hour after injection in the group treated with AR545C that normalized after 2 hours. The prolongation of bleeding time was significantly greater in the group treated with S-nitroso-AR545C, increasing almost 8-fold compared with the pretreatment value. The bleeding time shortened by the end of the experiment but was still prolonged at 2 hours after injection.
Effect of Intravenous AR545C or S-nitroso-AR545 Injection on Hemostatic Parameters in Rabbits
| . | Bleeding Time (s) . | ||
|---|---|---|---|
| Time (min) | 0 | 60 | 120 |
| (normal range) | (58-73) | ||
| AR545C | 62 ± 2 | 130 ± 11 | 90 ± 20 |
| S-nitroso-AR545C | 63 ± 5 | 483 ± 173-150 | 260 ± 103-150 |
| PT (s) | |||
| Time (min) | 0 | 60 | 120 |
| (normal range) | (10.8-11.2) | ||
| AR545C | 11.0 ± 0.1 | 10.6 ± 0.2 | 10.8 ± 0.3 |
| S-nitroso-AR545C | 11.0 ± 0.2 | 10.8 ± 0.1 | 10.7 ± 0.4 |
| PTT (s) | |||
| Time (min) | 0 | 60 | 120 |
| AR545C | 11.8 ± 0.93-151 | 11.9 ± 0.63-151 | 12.8 ± 0.13-151 |
| (normal range) | (10.1-13.3)3-151 | ||
| S-nitroso-AR545C | 15.5 ± 0.53-152 | 16.5 ± 0.23-152 | 15.2 ± 0.33-152 |
| (normal range) | (14.6-16.3)3-152 | ||
| Platelets (×109/L) | |||
| Time (min) | 0 | 60 | 120 |
| (normal range) | (355-468) | ||
| AR545C | 379 ± 22 | 403 ± 10 | 435 ± 12 |
| S-nitroso-AR545C | 446 ± 11 | 400 ± 18 | 399 ± 17 |
| . | Bleeding Time (s) . | ||
|---|---|---|---|
| Time (min) | 0 | 60 | 120 |
| (normal range) | (58-73) | ||
| AR545C | 62 ± 2 | 130 ± 11 | 90 ± 20 |
| S-nitroso-AR545C | 63 ± 5 | 483 ± 173-150 | 260 ± 103-150 |
| PT (s) | |||
| Time (min) | 0 | 60 | 120 |
| (normal range) | (10.8-11.2) | ||
| AR545C | 11.0 ± 0.1 | 10.6 ± 0.2 | 10.8 ± 0.3 |
| S-nitroso-AR545C | 11.0 ± 0.2 | 10.8 ± 0.1 | 10.7 ± 0.4 |
| PTT (s) | |||
| Time (min) | 0 | 60 | 120 |
| AR545C | 11.8 ± 0.93-151 | 11.9 ± 0.63-151 | 12.8 ± 0.13-151 |
| (normal range) | (10.1-13.3)3-151 | ||
| S-nitroso-AR545C | 15.5 ± 0.53-152 | 16.5 ± 0.23-152 | 15.2 ± 0.33-152 |
| (normal range) | (14.6-16.3)3-152 | ||
| Platelets (×109/L) | |||
| Time (min) | 0 | 60 | 120 |
| (normal range) | (355-468) | ||
| AR545C | 379 ± 22 | 403 ± 10 | 435 ± 12 |
| S-nitroso-AR545C | 446 ± 11 | 400 ± 18 | 399 ± 17 |
Results are mean ± SEM values of 3 animals.
P < .001 compared with control (t = 0) or AR545C.
Measured with Thrombosil I or
Actin FS as described in Materials and Methods.
DISCUSSION
Several agents have been shown to block the interaction between vWF and its platelet receptor GPIb.8-12 Some of these compounds, such as monoclonal antibodies,5,9 synthetic peptides,27 and recombinant vWF fragments,12,13have been tested in various models of experimental thrombosis. In addition, it has previously been shown that NO or its S-nitroso-congeners exhibit antiplatelet properties by inhibiting platelet aggregation14,15 and adhesion.16
In our previous study,13 we showed that recombinant vWF fragment AR545C inhibited ristocetin- and botrocetin-induced platelet aggregation of human and rabbit platelets, respectively. AR545C also enhanced the thrombolytic effect of recombinant tissue-type plasminogen activator in a rabbit thrombosis model. In the present study, we evaluated the antiplatelet properties of the S-nitroso-derivative of AR545C, which should combine both antiadhesive actions with antiaggregating actions in the same molecule and target the delivery of NO to the site of vascular injury.
Our data show that S-nitroso-AR545C potentiated the antiplatelet effects of AR545C in the 3 systems studied: ristocetin- or botrocetin-induced platelet aggregation in vitro or ex vivo, ADP-induced platelet aggregation, and interaction of platelets with ECM under conditions of high shear. The superior antiplatelet effect of S-nitroso-AR545C can be attributed to the independent action of 2 moieties of the molecule: blocking platelet GPIb receptor through the AR545C moiety and elevating platelet cGMP through the -SNO moiety. Moreover, the data showed that these 2 effects are synergistic.
S-nitroso-AR545C inhibited ristocetin-induced platelet agglutination in a dose-dependent manner with an average IC50 = 0.02 ± 0.006 μmol/L; this concentration is one fifth of that for AR545C.13 Similarly, in the cone-plate viscometer analysis system, a dose-dependent inhibition of adhesion and aggregation on ECM was more notable at each concentration of S-nitroso-AR545C compared with AR545C, reaching a statistically significant difference at concentrations greater than 1.0 μmol/L (P < .05 by ANOVA). Moreover, 1.0 μmol/L of S-nitroso-AR545C resulted in complete inhibition of aggregate formation, whereas the same concentration of R545C had only a slight effect on aggregate formation.
As expected, AR545C showed no effect on ADP-induced platelet aggregation, because its effects are a consequence of competition with vWF for binding to GPIb. By contrast, a significant dose-dependent inhibition of ADP-induced platelet aggregation was observed with S-nitroso-AR545C. S-nitroso-AR545C abolished platelet aggregation completely at concentrations greater than 0.5 μmol/L, and the IC50 was 0.018 ± 0.002 μmol/L. Finally, the ex vivo efficacy of S-nitroso-AR545C was examined in rabbits. At concentrations one half that of AR545C, S-nitroso-AR545C was able to inhibit significantly botrocetin-induced platelet aggregation to a much greater extent and for a longer period of time than AR545C itself, probably reflecting the synergistic effect of NO with the vWF fragment. In addition, ADP-induced platelet aggregation was inhibited by S-nitroso-AR545C by almost 60%. Taken together, the potencies of the S-nitroso-AR545C in vitro and ex vivo appear to be significantly greater (3- to 10-fold and 4-fold, respectively) than those of AR545C.
The doses of AR545C or S-nitroso-AR545C used in this study had no effect on platelet count or plasma coagulation tests (PT, PTT); however, bleeding time was significantly prolonged, especially when rabbits were injected with the S-nitroso-AR545C, apparently because of its dual inhibitory effects on platelet function. Despite the prolongation in bleeding time, no bleeding was observed during the experiment or at necropsy of the animals. The prolongation of bleeding time observed in our study contrasts with the lack of effect on bleeding time reported by Azzam et al12 after injection of VCL in guinea pigs. This difference may stem from differences in the model or the compound used.
Most drugs interfering with platelet function exhibit a single antiplatelet action, such as inhibition of adhesion or of aggregation. In contrast, S-nitroso-AR545C manifests 2 independent but synergistic antiplatelet activities combined in the same molecule. This compound likely has 2 pharmacologically relevant mechanisms in target cells: it may act through interference of platelet binding to vWF and through NO-mediated intracellular soluble guanylyl-cyclase activation. The data from this study show that S-nitroso-AR545C exhibits significantly more potent antiplatelet activity than AR545C, and this effect seems to be attributed to its independent actions via GPIb- and NO-dependent pathways.
ACKNOWLEDGMENT
We thank Stephanie Tribuna and Ann Ward Scribner for excellent technical assistance. We are indebted to David Castel, DVM, for his assistance in experiments with rabbits.
Supported in part by National Institutes of Health (NIH) Grants No. HL53919, HL48743, HL53993, and by a Merit Review Award from the US Veterans Administration.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Aida Inbal, MD, Institute of Thrombosis & Hemostasis, Sheba Medical Center, Tel-Hashomer 52621, Israel.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal