Abstract
The high-affinity receptor for interleukin-3 (IL-3) is a complex of the IL-3–binding subunit (IL-3) and a larger β chain—βc, or, in the mouse, βc or its close relative βIL-3. There is evidence that the critical event that initiates signaling is not the approximation of the cytoplasmic domains of IL-3 and βIL-3, but is, rather, the formation of a β-β homodimer. Many of these studies involved the analyses of receptor chimeras where the cytoplasmic domains were derived from IL-3, βc or βIL-3, and the extracellular domains were derived from other cytokine receptors, such as the erythropoietin receptor (EpoR). However, evidence that the EpoR may also associate with other receptors clouds the interpretation of these experiments. Therefore, we reevaluated the structure of the functional IL-3R using chimeric receptors with extracellular domains derived not from members of the cytokine-receptor family, but from CD8 or CD16. We show, by expression of these chimeras in Ba/F3 or CTLL-2 cells, that mitogenic signals were only generated by heterodimerization of the cytoplasmic domains of IL-3 and βIL-3. Homodimers of either IL-3 or βIL-3, alone or in combination, were nonfunctional. Furthermore, the ability of heterodimers to stimulate mitogenesis correlated with their ability to induce tyrosine phosphorylation of JAK-2. These data suggest that the physiological activation of the IL-3R involves the generation of simple heterodimers of IL-3 and βIL-3.
THE CYTOKINE-RECEPTOR FAMILY can be divided into those which are homodimers of a single subunit (eg, receptors for growth hormone and erythropoietin [Epo]), and those which are heterodimers or higher-order oligomers made up of 2 or more different subunits (eg, interleukin-4 [IL-4] and IL-6).1,2 The receptors for IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) belong to the latter group. Each of these cytokines binds specifically with low or moderate affinity to a unique α chain termed, respectively, IL-3Rα, IL-5Rα, and GM-CSFRα. These complexes of the ligand and its respective α chain each bind with high affinity to a common beta chain (βc), which is required for activation of intracellular signals.3 In the mouse, while IL-3Rα, IL-5Rα, and GM-CSFRα and their ligands interact with the murine homolog of βc, complexes of IL-3 and IL-3Rα can also form a functional IL-3 receptor by interacting with an IL-3–specific β chain (βIL-3) that is the product of a relatively recent duplication of βc.4 5
Activation of JAKs follows rapidly after binding of cytokines to their receptors, and is essential for the initiation of intracellular signaling.6-8 Moreover, in some artificial systems in which the cytoplasmic domain of a transmembrane protein was replaced by JAK2, the induction of dimerization of the membrane-associated JAK was sufficient to deliver a proliferative signal.9,10 These observations suggest that ligand-induced dimerization or oligomerization of cytokine receptor subunits triggers signaling by bringing together JAK molecules associated with the receptor subunits, and allowing trans-phosphorylation and activation of JAKs.11
Experiments on several of the multi-subunit cytokine receptors have suggested that dimerization of their respective long chains which, like βc, have multiple potential docking sites for signaling molecules, is sufficient for the initiation of a proliferative signal. Thus, it has been shown that homodimerization of gp130,12,13 hβc,14-17 the IL-2Rβ,18 or the IL-4Rα19,20 are sufficient for the initiation of biochemical and biological events. These observations are consistent with the paradigm of the IL-6R where the function of the ligand-binding (α) chain is merely to induce dimerization of the β chain, which mediates all the intracellular signaling functions.21 However, because these observations have all involved overexpression of the receptor chains in cell lines, it is by no means clear that they accurately reflect physiological signaling processes. The situation in normal cells with smaller numbers of receptors and different levels of signal transduction molecules may be significantly different. In the case of IL-3 (or the closely related IL-5 or GM-CSF), it is unclear whether signaling is mediated by simple heterodimerization of the αIL-3 and βIL-3chains, implying an active role for αIL-3, or alternatively, by analogy with the receptor for IL-6, is mediated by homodimerization of the β chain. Truncations of the relatively short cytoplasmic domains of the IL-3Rα, IL-5Rα, and GM-CSFRα do not affect ligand binding, but, in contrast with results with the IL-6Rα chain, abolish signaling17,22-24 (and P.C. Orban, K.B. Leslie, unpublished data). Further, Stomski et al25 reported finding disulfide-linked heterodimers of human αIL-3 and βc after binding of IL-3. However, there is other evidence that βc homodimers might be both necessary and sufficient for transduction of the mitogenic signal of IL-3. The βc exhibits extensive homology with the Epo receptor (EpoR), which forms a homodimer in the active state.26,27 Observations that a chimera composed of the extracellular and transmembrane portion of the EpoR and the cytoplasmic portion of the mouse βIL-3 chain (Epo/βIL-3) conferred responsiveness to Epo in murine IL-3–dependent cell lines28-31 have been interpreted as showing that dimerization of the β chain was sufficient for signaling. Other evidence has come from the demonstration of the existence of “preformed” dimers of the βc in cell lines32 as well as in primary leukemic cells.33
However, a simple homodimer of the EpoR may not constitute a functional receptor, and may include additional subunits such as the steel ligand factor receptor (c-kit) and βc.34-37 Indeed, when cells are stimulated with Epo, βc undergoes tyrosine phosphorylation and is coimmunoprecipitated with the EpoR.34,35 Moreover, when expressed in the T-cell line CTLL-2, which does not express βc, neither EpoR nor a chimeric receptor consisting of the extracellular and transmembrane portion of the EpoR together with the cytoplasmic portion of βIL-3 supported ligand-induced proliferation.16,28 38 In contrast, however, when the αIL-3 and βIL-3 were transfected into CTLL-2 cells, they were capable of transducing a ligand-induced mitogenic signal.
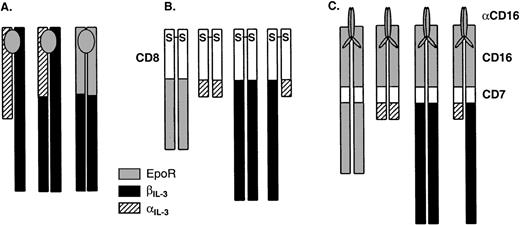
To avoid the confounding possibility that the extracellular component of a cytokine receptor chimera could qualitatively influence biochemical signaling, perhaps by recruiting additional receptor subunits to the receptor complex,34,36,37 39 we generated chimeras in which the cytoplasmic domains of mouse αIL-3and βIL-3 were fused to extracellular domains derived from proteins that were unrelated to cytokine receptors.
MATERIALS AND METHODS
Plasmids.
DNA manipulations were performed by standard methods. The plasmids pSut1 and pCD226 containing the mouse αIL-3 and mouse βIL-3 cDNAs, respectively, were the kind gift of Atsushi Miyajima (University of Tokyo, Tokyo, Japan). The plasmid pCd16.tm7.syk was the kind gift of Brian Seed (Massachusetts General Hospital, Boston, MA), and the plasmid pMV7F1.2, containing the human CD8α cDNA, was the kind gift of Dan Littman (The Skirball Institute, New York, NY). The plasmids pXM and pXMR129C, containing cDNAs encoding the wild-type and Cys-Cys mutated forms of the mouse EpoR, were the kind gift of Harvey Lodish (The Whitehead Institute, Cambridge, MA). All chimeric receptors were cloned into the expression vector pME18Δ, which was derived from pSut1 by removing the inserted cDNA. Polymerase-mediated amplification with Vent polymerase (New England Biolabs, Mississauga, Ontario, Canada) was used to incorporate restriction enzyme sites for subcloning and shuttling components of receptor chimeras. The anti-sense oligo GGA ATT CTC AGG CGT CTG TCA CAG GCG TCA CCTC was used to amplify and add anEcoRI site to the 3′ end of the cytoplasmic domain of αIL-3. Similarly, the antisense oligo GGA ATT CAG ACT CAG CAC ACC TTC CCA GAC TGG CTAT was used to amplify and introduce anEcoRI site the 3′ end of βIL-3, and the anti-sense oligo TGA ATT CGT CCT AGG AGC AGG CCA CA to amplify and introduce an EcoRI site to the 3′ end of the EpoR.
For construction of the EpoR/βIL-3 chimera, the extracellular domain of the mouse EpoR was amplified using the sense oligo TGA ATT CAT CAT GGA CAA ACT CAG GGT G and the anti-sense oligo CCC GAT ATC CAG GTC GCT AGC GGT CAG, adding an EcoRV site to the 3′ end. The complementary portion (transmembrane and cytoplasmic domain) of the βIL-3 sequence was amplified using the sense oligo CCC GAT ATC GAC TGG GTG ATG CCC ACG adding anEcoRV site, and the antisense oligo as above. After subcloning and sequencing, chimeric cDNAs were formed by digestion withEcoRV and ligation of the 2 subclones.
For construction of the αIL-3/βIL-3chimera, the extracellular portion of the αIL-3 cDNA was amplified using the sense oligo CCC GAA TTC ATG GCC GCC AAC CTG TGG CTC, which adds an EcoRI site for subcloning, and the anti-sense oligo CCC GAT ATC CTT CAC AGG CAT CAC CTC, which adds anEcoRV site to the 3′ end. The amplified fragment was subcloned, digested with EcoRV, and ligated to the complementary (transmembrane and cytoplasmic) portions of the βIL-3 cDNA (see above).
To generate CD8 chimeras, sense oligos were designed to addEcoRV sites immediately 5′ of the transmembrane portion of the cDNAs of αIL-3 (CCC GAT ATC AAG ACA GCC TTG GTG ACT TCA GTG), and the EpoR (CCC GAT ATC CCT CTC ATC TTG ACG CTG) and the βIL-3 (see above). Amplified fragments were cloned and sequenced before ligation to the extracellular portion of the CD8α cDNA, which has an endogenous EcoRV site immediately 5′ of the transmembrane domain sequence.
CD16/7 chimeras were generated by replacing the portion encoding syk in the plasmid pCd16.tm7.syk with the appropriate cytokine receptor cytoplasmic domain. The αIL-3 cytoplasmic domain was amplified using a sense oligo CAC CAT GGG ATC CAC GCG TAA GTC GCT GCT CTA CCG CCTG to introduce an MluI site for fusion to the CD16/7 extracellular and transmembrane domains. Similarly, the βIL-3 cytoplasmic domain was amplified using the sense oligo GGA ATT CAC GCG TGT TTA TGG GTA CAG GACA to introduce anMluI site. To create the CD16/7/EpoR chimera a self-complementary oligo pair with SalI and BglII compatible ends (sense: TCG ACG CGT TCC CAC CGC CGG ACT CTG CAG CAG AAG ATC, and anti-sense: GAT CTT CTG CTG CAG AGT CCG GCG GTG GGA ACG CGT CGA) was introduced into a Bluescript subclone (Stratagene, La Jolla, CA) containing the cytoplasmic portion of the EpoR, which was digested with XhoI and BglII, so as to introduce theMluI site to the 5′ end of the EpoR cytoplasmic domains.
Cell culture.
Ba/F3 cells and transfected clones were maintained in RPMI 1640 medium (Stem Cell Technologies, Vancouver, BC, Canada), supplemented with 10% fetal calf serum (FCS) (Intergen, Purchase, NY), 50 μmol/L 2-mercaptoethanol, and 2% (vol/vol) 10X concentrated WEHI 3B-conditioned medium as a source of IL-3. CTLL-2 cells and transfected clones were maintained in Dulbecco’s modified Eagle’s medium (Stem Cell Technologies), supplemented with 10% FCS, 50 μmol/L 2-mercaptoethanol, and 3% (vol/vol) IL-2–containing conditioned media derived from AgX063 cells transfected with the murine IL-2 cDNA.40
Transfections and screening of protein expression.
Ba/F3 and CTLL-2 cells were washed twice in phosphate-buffered saline (PBS) and 1 × 107 cells were resuspended in 800 μL of transfection buffer (25 mmol/L HEPES, 0.75 mmol/L Na2HPO4, 140 mmol/L KCl, 5 mmol/L NaCl, 2 mmol/L MgCl2, 0.5% Ficoll 400 [Pharmacia Biotech, Uppsala, Sweden]). For each transfection, cells were mixed with 1 μg of linearized pPGKNeo or pPGKPuro together with 15 μg of the linearized expression vector of interest, and subjected to electroporation using a Bio-Rad gene pulser (Bio-Rad, Mississauga, Ontario, Canada) at 960 μF and 270 V. After transfection, the cells were cultured in the appropriate media for 48 hours and then transferred to selection media in 96-well plates. Individual clones of neomycin- or puromycin-resistant clones were tested for expression of the appropriate receptor chain by flow cytometry. Either OKT8 (American Type Culture Collection [ATCC], Rockville, MD) or Leu2A (Becton Dickinson, Mississauga, Ontario, Canada) were used to detect cell-surface expression of human CD8α. 3G8 (Cedar Lane, Hornby, Ontario, Canada) was used to detect cell-surface expression of human CD16. 9D3 (Alice Mui, DNAX, Palo Alto, CA) was used to detect expression of βIL-3. Rabbit polyclonal antiserum raised against the extracellular domain of IL-3Rα was used to detect expression of the IL-3Rα.41
Cell proliferation assays.
Proliferation of cells was assessed by either [3H]-thymidine incorporation into de novo synthesized DNA, or 3-[Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) incorporation. Cells were washed 3 times with HEPES-buffered saline solution (HBSS) supplemented with 2% (vol/vol) FCS. Cells were plated at 1,000 cells/well in a Terasaki microtiter plate (Disposable Products, Adelaide, SA, Australia) for [3H]-thymidine incorporation or at 10,000 cells/well in a 96-well plate for MTT assays. Chemically synthesized IL-3 (Ian Clark-Lewis, The Biomedical Research Centre, Vancouver, BC, Canada) was used to assess maximal proliferation of Ba/F3 cells. Recombinant murine IL-2 (Genzyme, Cambridge, MA) was used to assess maximal proliferation of CTLL-2 cells. Recombinant human Epo was a kind gift from Dr Ross Hardison (Pennsylvania State University, State College, PA). For use in bioassays, the 3G8 (anti-CD16) antibodies were dialyzed against RPMI to remove azide and were added in serial dilutions to cells. Cells were incubated at 37°C for 40 hours, and then pulsed for a further 8 hours with either [3H]-thymidine at a final concentration of 15 μCi/mL, or 0.75 μg/mL MTT (Sigma, Oakville, Ontario, Canada). In the [3H]-thymidine uptake assays, cells were harvested onto filters and counted in a scintillation counter. In MTT assays, the reaction product was solubilized by addition of 100 μL of 10% sodium dodecyl sulfate (SDS), 50% dimethyl formamide (DMF), pH 4.5, and the plates were read at OD550 using a BioTek plate reader (BioTek, Winooski, VT).
Cell-surface biotinylation.
Cells were washed twice in HBSS and resuspended at 2 × 107 cells/mL in HBSS supplemented with 0.8 mg/mL Sulfo-NHS-Biotin (Pierce, Rockford, IL). After 15 minutes on ice, the cells were washed 3 times with 50 mL of HBSS supplemented with 10% FCS to quench the biotinylation reaction, followed by 1 wash in HBSS. The cells were lysed in lysis buffer (20 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 2 mmol/L EDTA, 1 μmol/L phenylmethylsulfonyl fluoride, 2 μg/mL leupeptin, 0.7 μg/mL pepstatin, 10 μg/mL aprotinin, 10 μg/mL soybean trypsin inhibitor). Before specific immunoprecipitation, lysates were “precleared” by absorption with protein A-Sepharose (Pharmacia, Uppsala, Sweden). Chimeric receptors with the extracellular domain of human CD8α were immunoprecipitated with OKT8, followed by adsorption onto protein A-Sepharose. Beads were washed 3 times with lysis buffer, boiled in SDS sample buffer, and the eluate was subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. The surface-biotinylated material precipitated with anti-CD8 was detected by immunoblotting with streptavidin-conjugated horseradish peroxidase (HRP; Calbiochem, La Jolla, CA).
Cell stimulations.
To analyze the biochemical effects of using anti-CD16 antibodies to generate homodimers or heterodimers of the cytoplasmic domains of the IL-3Rα or β, the respective transfectants were placed in RPMI, 10% FCS, 0.2% WEHI-3B for 16 hours, washed 3 times with serum-free RPMI supplemented with 20 mmol/L HEPES (SFM), and incubated in SFM at 1 × 107 cells/mL at 37°C for 1 hour. The cells were then left untreated as a control or stimulated with synthetic IL-3 (10 μg/mL) for 10 minutes, or anti-CD16 as follows. The monoclonal anti-CD16 (3G8, 1 μg/mL) was allowed to bind to the cells on ice for 15 minutes, after which cells were washed once with SFM, and resuspended in 1 mL of SFM containing 1 μg/mL secondary goat α-mouse Ig (DAKO A/S, Glostrup, Denmark). The cells were then transferred to 37°C for 10 minutes and lysed in lysis buffer supplemented with phosphatase inhibitors (1 mmol/L sodium orthovanadate, 1 mmol/L sodium molybdate, 10 mmol/L sodium fluoride). Lysates were then incubated with antisera against JAK-2 (Upstate Biotechnology Inc, Lake Placid, NY), followed by adsorption onto protein A-Sepharose. Beads were washed and boiled as above, and the eluates were subjected to SDS-PAGE and immunoblotting with 4G10 (Upstate Biotechnology Inc) to detect tyrosine-phosphorylated JAK-2 and, after stripping, with antisera to JAK-2 to assess equivalency of loading.
RESULTS
Epo-mediated homodimerization of βIL-3 leads to mitogenesis in Ba/F3 cells.
Given that the discrepancies in the literature about the function of homodimers of the cytoplasmic domain of βIL-3 may relate to differences between clones of cell lines, we first repeated the experiments of expressing full-length EpoR or the EpoR/βIL-3 chimera using our line of IL-3–dependent Ba/F3 cells and IL-2–dependent CTLL-2 cells (see Fig 1 for schematic diagrams of all chimeric receptors used in this work). Like others28,31 we observed that the EpoR/βIL-3 chimera was capable of transducing a proliferative response to Epo in Ba/F3 cells (Fig 2). We noted, however, that untransfected Ba/F3 cells displayed some small but reproducible response to higher concentrations of Epo, consistent with the observations of Damen et al,42 and suggesting that Ba/F3 cells express some endogenous EpoR. Expression of the R129C mutant of the EpoR in Ba/F3 cells, which is expressed as a homodimer by virtue of a Cys-Cys bond in the extracellular region,26 also conferred factor independence to BaF/3 cells (data not shown), as reported by others.16,26 In contrast, when these various constructs were expressed in IL-2–dependent CTLL-2 cells, which do not express any of the receptor chains known to be involved in Epo or IL-3 signaling, we failed to obtain clones that responded to Epo; nor, in the case of the R129C mutant, did we obtain factor-independent CTLL-2 transfectants (data not shown). These differences between Ba/F3 and CTLL-2 cells are consistent with previous observations.16,28 38
Schematic representation of the chimeric receptors used in this study. (A) Receptors that are inducibly dimerized after addition of a cytokine. The transmembrane (TM) segment is derived from the same cytokine receptor as the cytoplasmic domain in each case. (B) Receptors that exist as constitutive dimers by virtue of the hCD8 extracellular domain, which forms a disulfide-linked dimer. The transmembrane portion is derived from the same cytokine receptor as the cytoplasmic domain in each case. (C) Receptors that are inducibly dimerized after addition of an antibody against CD16. The transmembrane portion is derived from CD7.
Schematic representation of the chimeric receptors used in this study. (A) Receptors that are inducibly dimerized after addition of a cytokine. The transmembrane (TM) segment is derived from the same cytokine receptor as the cytoplasmic domain in each case. (B) Receptors that exist as constitutive dimers by virtue of the hCD8 extracellular domain, which forms a disulfide-linked dimer. The transmembrane portion is derived from the same cytokine receptor as the cytoplasmic domain in each case. (C) Receptors that are inducibly dimerized after addition of an antibody against CD16. The transmembrane portion is derived from CD7.
Ba/F3 cells transfected with the full-length EpoR or the EpoR/βIL3 chimera proliferate in response to Epo. Cells were washed free of IL-3 and incubated with indicated concentrations of Epo. After 40 hours, the cells were pulsed with MTT for a further 4 hours, and MTT reduction was measured as optical density at 550 nmol/L (OD550) of solubilized cells. Data are plotted as the percentages of the maximal response of cells cultured in IL-3. Error bars represent SEM of triplicate samples. Similar results were obtained with several individual clones.
Ba/F3 cells transfected with the full-length EpoR or the EpoR/βIL3 chimera proliferate in response to Epo. Cells were washed free of IL-3 and incubated with indicated concentrations of Epo. After 40 hours, the cells were pulsed with MTT for a further 4 hours, and MTT reduction was measured as optical density at 550 nmol/L (OD550) of solubilized cells. Data are plotted as the percentages of the maximal response of cells cultured in IL-3. Error bars represent SEM of triplicate samples. Similar results were obtained with several individual clones.
Homodimers of βIL-3 fail to stimulate mitogenesis in CTLL-2 cells.
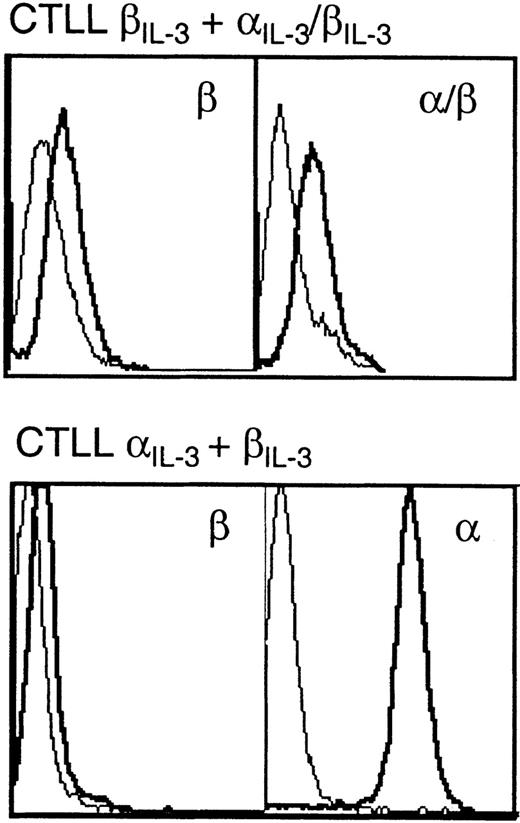
One approach to determining whether the generation of simple dimers of the βIL-3 is sufficient to generate signals is to investigate the activity of dimers of the cytoplasmic portion of βIL-3 formed when IL-3 brings together βIL-3 and a chimera of the extracellular portion of αIL-3 and the cytoplasmic domain of βIL-3. Clones of CTLL-2 cells were derived that expressed either full-length αIL-3 alone, full-length βIL-3 alone, both full-length chains together, or the full-length βIL-3 and the chimeric αIL-3/βIL-3 together. Expression of the exogenous receptor chains was assessed by flow cytometry (Fig 3). As shown in Table 1, CTLL-2 clones that expressed both full-length αIL-3 and βIL-3 proliferated in response to IL-3, whereas clones that expressed either chain alone, the αIL-3/βIL-3 chimera alone, or the full-length βIL-3 together with the αIL-3/βIL-3 chimera, showed no growth in response to IL-3. These data suggested that simple dimers of the cytoplasmic domain of βIL-3 were not sufficient for the generation of mitogenic signals, at least in these cells.
Flow cytometric analyses of expression of chimeric receptors. CTLL-2 cells expressing both βIL-3 and IL-3/βIL-3 (top), or IL-3and βIL-3 (bottom) were stained with antibodies against the extracellular domain of IL-3 or βIL-3. Thin lines represent results with cells treated with fluorescein isothiocyanate (FITC)-coupled secondary antibody alone; thick lines represent results with cells incubated with anti-receptor antibodies and then FITC-coupled secondary antibody. CTLL-2 cells bearing IL-3 alone or βIL-3 alone displayed levels of expression similar to those shown for CTLL IL-3 + βIL-3.
Flow cytometric analyses of expression of chimeric receptors. CTLL-2 cells expressing both βIL-3 and IL-3/βIL-3 (top), or IL-3and βIL-3 (bottom) were stained with antibodies against the extracellular domain of IL-3 or βIL-3. Thin lines represent results with cells treated with fluorescein isothiocyanate (FITC)-coupled secondary antibody alone; thick lines represent results with cells incubated with anti-receptor antibodies and then FITC-coupled secondary antibody. CTLL-2 cells bearing IL-3 alone or βIL-3 alone displayed levels of expression similar to those shown for CTLL IL-3 + βIL-3.
CTLL-2 Cells Expressing βIL-3 and the IL-3/βIL-3 Chimera Fail to Survive in the Presence of IL-3
| Cell Type . | Viability in IL-2 . | Viability in IL-3 . |
|---|---|---|
| CTLL-2 | + | − |
| αIL-3 | + | − |
| βIL-3 | + | − |
| αIL-3 + βIL-3 | + | + |
| βIL-3 + αIL-3/βIL-3 | + | − |
| Cell Type . | Viability in IL-2 . | Viability in IL-3 . |
|---|---|---|
| CTLL-2 | + | − |
| αIL-3 | + | − |
| βIL-3 | + | − |
| αIL-3 + βIL-3 | + | + |
| βIL-3 + αIL-3/βIL-3 | + | − |
Cells were cultured in IL-2 or IL-3 in triplicate for 4 days and were scored for viability by light microscopy. + represents >99% viable cells and − represents the absence of any viable cells. In no cases were any intermediate responses observed.
Constitutive heterodimers of αIL-3 and βIL-3, but not homodimers of βIL-3, stimulate mitogenesis in Ba/F3 and CTLL-2 cells.
Given that the use of extracellular domains of growth factor receptors in such chimeric proteins might be difficult to interpret because of the possibility of the recruitment of cytokine-receptor family subunits,34,36,37,39,43 we next generated chimeras in which the extracellular regions of an unrelated cell-surface molecule, human CD8α,44,45 were fused with the transmembrane and cytoplasmic portions of the respective IL-3 and Epo receptor chains (see Fig 1). Human CD8α has been shown to spontaneously form disulfide-linked dimers at the cell surface.45 Chimeric receptor chains—CD8/EpoR, CD8/αIL-3, and CD8/βIL-3—were each expressed in Ba/F3 cells, as confirmed by flow cytometric analysis (Fig4A). In addition, we derived Ba/F3 clones that coexpressed both the CD8/αIL-3 and CD8/βIL-3 chimeras. To confirm expression of both of these chimeras in the same clone, we biotinylated the cell surface, lysed the cells, and immunoprecipitated the chimeric proteins with an antibody against CD8. The presence of the biotinylated chimeras in the anti-CD8 immunoprecipitates was assessed by SDS-PAGE and blotting with streptavidin-conjugated peroxidase. As shown in Fig 4B, biotinylated molecules corresponding to the predicted sizes of both chimeras were present.
Heterodimerization of the cytoplasmic domain of βIL-3 and IL-3 is required to induce mitogenesis in Ba/F3 cells. (A) Flow cytometric analyses confirming expression of the CD8/IL-3R chimeric receptors in Ba/F3 cells. Thin lines represent controls treated with FITC-coupled secondary antibody alone; thick lines represent results with cells stained with CD8 and FITC-coupled secondary antibody. The bottom righthand panel represents the CD8 signal in cells expressing both CD8/IL-3 and CD8/βIL-3. (B) Immunoprecipitation of biotinylated cell-surface molecules to confirm the expression of CD8/IL-3 and CD8/βIL-3 in doubly transfected cells. Lane 1, control Ba/F3; lane 2, Ba/F3 transfected with CD8/βIL-3; lane 3, Ba/F3 transfected with CD8/IL-3; lane 4, Ba/F3 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation by transfectants incubated in the absence of IL-3. Cells were cultured with or without IL-3 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-3. Error bars represent the SEM of triplicate samples.
Heterodimerization of the cytoplasmic domain of βIL-3 and IL-3 is required to induce mitogenesis in Ba/F3 cells. (A) Flow cytometric analyses confirming expression of the CD8/IL-3R chimeric receptors in Ba/F3 cells. Thin lines represent controls treated with FITC-coupled secondary antibody alone; thick lines represent results with cells stained with CD8 and FITC-coupled secondary antibody. The bottom righthand panel represents the CD8 signal in cells expressing both CD8/IL-3 and CD8/βIL-3. (B) Immunoprecipitation of biotinylated cell-surface molecules to confirm the expression of CD8/IL-3 and CD8/βIL-3 in doubly transfected cells. Lane 1, control Ba/F3; lane 2, Ba/F3 transfected with CD8/βIL-3; lane 3, Ba/F3 transfected with CD8/IL-3; lane 4, Ba/F3 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation by transfectants incubated in the absence of IL-3. Cells were cultured with or without IL-3 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-3. Error bars represent the SEM of triplicate samples.
Ba/F3 cells expressing the CD8/EpoR chimera grew independently of exogenous IL-3 or Epo (Fig 4C). This was despite the low levels of expression of the chimeric CD8/EpoR protein as judged by our inability to detect its expression by flow cytometry (Fig 4A). This observation suggests that the CD8 domains formed disulfide-linked dimers as predicted, and that the generation of simple dimers of the EpoR cytoplasmic domain in these cells was sufficient for their proliferation and survival. In contrast, neither the Ba/F3 cells expressing CD8/αIL-3 alone, nor those expressing CD8/βIL-3 alone, were capable of factor-independent growth, though these clones continued to respond normally to IL-3. On the other hand, all clones that expressed both the CD8/αIL-3 and CD8/βIL-3 chimeras showed factor-independent growth. Clones expressing both chimeric receptors were obtained by transfecting a series of clones that expressed one chimeric chain, and were thus not factor-independent with the second CD8 chimera. Other clones were generated by cotransfection of parental cells with both chimeric chains simultaneously. All clones expressing one CD8 chimera could be rendered factor-independent by expression of the alternate CD8 chimera. This result also showed that, while the clones expressing the individual CD8 chimeras remained factor-dependent, they were nonetheless fully capable of factor-independent growth when transfected with both chimeras.
These experiments with CD8/αIL-3 and CD8/βIL-3 chimeras were repeated in CTLL-2 cells with identical results (Fig 5A through C). These data clearly indicate that simple dimerization of βIL-3, at least when expressed at the levels we observed, is not sufficient to promote survival or mitogenesis in either cell line. In contrast, both cell types reproducibly exhibited factor-independent growth when the cytoplasmic domains of αIL-3 and βIL-3 were heterodimerized. These results suggest that heterodimers of αIL3 and βIL3 cytoplasmic domains are significantly more efficient in initiating signaling than dimers of βIL-3.
Heterodimerization of the cytoplasmic domain of βIL3 and IL3 induces mitogenesis in CTLL-2 cells. (A) Flow cytometric analysis confirming expression of the hCD8 chimeric receptors. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD8 and FITC-coupled secondary antibody. (B) Immunoprecipitation of surface biotinylated proteins confirming expression of both CD8/ and CD8/β in doubly transfected cells as described in the legend to Fig 4B. Lane 1, parental CTLL-2; lane 2, CTLL-2 transfected with CD8/βIL-3; lane 3, CTLL-2 transfected with CD8/IL-3; lane 4, CTLL-2 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation of transfectants in the absence of IL-2. Cells were cultured with or without IL-2 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-2. Error bars represent the SEM of triplicate samples.
Heterodimerization of the cytoplasmic domain of βIL3 and IL3 induces mitogenesis in CTLL-2 cells. (A) Flow cytometric analysis confirming expression of the hCD8 chimeric receptors. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD8 and FITC-coupled secondary antibody. (B) Immunoprecipitation of surface biotinylated proteins confirming expression of both CD8/ and CD8/β in doubly transfected cells as described in the legend to Fig 4B. Lane 1, parental CTLL-2; lane 2, CTLL-2 transfected with CD8/βIL-3; lane 3, CTLL-2 transfected with CD8/IL-3; lane 4, CTLL-2 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation of transfectants in the absence of IL-2. Cells were cultured with or without IL-2 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-2. Error bars represent the SEM of triplicate samples.
Inducible heterodimers of αIL-3 and βIL-3, but not homodimers of βIL-3, stimulate mitogenesis in Ba/F3 cells.
These data showed a requirement for heterodimerization of the cytoplasmic domains of αIL-3 and βIL-3 and contradicted a body of evidence suggesting that the dimerization of the cytoplasmic domain of βIL-3 is sufficient for mitogenic signaling.28-31 Therefore, we investigated this question using another system that involved the use of chimeras consisting of the extracellular portion of the human CD16 molecule, the transmembrane segment of human CD7, and the cytoplasmic portions of either the EpoR, αIL-3, or βIL-3. In this system, the chimeric proteins are expressed as monomers but dimerization can be induced using a monoclonal antibody (MoAb) (3G8) specific for the extracellular domain of CD16.10 46 These chimeras were expressed alone or together in Ba/F3 cells, and expression was confirmed by flow cytometry (Fig 6A). Proliferative responsiveness to the MoAb to hCD16 (3G8) was assessed by [3H]-thymidine incorporation (Fig 6C and D). Cells expressing only CD16/7/αIL3 or only CD16/7/βIL3 failed to respond to any concentration of the dimerizing antibody. However, in keeping with the results obtained with the CD8 chimeras, those cells that expressed both CD16/7/αIL-3 and CD16/7/βIL-3 exhibited proliferative responses to the dimerizing antibody.
Induction of heterodimerization of the cytoplasmic domains of βIL3 and IL3 is mitogenic in Ba/F3 cells. Flow cytometric analysis confirming expression of (A) the CD16/7 chimeric receptors. The bottom righthand panel represents the CD16 signal obtained in cells expressing both CD16/7/ and CD16/7/β. (B) The expression of CD16/7/ and CD8/β. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD16 and FITC-coupled secondary antibody. (C and D) [3H]-thymidine incorporation of Ba/F3 cells transfected with the indicated CD16/7 chimeras in response to addition of the CD16 antibody, 3G8. Cells were cultured with increasing amounts of 3G8 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent maximal [3H]-thymidine incorporation observed in IL-3.
Induction of heterodimerization of the cytoplasmic domains of βIL3 and IL3 is mitogenic in Ba/F3 cells. Flow cytometric analysis confirming expression of (A) the CD16/7 chimeric receptors. The bottom righthand panel represents the CD16 signal obtained in cells expressing both CD16/7/ and CD16/7/β. (B) The expression of CD16/7/ and CD8/β. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD16 and FITC-coupled secondary antibody. (C and D) [3H]-thymidine incorporation of Ba/F3 cells transfected with the indicated CD16/7 chimeras in response to addition of the CD16 antibody, 3G8. Cells were cultured with increasing amounts of 3G8 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent maximal [3H]-thymidine incorporation observed in IL-3.
Coincident formation of homodimers of cytoplasmic domains of αIL-3 and βIL-3is not mitogenic.
It was conceivable that the mitogenic responses to the anti-CD16 antibody, observed in cells expressing both CD8/αIL-3 and CD8/βIL-3, reflected a requirement for signals generated by the coincident formation of independent homodimers of αIL-3 or βIL-3 cytoplasmic domains. To investigate this possibility, we derived clones of Ba/F3 cells expressing both CD8/αIL-3 and CD16/7/βIL-3. Expression of both receptor chimeras was confirmed by flow cytometry (Fig 6B). In these cells, CD8/αIL-3 was constitutively dimerized and anti-CD16 antibodies were used to generate homodimers of the cytoplasmic domain of βIL-3. These clones were derived from the same CD8/αIL-3-expressing clones that generated factor-independent subclones when CD8/βIL-3 was also expressed (Fig 4C). However, addition of anti-CD16 did not induce proliferation at any dose tested (Fig 6D).
Mitogenic effects of heterodimers of the cytoplasmic domains of αIL-3 and βIL-3correlate with tyrosine phosphorylation of JAK-2.
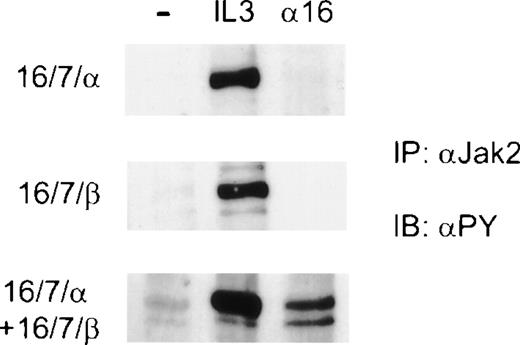
Transmission of a proliferative signal through the IL-3 receptor is known to involve the tyrosine phosphorylation and activation of JAK-2.6 11 Therefore, we assessed phosphorylation of JAK-2 after antibody-induced dimerization of chimeric receptors in Ba/F3 cells expressing various combinations of CD16/7 chimeras. Clones expressing a single CD16/7 chimera (αIL-3 or βIL-3) were transfected with the alternate CD16/7 chimeras. Dimerization of CD16/7/αIL-3 alone or CD16/7/βIL-3 alone failed to stimulate phosphorylation of JAK-2 (Fig 7). However, when dimerization of CD16 extracellular domains was induced in cells expressing both CD16/7/αIL-3 and CD16/7/βIL-3, phosphorylation of JAK-2 was clearly evident (Fig 7). These data indicated that homodimerization of the cytoplasmic domains of either αIL-3 or βIL-3 alone was ineffective in activating JAK-2, and that only the generation of heterodimers of the cytoplasmic domains of αIL-3 and βIL-3resulted in tyrosine phosphorylation of JAK-2. Furthermore, activation of JAK-2 in cells expressing the various chimeras correlated with their ability to proliferate.
Antibody-induced heterodimerization of the cytoplasmic domains of IL-3 and βIL-3 induces tyrosine phosphorylation of JAK-2. Factor- and serum-starved cells were incubated on ice with anti-CD16 antibodies for 10 minutes (16). Cells were washed once with RPMI, and after the addition of secondary goatmouse, the cells were stimulated at 37°C for 10 minutes. Alternatively, the cells were stimulated with IL-3 for 10 minutes or left unstimulated as a control (−). Cell lysates were subjected to immunoprecipitation (IP) with antibodies against JAK-2, and the eluates were resolved by SDS-PAGE. The membranes were immunoblotted (IB) with 4G10 (PY) to detect phosphorylation on tyrosine.
Antibody-induced heterodimerization of the cytoplasmic domains of IL-3 and βIL-3 induces tyrosine phosphorylation of JAK-2. Factor- and serum-starved cells were incubated on ice with anti-CD16 antibodies for 10 minutes (16). Cells were washed once with RPMI, and after the addition of secondary goatmouse, the cells were stimulated at 37°C for 10 minutes. Alternatively, the cells were stimulated with IL-3 for 10 minutes or left unstimulated as a control (−). Cell lysates were subjected to immunoprecipitation (IP) with antibodies against JAK-2, and the eluates were resolved by SDS-PAGE. The membranes were immunoblotted (IB) with 4G10 (PY) to detect phosphorylation on tyrosine.
DISCUSSION
We have shown that the transduction of mitogenic signals from the murine IL-3 receptor requires the heterodimerization of the cytoplasmic domains of the 2 receptor components αIL-3 and βIL-3. Our experiments were performed in murine cells well characterized with respect to endogenous cytokine receptor expression, and used murine cytokine receptor components to minimize the likelihood of confounding molecular interactions. We obtained qualitatively identical results using two different means of generating dimers of the αIL-3 and βIL-3 cytoplasmic domains (Figs 4 and 6). In the case of CD8, dimerization was achieved via a single constitutive disulfide bond and, in the case of CD16, through addition of a specific MoAb. The use in these chimeras of the extracellular domains of CD8 or CD16, rather than those of a cytokine receptor family member such as the EpoR, obviated the possibility of recruitment of additional cytokine receptor subunits, which clouded the interpretation of previously reported experiments with chimeric receptors in which the extracellular domains were derived from subunits of cytokine receptors. Significantly, the same results were obtained from experiments performed in IL-3–dependent Ba/F3 cells and in IL-2–dependent CTLL-2 cells (Figs 4 and 5).
We could find no circumstance in which homodimerization of either the cytoplasmic domains of αIL-3 or βIL-3resulted in growth. Thus, neither constitutive dimerization of the βIL-3 cytoplasmic region in a CD8/βIL-3chimera nor the anti-CD16–induced dimerization of CD16/7/βIL-3 supported proliferation of Ba/F3 or CTLL-2 cells (Figs 4C, 5C, and 6D). All of these cells expressed chimeras that were potentially functional, because derivatives of these clones transfected with respective CD8/αIL-3 or CD16/7/αIL-3 chimeras proliferated spontaneously or in response to anti-CD16 antibody. Likewise, in CTLL-2 cells, the generation of βIL-3 cytoplasmic domain homodimers by addition of IL-3 to cells expressing βIL-3 and an αIL-3/βIL-3 chimera did not result in IL-3–dependent proliferation (Table 1), whereas all clones we obtained that expressed both βIL-3 and full-length αIL-3 proliferated in the presence of IL-3. In all cases, heterodimerization of the αIL-3 and βIL-3cytoplasmic domains resulted in proliferation (Figs 4C, 5C, and 6D). Moreover, the simultaneous presence in the same cells of homodimers of the αIL-3and βIL-3 cytoplasmic domains was insufficient for mitogenesis (Fig 6D).
Our findings that coexpression of CD8/αIL-3 and CD8/βIL-3 results in mitogenesis suggest not only that dimerization of the α and β cytoplasmic domains is required, but also that simple heterodimers rather than higher-order oligomeric complexes may be sufficient. Furthermore, dimerization of CD16 receptor chimeras was induced by addition of an MoAb that can only bind to 1 epitope on each CD16 monomer. Thus, each divalent antibody can only link 2 CD16/cytoplasmic domain receptor chimeras. Our data suggest that all that is required of the extracellular domains for mitogenic signaling is that they generate a simple heterodimer. However, we cannot exclude the possibility that the cytoplasmic domains of α and β heterodimers drive the formation of higher-order multimers.
Importantly, we found that the ability of the various clones to grow correlated with the tyrosine phosphorylation of JAK-2, the key step in the biochemical events that lead from cytokine receptor activation to proliferative responses.6,7 47 Thus, cell lines expressing either 16/7/αIL-3 or 16/7/βIL-3 showed no detectable JAK-2 phosphorylation when antibody-mediated homodimerization was induced by anti-CD16. In contrast, when the complementary CD16/7 chimera was also expressed in these lines, addition of anti-CD16 resulted in phosphorylation of JAK-2 (Fig 7).
Our data on the failure of homodimerized βIL-3 to result in growth (Table 1, Figs 4C, 5C, and 6D) or biochemical changes associated with growth (Fig 7) contrast with a series of studies reporting that the induction of homodimerization of the cytoplasmic domain of βc or βIL-3 resulted in proliferation.15,16,28 However, in all these cases the extracellular domains of the chimeric receptors were derived from cytokine receptors. Our results and those of others35 show that the EpoR, or chimeras that include the extracellular domain of EpoR, and the cytoplasmic domain of βc or βIL-3 function in Ba/F3 cells (Fig 2) but not in CTLL-2 cells (data not shown). Together, these data indicate a requirement for an additional molecule, absent in CTLL-2 cells, for the function of the EpoR. Thus, in Ba/F3 cells, we observed that the EpoR/βIL-3 chimera gave a mitogenic signal in the presence of Epo, or when the chimera was constitutively dimerized by virtue of an EpoR point mutation (Fig 2 and data not shown).28,31 However, in CTLL-2 cells neither chimera was functional (data not shown), suggesting that Ba/F3 cells express an additional protein that was required for the function of chimeras with EpoR extracellular domains and is not present in CTLL-2 cells. Therefore, our results support the notion that chimeras of the cytoplasmic domain of βIL-3 and the extracellular portion of the EpoR are functional only if additional membrane-associated molecules are recruited. As discussed, there is evidence that the activated EpoR interacts functionally with other receptors not present in CTLL-2 cells, including c-kit and βc.34-37
There is one report of homodimerization of βc leading to mitogenesis in the likely absence of the recruitment of other subunits of the cytokine receptor family. Patel et al48 generated chimeras of the cytoplasmic domains of βc or GM-CSFRα using as the extracellular domains, the leucine-zipper portions from c-fos and c-jun. In keeping with our observations, they showed that heterodimers of the GM-CSFRα and βc cytoplasmic domains delivered the strongest mitogenic signal. However, in contrast to our results, they also observed a weak mitogenic signal from homodimers of the β chain, and a still weaker, but detectable, signal from α chain homodimers.
What, aside from recruitment of other cytokine receptor subunits, might explain the differences between our data, which imply that heterodimers of the cytoplasmic domains of αIL-3 and βIL-3 are necessary for transduction of proliferative signals, and those that indicate that homodimers of βc, βIL-3,28-31 and even αIL-3,48 can generate signals? The most parsimonious explanation would be that at sufficient levels of expression, in cells that also express at sufficient levels key intracellular signaling proteins, homodimers might generate mitogenic signals, but at lower levels of efficiency than α/β heterodimers. In the simplest model, JAK-2 is associated to the same degree with both αIL-3 and βIL-3, and homodimerization of either αIL-3 or βIL-3 could then lead to apposition and trans-activation of JAK-2. The generation of mitogenic signals from the homodimers might depend on unphysiologically high levels of expression present in transfected cell lines, and at physiological levels of expression, only the generation of simple heterodimers of αIL-3 and βIL-3 activate signal transduction. It could additionally be postulated that steric considerations, or differences in the affinity of JAK-2 for the different dimers could mean that the relative efficiency of JAK-2 activation decreases in the hierarchy of αIL-3βIL-3 >> βIL-3βIL-3 > αIL-3αIL-3.
Several lines of evidence support an active role for the cytoplasmic domain of αIL-3 in signal transduction. First, deletion or mutation of the short cytoplasmic tails of the IL-3R, IL-5R, and GM-CSFRα chains abrogates signal transduction triggered by the receptor ligand17,22-24,49 (and P.C. Orban, K.B. Leslie, unpublished data). In support of a direct role of these α subunits in recruitment and activation of JAK-2, Ogata et al50 have shown that JAK-2 can bind to IL-5Rα. Likewise, we have shown that the cytoplasmic domain of IL-5Rα binds directly to JAK-2 (P. Orchansky, J.W. Schrader, manuscript in preparation). Together with evidence that βc also binds JAK-2,51 these data fit well with the model that both αIL-3 and βIL-3 contribute to recruitment of 2 molecules of JAK-2 to a heterodimeric complex. The notion of a hierarchy of efficiency of activation of JAK-2 of αIL-3βIL-3 >> βIL-3βIL-3 > αIL-3αIL-3 correlates well with the elegant experiments of Patel et al48 with overexpressed proteins. We contend, however, that at the levels of receptor expression found in primary cells, typically of the order of a few hundred high-affinity receptors per cell, only αIL-3βIL-3heterodimers generated by the presence of physiological ligand would deliver sufficient signal to trigger mitogenesis.
ACKNOWLEDGMENT
We thank Sheila Ayres for technical assistance. We thank Harvey Lodish for the EpoR cDNA, Atsushi Miyajima for the IL-3Rα and βIL-3 cDNAs, Dan Littman for the CD8α cDNA, Brian Seed for the CD16/7 cDNA, Alice Mui for 9D3, and Ross Hardison for Epo.
P.C.O. and M.K.L. contributed equally to this work.
Supported by the Medical Research Council of Canada and the Protein Engineering Network of Centres of Excellence.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John W. Schrader, MB, PhD, The Biomedical Research Centre, 2222 Health Sciences Mall, University of British Columbia, Vancouver, BC V6T 1Z3, Canada; e-mail:john@brc.ubc.ca.


![Fig. 4. Heterodimerization of the cytoplasmic domain of βIL-3 and IL-3 is required to induce mitogenesis in Ba/F3 cells. (A) Flow cytometric analyses confirming expression of the CD8/IL-3R chimeric receptors in Ba/F3 cells. Thin lines represent controls treated with FITC-coupled secondary antibody alone; thick lines represent results with cells stained with CD8 and FITC-coupled secondary antibody. The bottom righthand panel represents the CD8 signal in cells expressing both CD8/IL-3 and CD8/βIL-3. (B) Immunoprecipitation of biotinylated cell-surface molecules to confirm the expression of CD8/IL-3 and CD8/βIL-3 in doubly transfected cells. Lane 1, control Ba/F3; lane 2, Ba/F3 transfected with CD8/βIL-3; lane 3, Ba/F3 transfected with CD8/IL-3; lane 4, Ba/F3 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation by transfectants incubated in the absence of IL-3. Cells were cultured with or without IL-3 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-3. Error bars represent the SEM of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722004ax.jpeg?Expires=1767725394&Signature=R0nwdBgOVJktOWbbZVYcgggv-3elZnCfnS6zv0HvncQPZBYjHO1JYAn9CzaI4LL5pX8YwnCsqevi4qdcl5DKlInE~JYSMYQgdSPZ3N2vRqLjLp0nyp~c6OCUwwWyY6ZoWYRRIfXbv-PBEf8YKuTXwurpgAsv9xknCdjsZeo~yb2XHIeCIVEiFoEQDRhvgVDprUaswKMRjO6Pp04v4YGkwbCSgdD5itmXvD600TlnIKQUsugcrAbmQigMkL6AdyAv11WMDB4TmJ5COI3uh9XIDOzk5Zx~OVABWw9FVRrkDOgxuUJR-YMAvxMRakm7s1sxU2xEqyGgFg4SFIPmNzvdJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Heterodimerization of the cytoplasmic domain of βIL-3 and IL-3 is required to induce mitogenesis in Ba/F3 cells. (A) Flow cytometric analyses confirming expression of the CD8/IL-3R chimeric receptors in Ba/F3 cells. Thin lines represent controls treated with FITC-coupled secondary antibody alone; thick lines represent results with cells stained with CD8 and FITC-coupled secondary antibody. The bottom righthand panel represents the CD8 signal in cells expressing both CD8/IL-3 and CD8/βIL-3. (B) Immunoprecipitation of biotinylated cell-surface molecules to confirm the expression of CD8/IL-3 and CD8/βIL-3 in doubly transfected cells. Lane 1, control Ba/F3; lane 2, Ba/F3 transfected with CD8/βIL-3; lane 3, Ba/F3 transfected with CD8/IL-3; lane 4, Ba/F3 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation by transfectants incubated in the absence of IL-3. Cells were cultured with or without IL-3 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-3. Error bars represent the SEM of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722004bw.jpeg?Expires=1767725394&Signature=J3d8sXVI09iOMd9RH~vFGnYh5Lk6jlfV0V~8L450HZxsqhRrnFRa9VFv1~R5oAj0Wx3ktfVV9UeDBAyZwsjgCAi96dEuPf-k0SLzOjnBiygHaSJ8I6VqR-GAYaZ91xLeEKjHF40UgkDc~kAC6xcP9n1LnCcijs-xePDjgURjeKbcZUMi9j2T~RCbiZcHSvRdH1y1208XXwXxWnG6An9jniDMc0-B0HN6z1ek0GeQPXEQ2sEvY22IC1gAlVYIhFMXvH4LsBliJdD6YW1JdJ0j1qimDAgEfIzokDp~BTkuDfDx0RUXBoGtonxseJrkgZohXVqv28r0QCMumeymxT4yNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Heterodimerization of the cytoplasmic domain of βIL-3 and IL-3 is required to induce mitogenesis in Ba/F3 cells. (A) Flow cytometric analyses confirming expression of the CD8/IL-3R chimeric receptors in Ba/F3 cells. Thin lines represent controls treated with FITC-coupled secondary antibody alone; thick lines represent results with cells stained with CD8 and FITC-coupled secondary antibody. The bottom righthand panel represents the CD8 signal in cells expressing both CD8/IL-3 and CD8/βIL-3. (B) Immunoprecipitation of biotinylated cell-surface molecules to confirm the expression of CD8/IL-3 and CD8/βIL-3 in doubly transfected cells. Lane 1, control Ba/F3; lane 2, Ba/F3 transfected with CD8/βIL-3; lane 3, Ba/F3 transfected with CD8/IL-3; lane 4, Ba/F3 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation by transfectants incubated in the absence of IL-3. Cells were cultured with or without IL-3 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-3. Error bars represent the SEM of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722004cx.jpeg?Expires=1767725394&Signature=jZBBK6N1zQjdVTdT9nWXcA~gdrBzJYTiWDi-dKbbb2pwqYbU5WZpVRWih5FXPTYqnItkQ4BOuuYcRj9fk9SfIlZfbQIMy6cOU4ptqjArfNYgvziP-ZD5s0r0Vkq4FuElCSN6SGGWPMoRoIt838q4F6lxwUdXIIvhi1tUB2NkurApp~dZXHkMA8lBBAf64Q4tQadvXU1EARse7CoSNG~RYYGg6mzuy~WZTvK2xbTdpuNjs-QNWGDq8iIViOftJst60-ydF96zmnExJba-HY1SlwtViLJuYhU8YplzKY0r6Y~8f3sW67AwwC3LXctZFn11RHDJ~pRZOFgPnn2OugeUUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Heterodimerization of the cytoplasmic domain of βIL3 and IL3 induces mitogenesis in CTLL-2 cells. (A) Flow cytometric analysis confirming expression of the hCD8 chimeric receptors. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD8 and FITC-coupled secondary antibody. (B) Immunoprecipitation of surface biotinylated proteins confirming expression of both CD8/ and CD8/β in doubly transfected cells as described in the legend to Fig 4B. Lane 1, parental CTLL-2; lane 2, CTLL-2 transfected with CD8/βIL-3; lane 3, CTLL-2 transfected with CD8/IL-3; lane 4, CTLL-2 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation of transfectants in the absence of IL-2. Cells were cultured with or without IL-2 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-2. Error bars represent the SEM of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722005ax.jpeg?Expires=1767725394&Signature=O8JSoX2gFf0C8U5RF9dC7UNbtUrxC1pTP4vBM1PSU2uQeQMgDVbOzvDUhu8AWRZMFJ9rGdPmg93M54LQdGyKoq3N61JnPOfMBp30vFalMgJSbAjoBEUF9APlt7tbZMoI7nSyqx7-FlfDUgoQKLDaVzdt-AVVwxS365dsjz1SuLpdisaxVusQlDSla99SfWk5G3zowX5HNo6yCY4tpdbvl0PS4CsS7xqPmohb24xM3HfSaFRfrmib6DQTcA1sV-SIVLM48Bt~EImyqt51W0VM3ubjTpgiC~VAUJVH-3GcHpJ1GYO0nXWD~pDSXYGuEpETjTvvOGT-XJzNExldwnmXoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Heterodimerization of the cytoplasmic domain of βIL3 and IL3 induces mitogenesis in CTLL-2 cells. (A) Flow cytometric analysis confirming expression of the hCD8 chimeric receptors. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD8 and FITC-coupled secondary antibody. (B) Immunoprecipitation of surface biotinylated proteins confirming expression of both CD8/ and CD8/β in doubly transfected cells as described in the legend to Fig 4B. Lane 1, parental CTLL-2; lane 2, CTLL-2 transfected with CD8/βIL-3; lane 3, CTLL-2 transfected with CD8/IL-3; lane 4, CTLL-2 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation of transfectants in the absence of IL-2. Cells were cultured with or without IL-2 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-2. Error bars represent the SEM of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722005bw.jpeg?Expires=1767725394&Signature=BqmNQfEe4N01rsR-dq~2luYgHF9RU1PRRLfIOCPvaMEYvPXASucf6mq~m44OHwW9G0XyYn6shHSj3aRq9IkP-XZZP4sC7NPXE~y8SGQrNMBZWkMchuICzCcA9IDJZU395Bz-LswhDMSu02ldRmgA5Tf0EsUnJV5yx5rCQVyq7g9hpSqQKvRP8For3IU7ifIokl5wCIFyuU4uZy5rDwoXB~O0zjk5-E42Fto2yniJdTA57t4Mwmp0Ab-50JQIyCVygSKBqIg6oxPSTv6IqNc9DLOGw5i4WdKmqrBooNfjqHgO~~Ecmou3bq7PfmZ7OHRv1b~rR--n0OxFNEmz1IVDwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Heterodimerization of the cytoplasmic domain of βIL3 and IL3 induces mitogenesis in CTLL-2 cells. (A) Flow cytometric analysis confirming expression of the hCD8 chimeric receptors. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD8 and FITC-coupled secondary antibody. (B) Immunoprecipitation of surface biotinylated proteins confirming expression of both CD8/ and CD8/β in doubly transfected cells as described in the legend to Fig 4B. Lane 1, parental CTLL-2; lane 2, CTLL-2 transfected with CD8/βIL-3; lane 3, CTLL-2 transfected with CD8/IL-3; lane 4, CTLL-2 transfected with CD8/IL-3 and CD8/βIL-3. (C) [3H]-thymidine incorporation of transfectants in the absence of IL-2. Cells were cultured with or without IL-2 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent of maximal incorporation observed in IL-2. Error bars represent the SEM of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722005cx.jpeg?Expires=1767725394&Signature=4ABBbobm3mEAvcV4QqtAQ-2sH8xWFeLEFFBQfRSZYPHI2XOwoRWOunPVTNAUs~ND2UcTJbhPCPipodq2iGn49fGR-O4qZuqHDP0cdkzFNrOWgiL2I16~97tQ3GTk8sddCDGxk2oWJWXaBthg-sAHYX3dm2WbLUmasiK05YtUwfPxFkdJH1Z4XxbgSS585a4nadXM4JajaOBhM9e~q~bGx2wYlVqdtl6pyDDZlVWtg7Zo1oupN4uycb5AwiJrvzKp0LDwqMuYe6fu3xK5DRvseXb6K7nczYXYHxJvz0rioZ77Tj1FhvLTrdbByYMu6OcdEFfc9~c3VV65E~VveNttCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Induction of heterodimerization of the cytoplasmic domains of βIL3 and IL3 is mitogenic in Ba/F3 cells. Flow cytometric analysis confirming expression of (A) the CD16/7 chimeric receptors. The bottom righthand panel represents the CD16 signal obtained in cells expressing both CD16/7/ and CD16/7/β. (B) The expression of CD16/7/ and CD8/β. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD16 and FITC-coupled secondary antibody. (C and D) [3H]-thymidine incorporation of Ba/F3 cells transfected with the indicated CD16/7 chimeras in response to addition of the CD16 antibody, 3G8. Cells were cultured with increasing amounts of 3G8 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent maximal [3H]-thymidine incorporation observed in IL-3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722006ax.jpeg?Expires=1767725394&Signature=ZGJviunY7~4iwXbjVv72dFZzBobo6dpPsksJJ-V85X7Qawh7kP6iBGNTqIViMoyfX9qCWU32MGXOkGmoezUUhiUdhWVssFjxC4fu4eLRKOC1dWQxmkbIAg12ioyP9GAOwCEHPA0GcMMyaBcLuF5vq845DknrjRAgZwJx0X7bI9qWbMsLUBz94c5IqqGM5e3oMZNns7Cwg9COV6xExCZe6MKVkw1sNsRAqmrxEpfWv27MldCa73PrwtosuJpRUjBxhyJImM0Z0b5bIC0Sd3cNkl5JgT~e2BK3q5Jb8LBcondlblGuyl1yXmJDdT5Z7eau60ag28wzHxPKk16hZqQ54Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Induction of heterodimerization of the cytoplasmic domains of βIL3 and IL3 is mitogenic in Ba/F3 cells. Flow cytometric analysis confirming expression of (A) the CD16/7 chimeric receptors. The bottom righthand panel represents the CD16 signal obtained in cells expressing both CD16/7/ and CD16/7/β. (B) The expression of CD16/7/ and CD8/β. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD16 and FITC-coupled secondary antibody. (C and D) [3H]-thymidine incorporation of Ba/F3 cells transfected with the indicated CD16/7 chimeras in response to addition of the CD16 antibody, 3G8. Cells were cultured with increasing amounts of 3G8 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent maximal [3H]-thymidine incorporation observed in IL-3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722006bx.jpeg?Expires=1767725394&Signature=DTgVRbakrmu8CpElnWelDjsNoe9xEFIz5lNMB6Ot7mYKEaOxoi2kNi6BXeNYjHn8OKJy-KkTTO54fEjp7IYY6C5-R2uGiTjl0aqrqZgkawawgMLbe3~LP7UQOfPwrYPOTunlrueWJqrcH5k5nPyveRLAxrRnkot73sPAkJ9seBOYez59k-rkoGcihaEeC7saEbE2eKm17qfsvJFcf~kIGvnYtGdWloQfQ6HGRufO~0hqOI0ouMlvmQgJNlT19D955CRe7GkrD~35BAvlGjmh5OArMNblZpdlVTeiMAKP0LaaUengk5Dr5P-S9ZROQ9eMlfUnh6xukFG2Czv5hry6FA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Induction of heterodimerization of the cytoplasmic domains of βIL3 and IL3 is mitogenic in Ba/F3 cells. Flow cytometric analysis confirming expression of (A) the CD16/7 chimeric receptors. The bottom righthand panel represents the CD16 signal obtained in cells expressing both CD16/7/ and CD16/7/β. (B) The expression of CD16/7/ and CD8/β. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD16 and FITC-coupled secondary antibody. (C and D) [3H]-thymidine incorporation of Ba/F3 cells transfected with the indicated CD16/7 chimeras in response to addition of the CD16 antibody, 3G8. Cells were cultured with increasing amounts of 3G8 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent maximal [3H]-thymidine incorporation observed in IL-3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722006cx.jpeg?Expires=1767725394&Signature=kixxjcE4QdWW~g2nlsuqwrJj63QD4OLHaToGzwXFNP1PIzI8AKQO86CMoQS~hchPbZ1IT-gq6Cdk8wygaR0S1nr6KsmH3HCEWOpucoTcrbKRYwCeqfEXGHBIZq5xYjJ6objxLYOJqHpHl6aa1B2cmmdLOwQTG4ni2ZSZNqfcFX88m4eChnwKQhkExjWDI98o18VoIfXJSZxK-mSZkMr7AJnV8qQGqIvsOWINxNQFbRTRpX-s4L0YY~UutobwTdLYjnGsu9lpqTSLO8IgOxYf-rT6Nt2IwaMRzBnHYokUBnd0MKb7BxGuFxq68IAjxyZWdYPSCf0OgDL9-V5rftTGMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Induction of heterodimerization of the cytoplasmic domains of βIL3 and IL3 is mitogenic in Ba/F3 cells. Flow cytometric analysis confirming expression of (A) the CD16/7 chimeric receptors. The bottom righthand panel represents the CD16 signal obtained in cells expressing both CD16/7/ and CD16/7/β. (B) The expression of CD16/7/ and CD8/β. Thin lines represent results with cells treated with FITC-coupled secondary antibody alone; thick lines represent results with cells treated with CD16 and FITC-coupled secondary antibody. (C and D) [3H]-thymidine incorporation of Ba/F3 cells transfected with the indicated CD16/7 chimeras in response to addition of the CD16 antibody, 3G8. Cells were cultured with increasing amounts of 3G8 for 40 hours and then incubated for an additional 8 hours in the presence of [3H]-thymidine. Data are plotted as the percent maximal [3H]-thymidine incorporation observed in IL-3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1614/5/m_blod41722006dx.jpeg?Expires=1767725394&Signature=jGd7egjNkAy4dmi43fu9n6R~W0dC7J5Eeikq6LivQ9Feom-ZnyEW2ApoSP1ZVHl1nhUOM9HPSAV5r6B0PdjN7xiiIJRuDAFbv~9fwzcZlXu4mZlCU0~Qlt9Zmod0dNw3Xm3ydqho8swsiOxRpvBqqxWUNJgFmQXPMpwWZtzt8chSBB6BlsZurZITpY79q9JrtpKIxbrXpZVirosxV6QLC4LwkCwCam8Z3EqUwWA7PloJCb0tnIaYqUJarAxTYG~c3opm4HGAUwq7k4C~~cGCw4QhGASQW1Oe8ZrUnDb1emB5af02C5ZDvT9pT1edpvf9455t4f~JM0FLpDjN3~KMgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal