Abstract
In several erythroleukemia cell lines, activation of mitogen-activated protein kinases (MAPK) by phorbol esters or megakaryocyte growth and development factor (MGDF) is required for induction of megakaryocytic phenotype and growth arrest. To support this model, we have examined the effect of a specific inhibitor of this pathway (PD98059) on human CD34+ hematopoietic progenitors isolated from cord blood (CB), induced to differentiate along the megakaryocytic lineage in liquid cultures supplemented with rhuMGDF. RhuMGDF induced a sustained activation of MAPK in megakaryocytes and this activation was completely inhibited in the presence of low concentrations of PD98059 (6 to 10 μmol/L). At this concentration, PD98059 induced an increase in cell proliferation, resulting in accumulation of viable cells and a prolongation of the life time of the cultures. This increase correlated with an increase in DNA synthesis rather than with a reduction in apoptosis. This effect was combined with developmental changes indicative of delayed megakaryocytic differentiation: (1) PD98059-treated cells tended to retain markers of immature progenitors as shown by the increased proportion of both CD34+ and CD41+CD34+ cells. (2) PD98059-treated cultures were greatly enriched in immature blasts cells. (3) PD98059 increased megakaryocytic progenitors able to form colonies in semisolid assays. Thus, the MAPK pathway, although not required for megakaryocyte formation, seems to be involved in the transition from proliferation to maturation in megakaryocytes. Inhibition of MAPK activation also led to an increase in the number and size of erythroid colonies without affecting granulocyte/macrophage progenitor numbers suggesting that, in addition to the megakaryocytic lineage, the MAPK pathway could play a role in erythroid lineage differentiation.
HEMATOPOIESIS is an ordered and tightly regulated process of proliferation and differentiation leading to the generation of mature blood cells from a small number of totipotent stem cells. The regulatory system governing megakaryocytopoiesis and platelet production follows this general scheme, involving the committment of mutipotential progenitors to megakaryocyte precursors and final maturation leading to the formation of platelets. In their development, megakaryoblasts undergo a series of transformations such as expression of lineage-specific antigens, several rounds of endomitotic replication resulting in large polyploid cells, and the development of an extensive system of demarcation membranes, which delimits platelet territories.1 Both these proliferative and maturative steps have been shown to be controlled in vitro and in vivo by the c-Mpl receptor and its cognate ligand thrombopoietin (TPO), also known as megakaryocyte growth and development factor (MGDF).2-6 In addition to its effects on megakaryopoiesis and thrombopoiesis, MGDF supports the survival and proliferation of human and murine primitive hematopoietic cells.7 8
Cells in which the balance between proliferation and differentiation is altered are more prone to leukemogenesis. Thus, it is of considerable interest to determine the molecular mechanisms controlling this choice. Because growth factors play a central role in these processes, great efforts have been made at elucidating the signal transduction pathways linking receptors at the cell surface to gene transcription in the nucleus. The classical mitogen-activated protein kinase (MAPK) (also known as extracellular-regulated kinase, [ERK]) pathway is the major of these mechanisms. ERKs are activated by most growth factors and have been shown to be a key regulator of both proliferation and differentiation in different cell types. Constitutive activation of MAPK is sufficient to transform fibroblasts, while inhibition of this pathway blocks Ras-mediated cell transformation and growth factor-induced mitogenesis.9,10 However, recent observations have also underscored the essential role of this pathway in cell differentiation.10-13 In hematopoietic cells, the MAPK pathway has been shown to control the differentiation of immature thymocytes downstream of the T-cell receptors14,15 and to regulate early development of B cells.16
ERK are serine/threonine kinases whose activation itself involves the dual phosphorylation of two crucial threonine and tyrosine residues. This phosphorylation occurs on sequential activation of p21Ras and the protein kinases Raf and MAPK kinases, MEK 1 and MEK2 (reviewed in Dhanasekaran et al17). Once activated, ERKs phosphorylate a variety of cytosolic proteins including other kinases such as pp90Rsk18 and phospholipase A2.19 On activation, ERKs also translocate to the nucleus,13,20where they phosphorylate transcription factors such as Elk-1, leading to the transcription of c-Fos.17 21
Mpl is a member of the cytokine receptor superfamily, which includes the receptors for erythropoietin (Epo), granulocyte-macrophage colony-stimulating factor (GM-CSF), and most interleukins.2A number of receptors of this family, including Mpl, promote both proliferation and differentiation signals when they are introduced in various cell lines.22-27 We have previously shown that these functions are separately controlled by distinct regions of Mpl cytoplasmic domain.22 This scheme applies also to other cytokine receptors.24-27 ERK activation has been reported for most of these receptors and has been shown either necessary or dispensable for proliferation.28 Correlations between the loss of maturation effect on mutation of the intracellular domain of the receptors and ERK activation have been described in some instances. The MAPK pathway was shown to be dispensable for GM-CSF–induced myeloid differentiation of the WT19 cell line.27 By contrast, we have previously shown that MGDF induces a sustained activation of ERK in UT7 cells in which we introduced Mpl (UT7-Mpl) and that this prolonged activation is required for MGDF-induced increase in megakaryocytic-specific antigens, CD41 and CD42b.29
The importance of the MAPK pathway in megakaryocytic differentiation was corroborated in several erythroleukemia cell lines where increase in CD41 can be induced on introduction of constitutively activated mutants of MEK.30-32 Interestingly, this same pathway was found to repress erythroid differentiation, suggesting that MAPK might balance the commitment to megakaryocyte versus erythroid cell lineage from a common ancestor. However, bipotent cell lines can neither mimic differentiation and proliferative capacities of hematopoietic progenitors nor reflect all stages of megakaryocytic differentiation. In addition, 1 study has reported the existence of significant differences in MGDF-induced signaling between megakaryocytes and established cell lines.33 Likewise, sustained activation of the MAPK pathway has been shown to lead to exactly opposite effects on proliferation in primary and transformed fibroblasts.34This prompted us to analyze in the present study the effects of a specific MEK inhibitor, PD98059,35 on freshly isolated primary human CD34+ hematopoietic progenitor cells induced to differentiate along the megakaryocytic lineage in liquid cultures supplemented with rhuMGDF.
MATERIALS AND METHODS
Growth factors.
Pegylated-human recombinant megakaryocyte growth and differentiation factor (PEG-rhu-MGDF) was a generous gift from Amgen (Thousand Oaks, CA). Human erythopoietin (specific activity, 120,000 U/mg) was provided by Dr M. Brandt (Boehringer, Mannheim, Germany). Recombinant stem cell factor (SCF) was from Amgen and interleukin-3 (IL-3), IL-6, GM-CSF were obtained from Dr Shimosaka (Kirin Brewery Co, Tokyo, Japan).
Isolation of CD34+progenitors from cord blood.
Umbilical cord blood (CB) units from normal full-term deliveries were obtained, after informed consent of the mothers, from the Obstetrics Unit of Hôpital Saint-Vincent de Paul, Paris, France, and collected in placental blood collection bags (Maco Pharma, Tourcoing, France) by the Centre d’Hémobiologie Périnatal. The mean CB total volume collected was 85 mL. CB units were diluted with 50 mL phosphate-buffered saline (PBS; GIBCO-BRL, Life Technologies, Cergy-Pontoise, France) containing 0.8% bovine serum albumine (BSA; StemCell Technologies, TEBU, Le Perray-en-Yvelines, France), then submitted to Ficoll density gradient (Lymphocyte separation medium; Eurobio, Les Ulis, France). Low-density cells were recovered and enriched for CD34+ cells by 2 cycles of positive selection using anti-CD34 antibody (QBEND/10) and magnetic cell sorting on Midi-MACS then Mini-MACS columns (CD34 isolation kit; Miltenyi Biotech, TEBU). CD34+-enriched cells were numbered and viability was determined by trypan blue exclusion.
The purity of the CD34-selected cells was determined after each isolation by fluorescence-activated cell sorting (FACS) analysis using a monoclonal antibody directed against CD34 directly conjugated to phycoerythrin. These preparations usually averaged about 85% to 90% CD34+ cells.
Liquid suspension cultures.
To avoid interference of serum, purified CD34+ cells (4 × 104 to 105/mL) were cultured in serum-free Iscove’s modified Dulbecco’s medium (IMDM) (GIBCO-BRL) supplemented with 15% of a commercial mixture BSA, insulin, and transferrin (BIT 9500; StemCell Technologies), and 100 ng/mL PEG-rhuMGDF in the presence of various concentrations (3 to 20 μmol/L) PD9805935 diluted in dimethyl sulfoxide (DMSO) or the equivalent volumes of DMSO alone as controls. Every 3 days, viable cells were scored by Trypan blue dye exclusion and a fraction of them was harvested for further analysis (see below). The remaining cells were amplified with fresh serum-free IMDM containing PEG-rhuMGDF and either 6 μmol/L MEK inhibitor or DMSO. Cell density was reajusted to 1 to 4 × 105cells/mL.
Cell labeling and flow cytometry analysis.
Immunophenotyping was essentially performed as described.22Briefly, CD34+ cells were washed twice in PBA (PBS containig 1% BSA and 0.2% sodium azide) and incubated for 30 minutes at 4°C with 1 mg of normal goat aggregated IgG (Sigma, St Louis, MO) per mL to saturate Fc receptors. After 1 wash in PBA, cells were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin-labeled (R-PE) mouse antibodies directed against the following antigens: CD34, CD41, CD61 GpA (Immunotech, Marseille, France) or CD42b (Dako, Glostrup, Denmark), applied at the recommended concentrations. Control stainings were performed with isotype-matched control antibodies. Incubation was performed for 30 minutes on ice. After staining, the cells were washed twice and resuspended in PBA containing 1% formaldehyde. Fluorescence was analyzed on an ELITE flow cytometer (Coulter Corp, Miami, FL). The percentage of positive cells and the mean fluorescence intensity (MFI), a measure of the relative density of the antigen on the surface of stained cells, were determined for each sample. In some experiments, CB hematopoietic progenitors were stained simultaneously with R-PE–anti-CD34 and either FITC-anti–CD41 or FITC-anti–CD42b antibodies, or FITC-anti–CD34 and R-PE–anti-CD41. Spillover of green fluorescence into the red fluorescence was compensated electronically for each experiment by using single-stained samples.
To determine the frequency of apoptotic cells within the cultures, cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) and incubated on ice with FITC-labeled annexin V at the recommended concentration (Coulter) for 10 minutes. FACS analysis was performed immediately.
DNA synthesis.
DNA synthesis was measured by thymidine incorporation as follows: CD3+ progenitor cultures performed in the presence or absence of MEK inhibitor were harvested at regular intervals (between 6 and 15 days) and triplicate samples of 2 × 104 cells were plated directly in 96-well plates. A total of 2 μCi [3H]thymidine was then added and the amount of radioactivity incorporated in cells was determined 15 hours later.
Quantification of clonogenic progenitors in semisolid culture assays.
The presence of erythroid (burst-forming unit-erythroid [BFU-E]), colony-forming unit granulocyte/macrophage (CFU-GM), and colony-forming unit-megakaryocytic (CFU-MK) progenitors was evaluated every 3 to 4 days along the culture. For erythroid and GM progenitors, previously described methylcellulose assays were used36: aliquots of cells, which had been cultured in the presence or absence or PD98059 were seeded at 500 to 5,000 cells/mL depending on the day of culture, in StemGEM 1d methylcellulose medium. This medium contains Epo, IL-3, IL-6, SCF, G-CSF, GM-CSF, IL-11 and allows the growth of all types of erythroid and myeloid progenitors (StemBio Research, CNRS, Villejuif, France). Cultures were performed in duplicate and incubated in fully humidified atmosphere with 5% CO2 in air at 37°C. Colonies were scored after 14 days. Megakaryocyte progenitors were cultured using serum-free fibrin clot, as described.6Briefly, cells (1 × 105/mL) were suspended in IMDM suplemented with the BIT 9500 mixture, 20 ng/mL PEG-rhuMGDF, and 50 ng/mL SCF. Fibrin clot formation was obtained by adding 1 mg/mL plasma fibrinogen, 0.01 mol/L ε-aminocaproic acid, and 7 IU/mL thrombin (Thrombiprest; Diagnostica Stago, Asnières, France). Cultures were incubated for 12 days at 37°C in a 5% CO2 95% air humidified incubator. Colonies were quantified by indirect immunophosphatase alkaline labeling after staining with anti-CD61 monoclonal antibody (Y2-51; Dako). Dishes were scanned completely under an inverted microscope at 40× or 100× magnification. Colonies are defined as 1 or more CD61+ megakaryocyte.
Assays for MAPK activation.
ERK activity was measured by immunoblotting of whole-cell extracts with an activation-specific polyclonal antibody, which recognizes the dual phosphorylated forms of p42 ERK2 and p44 ERK1 (Promega Corp, Madison, WI). Total ERK amounts were determined by reprobing the same membranes with an anti-ERK1 antibody recognizing both ERK1 and ERK2 (SC-93; Santa Cruz Biotechnology Inc, Santa Cruz, CA). Whole-cell lysates were prepared as described29 from 7- to 11-day cultures of CD34+ cells. Cells were either lysed directly or washed 3 times and deprived of rhuMGDF by incubation for 5 hours in cytokine-free medium before stimulation. Stimulation was performed for 30 minutes and 1 hour at 37°C with 100 ng/mL rhuMGDF, in the presence or absence of 6 μmol/L PD98059 or the equivalent amount of DMSO as control.
Morphological studies.
Cells were collected every 2 or 3 days of culture and concentrated on glass slides by cytocentrifugation. Cell morphology was examined after May-Grünwald-Giemsa (MGG) staining.
Statistical analysis.
Statistical differences in cell expansion and apoptosis between the subfractions of cells grown in the presence or absence of PD98059 were analyzed using Fischer-Snedecor test applied to analysis of variance (ANOVA).
RESULTS
MGDF-mediated MAPK activation in primary megakakaryocytic cells derived from CB CD34+ progenitors.
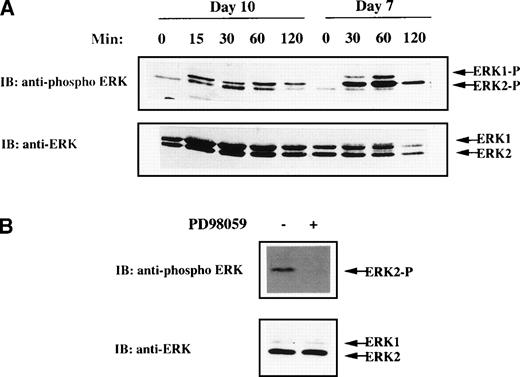
MGDF has been shown to drive unilineage differentiation along the megakaryocytic pathway and to lead to almost pure megakaryocytes in suspension cultures.1,3-6 To determine whether MAPK pathway activation could be of relevance in MGDF-mediated functions in megakaryocytes, we first analyzed whether rhuMGDF was able to trigger MAPK activation in physiological conditions, ie, primary normal megakaryocytes. CD34+ cells freshly isolated from human umbilical CB were cultured in suspension in serum-free medium containing only rhuMGDF as growth factor (as described in Materials and Methods). After 10 days, about 85% of the cells were positive for the megakaryocytic-specific antigen CD41 (see below). No phosphorylated ERK could be detected when lysates from these cells were directly prepared and loaded on gel (data not shown). However, rhuMGDF was able to trigger MAPK activation in CD41+ cells starved of cytokine by a 5-hour incubation in rhuMGDF-free medium before stimulation (Fig 1A). In some experiments, depending on the donor sample, a unique band corresponding to phosphorylated ERK2 was observed (see Fig 1B). This was probably due to the far greater abundance of ERK2 than ERK1 in cells from these donnors, as shown by immublotting with an antibody recognizing both active and inactive ERK1 and ERK2 (Fig 1A and B). Active ERK2 was still detected after 120 minutes of stimulation, indicating that rhuMGDF induced a sustained MAPK activation in primary megakaryocytes. This activation, however, decayed more rapidly than that previously reported in the UT7-Mpl megakaryoblastic cell line stimulated with MGDF.29 This might be due to some downregulation of ERKs occuring during the several days of preculture of CD34+ cells in the presence of high concentrations of MGDF.
MAPK activation in CB-derived megakaryocytes. (A) Kinetic of ERK activation in day 7 and day 10 cells derived from purified CD34+ cells grown in the presence of rhuMGDF. Cells were deprived of growth factor by incubation for 5 hours in cytokine-free medium and stimulated for the indicated times with 100 ng/mL rhuMGDF. (B) Inhibition of rhuMGDF-induced ERK activation by PD98059. Cells were grown for 10 days with rhuMGDF, starved, and stimulated for 60 minutes at 37°C with 100 ng/mL of rhuMGDF in the presence of 6 μmol/L PD98059 (+) or the equivalent amount of DMSO as control (−). ERK activity was detected by immunoblotting whole-cell lysates with antiphospho ERK antibody. The amount of sample loaded in each lane was verified by immunoblotting the same membranes with an antibody recognizing both active and inactive ERK1 and ERK2.
MAPK activation in CB-derived megakaryocytes. (A) Kinetic of ERK activation in day 7 and day 10 cells derived from purified CD34+ cells grown in the presence of rhuMGDF. Cells were deprived of growth factor by incubation for 5 hours in cytokine-free medium and stimulated for the indicated times with 100 ng/mL rhuMGDF. (B) Inhibition of rhuMGDF-induced ERK activation by PD98059. Cells were grown for 10 days with rhuMGDF, starved, and stimulated for 60 minutes at 37°C with 100 ng/mL of rhuMGDF in the presence of 6 μmol/L PD98059 (+) or the equivalent amount of DMSO as control (−). ERK activity was detected by immunoblotting whole-cell lysates with antiphospho ERK antibody. The amount of sample loaded in each lane was verified by immunoblotting the same membranes with an antibody recognizing both active and inactive ERK1 and ERK2.
The MEK inhibitor increases rhuMGDF-induced proliferation of CD34+ cells.
The results described with K56230,31 and UT7-Mpl cell lines (unpublished data, March 1998) have suggested that the MAPK pathway could play a role in cell fate decision towards erythroid or megakaryocytic lineages. To explore this hypothesis, the MEK inhibitor was added to CB-derived CD34+ progenitors cultured in the presence of rhuMGDF. In a first set of experiments, we determined the optimal concentration of PD98059 that can be used in this system. Based on the dose (10 μmol/L), which inhibits rhuMGDF-induced differentiation in the UT7-Mpl model,29 concentrations of PD98059 ranging from 3 to 20 μmol/L were tested on CB CD34+ cell cultures (not shown). The concentration of 6 μmol/L was found both to fully inhibit MAPK activation triggered by rhuMGDF in this system (Fig 1B) and to be devoid of toxicity. This concentration was used in all of the following studies.
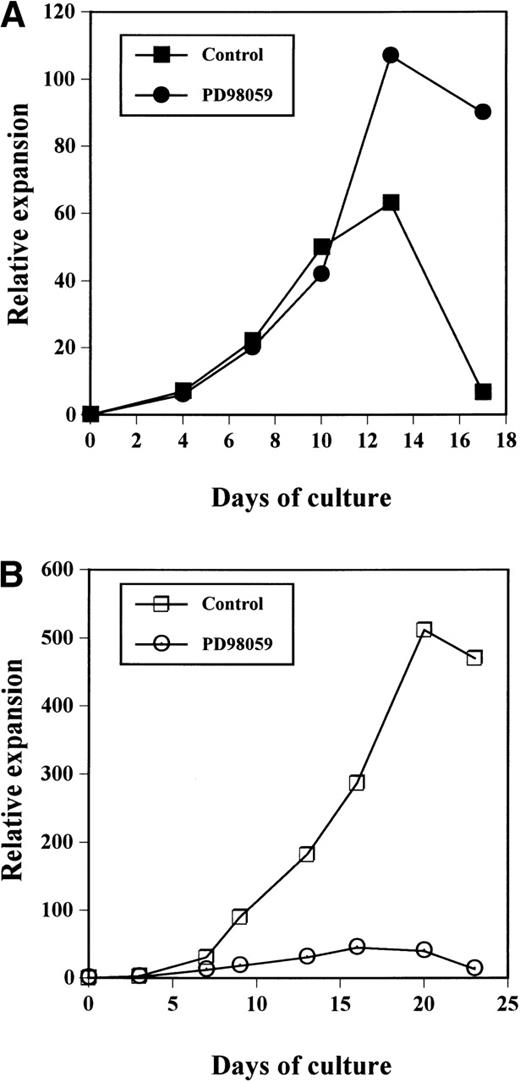
As shown in Fig 2A, the cultures treated with the MEK inhibitor always contained more viable cells than control cultures, after day 10. In both cultures, expansion was observed until around day 12. After this time point, the number of viable cells in control cultures declined quickly and a massive cell death occured usually around day 15; at day 15 to 18, all of the cells were dead. By contrast, in PD98059-treated samples, although proliferation reached a maximum around day 12, the total number of viable cells remained almost constant or declined slowly until day 18. Eventually, all PD98059-treated cells were dead by day 22. Thus, the difference in viable cell numbers between the 2 cultures culminated at the end of the cultures where PD98059-treated samples contained up to 13-fold more cells at day 17 than controls. The relative expansions of the cells after 17 days of culture with rhuMGDF in cultures containing PD98059 (58-fold ± 20-fold, mean ± standard deviation [SD], n = 7) or the corresponding volume of solvent (10-fold ± 5-fold, mean ± SD, n = 8) were significantly different (P < .01).
Relative expansion of CD34+ cells in the presence (•, ○) or absence (▪, □) of 6 μmol/L PD98059 induced by MGDF (A) or a mixture of SCF + IL-3 +IL-6 (B). The data have been normalized to the number of purified CD34+ cells seeded on day 0. The results presented are those from a representative experiment of 8 (A) or 3 (B) performed.
Relative expansion of CD34+ cells in the presence (•, ○) or absence (▪, □) of 6 μmol/L PD98059 induced by MGDF (A) or a mixture of SCF + IL-3 +IL-6 (B). The data have been normalized to the number of purified CD34+ cells seeded on day 0. The results presented are those from a representative experiment of 8 (A) or 3 (B) performed.
Thus, the presence of the MEK inhibitor resulted in prolongation of the cultures in the presence of MGDF. This negative effect of the MAPK pathway on the proliferation seemed specific for rhuMGDF responses. Indeed, in cultures of CB CD34+ cells performed in the presence of a mixture of either SCF + IL-3 + IL-6, the same concentration of PD98059 inhibited cell proliferation (Fig 2B). The MEK inhibitor has no effect on cell growth induced by a mixture of G-CSF and FLT3-ligand (data not shown).
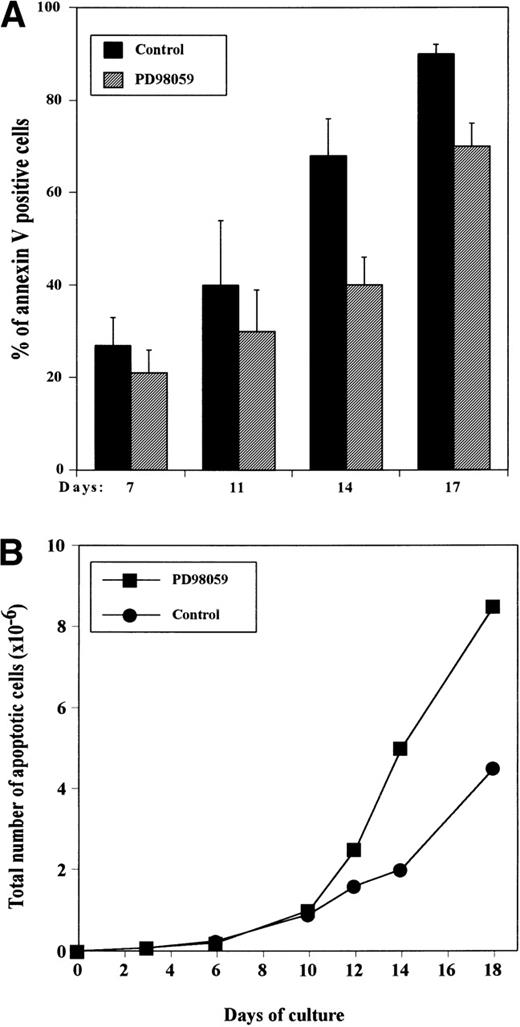
To determine whether the difference in absolute viable cell numbers observed between PD98059-treated and control cultures was due to a blockage of apoptosis, cells were stained at various time intervals with annexin V coupled to FITC. Figure 3shows that although still expanding, day 7 cultures already contained a significant number of apoptotic cells, and this proportion increased gradually during the culture. In controls, consistent with the drop in viable cell numbers, at day 16 to 17, almost all of the cells have entered the apoptotic process (Fig 3A). Although significantly (P < .05) inferior to that of control, the frequency of apoptotic cells was quite high in PD98059-treated cultures reaching about 70% at day 16 to 17 (Fig 3A). This results in a higher absolute number of apoptotic cells in cultures containing the inhibitor (Fig3B). From these data, the rate of apoptosis of the cells in 2 days (between days 10 and 12 or 12 and 14) was estimated by the following ratio: (absolute number of new apoptotic cells generated in between days 10 to 12 [or 12 to 14])/total number of viable cells on day 10 [or 12])×100. We found that about 30% of the cells were dying during these 2-day periods in both control and PD98059-treated cultures. These results suggest that inhibition of apoptosis is probably not the mechanism responsible for the increased proliferation of PD98059-treated cells.
Appearance of apoptotic cells in the cultures as measured by annexin V staining. CD34+ cells were grown with rhuMGDF in the absence or presence of 6 μmol/L PD98059 and stained with annexin V-FITC at the indicated days. The percentage of annexin V–positive cells was determined by FACS analysis. (A) Frequency of apoptotic cells in control cultures (▪) and cultures containing the inhibitor (▨). The results represent mean ± SD from 4 independent experiments. (B) Total number of apoptotic cells in the presence (▪) or absence (•) of PD98059. The results from a representative experiment are shown.
Appearance of apoptotic cells in the cultures as measured by annexin V staining. CD34+ cells were grown with rhuMGDF in the absence or presence of 6 μmol/L PD98059 and stained with annexin V-FITC at the indicated days. The percentage of annexin V–positive cells was determined by FACS analysis. (A) Frequency of apoptotic cells in control cultures (▪) and cultures containing the inhibitor (▨). The results represent mean ± SD from 4 independent experiments. (B) Total number of apoptotic cells in the presence (▪) or absence (•) of PD98059. The results from a representative experiment are shown.
We therefore assessed whether PD98059 could affect the capacity of the cells to enter S phase. For that, DNA synthesis was tested by measuring incorporation of [3H]thymidine into the cells at regular intervals. The kinetic profile of incorporation was exactly inverse of that of apoptosis: in the presence or absence of inhibitor, incorporation was maximun around day 8 and declined gradually thereafter (Fig 4). However, DNA synthesis was greatly increased in the presence of the MEK inhibitor, reaching up to 4-fold. By days 12 to 15, [3H]thymidine incorporation was still significant in PD98059-treated cells, while it had reached background levels in control cells.
Effect of the MEK inhibitor on DNA synthesis capacity of CD34+ cells grown with rhuMGDF. Cells from cultures performed in the absence (▪) or presence (▨) of 6 μmol/L PD98059 were harvested at the indicated days and DNA synthesis was assessed by measuring [3H]thymidine incorporation. Results are mean ± SD of triplicate determinations from a representative experiment of 3 performed. The relative increase in [3H]thymidine incorporation in PD98059-treated cells as compared with control DMSO-treated cells is indicated below the bars for each time point.
Effect of the MEK inhibitor on DNA synthesis capacity of CD34+ cells grown with rhuMGDF. Cells from cultures performed in the absence (▪) or presence (▨) of 6 μmol/L PD98059 were harvested at the indicated days and DNA synthesis was assessed by measuring [3H]thymidine incorporation. Results are mean ± SD of triplicate determinations from a representative experiment of 3 performed. The relative increase in [3H]thymidine incorporation in PD98059-treated cells as compared with control DMSO-treated cells is indicated below the bars for each time point.
Globally, these data suggest that the augmentation cell numbers in cultures treated with the MEK inhibitor results mainly from an increased mitosis capacity of the cells. Thus in CD34+progenitors, the MAPK pathway functions as a negative regulator of rhuMDGF-induced mitogenesis.
Phenotypic analysis of cultured progenitors in the presence or absence of MEK inhibitor.
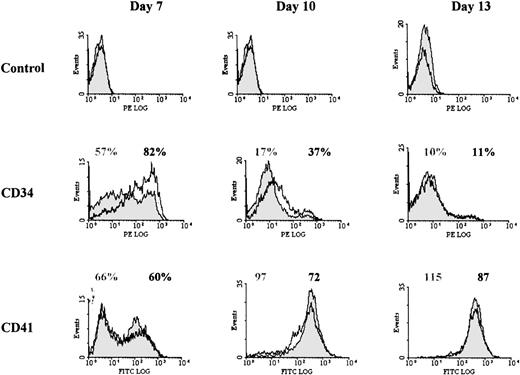
Previous studies have shown that during differentiation of megakaryocytic progenitors, the proliferative capacity of progenitors decreases as a continuum, as cell surface phenotype change from CD34+CD41+/CD61+ cells to CD34−CD41+/CD61+cells.37 38 Therefore, we analyzed whether the increased proliferation in PD98059-treated cultures could be due to the presence of more immature progenitors, and in greater numbers, than in control cultures. Surface expression of CD34 and of the megakaryocytic markers CD41, CD61, and CD42b were analyzed at regular time intervals in cultures treated or not with PD98059.
As shown in Table 1 and Fig 5, and as previously described,37 38 CD41 appeared early in the culture, while CD42b was expressed later during megakaryocytic differentiation. By contrast with what had been observed with cell lines, no major difference in expression of these markers was observed between cultures containing PD98059 or control. The mean fluorescence intensity of anti-CD41 staining was only slightly decreased (by 21% ± 10% at day 10, mean ± SD, n = 5) in cells treated with the inhibitor. However, the 2 cultures clearly differed by the surface expression of CD34 (Fig 5 and Table 1): both the proportion of CD34+cells and the level of expression of CD34 on the positive cells were consistently increased in PD98059-treated cultures (58% ± 26% and 51% ±15% of increase in CD34+ cell number and MFI, respectively, at day 7, mean ± SD, n = 5). This result suggested that inhibition of the MAPK pathway in this system delayed rhuMGDF-induced megakaryocytic differentiation.
Effect of the MEK Inhibitor on Cell Surface Markers
| Days . | Treatment* . | % of Positive Cells . | ||
|---|---|---|---|---|
| CD34 . | CD41 . | CD42b . | ||
| 4 | − + | 77 ± 24 80 ± 18 | 40 ± 19 39 ± 17 | ND ND |
| 7 | − + | 43 ± 17 60 ± 23 | 63 ± 12 63 ± 15 | 24 ± 7 23 ± 5 |
| 10 | − + | 17 ± 1 32 ± 7 | 84 ± 13 87 ± 9 | 74 ± 4 74 ± 4 |
| 13 | − + | 7 ± 5 9 ± 6 | 83 ± 18 85 ± 15 | 71 ± 13 75 ± 6 |
| Days . | Treatment* . | % of Positive Cells . | ||
|---|---|---|---|---|
| CD34 . | CD41 . | CD42b . | ||
| 4 | − + | 77 ± 24 80 ± 18 | 40 ± 19 39 ± 17 | ND ND |
| 7 | − + | 43 ± 17 60 ± 23 | 63 ± 12 63 ± 15 | 24 ± 7 23 ± 5 |
| 10 | − + | 17 ± 1 32 ± 7 | 84 ± 13 87 ± 9 | 74 ± 4 74 ± 4 |
| 13 | − + | 7 ± 5 9 ± 6 | 83 ± 18 85 ± 15 | 71 ± 13 75 ± 6 |
Results are means ± SD from 5 independent experiments.
Abbreviation: ND, not determined.
CB cells were cultured with rhuMGDF in the absence (−) or presence of 6 μmol/L PD98059 (+). At the indicated days, cells were stained with either anti-CD34, anti-CD41, or anti-CD42b antibodies coupled to FITC.
Effect of the MEK inhibitor on CD34 and CD41 cell surface expression. Representative histograms from 1 of at least 4 experiments performed with blood from different donors are shown for each marker. Cells from control cultures (filled histograms) or cultures containing 6 μmol/L PD98059 (open histograms) were harvested at the indicated days and stained separately with either anti-CD34 antibody coupled to R-PE or anti-CD41 antibody coupled to FITC. In some experiments, labeling was performed with FITC-anti-CD34 and R-PE-anti-CD41 antibodies and similar results were obtained. For each marker, the percentage of positive cells (or the MFI for CD41 at days 10 and 13) are indicated: gray labeling, controls; black labeling, PD98059-treated samples.
Effect of the MEK inhibitor on CD34 and CD41 cell surface expression. Representative histograms from 1 of at least 4 experiments performed with blood from different donors are shown for each marker. Cells from control cultures (filled histograms) or cultures containing 6 μmol/L PD98059 (open histograms) were harvested at the indicated days and stained separately with either anti-CD34 antibody coupled to R-PE or anti-CD41 antibody coupled to FITC. In some experiments, labeling was performed with FITC-anti-CD34 and R-PE-anti-CD41 antibodies and similar results were obtained. For each marker, the percentage of positive cells (or the MFI for CD41 at days 10 and 13) are indicated: gray labeling, controls; black labeling, PD98059-treated samples.
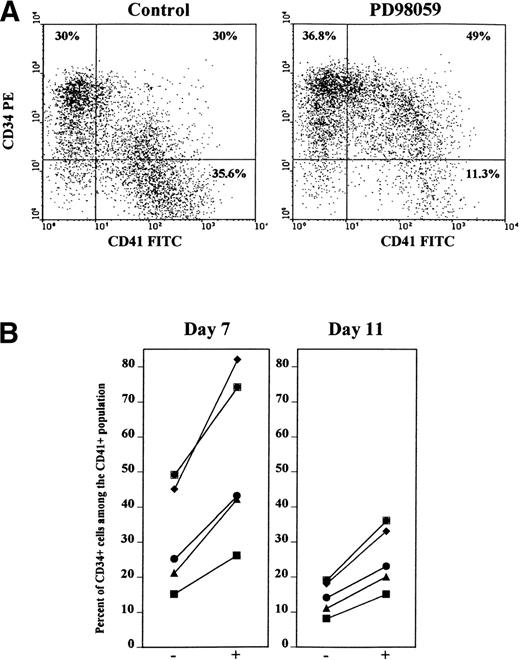
To further assess this possibility, cells were stained simultaneously with anti-CD34 and anti-CD41 antibodies. As shown in Figs 6A and 7, as for CD34+ cells, the frequency of doubly stained CD34+CD41+ cells was greatly increased (71% ± 14% of increase, mean ± SD, n = 5) in cultures containing the MEK inhibitor, as compared with control cultures. By contrast the number of CD34+CD41− cells remained almost unchanged (Fig 6A, 16% ± 12% of increase, mean ± SD, n = 5). Figure 6B shows that the relative proportion of double-stained CD34+CD41+ cells among the CD41+cells was greatly augmented by the MEK inhibitor in 5 of 5 independent experiments. On average, 30% of day 7-CD41+ cells expressed CD34 in control cultures, while this proportion averaged 58% in the presence of PD98059. After 11 days of culture, the expression of CD34 dropped, but the same phenomenon was still observed (Fig 6B). Likewise, the proportion of double-positive CD34+CD42b+ cells was greatly enriched after treatment with the MEK inhibitor (44% v 75% of day 7-CD42b cells also expressing CD34 were found in control and PD98059-treated cultures, respectively).
Increased frequency of double-stained CD34+CD41+ cells in PD98059-treated cultures. Cells from cultures containing or not 6 μmol/L PD98059 were harvested at different time intervals and stained simultaneously with R-PE-anti-CD34 and FITC-anti-CD41 (or FITC-anti-CD34 and R-PE-anti-CD41) antibodies. (A) FACS dot plot from a representative experiment on day 7 cells. (B) Percent of CD34+ cells among the CD41+ cell population after 7 and 11 days of culture in the absence (−) or in the presence (+) of PD98059 in 5 independent experiments.
Increased frequency of double-stained CD34+CD41+ cells in PD98059-treated cultures. Cells from cultures containing or not 6 μmol/L PD98059 were harvested at different time intervals and stained simultaneously with R-PE-anti-CD34 and FITC-anti-CD41 (or FITC-anti-CD34 and R-PE-anti-CD41) antibodies. (A) FACS dot plot from a representative experiment on day 7 cells. (B) Percent of CD34+ cells among the CD41+ cell population after 7 and 11 days of culture in the absence (−) or in the presence (+) of PD98059 in 5 independent experiments.
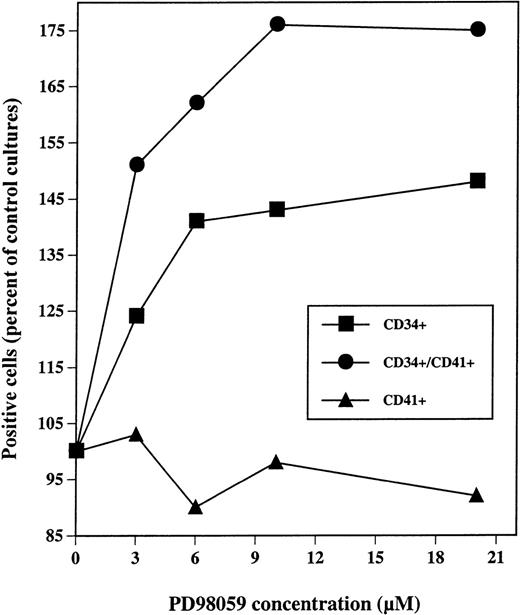
Dose-response analysis of PD98059 effect on the presence of CD34+, CD41+, and CD41+CD34+ cells in MGDF-induced cultures. CD34+ cells were grown with rhuMGDF in the presence of the indicated concentrations of PD98059 diluted in DMSO or the equivalent volumes of DMSO alone. After 7 days, cells were labeled with FITC-anti-CD41 and R-PE-anti-CD34 antibodies either separately or simultaneously. Results are expressed as a percent of the frequency of positives cells for each marker obtained with control cells.
Dose-response analysis of PD98059 effect on the presence of CD34+, CD41+, and CD41+CD34+ cells in MGDF-induced cultures. CD34+ cells were grown with rhuMGDF in the presence of the indicated concentrations of PD98059 diluted in DMSO or the equivalent volumes of DMSO alone. After 7 days, cells were labeled with FITC-anti-CD41 and R-PE-anti-CD34 antibodies either separately or simultaneously. Results are expressed as a percent of the frequency of positives cells for each marker obtained with control cells.
As shown in Fig 7, maximum increases in the numbers CD34+and CD34+CD41+ cells were obtained with a concentration of 6 to 10 μmol/L of PD98059.
Taken together, these results suggest that the megakaryocytic CD41 or CD42b positive cells present in the cultures containing the MEK inhibitor are in a more immature differentiation state than their control counterparts.
Enhanced megakaryocytic and erythroid colony formation in the presence of the MEK inhibitor.
The presence of progenitors was evaluated all along the culture by the capacity of the cells to derive clonogenic colonies in semisolid assays. Cells grown with rhuMGDF in the presence or absence of MEK inhibitor, were harvested after various days in culture, and were plated in the absence of inhibitor with a cocktail of cytokines allowing the growth of either erythroid and GM or MK colonies. Colonies were counted 12 to 14 days later.
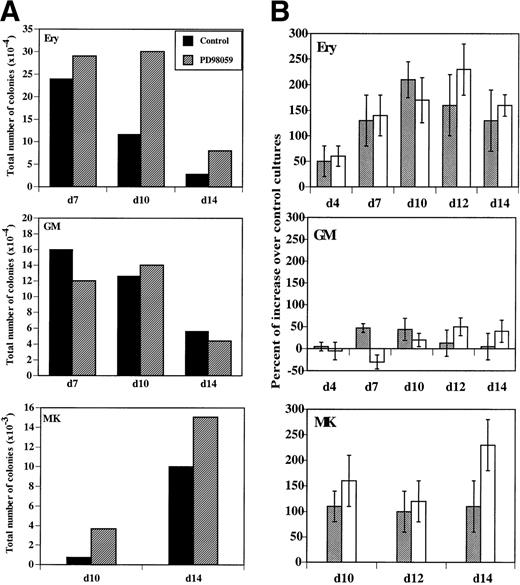
MK colonies were derived from cells, which had received the MEK inhibitor during the liquid cultures and in greater number than in controls (Fig 8A). Both the relative (number of colonies/1,000 cells) and the absolute number of colonies present in the cultures were increased in the presence of PD98059 (Fig8A and B). This increase was consistently observed in 5 of 5 independent experiments. The median number of MK colonies/1,000 cells formed with cells cultured 12 days without and with PD98059 was respectively, 1.9 (range, 0.4 to 4) and 3.9 (range, 1.1 to 7). The increased clonogenic capacity of PD98059-treated cells confirms that activation of the MAPK pathway negatively regulates proliferation of megakaryocytic progenitors.
Evolution of erythroid (Ery), megakaryocytic (MK), and GM clonogenic progenitors in MGDF-induced cultures. Clonogenic progenitors in cultures performed in the absence or the presence of 6 μmol/L PD98059 were quantified at the indicated days by semisolid culture assays as described in Materials and Methods. (A) Total number of colonies formed with control (▪) or PD98059-treated cultures (▨). The results from a representative experiment, where determinations were done in duplicate, are shown. (B) Percent of increase in relative (▩) or total (□) colony numbers obtained with PD98059-treated cells as compared with control cells. Results are mean ± SD of at least 4 independent experiments for each type of colony.
Evolution of erythroid (Ery), megakaryocytic (MK), and GM clonogenic progenitors in MGDF-induced cultures. Clonogenic progenitors in cultures performed in the absence or the presence of 6 μmol/L PD98059 were quantified at the indicated days by semisolid culture assays as described in Materials and Methods. (A) Total number of colonies formed with control (▪) or PD98059-treated cultures (▨). The results from a representative experiment, where determinations were done in duplicate, are shown. (B) Percent of increase in relative (▩) or total (□) colony numbers obtained with PD98059-treated cells as compared with control cells. Results are mean ± SD of at least 4 independent experiments for each type of colony.
More surprisingly, a similar consistent increase in erythroid colonies was also found (Fig 8A and B). There was a wide interindividual variation, but in every case (6 of 6 independent experiments) inhibition of the MAPK pathway resulted in an augmentation of the number of erythroid colonies: median 5.1 (range, 0.8 to 10) and 10.4 (range, 2.3 to 28) colonies/1,000 cells for control and PD98059-treated cells, respectively, after 10 days of culture. Moreover, the average size of the erythroid colonies was larger in the presence of PD98059, as compared with controls (data not shown), suggesting the presence of more immature erythroid progenitors in these cultures.
By contrast, inhibition of MAPK activation did not significantly alter the formation of GM colonies (Fig 8A and B): the median number of GM colonies/1,000 cells was 11 (range, 0.25 to 30) and 14 (range, 0.5 to 31) in the absence or presence of PD98059, respectively. To further examine whether the MAPK pathway could influence the formation of colonies other than erythroid or megakaryocytic, CD34+cells were cultured with G-CSF and Flt-3 ligand, conditions allowing exclusively the development of granulocytic progenitors. In these conditions, no difference was observed in the number of colonies developed between cultures containing the PD98059 or control (data not shown).
Morphological examination of cells from liquid cultures.
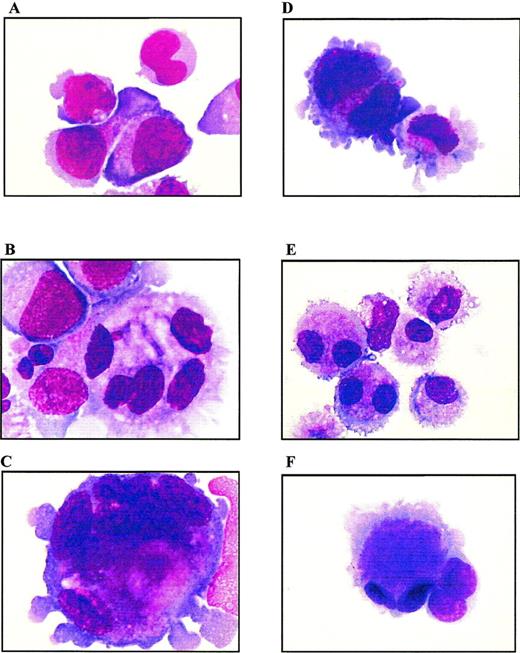
Cells from both PD98059-treated cultures or untreated controls were examined at days 7, 10, 13, and 17 of culture by MGG staining. Analysis was performed on cytocentrifugation products of 3 independent experiments. Whereas day 7 cells from both PD98059-treated or control cultures displayed a similar immature blastic morphology (not shown), striking differences appeared from day 10 (Fig 9A and D). At day 13, control cultures contained a majority of mature micromegakaryocytes (more than 90%) and only 6% of blast cells (Fig 9E and F). By contrast, MEK-treated cultures showed a slower differentiation with the presence, at day 13, of 55% of blast cells and only 45% of mature megakaryocytes and micromegakaryocytes (Fig 9B and C). In these cultures, the presence of undifferentiated blast cells was observed until day 17 (not shown). Examination of cells at the end of the culture (from day 17) showed drastic differences between PD98059-treated cells and controls, in agreement with the proliferation curves shown Fig 2: a mixture of immature and mature living cells was maintained in the presence of the MEK inhibitor, whereas massive cell necrosis was observed in controls (not shown).
Morphologic analysis of cells from liquid cultures. MGG staining of cytocentrifugated cells from PD98059-treated (A to C) or control (D to F) cultures, at day 10 (A and D) and day 13 (B, C, E, and F); original magnification × 100.
Morphologic analysis of cells from liquid cultures. MGG staining of cytocentrifugated cells from PD98059-treated (A to C) or control (D to F) cultures, at day 10 (A and D) and day 13 (B, C, E, and F); original magnification × 100.
On the other hand, PD98059-treated cultures contained much more megakaryoblastic cells of larger size and with a polylobulated nucleus than controls: the number of polyploid cells averaged 10% and 30% in control and PD98059-treated cultures, respectively. These results are in agreement with previous studies39 40 showing that CB-derived megakaryocytes exhibit a high proliferative capacity without maturation to hyperploid cells. Among the polyploid cells, differences in maturation clearly appeared between the 2 cultures. Indeed, more than half of the polyploid cells present in PD98059-treated cultures had a blastic immature morphology, while more than 95% of polyploid cells from control cultures were mature megakaryocytes. Measurement of cell DNA content after propidium iodide staining confirmed that megakaryocytes from control cultures have a low ploidy with most of the cells retaining 2N and 4N ploidy status throughout the culture period (data not shown). No major difference in the frequency of polyploid cells between control cultures and those containing PD98059 could be detected by FACS analysis. The discrepancy between these data and those obtained from morphological examination is probably due to the relatively low number of polyploid cells even in PD98059-treated cultures and to the high heterogenity in the ploidy class among these cells, so that the number of cells in a given ploidy class might be too low to be detected by FACS analysis.
DISCUSSION
Investigation on the function of the MAPK pathway has been greatly aided recently by the discovery of inhibitors acting specifically on MEK1 and 2 and preventing efficiently ERK activation when added to intact cells.35 Such inhibitors are now widely used to study the role of ERK in various cell lines or primary cells. Here, we show that the MEK inhibitor can be added to long-term cultures of normal human hematopoietic CD34+ progenitors grown in the presence of MGDF, without displaying toxicity. A low concentration of PD98059 (6 to 10 μmol/L) was found to fully inhibit ERK activation induced by MGDF. At this concentration, PD98059 increased MGDF-induced progenitor proliferation, while delaying their megakaryocytic differentiation. Previous studies have shown that at concentrations up to 50 μmol/L, PD98059 inhibits the MEK kinases without affecting a variety of other kinase activities, including the related JNK and p38 MAPK pathways in different cellular models.35,41 Very recently, however, PD98059 has been shown to act as a direct inhibitor of cyclooxygenases.42 Indomethacin, at a dose currently used to inhibit both cyclooxygenase-1 and -2,42 had no effect on MGDF-induced proliferation of CD34+ progenitors (data not shown), ruling out that inhibition of cyclooxygenase might be responsible for the observed effect of PD98059. Previous studies in cell lines have shown that PD98059 and dominant-negative mutant of MEK have a similar inhibitory effect on megakaryocytic differentiation.30,31 In addition, a very recent study has reported that the megakaryocytic-specific enhancer of the α2 integrin gene is directly controlled by MAPKs,43 further suggesting a role of the MAPK pathway in megakaryocytic differentiation. These data support the results described here and suggest that the delay in megakaryocytic differentiation induced by PD98059 is most probably related to its ability to inhibit MAPK activation, although we cannot definitively eliminate an effect of PD98059 on unknown processes.
One of the most surprising findings of this study is the increase in proliferation of the cells in response to MGDF on treatment with the MEK inhibitor. This effect seems to be mainly due to an increase in DNA synthesis rather than to a reduction in the proportion of cells in apoptosis. This phenotype is at odds with the well-admitted role played by the MAPK signaling modules in mitogenesis induced by growth factors and in the aberrant cell proliferation associated with neoplastic transformation in mammals.9,10,17 However, an antiproliferative role of the MAPK pathway is not unprecedented. Several studies have reported that activation of the MAPK pathway by growth factors or overexpression of constitutively activated forms of kinases of this pathway can lead to cell-cycle arrest, senescence, or even apoptosis.34,44-47 In a given cellular context, the ultimate cellular response to activation of the MAPK pathway has been shown to depend on the strength and duration of the signal: transient or acute activation may contribute to cell-cycle progression, whereas a chronic high activity may result in cell-cycle arrest.34,45-48 MGDF was found to induce sustained ERK activation both in erythromegakaryocytic leukemia cell lines29 and here in normal megakaryocytes derived from CB progenitors. ERK activation has been reported for most members of the cytokine receptors and works on hematopoietic cell lines or normal progenitors have documented the requirement for this pathway in proliferation27,28,49-52 and/or survival52,53in response to EPO, GM-CSF, IL-3, or G-CSF. However, the sustained or transient nature of the MAPK activity was not reported in these models. Interestingly, the pro-proliferative effects of the MEK inhibitor were found only in cultures of CB progenitors performed in the presence of MGDF: PD98059 was found to inhibit cell proliferation in cultures containing a mixture of IL-3, SCF, and IL-6, and it had no effect on cell numbers in cultures grown with G-CSF plus Flt3-ligand. Likewise, in the UT7-Mpl cell line, PD98059 inhibits cell growth in the presence of either GM-CSF or EPO, while it enhances MGDF-induced DNA synthesis29 (and unpublished data, December 1996). In this system, both GM-CSF and EPO can induce MAPK activation, but this activation is more transient and less intense than that triggered by MGDF.29 54 Thus, the use of the MAPK pathway to transduce events other than proliferation or prevention of apoptosis seems to be specific for Mpl and does not reflect a general feature of cytokine receptor signaling.
Very recently, it has been shown that the ultimate cellular response of constitutive activation of the MAPK cascade, cell-cycle arrest or transformation, is dependent on the integrity of the cell-cycle machinery signal, ie, that it can be opposite whether the context is a normal or an immortalized cell.34 The increase in MGDF-induced thymidine incorporation and long-term cell growth induced by the MEK inhibitor in both normal hematopoietic progenitors and leukemic cell lines indicates that the antiproliferative function of the MAPK pathway in cells of the megakaryocytic lineage is not specifically linked to a normal cellular context.
Apart from the MAPK pathway, MGDF has been shown to trigger phosphorylation and activation of many transducing molecules including members of the JAK and STAT family, phosphatidylinositol 3-kinase (PI3-K) or the ERK-related JNK and p38 MAPK.33,55-59 A recent study60 has shown that dominant negative forms of STAT5, but not those of STAT1 or STAT3, were able to reduce MGDF-induced proliferation of F-36-P cells expressing Mpl, suggesting that STAT5 could be involved, at least partially, in mitogenesis induced by MGDF. An inhibition of similar magnitude was also detected on expression of a dominant negative mutant of Ras in these cells.60 Among the Ras effectors, PI3-K is a good canditate that might participate in MGDF mitogenic response, as this kinase has recently been shown to couple growth factor receptors to the cell-cycle machinery and antiapoptotic signals.46 61 Further studies will be required to determine whether 1 or several of these signaling cascades are essential for MGDF-induced proliferation and/or survival of megakaryocytes.
In most cell lines, cell-cycle arrest is combined with differentiation.22-24,30,31,47,48 Likewise, it has been reported that megakaryocytic progenitor cells represent a continuum of cells from high to low proliferative capacities associated with changes in the surface phenotype.37,38 Notably, a decrease in progenitor proliferation has been described on the loss of the CD34 marker of immature progenitors, ie, on transition from the CD34+CD61+ to the CD34−CD61+ phenotype.37Accordingly, the increase in proliferation of the cells observed in cultures containing the MEK inhibitor was combined with several developmental changes indicative of a delay in the differentiation process. First, although the MEK inhibitor mostly did not affect the expression of megakaryocytic markers CD41 or CD42b, PD98059-treated cells tended to retain markers of immature progenitors, as shown by the increased proportion of both CD34+ and double-positive CD41+CD34+ cells in cultures containing the MEK inhibitor, as compared with control cultures. Second, morphologic studies clearly showed that the cultures containing the MEK inhibitor were greatly enriched in immature blast cells even after extended culture (days 17 to 21), while control cultures presented a majority of mature megakaryocytes around day 13. This analysis also showed an increase in polyploid cells of large size in PD98059-treated cells. Although surprising, this observation fits well with a previous study showing that cytoplasmic and nuclear maturation are dissociated, and that polyploid cells can be found among immature megakaryocytic precursors: despite their more immature state, the CD34+CD41+ and CD34+CD42b+ cell populations were found to contain a higher proportion of polyploid cells than the CD34−CD41+ and CD34−CD42b+ populations, respectively.37 Thus, this suggests that the expression of CD34 is related to the ability of megakaryocytic precursors to accomplish DNA synthesis, either mitosis or endomitosis. The higher polyploidization capacity of CD34+CD41+ cells may be explained by the presence, among the CD34−CD41+ cells of micromegakaryocytes, ie, mature megakaryocytes, which have no endomitotic capacity. Supporting this possibility, we observed a majority of mature small megakaryocytes in control cultures. These results are in agreement with the low ploidy of megakaryocytes derived ex vivo from CB, even in the presence of MGDF.39 40 Finally, the delayed maturation of megakaryocytes induced by the MEK inhibitor was also supported by the increased megakaryocytic colony formation with cells derived from cultures treated with PD98059. Thus, the MAPK pathway, although not required for megakaryoblast formation, may regulate the transition from proliferation to maturation in this lineage
Consistently higher numbers of erythroid colonies of large size were formed with PD98059-treated cells, indicating the presence of erythroid progenitors both more numerous and more immature in these cultures than in control cultures. This result suggests that the MEK inhibitor could affect the differentiation of erythroid progenitors generated in the cultures and/or the differentiation of a bipotent erythromegakaryocytic progenitor.62 The absence of a consistent alteration in GM colony numbers in the presence of PD98059 supports this possibility. Our experiments did not allow direct assessment of erythroid differentiation. However, because erythroid differentiation cannot be studied in the presence EPO alone and requires cytokine mixtures, the involvement of the MAPK pathway in erythroid lineage differentiation might not be easily detected in such a culture system.
During T-cell development, activation of the ERK pathway has been shown to influence commitment by favoring the differentiation into the CD4 versus the CD8 lineage.63 Studies performed with bipotent erythromegakaryocytic cell lines have shown that activation of MAPK pathway in K562 cells, while increasing megakaryocytic-specific antigens, suppresses expression of hemoglobin, a key marker of erythroid differentiation.30 Conversely, inhibition of MAPK activity, even at the basal level, results in increased expression of globins and prevents phorbol myristate acetate (PMA)-induced downregulation of erythroid markers in K562 cells. In UT7 cells, increased surface expression of GpA was induced even in the presence of GM-CSF on inhibition of basal ERK activity (F. Porteu, unpublished data, March 1998). Thus, it was suggested that MAPK might also control erythroid versus megakaryocytic lineage commitment. The present study does not support this hypothesis. Indeed, first, PD98059 did not prevent the commitment towards the megakaryocytic lineage. Second, no cells expressing the erythroid marker, GpA, were generated in the cultures grown with TPO in the absence of EPO.
The best characterized of MAPK targets are members of the ETS and AP-1 families of transcription factors. Phosphorylation has been shown to play a critical role in modulating activity of transcription factors.64 ERKs also phosphorylate and regulate the activities of transcription factors of the STAT family65and of various cytosolic molecules involved in signal transduction such as kinases18,19 or effectors of apoptosis.66Further studies are currently underway to characterize ERK-specific targets in megakaryocytes.
ACKNOWLEDGMENT
We thank Amgen for providing PEG-rhuMGDF and SCF. We also greatfully aknowledge Dr I. Dusanter-Fourt for critical review of the manuscript and I. Bouchaert (ICGM) for help in cytometry analysis.
Supported by INSERM and by the Comité de Paris from the Ligue Nationale Contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Françoise Porteu, PhD, ICGM, INSERM U363, Hôpital Cochin, 27 rue du Fg St Jacques, 75014, Paris, France; e-mail: porteu@cochin.inserm.fr.

![Fig. 4. Effect of the MEK inhibitor on DNA synthesis capacity of CD34+ cells grown with rhuMGDF. Cells from cultures performed in the absence (▪) or presence (▨) of 6 μmol/L PD98059 were harvested at the indicated days and DNA synthesis was assessed by measuring [3H]thymidine incorporation. Results are mean ± SD of triplicate determinations from a representative experiment of 3 performed. The relative increase in [3H]thymidine incorporation in PD98059-treated cells as compared with control DMSO-treated cells is indicated below the bars for each time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1601/5/m_blod41719004x.jpeg?Expires=1767721341&Signature=DNOReQDUmGfC9v5Zyosik14qbctaqFs-cbs36sPagY1--KogAzNUwUsDS3-JZcSP8wQPoXQH3YmYhGTzP1wUq5zbWKNTgcYAecz~zZ~GyxxYHzNQDGP2pH1QCWw7P8kZUh8nWdP9qNU0kxnjUmAdM~5QvhUf~R~BTxgYX723heUvX8kROIuO5TUZLoZ4toyiI8kTnCYog0XfTqIrMZC1D6VbjlxvwlZi8RO~~Od9x9mlCuxbETAjRJ-HCun3XtMNHkcB6QXZpN4h5ATS1Q7XY3mrAAYybyp0AxhRyBTKtStgBpxWZw8F5YE89VOUpJ7OOliQJSXi1oPyLhFe7vsQFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal