Abstract
Several clinical studies have demonstrated an inverse relationship between circulating levels of estrogen and tissue-type plasminogen activator (t-PA). The present study was designed to test the hypothesis that estrogens lower plasma levels of t-PA by increasing its clearance from the bloodstream. 17-Ethinyl estradiol (EE) treatment resulted in a significant increase in the clearance rate of recombinant human t-PA in mice (0.46 mL/min in treated mice v 0.32 mL/min in controls; P < .01). The clearance of endogenous, bradykinin-released t-PA in rats was also significantly increased after EE treatment (area under the curve [AUC], 24.9 ng/mL · min in treated animals v 31.9 ng/mL · min in controls; P < .05). Two distinct t-PA clearance systems exist in vivo: the low-density lipoprotein receptor-related protein (LRP) on liver parenchymal cells and the mannose receptor on mainly liver endothelial cells. Inhibition of LRP by intravenous injection of receptor-associated protein (RAP) as a recombinant fusion protein with Salmonella japonicum glutathione S-transferase (GST) significantly retarded t-PA clearance in control mice (from 0.41 to 0.25 mL/min; n = 5, P < .001) and EE-treated mice (from 0.66 to 0.35 mL/min; n = 5, P < .005), but did not eliminate the difference in clearance capacity between the 2 experimental groups. Similar results were obtained in mice in which LRP was inhibited via overexpression of the RAP gene in liver by adenoviral gene transduction. In contrast, administration of mannan, a mannose receptor antagonist, resulted in identical clearances (0.22 mL/min in controls and 0.24 mL/min in EE-treated mice). Northern blot analysis showed a 6-fold increase in mannose receptor mRNA expression in the nonparenchymal liver cells of EE-treated mice, whereas the parenchymal LRP mRNA levels remained unchanged. These findings were confirmed at the protein level by ligand blotting and Western blotting analysis. Our results demonstrate that EE treatment results in increased plasma clearance rate of t-PA via induction of the mannose receptor and could explain for the inverse relationship between estrogen status and plasma t-PA concentrations as observed in humans.
THE FIBRINOLYTIC SYSTEM, which is responsible for the dissolution of fibrin in the circulation, requires precise regulation to ensure that it is neither deficient nor excessive. The physiological importance of the system in humans is demonstrated by associations between impaired fibrinolysis and thrombotic events on the one hand and between excessive fibrinolysis and bleeding complications on the other. Clinical and epidemiological studies suggest that lowered fibrinolytic activity, resulting in enhanced fibrin deposition, is a significant contributor to the development of atherothrombosis (for a review, see Emeis et al1 and references therein).
Tissue-type plasminogen activator (t-PA) is a key enzyme in the fibrinolytic process that converts the inactive proenzyme plasminogen to plasmin, a broad-spectrum protease that cleaves fibrin. Human t-PA is also of particular pharmacological interest because of its value in the treatment of thromboembolic disorders.2 t-PA activity in blood is regulated at several levels, including controlled synthesis and release of t-PA from the vascular endothelium,3 the presence of the physiological inhibitor plasminogen activator inhibitor type-1 (PAI-1),4 and rapid hepatic clearance of t-PA.5 It is well-documented that the synthesis of t-PA (and PAI-1) is influenced by a variety of exogenous factors, such as hormones, cytokines, growth factors, and vasoactive compounds.6 Little is known about the regulation of hepatic clearance capacity. Changes in t-PA clearance rate under various physiological and experimental conditions are usually attributed to the more rapid clearance of t-PA as compared with that of t-PA/PAI-1 complexes7 and/or to changes in the liver blood flow.8
There are several indications of an inverse correlation between plasma estradiol levels and plasma t-PA levels. Firstly, lower plasma concentrations of t-PA are found in women as compared with men.9-11 Secondly, the use of estrogens by women on oral contraceptives or hormone replacement significantly decreases plasma t-PA levels.11-13 Thirdly, the administration of estradiol (in combination with cyproterone acetate) to male-to-female transsexual subjects markedly reduces circulating t-PA concentrations.14 To estimate basal t-PA synthesis in the transsexual subjects, a venous occlusion test was performed. No significant difference in increment in venous plasma t-PA levels in response to occlusion of the upper arm was found in the subjects before and after hormone treatment (Emeis and Giltay, unpublished observations), suggesting that estradiol lowers circulating t-PA levels by increasing t-PA clearance rather than altering t-PA synthesis. In line with this, in vitro studies using cultured human vascular endothelial cells failed to demonstrate a direct effect of estrogens on t-PA synthesis.15 In the present communication, we report that 17α-ethinyl estradiol (EE) treatment enhances the clearance rate of endogenous t-PA in rats and of exogenously administered recombinant human t-PA in mice. Two major t-PA receptors exist in liver, the low-density lipoprotein (LDL) receptor-related protein (LRP) on predominantly liver parenchymal cells and the mannose receptor mainly on liver endothelial cells.5 By using specific receptor antagonists, we show that the increase in clearance of t-PA in EE-treated mice occurs via the mannose receptor. The role of the mannose receptor in the clearance of t-PA is further substantiated by demonstrating an EE-increased mannose receptor expression at the mRNA and protein level. These results provide an explanation for the inverse relationship between estradiol and plasma t-PA concentrations as observed in clinical studies.
MATERIALS AND METHODS
Materials.
Recombinant human t-PA expressed in Chinese hamster ovary cells (Activase) was obtained from Genentech (San Francisco, CA). 17α-Ethinyl estradiol, bradykinin, mannan, and peanut (arachis) oil were purchased from Sigma Chemical Co (St Louis, MO). Hypnorm (10 mg/mL fluanison and 0.315 mg/mL fentanyl citrate) was from Janssen Pharmaceutica (Tilburg, The Netherlands) and Midazolam (5 mg/mL) from Roche (Mijdrecht, The Netherlands). Collagenase (type IV) was purchased from Boehringer Mannheim (Mannheim, Germany). The mouse monoclonal human mannose receptor antibodies were prepared in our laboratory.16 Enzyme immunoassay kits for determination of human t-PA antigen (Thrombonostika t-PA) were obtained from Organon Teknika (Boxtel, The Netherlands). Materials used in the rat t-PA immunoassay (enzyme-linked immunosorbent assay [ELISA]) have been described previously.17 Receptor-associated protein (RAP) was produced as a recombinant fusion protein withSalmonella japonicum glutathione S-transferase (GST) and purified according to a combination of the methods described by Herz et al18 and Warshawsky et al.19 The RAP-GST plasmid was kindly provided by Dr J. Herz (University of Texas, Dallas, TX). Human trypsin-activated α2-macroglobulin was prepared as described by Ziere et al.20 Other materials used have been specified in the methods described or in the related references.
Preparation of recombinant adenoviral vectors.
The recombinant adenoviral vectors expressing either the RAP gene (Ad-RAP) or the β-galactosidase gene (Ad-β-Gal) under the control of cytomegalovirus (CMV) promoter were kindly provided by Drs T. Willnow and J. Herz, respectively.21,22 The generation of these vectors and the propagation and titration of the recombinant adenovirus have been described previously.23For in vivo adenovirus infection, the virus was subjected to 2 rounds of purification by CsCl gradient centrifugation followed by extensive dialysis against a buffer containing 25 mmol/L Tris-HCl, 137 mmol/L NaCl, 5 mmol/L KCl, 0.73 mmol/L Na2HPO4, 0.9 mmol/L CaCl2, 0.5 mmol/L MgCl2, pH 7.45, at 4°C. After dialysis, mouse serum albumin was added to 0.2% (wt/vol) and glycerol to 10% (vol/vol), and aliquots of the virus stocks were frozen in liquid N2 and stored at −80°C. Routinely, virus titers of the stocks varied from 1 to 5 × 1010 plaque-forming units (PFU)/mL. PFU (3 × 109) in a total volume of 200 μL (diluted with phosphate-buffered saline [PBS]) were injected into the tail vein of LDL receptor (LDLR)-deficient mice. t-PA clearance studies were performed at day 5 after virus injection.
Animals.
C57 black6/B6 mice and Wistar rats were obtained from Iffa-Credo (Someren, The Netherlands). LDLR-deficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME). For experiments, male rats (8 to 12 weeks old) and male mice (8 to 14 weeks old) were used. The animals were housed as an experimental group with a 12-hour light cycle and free access to drinking water and standard (chow) diet. All experimental procedures were performed in accordance with The Netherlands law on experiments with animals.
Estradiol treatment.
Rats and mice were injected 4 times subcutaneously with EE in arachis oil (5 mg/kg body weight/day), either during 4 consecutive days or on days 1, 4, 5, and 6. The LDLR-deficient mice (which were used in parallel for very low-density lipoprotein clearance studies) received 3 subcutaneous injections of 100 μg EE in arachis oil (1 injection every 2 weeks); control animals received arachis oil only. Experiments were always performed 1 day after the last estradiol injection in case of the short-term treatment or 2 weeks after the third estradiol injection in case of the long-term treatment of LDLR-deficient mice. No differences in t-PA clearance characteristics were observed for the various EE treatment protocols.
Clearance of bradykinin-released t-PA in rats.
EE- or vehicle-treated rats were anesthetized with intraperitoneal Nembutal (60 mg/kg body weight) and cannulated, and bradykinin (50 μg/kg body weight) was injected as a bolus into the vein of the penis. Blood was collected immediately before and at different time intervals (1 to 10 minutes) after bradykinin injection through a carotid artery cannula, and citrated plasma was prepared. Rat t-PA antigen concentrations were determined in citrated plasma by ELISA.17
Plasma clearance of exogenous t-PA in mice; effect of inhibitors.
EE- or vehicle-treated mice were anesthetized by injection of 60 μL Hypnorm/30 g body weight and 40 μL Midazolam/30 g body weight. Fifteen micrograms of recombinant human t-PA in 200 μL sterile saline was injected into a tail vein. Blood (35 to 40 μL) was collected immediately after t-PA injection and at different time intervals (1 to 10 minutes) thereafter, and citrated plasma was prepared. Human t-PA antigen levels were determined in citrated plasma by ELISA. In studies in which mannan (5 mg/kg body weight) was administered, competitor was injected 1 to 3 minutes before administration of t-PA. RAP-GST (40 mg/kg body weight) was preinjected 1 minute before t-PA injection. In case of Ad-RAP or Ad-β-Gal infections, LDLR-deficient mice were injected with 3 × 109 PFU of virus in a total volume of 200 μL (diluted with PBS) into a tail vein, 5 days before the t-PA clearance studies. At the end of experiments, livers were rapidly removed and immediately frozen in liquid nitrogen for preparation of total RNA or membrane fragments.
Isolation of liver cells.
Mouse parenchymal and nonparenchymal liver cells were isolated by a procedure similar to one developed for rats.24 In short, the liver was preperfused for 8 minutes with Ca2+-free Hanks’ buffer, followed by a 8 minutes perfusion with Hanks’ buffer containing 0.05% (wt/vol) collagenase (flow rate, 14 mL/min). The resulting cell suspension was filtered, and parenchymal and nonparenchymal cells were subsequently separated by differential centrifugation and density gradient centrifugation as described in detail earlier.24
Isolation of total RNA and Northern blot analysis.
RNA from liver was prepared following the procedure of Chomczynski and Sacchi.25 In short, frozen liver samples were triturated in liquid nitrogen in a mortar, resuspended at 100 mg/mL in guanidinium thiocyanate/phenol-chloroform extraction buffer, and homogenized mechanically using a motor-driven Potter-Elvehjem homogenizer (10 strokes at 0°C). Total RNA was isolated and electrophoresed in a 1% (wt/vol) agarose gel under denaturing conditions using 1 mol/L formaldehyde, blotted, and hybridized as described previously.26 The following cDNA fragments were used as probes in the hybridization experiments: a 6-kb XhoI-EcoRI fragment of the human LRP cDNA,27 a 5.1-kb full-length EcoRI fragment of the mouse mannose receptor28 (kindly provided by Dr R. Ezekowitz, Harvard University, Boston, MA), and a 1.2-kb Pst I fragment of a rat glyceraldehyde-3-phosphate dehyrogenase (GAPDH) cDNA provided by Dr R. Offringa (Leiden University, Leiden, The Netherlands).
Preparation of membrane fragments and ligand-blotting analysis.
Frozen liver samples were triturated in liquid nitrogen in a mortar and solubilized (at 60 mg/mL) in PBS containing 0.05% (vol/vol) Tween 20. After homogenizing using a motor-driven Potter-Elvehjem homogeniser (10 strokes at 0°C), debris and nuclei were removed by centrifugation at 1,000g for 15 minutes. The supernatants containing the membrane fragments were stored at −20°C until use. Samples (6 μL) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 5% gel using an SDS-concentration of 0.1% (wt/vol). After electrophoresis, the proteins were transferred electophoretically to Protan nitrocellulose filters (Schleicher and Schuell, Dassel, Germany) in a buffer of 25 mmol/L Tris-HCl, 190 mmol/L glycine, pH 8.6, 20% (vol/vol) methanol, and 0.1% (wt/vol) SDS at 1 mA/cm2 overnight, using a Pharmacia-LKB semi-dryblott apparatus (Pharmacia-LKB, Uppsala, Sweden). The filters were blocked with 10 mmol/L Tris-HCl, pH 8.0, containing 150 mmol/L NaCl, 1% (wt/vol) skim milk, and 0.1% (vol/vol) Tween-20 for 1.5 hours, followed by incubation with 125I-GST-RAP at a concentration of 2 nmol/L in 10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.5% (wt/vol) skim milk, and 0.1% (vol/vol) Tween-20 at room temperature for 3 hours. After incubation, the filters were washed extensively with 30 mL 10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, and 0.1% (vol/vol) Tween-20 for 3 hours. Bound ligand was visualized by autoradiography.
Western blotting.
For Western blotting, samples (7.5 μL) of membrane fragments were diluted 1 to 1 in Laemmli incubation buffer (50 mmol/L Tris-HCl pH 6.8, 1% [wt/vol] SDS, 10% [vol/vol] glycerol, and 8 mol/L urea) and separated on a 5% to 18% (wt/vol) Laemmli gel. After electrophoresis, the proteins were transferred to Protan nitrocellulose in a buffer of 192 mmol/L glycine, 25 mmol/L Tris-HCl (pH 8.3), and 10% (vol/vol) methanol at 300 mA/cm2 overnight using a wet-blot apparatus (Hoefer Scientific Instruments, San Francisco, CA). The filters were blocked with 1% (wt/vol) skim milk in buffer A consisting of 0.5 mol/L NaCl, 20 mmol/L Tris-HCl (pH 7.5), and 0.05% (vol/vol) Tween 20 for 0.5 hour at room temperature, followed by incubation with a mouse mannose receptor monoclonal antibody (MoAb 15.2)16 at a concentration of 1 μg/mL in buffer A for 1.5 hours at room temperature. Next, the blots were washed 3 times with buffer A and incubated for 1.5 hour at room temperature with rabbit antimouse RaM-PO (1:5,000; Nordic, Tilburg, The Netherlands) as a conjugate. Finally, the blots were stained with the peroxidase substrate BM-blue (Boehringer Mannheim, Mannheim, Germany).
Statistics.
The unpaired Student’s t-test was used to determine statistical significance of the values obtained.
RESULTS
Effect of estrogen treatment on plasma clearance of t-PA in rats and mice.
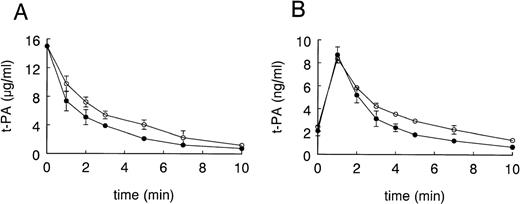
To evaluate whether estrogen treatment influences the clearance of t-PA, we pursued 2 approaches. Firstly, we determined the disappearance of exogenous recombinant human t-PA in EE-treated and control mice. As shown in Fig 1A for a bolus injection of 15 μg of t-PA, clearance of t-PA was significantly faster in EE-treated mice than in control mice (0.47 ± 0.04 mL/minv 0.33 ± 0.04 mL/min, respectively; n = 5, P< .01). Secondly, we determined the effect of EE on the clearance of endogenous, bradykinin-released t-PA.29 30 For practical reasons of blood sampling, these studies were performed in rats (Fig 1B). At baseline, before the infusion of bradykinin, plasma t-PA antigen levels were 2.0 ± 0.14 ng/mL and 2.5 ± 0.04 ng/mL in EE-treated and control rats, respectively (n = 6, P < .05). Plasma t-PA antigen levels peaked in both experimental groups 1 minute after bradykinin injection and peak levels were not significantly different in the EE-treated rats (8.7 ng/mL) versus the control rats (8.4 ng/mL), indicating that bradykinin had induced similar amounts of t-PA in both groups. t-PA antigen subsequently decreased faster in the EE-treated group than in the control group (area under the curve [AUC] of 24.9 ± 3.4 ng/mL · min and 31.9 ± 2.6 ng/mL · min, respectively; P < .05). Analysis of the clearance curve showed that release of t-PA was completed at 1 minute. On the assumption that the biosynthesis of t-PA is unaffected, we can calculate from the observed change in clearance and a plasma t-PA value in control rats of 2.5 ± 0.04 ng/mL a steady-state plasma t-PA concentration after estradiol treatment of 1.95 ± 0.23 ng/mL as compared with an experimentally determined value of 2.0 ± 0.14 ng/mL in EE-treated rats. The observed change in plasma t-PA clearance therefore fully explains the observed decrease in plasma t-PA concentration after EE treatment of the rats.
Effect of EE treatment on plasma clearance of exogenous human t-PA in mice and endogenous, bradykinin-released t-PA in rats. As described in Materials and Methods, mice received injections into a tail vein with 15 μg of recombinant human t-PA (A) or rats received injections into a tail vein with 50 μg/kg body weight bradykinin (B), after pretreatment of the animals with EE or vehicle. Blood samples were collected at the indicated times and t-PA antigen concentrations were determined by ELISA. Data points are the mean ± SD and represent 1 of 3 similar experiments performed in 5-fold (A) and 1 experiment performed in triplicate (B). (○) Vehicle; (•) EE.
Effect of EE treatment on plasma clearance of exogenous human t-PA in mice and endogenous, bradykinin-released t-PA in rats. As described in Materials and Methods, mice received injections into a tail vein with 15 μg of recombinant human t-PA (A) or rats received injections into a tail vein with 50 μg/kg body weight bradykinin (B), after pretreatment of the animals with EE or vehicle. Blood samples were collected at the indicated times and t-PA antigen concentrations were determined by ELISA. Data points are the mean ± SD and represent 1 of 3 similar experiments performed in 5-fold (A) and 1 experiment performed in triplicate (B). (○) Vehicle; (•) EE.
Effect of RAP and mannan on the plasma clearance of t-PA in mice.
The clearance of t-PA from the circulation is mainly mediated via 2 independent receptor systems, the LRP and the mannose receptor. To gain more insight into the receptor system responsible for the EE-induced increase in t-PA clearance, we blocked uptake of t-PA by each of the 2 receptor systems by specific antagonists.
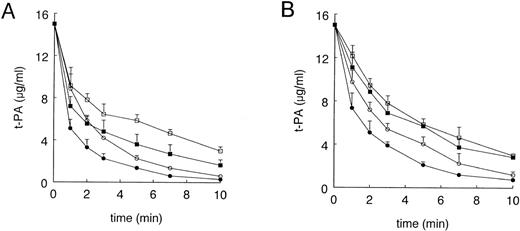
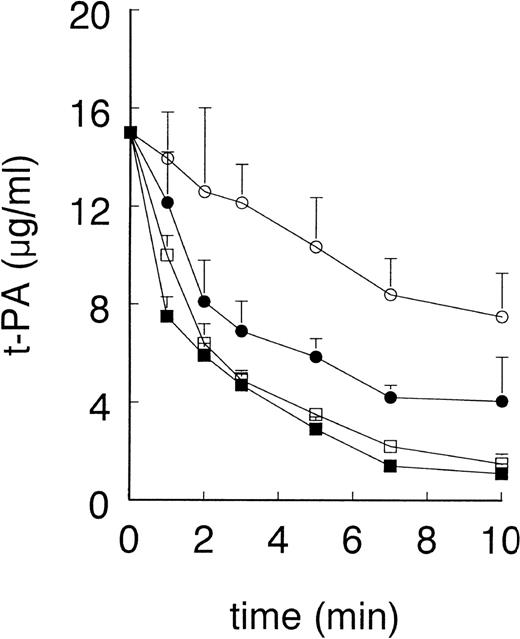
Preinjection of GST-RAP (40 mg/kg), a dose previously shown to block t-PA clearance via LRP in rats,31 reduced t-PA clearance in control mice (from 0.41 ± 0.02 mL/min to 0.25 ± 0.02 mL/min, n = 4) and in EE-treated mice (from 0.66 ± 0.08 mL/min to 0.35 ± 0.05 mL/min, n = 5), but the difference in clearance of t-PA between the 2 experimental groups remained maintained (Fig 2A). Because GST-RAP is very rapidly cleared from the blood circulation,20 we also evaluated the functional effect of RAP that was overexpressed in the liver of mice using an adenoviral gene transfer technique.32 As a control for LRP blockade, we measured the clearance of radiolabeled α2-macroglobulin, a specific ligand for LRP. In accordance with the data shown by Narita et al,32 5 days after injection with AdCMV-RAP, mice were unable to clear α2-macroglobulin from the circulation, whereas the control, Ad-LacZ–infected animals rapidly cleared this ligand for LRP (data not shown). Also, in the Ad-RAP–infected mice clearance of t-PA was increased in EE-treated mice as compared with control animals (0.24 ± 0.04 mL/min v 0.14 ± 0.02 mL/min, n = 3, P< .01; Fig 3).
Effect of GST-RAP and mannan on the plasma clearance of t-PA in control and EE-treated mice. As described in Materials and Methods, EE- or vehicle-treated mice received injections into a tail vein with 15 μg of recombinant human t-PA, 1 minute after preadministration of 40 mg/kg body weight GST-RAP or PBS (A) or 1 to 3 minutes after preadministration of mannan or PBS (B). Blood samples were collected at the indicated times and t-PA antigen concentrations were determined by ELISA. Data points are the mean ± SD of 4 or 5 mice in each treatment group. (A) (○) Vehicle; (•) EE; (□) vehicle and GST-RAP; (▪) EE and GST-RAP. (B) (○) Vehicle; (•) EE; (□) vehicle and mannan; (▪) EE and mannan.
Effect of GST-RAP and mannan on the plasma clearance of t-PA in control and EE-treated mice. As described in Materials and Methods, EE- or vehicle-treated mice received injections into a tail vein with 15 μg of recombinant human t-PA, 1 minute after preadministration of 40 mg/kg body weight GST-RAP or PBS (A) or 1 to 3 minutes after preadministration of mannan or PBS (B). Blood samples were collected at the indicated times and t-PA antigen concentrations were determined by ELISA. Data points are the mean ± SD of 4 or 5 mice in each treatment group. (A) (○) Vehicle; (•) EE; (□) vehicle and GST-RAP; (▪) EE and GST-RAP. (B) (○) Vehicle; (•) EE; (□) vehicle and mannan; (▪) EE and mannan.
Effect of preadministration of AdCMV-RAP on plasma clearance of t-PA in control and EE-treated mice. As described in Materials and Methods, EE- and vehicle-treated mice were injected with AdCMV-RAP or AdLacZ. Five days after virus administration, mice were injected with 15 μg recombinant human t-PA. Blood samples were collected at the indicated times and plasma t-PA concentrations were determined by ELISA. Data points are the mean ± SD of 3 mice in each treatment group. (○) Vehicle and AdCMV-RAP; (•) EE and AdCMV-RAP; (□) vehicle and AdLacZ; (▪) EE and AdLacZ.
Effect of preadministration of AdCMV-RAP on plasma clearance of t-PA in control and EE-treated mice. As described in Materials and Methods, EE- and vehicle-treated mice were injected with AdCMV-RAP or AdLacZ. Five days after virus administration, mice were injected with 15 μg recombinant human t-PA. Blood samples were collected at the indicated times and plasma t-PA concentrations were determined by ELISA. Data points are the mean ± SD of 3 mice in each treatment group. (○) Vehicle and AdCMV-RAP; (•) EE and AdCMV-RAP; (□) vehicle and AdLacZ; (▪) EE and AdLacZ.
To examine the contribution of the mannose receptor to the EE-induced t-PA clearance, we blocked this receptor system by preinjection of mice with mannan, a mannose receptor ligand. Preadministration of mannan (5 mg/kg), a dose previously shown to block clearance of ovalbumin, a mannose-terminated glycoprotein, reduced plasma clearance of t-PA from 0.33 ± 0.04 mL/min to 0.22 ± 0.01 mL/min in control mice and from 0.47 ± 0.04 mL/min to 0.25 ± 0.02 mL/min in EE-treated mice (Fig 2B). Thus, in contrast to RAP-blockade of LRP, preadministration of mannan abolished the faster clearance of t-PA in EE-treated animals (Fig 2B). These results indicate that the EE-increased t-PA clearance is mediated via the mannose receptor system.
Effect of EE-treatment on hepatic mannose receptor and LRP mRNA levels.
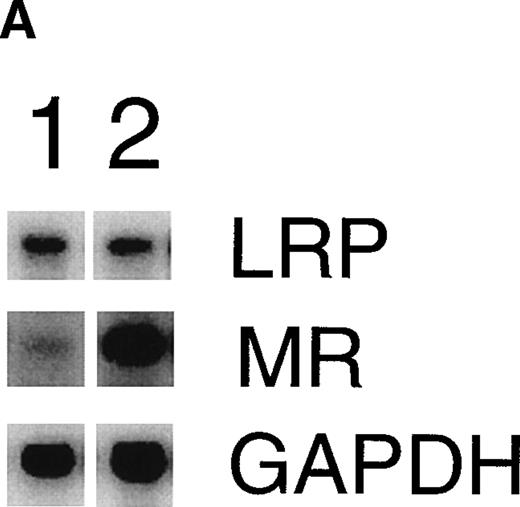
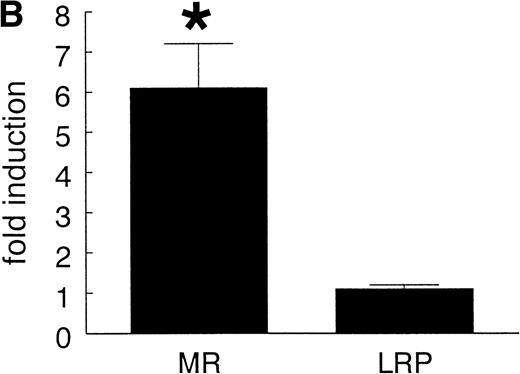
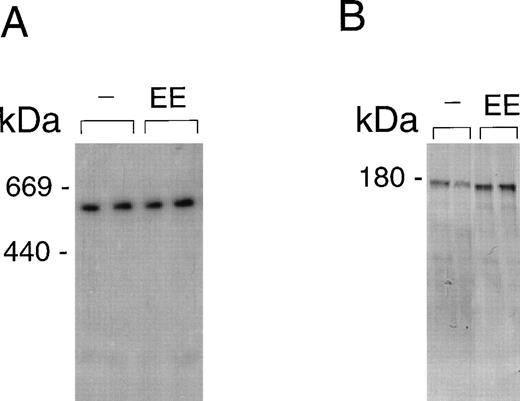
Consistent with earlier observations in rats,33 we found hepatic LRP mRNA expression in mice almost exclusively in hepatocytes, whereas the mannose receptor mRNA was demonstrated mainly in the nonhepatocyte (Kupffer cell/endothelial cell) fraction (data not shown). To further substantiate the EE-induced mannose receptor capacity, we performed Northern blotting studies to compare mannose receptor mRNA expression in livers from control and EE-treated mice. EE treatment strongly increased liver mannose receptor levels (6.1- ± 1.1-fold, n = 3, P < .05), but not LRP mRNA levels (1.1- ± 0.1-fold induction, n = 3, not significant; Fig 4). These effects of EE treatment on the gene level were confirmed at the protein level. As shown in Fig 5, Western blotting showed an increase in 180-kD mannose receptor protein signal in a liver membrane preparation of EE-treated animals as compared with that of control animals. The amount of 600-kD LRP protein, as determined by ligand blotting with 125I RAP, showed no significant difference between the two groups.
Effect of EE treatment on hepatic LRP and mannose receptor mRNA expression in mice. (A) Total RNA was extracted from livers from vehicle- (lane 1) or EE-treated mice (lane 2) and analyzed by Northern blotting for LRP and mannose receptor (MR) mRNA levels. As a control for equal loading, the blots were probed with the cDNA for GAPDH mRNA. (B) The signals for LRP and MR mRNA were quantified by phosphoimager analysis and adjusted for the corresponding GAPDH mRNA signals. The results shown are the amounts of hepatic LRP and MR mRNA in EE-treated mice relative to those found in vehicle-treated mice. Data are expressed as the mean ± SD of 3 independent experiments consisting of at least 4 animals in each treatment group.
Effect of EE treatment on hepatic LRP and mannose receptor mRNA expression in mice. (A) Total RNA was extracted from livers from vehicle- (lane 1) or EE-treated mice (lane 2) and analyzed by Northern blotting for LRP and mannose receptor (MR) mRNA levels. As a control for equal loading, the blots were probed with the cDNA for GAPDH mRNA. (B) The signals for LRP and MR mRNA were quantified by phosphoimager analysis and adjusted for the corresponding GAPDH mRNA signals. The results shown are the amounts of hepatic LRP and MR mRNA in EE-treated mice relative to those found in vehicle-treated mice. Data are expressed as the mean ± SD of 3 independent experiments consisting of at least 4 animals in each treatment group.
LRP and mannose receptor expression in livers from control and EE-treated mice. Membrane fragments were isolated from livers of mice treated for 4 days with vehicle (−) or EE. Solubilized membrane proteins were subjected to SDS-PAGE and transferred to a nitrocellulose membrane, as described in Materials and Methods. LRP (A) was visualized by incubating the blot with 125I GST-RAP. MR (B) was incubated with an MoAb against the MR and visualized as described in Materials and Methods. Equal loading was controlled for by Ponceau coloring of the blots.
LRP and mannose receptor expression in livers from control and EE-treated mice. Membrane fragments were isolated from livers of mice treated for 4 days with vehicle (−) or EE. Solubilized membrane proteins were subjected to SDS-PAGE and transferred to a nitrocellulose membrane, as described in Materials and Methods. LRP (A) was visualized by incubating the blot with 125I GST-RAP. MR (B) was incubated with an MoAb against the MR and visualized as described in Materials and Methods. Equal loading was controlled for by Ponceau coloring of the blots.
DISCUSSION
The present study demonstrates that EE administration significantly increases the clearance of endogenous, bradykinin-released t-PA in rats and that of exogenously administered recombinant human t-PA in mice. This increased t-PA clearance after EE treatment is most likely the result of an increase in mannose receptor-mediated clearance. Firstly, blocking of the LRP either by injection of GST-RAP or by overexpression of RAP by adenoviral gene transduction did not eliminate the difference in clearance between control and EE-treated mice. Secondly, in the presence of the mannose receptor antagonist mannan, t-PA clearance in control and EE-treated mice became identical. Thirdly, EE treatment of mice induced a 6-fold increase in liver mannose-receptor mRNA expression, whereas the amount of LRP mRNA in the EE-treated animals did not differ from that in control animals. These findings were confirmed at the protein level by Western blotting and ligand blotting.
Although the experiments were all performed in mice and rats, the results are likely to be relevant to observed changes in t-PA levels after estrogen administration in humans and also to observed gender differences in plasma t-PA concentrations because of the similarities between rodent and human t-PA clearance systems. In both rodents and humans, the main clearance organ of t-PA is the liver.34,35Also, the distribution of LRP and mannose receptor over the different liver cell types is similar in rodents and humans. Both in human liver and rat liver, LRP is mainly on hepatocytes, whereas the mannose receptor is present on liver endothelial cells and Kupffer cells.36-38 Our data in mice are in line with this, showing expression of LRP in hepatocytes and of mannose receptor in nonparenchymal cells.
An increased clearance of t-PA by estrogens as found in this study explains, at least in part, the inverse association between plasma t-PA levels and estrogens as observed in several clinical studies. In a cross-sectional study, t-PA (and PAI-1) antigen levels were lower in premenopausal women than in age-matched men, with the sex difference disappearing after menopause.11 t-PA and PAI-1 antigen levels were significantly higher in postmenopausal than in premenopausal women.11,39 Studies on the use of oral contraceptives,12,13 hormone replacement therapy,11 or studies with male-to-female transsexuals14 also show an inverse association between plasma t-PA concentrations and estrogen. The possibility that estrogens decrease plasma t-PA levels directly by decreasing endothelial t-PA production is not very likely. As a follow-up of our study with male-to-female transsexual subjects,14 we performed venous occlusion tests in a very similar group of subjects to estimate t-PA synthesis before and after hormone treatment. Whereas a decrease in basal plasma t-PA levels after estrogen treatment was confirmed, no significant difference in increment in venous plasma t-PA levels in response to occlusion of the upper arm was found (Emeis and Giltay, manuscript submitted). In accordance with this finding, in vitro studies with cultured human endothelial cells have shown that EE and 17-β estradiol do not influence t-PA synthesis.15However, we cannot exclude the possibility that other mechanisms also contribute to the decreased t-PA antigen levels after estrogen treatment. For instance, estrogen administration is usually associated with lower plasma PAI-1 levels.13,14,40,41 As a consequence, less t-PA/PAI-1 complex is formed, which may result in faster clearance of unbound t-PA.7
Our finding that estrogens increase mannose receptor expression is able to explain the lower levels of other mannose receptor ligands in women as compared with men, like β-glucuronidase and N-acetyl-β glucosaminidase.42,43 An effect of estrogen on the concentration of blood components via altered clearance is not unique for t-PA. Treatment of rats with EE leads to a marked increase in the number of LDL-receptors, resulting in lower plasma LDL-cholesterol levels as a result of increased clearance.44
Although the main point in this study is that upregulation of the mannose receptor in nonparenchymal liver cells explains increased t-PA clearance after estrogen administration, this effect of estrogen on mannose receptor expression may also have consequences for other cell types that express the mannose receptor, including tissue macrophages, dendritic cells, and sperm cells, and thus may have more general physiological importance also. In addition to the clearance of glycoproteins, the mannose receptor functions to mediate the phagocytosis of infectious agents, participates in antigen presentation, is involved in the homing of lymphocytes to the spleen, and plays a role in sperm fertility, and recent advances include evidence that the mannose receptor is associated with a signal transduction pathway leading to cytokine production.5,45 An increase in mannose receptor expression by estrogens may impact on these functions. However, it is uncertain whether the regulation of expression of the mannose receptor in other cell types in similar to that in nonparenchymal liver cells.5
The molecular mechanism underlying the EE-induced mannose-receptor expression in mice was not the subject of the present study, but most probably involves estrogen receptor-mediated stimulation of mannose-receptor gene transcription. The recent description of a second estrogen receptor, named ERβ,46,47 brings up many questions regarding the possibly distinct biological roles for the 2 estrogen receptor subtypes (α and β), their tissue distribution, and their ligand selectivities. In view of this, the regulation of the LDL receptor (mainly expressed in parenchymal cells) and the mannose receptor (in nonparenchymal cells) may involve different estrogen receptors, whether it be homodimeric forms of ERα or ERβ or heterodimers.48
In conclusion, the increased t-PA clearance after estrogen treatment via increased mannose receptor expression as shown in this study provides an explanation for the lowered t-PA levels associated with high estrogen status. The data presented in this study demonstrate for the first time that estrogens, besides their well-known effects on plasma lipid levels via changes in clearance receptors, are also able to modulate the hemostatic system via changes in clearance rate. Further research, including identification of the estrogen receptor(s) involved, is required to precisely analyze the estrogen-regulated mannose receptor gene expression at the molecular level.
ACKNOWLEDGMENT
The authors are grateful to Dr Thomas Willnow and Dr Joachim Herz for providing the adenovirus containing RAP and β-GAL cDNA. We thank Vivian Dahlmans, Marijke Voskuilen, and Richard van Veghel for excellent technical assistance.
Supported by grants from the Netherlands Heart Foundation (92.324) and the Netherlands Organization for Scientific Research, Council for Medical Research, Medical Sciences (903.39-117).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Teake Kooistra, PhD, Gaubius Laboratory, TNO Prevention and Health, PO Box 2215, 2301 CE Leiden, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal