Abstract
We determined the role of the heterophilic interaction of vβ3 integrin on endothelial cells with CD31 on leukocytes in mediating leukocyte-endothelial cell interactions. Preincubation of interleukin-4 (IL-4)–stimulated human umbilical vein endothelial cells (HUVECs) with anti-CD31 monoclonal antibodies (MoAbs) enhanced eosinophil adhesion to the IL-4–stimulated HUVECs, and the endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs was prevented by anti–vascular cell adhesion molecule-1 (VCAM-1) MoAb and anti–very late activation antigen-4 (VLA-4) MoAb, but not by anti–intercellular adhesion molecule-1 (ICAM-1) MoAb, anti–lymphocyte function-associated antigen-1 (LFA-1) MoAb, anti–P-selectin MoAb, or anti–E-selectin MoAb. CD31 stimulation of HUVECs increased the adhesive function of vβ3 integrin to its ligand RGD peptide, the binding of which reached a maximum at 10 minutes after the stimulation, and the CD31-induced vβ3 integrin activation on HUVECs was inhibited by inhibitors of protein kinase C and phosphatidylinositol 3 kinase (PI3-kinase). Furthermore, anti-vβ3 integrin MoAb and RGD peptide as well as soluble CD31 inhibited endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs. However, anti-vβ3 integrin MoAb had no significant inhibitory effect on the eosinophil adhesion to IL-4–stimulated or unstimulated HUVECs without CD31 stimulation of HUVECs. Finally, CD31 stimulation of eosinophils increased the adhesive function of 4β1 integrin (VLA-4) to its ligand fibronectin and their adhesion to IL-4–stimulated HUVECs in a VLA-4–dependent manner. These results indicate that CD31-mediated inside-out signaling activates vβ3 integrin on endothelial cells, that the heterophilic vβ3 integrin/CD31 interaction induces β1 integrin-mediated adhesion of eosinophils to endothelial cells, and that the heterophilic interaction itself is not significantly involved in firm adhesion of eosinophils to endothelial cells.
CD31 (PECAM-1) IS A CELL adhesion molecule of the Ig gene superfamily1 expressed on vascular endothelial cells, platelets, monocytes, neutrophils, and certain subsets of T lymphocytes.2-10 CD31 has both homophilic (CD31-CD31) and heterophilic (CD31-X) adhesive interactions,4,11 and it has recently been shown that αvβ3 integrin, which is expressed by endothelial cells12 and subsets of T cells,13,14 is a heterophilic ligand for CD31.15,16 CD31 has been implicated in many physiological events, especially leukocyte-endothelial cell interactions.17 It has been shown that CD31 is required for transendothelial migration of monocytes and neutrophils across cultured vascular endothelial cells in vitro.18 In vivo animal model studies have also shown that, using anti-CD31 antibodies, CD31 is involved in monocyte and neutrophil recruitment into sites of inflammation.19,20 Furthermore, several studies have shown that stimulation of CD31 with anti-CD31 antibodies increases adhesive function of β1 integrin on T cells10 and CD34+ hematopoietic progenitor cells21 and adhesive function of β2 integrin on monocytes, neutrophils,22 and natural killer cells,23suggesting that leukocyte CD31 may act as a signaling molecule. However, the functional importance of the heterophilic interaction of αvβ3 integrin on endothelial cells with CD31 on leukocytes has not yet been clarified in mediating leukocyte-endothelial cell interactions.
Therefore, to elucidate this issue, we studied the role of the heterophilic αvβ3 integrin/CD31 interaction in eosinophil adhesion to interleukin-4 (IL-4)–stimulated vascular endothelial cells. We also studied the role of CD31 in αvβ3 and α4β1 integrin activation of endothelial cells and eosinophils, respectively. Our results indicate that the heterophilic αvβ3 integrin/CD31 interaction forms an important amplifier loop for β1 integrin-mediated adhesion of eosinophils to endothelial cells, but that the heterophilic interaction itself is not significantly involved in firm adhesion of eosinophils to endothelial cells.
MATERIALS AND METHODS
Materials.
All the reagents were purchased from Sigma Chemical Co (St Louis, MO), unless otherwise stated. MCDB131 medium was purchased from Kurorera Kogyo Co (Tokyo, Japan). Percoll was purchased from Pharmacia Fine Chemicals (Uppsala, Sweden). Recombinant human IL-4 was obtained from Ono Pharmaceutical Co (Osaka, Japan).
Mouse antihuman CD31 monoclonal antibodies (MoAbs) NIH31-1 (IgG1) and NIH31-2 (IgG1)10,24 were a kind gift from Dr Y. Tanaka (University of Occupational and Environmental Health, Kitakyushu, Japan). Mouse antihuman vascular cell adhesion molecule-1 (VCAM-1) MoAb BBA6 (IgG1), antihuman intercellular adhesion molecule-1 (ICAM-1) MoAb 84H10 (IgG1), and antihuman very late activation antigen-4 (VLA-4) α-chain MoAb HP2.1 (IgG1) were purchased from Cosmo Bio Co (Tokyo, Japan). Mouse antihuman P-selectin MoAb AK4 (IgG1) and antihuman E-selectin MoAb 68-5H11 (IgG1) were purchased from Pharmingen (San Diego, CA). Mouse antihuman αvβ3 MoAb LM609 (IgG1)12 and antihuman αvβ5 MoAb P1F6 (IgG1) were purchased from Chemicon International Inc (Temecula, CA). Mouse antihuman VLA-5 α-chain MoAb KH/33 (IgG1) was purchased from Seikagaku Co (Tokyo, Japan). A mouse MoAb (IgG1) that recognizes keyhole limpet hemocyanin was purchased from Becton Dickinson Immunocytometry Systems (San Jose, CA) and used as a negative control. Recombinant human CD31 was purchased from R&D Systems (Minneapolis, MN).
Arg-Gly-Asp (RGD) peptide (ProNectin F), Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP) peptide, and Gly-Arg-Gly-Glu-Ser-Pro (GRGESP) peptide were purchased from Iwaki Glass (Chiba, Japan). Human fibronectin was purchased from Seikagaku Co. [51Cr]sodium chromate was purchased from Dupont NEN Research Products (Boston, MA). MACS microbeads conjugated with mouse antihuman CD16 MoAb were purchased from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany). Transwell cell culture chamber inserts (catalogue no. 3421) were purchased from Costar Corp (Cambridge, MA).
Culture of vascular endothelial cells.
Human umbilical vein endothelial cells (HUVECs) were isolated from fresh umbilical cords by trypsin digestion according to the method described by Jaffe et al.25 Briefly, the umbilical vein was rinsed with 50 mL of phosphate-buffered saline (PBS) and filled with 0.2% trypsin in PBS at room temperature for 15 minutes. Isolated HUVECs were cultured in MCDB131 medium supplemented with 10% fetal calf serum (FCS), basic fibroblast growth factor (10 ng/mL), heparin (90 μg/mL), penicillin (100 μg/mL), and streptomycin (100 U/mL) in a gelatin-coated 75-cm2 tissue culture flask at 37°C in a humidified atmosphere of 5% CO2 in air. The primary cultured HUVECs were harvested by trypsin digestion and seeded into a gelatin-coated 24-well tissue culture plate for use of adhesion assay. HUVECs were then cultured to confluence. Endothelial cells were identified by their typical cobblestone morphology and the presence of factor VIII-related antigen as detected by indirect immunofluorescence. Confluence was verified by phase-contrast microscopy inspection before use in experiments.
Purification of eosinophils.
Human eosinophils were purified from 100 mL of heparinized venous blood from normal individuals by Percoll density gradient centrifugation and a magnetic cell sorting using MACS anti-CD16 microbeads as described by Hansel et al.26 Briefly, after erythrocytes were sedimented in 1% (vol/vol) dextran in 0.9% NaCl, the leukocyte-rich cell suspension (5 × 107/mL) was overlayered on an isotonic Percoll solution (1.082 g/mL) and centrifuged at 1,000g for 30 minutes at 4°C. Granulocytes were obtained from the bottom of the Percoll solution and washed twice in cold Hanks’ balanced salt solution (HBSS) containing 2% FCS. Cells were then incubated with 100 μL of anti-CD16 MoAb MACS microbeads for 30 minutes at 4°C with occasional gentle mixing. Cells were then suspended in 500 μL of cold HBSS containing 2% FCS and applied to a separation column in a magnetic field. Pure eosinophils (purity >99%) were obtained by negative selection, because eosinophils are CD16− and pass through the column, and were suspended in HBSS.
Radiolabeling of eosinophils and HUVECs.
Purified eosinophils or HUVEC (3 × 107) were incubated with 0.4 mCi of sodium [51Cr] chromate in 1 mL of HBSS containing 5% FCS at 37°C for 30 minutes. Excess isotope was then washed off 3 times with HBSS containing 5% FCS and cells were resuspended in HBSS containing 5% FCS.
Adhesion assay.
HUVEC monolayers grown on the well of 24-well tissue culture plates were stimulated with IL-4 (100 ng/mL) for 8 hours at 37°C in 200 μL of MCDB131 medium containing 5% FCS in a humidified atmosphere of 5% CO2 in air. As a control, HUVECs were incubated with culture medium alone for 8 hours. After HUVECs were thoroughly washed with HBSS containing 5% FCS, 3 × 105 of51Cr-labeled eosinophils were incubated with HUVECs for 10 minutes at 37°C in 200 μL of HBSS containing 5% FCS. HUVECs were then gently washed 3 times with HBSS containing 5% FCS to remove the unbound cells. Adherent eosinophils were then lysed by the addition of 1 mL of HBSS containing 1% Triton X-100, and radioactivity of the cells was counted in a gamma counter. Each experiment was performed in triplicate, and the average of 3 determinations was used for subsequent calculations. Adherence of eosinophils to HUVECs was expressed as the percentage of radioactivity of total eosinophils added to each well.
In the first series of experiments, IL-4–stimulated or unstimulated HUVECs were preincubated with anti-CD31 MoAb (10 μg/mL), anti-αvβ3 integrin MoAb (10 μg/mL), or control mouse IgG (10 μg/mL) for 10 minutes at 37°C, then washed with HBSS containing 5% FCS, and used for adhesion assay. In the second series of experiments, after IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C and were then washed with HBSS containing 5% FCS, the adhesion assay of 51Cr-labeled eosinophils to HUVECs was performed in the presence of anti–VCAM-1 MoAb, anti–VLA-4 MoAb, anti–ICAM-1 MoAb, anti–lymphocyte function-associated antigen-1 (LFA-1) MoAb, anti–P-selectin MoAb, or anti–E-selectin MoAb (10 μg/mL each). In the third series of experiments, after IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG for 10 minutes at 37°C and were then washed with HBSS containing 5% FCS, the adhesion assay of 51Cr-labeled eosinophils to HUVECs was performed in the presence of anti-αvβ3 integrin MoAb (10 μg/mL) or GRGDSP peptide (or GRGESP peptide; 5 to 500 μg/mL). To further determine whether αvβ3 integrin/CD31 interaction between endothelial cells and eosinophils is involved in the endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated endothelial cells, we also examined the effect of soluble recombinant CD31 on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs. In these experiments, after IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG for 10 minutes at 37°C and were then washed with HBSS containing 5% FCS, the adhesion assay of 51Cr-labeled eosinophils to HUVECs was performed in the presence or absence of soluble recombinant CD31 (1 to 50 μg/mL).
Binding assay of αvβ3 integrin to RGD peptide.
To determine whether CD31 stimulation of endothelial cells increases the adhesive function of αvβ3 integrin to its ligand, we performed the binding assay of αvβ3 integrin on HUVECs to its ligand Arg-Gly-Asp (RGD) peptide.12 27 Briefly, wells of 24-well tissue culture plates were coated with 5 μg/mL ProNectin F or 1% bovine serum albumin (BSA; control) in PBS at room temperature for 2 hours. The wells were washed and subsequently coated with 1% BSA in PBS at room temperature for 1 hour to block nonspecific binding sites. After the wells were washed with HBSS, 51Cr-labeled HUVECs were added to the well at 5 × 105 cells/well in 200 μL of HBSS containing 1% BSA and were incubated in triplicate for 10 minutes at 37°C. Unbound cells were then washed off, and bound cells were lysed by the addition of 1 mL of HBSS containing 1% Triton X-100; radioactivity of the cells was counted in a gamma counter.
Because αvβ3, αvβ5, and α5β1 integrins on endothelial cells recognize the Arg-Gly-Asp (RGD) sequence present in extracellular matrix proteins such as vitronectin and fibronectin,27 28we first examined the effect of anti-αvβ3 integrin, anti-αvβ5 integrin, and anti-α5β1 integrin MoAbs on the binding of HUVECs to RGD peptide. After 51Cr-labeled HUVECs were preincubated with anti-CD31 MoAb (10 μg/mL) or control mouse IgG (10 μg/mL) for 10 minutes at 37°C and were then washed with HBSS containing 1% BSA, the binding assay of 51Cr-labeled HUVECs to RGD peptide was performed in the presence of anti-αvβ3 integrin MoAb, anti-αvβ5 integrin MoAb, or anti-α5β1 integrin MoAb (10 μg/mL each).
We also determined whether protein kinase C and phosphatidylinositol 3 kinase (PI3-kinase) are involved in the signal transduction for CD31-induced αvβ3 integrin activation on endothelial cells.51Cr-labeled HUVECs were preincubated with staurosporine (10−8 mol/L)29,30 or wortmannin (10−7 mol/L)31 32 for 10 minutes at 37°C and were then preincubated with anti-CD31 MoAb (10 μg/mL) or control mouse IgG (10 μg/mL) for 10 minutes at 37°C. After washing with HBSS containing 1% BSA, the 51Cr-labeled HUVECs were used for binding assay to RGD peptide.
Binding assay of VLA-4 to fibronectin.
To determine whether CD31 stimulation of eosinophils increases the adhesive function of α4β1 integrin (VLA-4) to its ligand, we performed the binding assay of VLA-4 on eosinophils to its ligand fibronectin. Briefly, wells of 24-well tissue culture plates were coated with 10 μg/mL fibronectin or 1% BSA (control) in PBS at 4°C overnight. The wells were washed and subsequently coated with 1% BSA in PBS at room temperature for 1 hour to block nonspecific binding sites. After the wells were washed with HBSS,51Cr-labeled eosinophils were added to the well at 5 × 105 cells/well in 200 μL of HBSS containing 1% BSA and were incubated in triplicate for 10 minutes at 37°C. Unbound cells were then washed off, bound cells were lysed by the addition of 1 mL of HBSS containing 1% Triton X-100, and the radioactivity of the cells was counted in a gamma counter. In these experiments, after 51Cr-labeled eosinophils were preincubated with anti-CD31 MoAb (10 μg/mL) or control mouse IgG (10 μg/mL) for 10 minutes at 37°C and were then washed with HBSS containing 1% BSA, the binding assay of 51Cr-labeled eosinophils to fibronectin was performed in the presence or absence of anti–VLA-4 MoAb (10 μg/mL).
Immunofluorescence.
After IL-4–stimulated HUVECs were incubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C and then washed with HBSS containing 5% FCS, HUVECs were fixed in acetone for 10 minutes at 4°C, dried, and rinsed with PBS. After blocking with 4% FCS in PBS, HUVECs were stained with anti-CD31 MoAb-fluorescein isothiocyanate (FITC; 10 μg/mL), anti-αvβ3 integrin MoAb-FITC (10 μg/mL), or control mouse IgG-FITC (10 μg/mL) for 60 minutes at room temperature, followed by washing with PBS.
Data analysis.
Data are summarized as the mean ± SD. The statistical analysis of the results was performed by the analysis of variance using Fisher’s least significant difference test for multiple comparisons. Pvalues less than .05 were considered significant.
RESULTS
Role of CD31 and αvβ3 integrin in eosinophil adhesion to vascular endothelial cells.
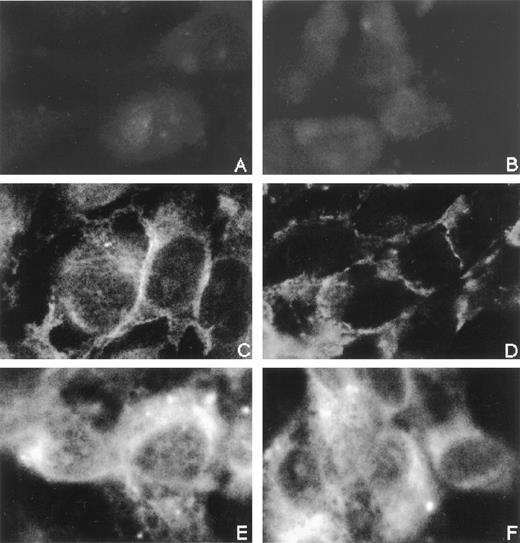
To determine the role of CD31/CD31 homophilic and αvβ3 integrin/CD31 heterophilic bindings in the adhesive interaction between eosinophils and vascular endothelial cells, we examined the effect of anti-CD31 MoAb and anti-αvβ3 integrin MoAb on eosinophil adhesion to IL-4–stimulated cultured vascular endothelial cells. In our preliminary experiments, CD31, but not αvβ3 integrin, was expressed on human eosinophils by flow cytometry to the same levels as human neutrophils and monocytes had (data not shown), whereas both CD31 and αvβ3 integrin were expressed on cultured HUVECs, as described previously.2-4,12 33 In addition, the distribution of αvβ3 integrin on HUVECs was not significantly affected by CD31 stimulation with anti-CD31 MoAb (Fig 1).
Immunofluorescence staining of CD31 and vβ3 integrin on IL-4–stimulated vascular endothelial cells. HUVEC monolayers grown on the well of 24-well tissue culture plates were stimulated with IL-4 (100 ng/mL) for 8 hours at 37°C. IL-4–stimulated HUVECs were then incubated with anti-CD31 MoAb NIH31-1 (right panels; B, D, and F) or control mouse IgG (left panels; A, C, and E) (10 μg/mL each) for 10 minutes at 37°C, fixed in acetone, and stained with anti-CD31 MoAb-FITC (C and D), anti-vβ3 integrin MoAb-FITC (E and F), or control mouse IgG-FITC (A and B) (10 μg/mL each) for 60 minutes at room temperature. (Original magnification × 1,000.)
Immunofluorescence staining of CD31 and vβ3 integrin on IL-4–stimulated vascular endothelial cells. HUVEC monolayers grown on the well of 24-well tissue culture plates were stimulated with IL-4 (100 ng/mL) for 8 hours at 37°C. IL-4–stimulated HUVECs were then incubated with anti-CD31 MoAb NIH31-1 (right panels; B, D, and F) or control mouse IgG (left panels; A, C, and E) (10 μg/mL each) for 10 minutes at 37°C, fixed in acetone, and stained with anti-CD31 MoAb-FITC (C and D), anti-vβ3 integrin MoAb-FITC (E and F), or control mouse IgG-FITC (A and B) (10 μg/mL each) for 60 minutes at room temperature. (Original magnification × 1,000.)
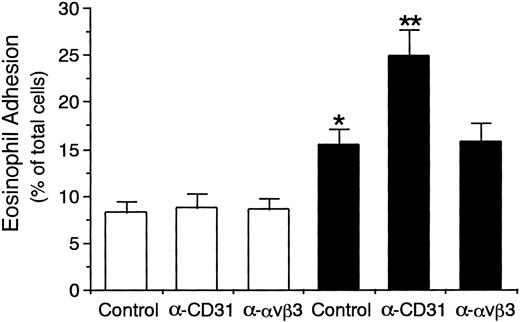
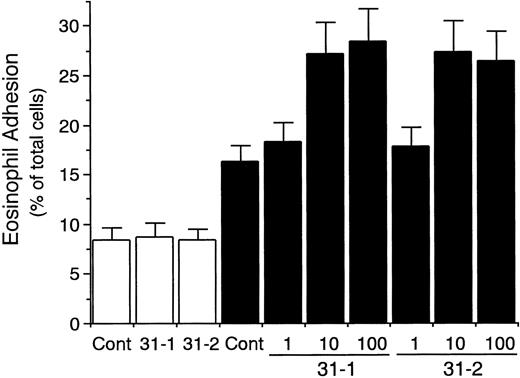
Eosinophil adhesion to unstimulated HUVECs was low (8.2% ± 1.2% of total eosinophils added, mean ± SD, n = 6 experiments; Fig 2). In contrast, eosinophil adhesion to HUVECs significantly increased after 8 hours of preincubation of HUVECs with IL-4 (100 ng/mL; 15.3% ± 1.8% of total eosinophils added, n = 6, P < .001; Fig 2), which was accompanied by the selective VCAM-1 expression on HUVECs (data not shown).34-36 Possible involvement of CD31 and αvβ3 integrin on endothelial cells in mediating the eosinophil adhesion to IL-4–stimulated HUVECs was then examined by using anti-CD31 MoAb and anti-αvβ3 integrin MoAb. Unexpectedly, preincubation of IL-4–stimulated HUVECs with anti-CD31 MoAb (NIH31-1) significantly enhanced eosinophil adhesion to the IL-4–stimulated HUVECs by 62% (24.9% ± 2.8% of total eosinophils added, n = 6, P < .001; Fig 2). In addition, anti-CD31 MoAb NIH31-1 and NIH31-2 (1 to 100 μg/mL) both dose-dependently increased eosinophil adhesion to IL-4–stimulated HUVECs (n = 3 to 6; Fig3). In contrast, preincubation of unstimulated HUVECs with anti-CD31 MoAb had no significant enhancing effect on the eosinophil adhesion to the unstimulated HUVECs (n = 6; Figs 2 and 3). On the other hand, anti-αvβ3 integrin MoAb (LM609) had no significant effect on the eosinophil adhesion to IL-4–stimulated or unstimulated HUVECs (n = 6 each; Fig 2). These results indicate that neither CD31/CD31 homophilic nor αvβ3 integrin/CD31 heterophilic interaction between endothelial cells and eosinophils is directly involved in eosinophil adhesion to endothelial cells and that CD31 stimulation of endothelial cells with anti-CD31 MoAb enhances eosinophil adhesion to IL-4–stimulated endothelial cells.
Effect of anti-CD31 and anti-vβ3 integrin antibodies on eosinophil adhesion to IL-4–stimulated vascular endothelial cells. HUVEC monolayers grown on the well of 24-well tissue culture plates were stimulated with or without IL-4 (100 ng/mL) for 8 hours at 37°C. IL-4–stimulated (▪) or unstimulated (□) HUVECs were preincubated with anti-CD31 MoAb NIH31-1 (10 μg/mL), anti-vβ3 integrin MoAb LM609 (10 μg/mL), or control mouse IgG (10 μg/mL) for 10 minutes at 37°C, washed, and then incubated with51Cr-labeled eosinophils (3 × 105) for 10 minutes at 37°C. After HUVECs were gently washed, adherent eosinophils were lysed by the addition of HBSS containing 1% Triton X-100 and the radioactivity of the cells was counted in a gamma counter. Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response to unstimulated HUVECs (*P < .001). **Significantly different from the mean value of the control response to IL-4–stimulated HUVECs (**P< .001).
Effect of anti-CD31 and anti-vβ3 integrin antibodies on eosinophil adhesion to IL-4–stimulated vascular endothelial cells. HUVEC monolayers grown on the well of 24-well tissue culture plates were stimulated with or without IL-4 (100 ng/mL) for 8 hours at 37°C. IL-4–stimulated (▪) or unstimulated (□) HUVECs were preincubated with anti-CD31 MoAb NIH31-1 (10 μg/mL), anti-vβ3 integrin MoAb LM609 (10 μg/mL), or control mouse IgG (10 μg/mL) for 10 minutes at 37°C, washed, and then incubated with51Cr-labeled eosinophils (3 × 105) for 10 minutes at 37°C. After HUVECs were gently washed, adherent eosinophils were lysed by the addition of HBSS containing 1% Triton X-100 and the radioactivity of the cells was counted in a gamma counter. Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response to unstimulated HUVECs (*P < .001). **Significantly different from the mean value of the control response to IL-4–stimulated HUVECs (**P< .001).
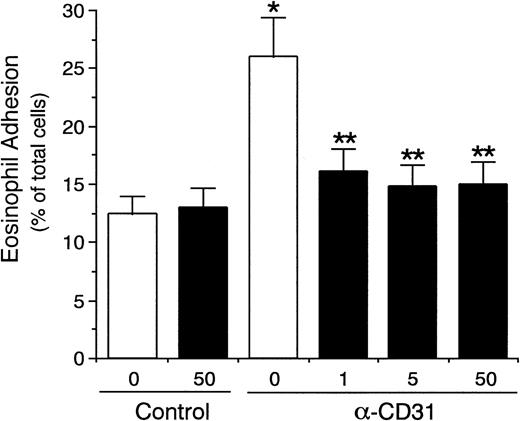
Dose-dependent effect of anti-CD31 antibodies on eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated (▪) or unstimulated (□) HUVECs were preincubated with anti-CD31 MoAb NIH31-1 (31-1) or NIH31-2 (31-2) (1, 10, and 100 μg/mL) or control mouse IgG (Cont; 10 μg/mL) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed. Data are the means ± SD for 3 to 6 experiments.
Dose-dependent effect of anti-CD31 antibodies on eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated (▪) or unstimulated (□) HUVECs were preincubated with anti-CD31 MoAb NIH31-1 (31-1) or NIH31-2 (31-2) (1, 10, and 100 μg/mL) or control mouse IgG (Cont; 10 μg/mL) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed. Data are the means ± SD for 3 to 6 experiments.
We also examined the effect of F(ab′)2 and Fab of anti-CD31 antibody on the CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs. F(ab′)2 of anti-CD31 MoAb similarly increased eosinophil adhesion to IL-4–stimulated HUVECs by 66% (n = 3), whereas the Fab fragments had no significant effect, indicating that dimerization of CD31 is required for the endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated endothelial cells.
CD31 stimulation of endothelial cells enhances eosinophil adhesion to IL-4–stimulated endothelial cells through VCAM-1/VLA-4 interaction.
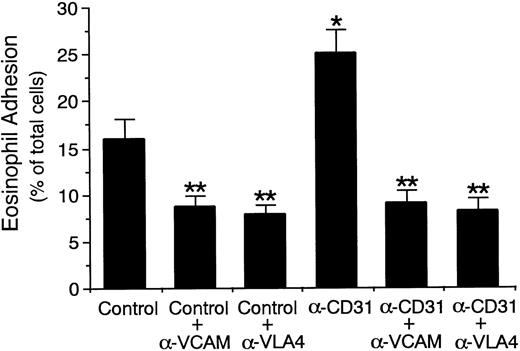
Because IL-4 selectively induces the expression of VCAM-1 on vascular endothelial cells34-36 and VLA-4 is a counter-receptor for VCAM-137 and is expressed on eosinophils,38-41we then examined whether VCAM-1/VLA-4 interaction is involved in the endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated endothelial cells. Eosinophil adhesion to IL-4–stimulated HUVECs was inhibited by the presence of anti–VCAM-1 MoAb and anti–VLA-4 MoAb by 45% and 51%, respectively (n = 5,P < .001; Fig 4). Endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs was abolished in the presence of anti–VCAM-1 MoAb or anti–VLA-4 MoAb to the same levels that anti–VCAM-1 MoAb and anti–VLA-4 MoAb decreased eosinophil adhesion to IL-4–stimulated, CD31-unstimulated HUVECs (n = 5, P < .001; Fig 4). However, the presence of anti–ICAM-1 MoAb, anti–LFA-1 MoAb, anti–P-selectin MoAb, or anti–E-selectin MoAb did not significantly affect the CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs (n = 5; data not shown). Therefore, endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated endothelial cells is mediated through VCAM-1/VLA-4 interaction, but not by ICAM-1, LFA-1, P-selectin, or E-selectin.
Effect of anti–VCAM-1 and anti–VLA-4 antibodies on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of 51Cr-labeled eosinophils to HUVECs was performed in the presence or absence of anti–VCAM-1 MoAb or anti–VLA-4 MoAb (10 μg/mL each). Data are the means ± SD for 5 experiments. *Significantly different from the mean value of the control response (*P < .001). **Significantly different from the mean value of the corresponding control response (**P < .001).
Effect of anti–VCAM-1 and anti–VLA-4 antibodies on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of 51Cr-labeled eosinophils to HUVECs was performed in the presence or absence of anti–VCAM-1 MoAb or anti–VLA-4 MoAb (10 μg/mL each). Data are the means ± SD for 5 experiments. *Significantly different from the mean value of the control response (*P < .001). **Significantly different from the mean value of the corresponding control response (**P < .001).
CD31 stimulation of endothelial cells increases the adhesive function of αvβ3 integrin to its ligand.
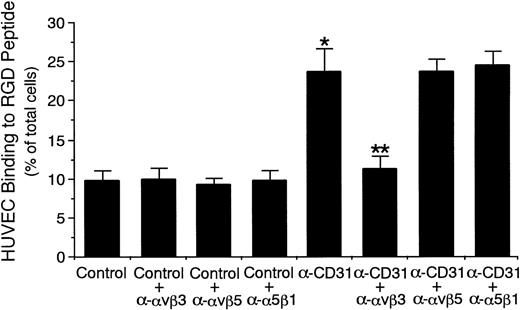
Because it has recently been shown that CD31 stimulation of leukocytes increases the adhesive function of β1 and β2 integrins on the surface,10,21-23,42 we determined whether CD31 stimulation of endothelial cells increases the adhesive function of αvβ3 integrin to its ligand Arg-Gly-Asp (RGD) peptide.12 27Incubation of unstimulated HUVECs with anti-CD31 MoAb significantly increased HUVEC binding to RGD peptide by 142% (control mouse IgG, 9.8% ± 1.3% v anti-CD31 MoAb, 23.8% ± 3.0% of HUVECs added, n = 6 experiments, P < .001; Fig 5), whereas incubation of unstimulated HUVECs with anti-CD31 MoAb had no significant effect on HUVEC binding to BSA-coated wells (control mouse IgG 2.7% ± 0.6%v anti-CD31 MoAb, 3.0% ± 0.4% of HUVECs added, n = 4). The presence of anti-αvβ3 integrin MoAb completely inhibited CD31-induced increase in the HUVEC binding to RGD peptide (11.3% ± 1.5% of HUVECs added, n = 6, P < .001; Fig 5). However, the presence of anti-αvβ5 integrin MoAb or anti–VLA-5 α-chain MoAb had no effect on the CD31-induced increase in HUVEC binding to RGD peptide (n = 6; Fig 5). In addition, we examined the expression of αvβ3 integrin on HUVECs incubated with anti-CD31 MoAb by flow cytometry. Incubation with anti-CD31 MoAb had no significant effect on the expression of αvβ3 integrin on HUVECs (data not shown). These data indicate that CD31 stimulation of endothelial cells selectively increases the adhesive function of αvβ3 integrin on the surface.
CD31 stimulation of endothelial cells increases the adhesive function of vβ3 integrin to RGD peptide.51Cr-labeled HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C and were then washed. 51Cr-labeled HUVECs (5 × 105) were then added to the RGD peptide-coated well of tissue culture plates and were incubated for 10 minutes at 37°C in the presence or absence of anti-vβ3 integrin MoAb, anti-vβ5 integrin MoAb, or anti-5β1 integrin MoAb (10 μg/mL each). After unbound cells were washed off, bound cells were lysed by the addition of HBSS containing 1% Triton X-100 and the radioactivity of the cells was counted in a gamma counter. Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response (*P < .001). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P < .001).
CD31 stimulation of endothelial cells increases the adhesive function of vβ3 integrin to RGD peptide.51Cr-labeled HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C and were then washed. 51Cr-labeled HUVECs (5 × 105) were then added to the RGD peptide-coated well of tissue culture plates and were incubated for 10 minutes at 37°C in the presence or absence of anti-vβ3 integrin MoAb, anti-vβ5 integrin MoAb, or anti-5β1 integrin MoAb (10 μg/mL each). After unbound cells were washed off, bound cells were lysed by the addition of HBSS containing 1% Triton X-100 and the radioactivity of the cells was counted in a gamma counter. Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response (*P < .001). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P < .001).
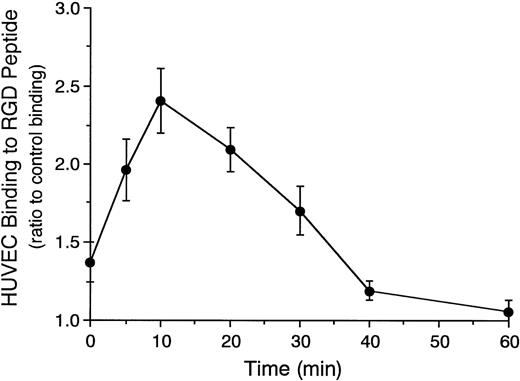
Time course of CD31-induced increase in the adhesive function of αvβ3 integrin on HUVECs to RGD peptide was then examined. CD31-induced HUVEC binding to RGD peptide rapidly increased and reached a peak at 10 minutes after the incubation of HUVECs with anti-CD31 MoAb (Fig 6), which was 2.4-fold greater than that of control MoAb-induced HUVEC binding at 10 minutes. After that, the CD31-induced HUVEC binding to RGD peptide gradually decreased and returned to the baseline at 40 minutes (Fig 6).
Time course of endothelial CD31-induced increase in the adhesive function of vβ3 integrin to RGD peptide.51Cr-labeled HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing the cells, the binding assay of 51Cr-labeled HUVECs to RGD peptide was performed for 10 to 60 minutes at 37°C. HUVEC binding to RGD peptide is expressed as the ratio of the binding of anti-CD31 MoAb-pretreated HUVECs to that of control IgG-pretreated HUVECs. Data are the means ± SD for 3 experiments.
Time course of endothelial CD31-induced increase in the adhesive function of vβ3 integrin to RGD peptide.51Cr-labeled HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing the cells, the binding assay of 51Cr-labeled HUVECs to RGD peptide was performed for 10 to 60 minutes at 37°C. HUVEC binding to RGD peptide is expressed as the ratio of the binding of anti-CD31 MoAb-pretreated HUVECs to that of control IgG-pretreated HUVECs. Data are the means ± SD for 3 experiments.
αvβ3 integrin/CD31 interaction mediates endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated endothelial cells.
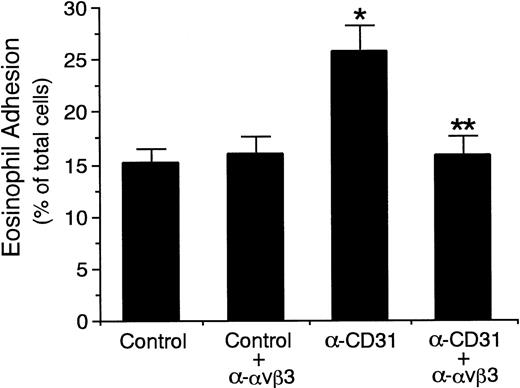
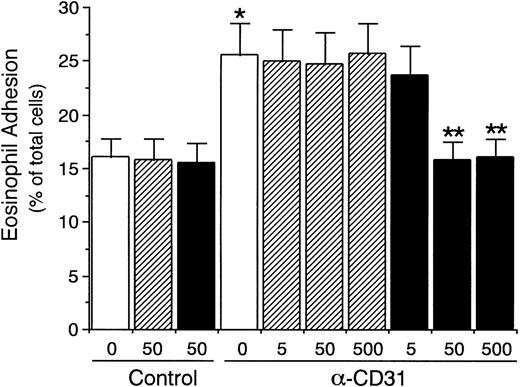
We then determined whether αvβ3 integrin/CD31 interaction between endothelial cells and eosinophils is involved in the endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated endothelial cells. As shown in Fig 7, the presence of anti-αvβ3 integrin MoAb significantly inhibited endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs by 94% (anti-CD31 MoAb, 25.9% ± 2.5%v anti-CD31 MoAb and anti-αvβ3 integrin MoAb, 15.8% ± 1.8% of total eosinophils added, n = 6, P < .001). GRGDSP peptide (50 to 500 μg/mL), but not GRGESP peptide, also completely inhibited endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs (n = 4, P < .005; Fig 8). Furthermore, soluble recombinant CD31 (1 to 50 μg/mL) significantly inhibited endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs by 77% to 87% (anti-CD31 MoAb, 26.1% ± 3.3% v anti-CD31 MoAb and soluble CD31 at 50 μg/mL, 14.9% ± 2.0% of total eosinophils added, n = 4, P < .005), whereas soluble CD31 had no significant inhibitory effect on the eosinophil adhesion to IL-4–stimulated HUVECs without CD31 stimulation of HUVEC (Fig 9). These results indicate that the increased αvβ3 integrin/CD31 heterophilic interaction between endothelial cells and eosinophils mediates the VLA-4–dependent eosinophil adhesion to IL-4–stimulated endothelial cells.
Effect of anti-vβ3 integrin antibody on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed in the presence or absence of anti-vβ3 integrin MoAb (10 μg/mL). Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response (*P < .001). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P < .001).
Effect of anti-vβ3 integrin antibody on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed in the presence or absence of anti-vβ3 integrin MoAb (10 μg/mL). Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response (*P < .001). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P < .001).
Effect of RGD peptide on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed in the presence of GRGDSP peptide (▪) or GRGESP peptide (control peptide; ▨; 5, 50, and 500 μg/mL each). Data are the means ± SD for 4 experiments. *Significantly different from the mean value of the control response (*P < .005). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P < .005).
Effect of RGD peptide on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed in the presence of GRGDSP peptide (▪) or GRGESP peptide (control peptide; ▨; 5, 50, and 500 μg/mL each). Data are the means ± SD for 4 experiments. *Significantly different from the mean value of the control response (*P < .005). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P < .005).
Effect of soluble recombinant CD31 on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed in the presence (▪) or absence (□) of soluble recombinant CD31 (1, 5, and 50 μg/mL). Data are the means ± SD for 4 experiments. *Significantly different from the mean value of the control response (*P < .005). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P< .005).
Effect of soluble recombinant CD31 on endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated vascular endothelial cells. IL-4–stimulated HUVECs were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing HUVECs, the adhesion assay of51Cr-labeled eosinophils to HUVECs was performed in the presence (▪) or absence (□) of soluble recombinant CD31 (1, 5, and 50 μg/mL). Data are the means ± SD for 4 experiments. *Significantly different from the mean value of the control response (*P < .005). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (**P< .005).
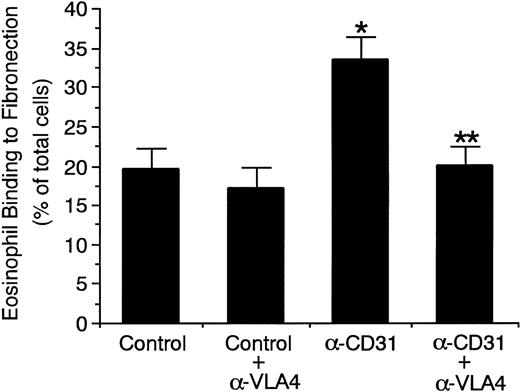
CD31 stimulation of eosinophils increases the adhesive function of α4β1 integrin (VLA-4) to its ligand.
To determine whether CD31 stimulation of eosinophils increases the adhesive function of α4β1 integrin (VLA-4) on the surface and thereby enhances eosinophil adhesion to IL-4–stimulated endothelial cells, we examined the adhesive function of VLA-4 on eosinophils to its ligand fibronectin. Stimulation of eosinophils with anti-CD31 MoAb significantly increased eosinophil binding to fibronectin by 71% (control mouse IgG, 19.6% ± 2.5% v anti-CD31 MoAb, 33.5% ± 2.9% of total eosinophils added, n = 4, P < .005; Fig 10). Anti–VLA-4 MoAb abolished the CD31-induced increase in eosinophil binding to fibronectin (20.1% ± 2.4% of total eosinophils added, n = 4, P < .005; Fig10). On the other hand, stimulation of eosinophils with anti-CD31 MoAb had no significant effect on eosinophil binding to BSA-coated wells (control mouse IgG, 1.9% ± 0.4% v anti-CD31 MoAb, 2.2% ± 0.3% of total eosinophils added, n = 4). In addition, anti-CD31 MoAb had no effect on the expression of VLA-4 on eosinophils (data not shown).
CD31 stimulation of eosinophils increases the adhesive function of 4β1 integrin (VLA-4) to fibronectin.51Cr-labeled eosinophils were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C and were then washed. 51Cr-labeled eosinophils (5 × 105) were then added to the fibronectin-coated well of tissue culture plates and were incubated for 10 minutes at 37°C in the presence or absence of anti–VLA-4 MoAb (10 μg/mL). After unbound cells were washed off, bound cells were lysed by the addition of HBSS containing 1% Triton X-100 and radioactivity of the cells was counted in a gamma counter. Data are the means ± SD for 4 experiments. *Significantly different from the mean value of the control response (*P < .005). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated eosinophils (**P < .005).
CD31 stimulation of eosinophils increases the adhesive function of 4β1 integrin (VLA-4) to fibronectin.51Cr-labeled eosinophils were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C and were then washed. 51Cr-labeled eosinophils (5 × 105) were then added to the fibronectin-coated well of tissue culture plates and were incubated for 10 minutes at 37°C in the presence or absence of anti–VLA-4 MoAb (10 μg/mL). After unbound cells were washed off, bound cells were lysed by the addition of HBSS containing 1% Triton X-100 and radioactivity of the cells was counted in a gamma counter. Data are the means ± SD for 4 experiments. *Significantly different from the mean value of the control response (*P < .005). **Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated eosinophils (**P < .005).
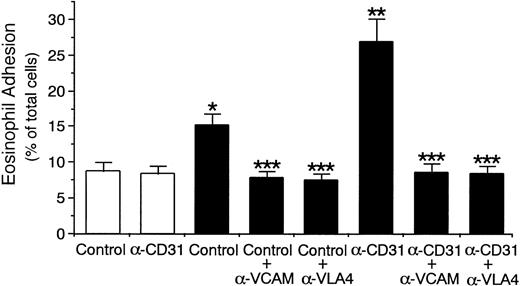
Stimulation of eosinophils with anti-CD31 MoAb also significantly increased eosinophil adhesion to IL-4–stimulated HUVECs by 77% (control mouse IgG, 15.2% ± 1.7% v anti-CD31 MoAb, 26.9% ± 3.2% of total eosinophils added, n = 6,P < .001; Fig 11). Anti–VLA-4 MoAb and anti–VCAM-1 MoAb inhibited CD31-induced increase in eosinophil adhesion to IL-4–stimulated HUVECs by 96% and 95%, respectively (n = 6, P < .001; Fig 11). These results indicate that CD31 stimulation of eosinophils increases the adhesive function of VLA-4 on the surface and thereby enhances eosinophil adhesion to IL-4–stimulated endothelial cells. In addition, the presence of anti-αvβ3 integrin MoAb or GRGDSP peptide did not significantly affect the eosinophil CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs (data not shown).
CD31 stimulation of eosinophils increases eosinophil adhesion to IL-4–stimulated endothelial cells through VLA-4/VCAM-1 interaction. 51Cr-labeled eosinophils were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing the cells, the adhesion assay of51Cr-labeled eosinophils to IL-4–stimulated (▪) or unstimulated (□) HUVECs was performed in the presence or absence of anti–VCAM-1 MoAb or anti–VLA-4 MoAb (10 μg/mL each). Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response to unstimulated HUVECs (*P < .001). **Significantly different from the mean value of the control response to IL-4–stimulated HUVECs (**P < .001). ***Significantly different from the mean value of the corresponding control response (***P < .001).
CD31 stimulation of eosinophils increases eosinophil adhesion to IL-4–stimulated endothelial cells through VLA-4/VCAM-1 interaction. 51Cr-labeled eosinophils were preincubated with anti-CD31 MoAb or control mouse IgG (10 μg/mL each) for 10 minutes at 37°C. After washing the cells, the adhesion assay of51Cr-labeled eosinophils to IL-4–stimulated (▪) or unstimulated (□) HUVECs was performed in the presence or absence of anti–VCAM-1 MoAb or anti–VLA-4 MoAb (10 μg/mL each). Data are the means ± SD for 6 experiments. *Significantly different from the mean value of the control response to unstimulated HUVECs (*P < .001). **Significantly different from the mean value of the control response to IL-4–stimulated HUVECs (**P < .001). ***Significantly different from the mean value of the corresponding control response (***P < .001).
Protein kinase C and PI3-kinase are involved in CD31-induced αvβ3 integrin activation in endothelial cells.
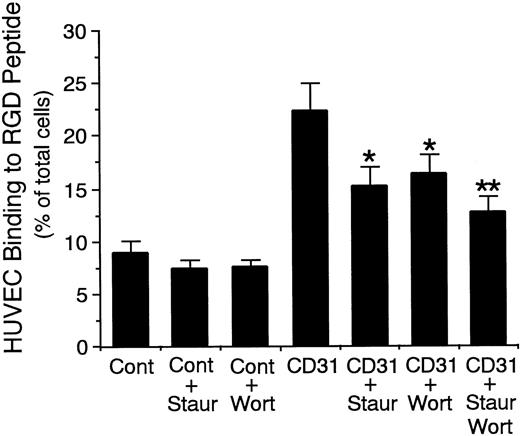
Finally, because it has been shown that, in hematopoietic cells, protein kinase C and PI3-kinase are involved in inside-out signaling of β1 integrin activation through the receptors,43-46 we determined whether these kinases are involved in the signal transduction for CD31-induced αvβ3 integrin activation on endothelial cells. Staurosporine (protein kinase C inhibitor; 10−8 mol/L)29,30 and wortmannin (PI3-kinase inhibitor; 10−7 mol/L)31 32inhibited CD31-induced increase in HUVEC binding to RGD peptide by 53% and 45%, respectively (n = 8 each, P < .001; Fig 12). In addition, the combination of staurosporine and wortmannin inhibited CD31-induced increase in HUVEC binding to RGD peptide by 71% (n = 6, P < .001; Fig 12). These results indicate that protein kinase C and PI3-kinase are involved in the CD31-mediated signaling for αvβ3 integrin activation on endothelial cells.
Effect of staurosporine and wortmannin on endothelial CD31-induced increase in the adhesive function of vβ3 integrin to RGD peptide. 51Cr-labeled HUVECs were preincubated with staurosporine (Staur; 10−8 mol/L), wortmannin (Wort; 10−7 mol/L), or the combination of them for 10 minutes at 37°C and were then preincubated with anti-CD31 MoAb (CD31) or control mouse IgG (Cont) (10 μg/mL each) for 10 minutes at 37°C. After washing the cells, the binding assay of 51Cr-labeled HUVECs to RGD peptide was performed for 10 minutes at 37°C. Data are the means ± SD for 6 to 8 experiments. *Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (*P < .001). **Significantly different from the mean value of the responses of anti-CD31 MoAb-pretreated HUVECs in the presence of staurosporine or wortmannin alone, respectively (**P < .05).
Effect of staurosporine and wortmannin on endothelial CD31-induced increase in the adhesive function of vβ3 integrin to RGD peptide. 51Cr-labeled HUVECs were preincubated with staurosporine (Staur; 10−8 mol/L), wortmannin (Wort; 10−7 mol/L), or the combination of them for 10 minutes at 37°C and were then preincubated with anti-CD31 MoAb (CD31) or control mouse IgG (Cont) (10 μg/mL each) for 10 minutes at 37°C. After washing the cells, the binding assay of 51Cr-labeled HUVECs to RGD peptide was performed for 10 minutes at 37°C. Data are the means ± SD for 6 to 8 experiments. *Significantly different from the mean value of the control response of anti-CD31 MoAb-pretreated HUVECs (*P < .001). **Significantly different from the mean value of the responses of anti-CD31 MoAb-pretreated HUVECs in the presence of staurosporine or wortmannin alone, respectively (**P < .05).
DISCUSSION
In this study, we show that CD31 stimulation activates αvβ3 integrin on endothelial cells and that the increased heterophilic interaction between αvβ3 integrin on endothelial cells and CD31 on eosinophils mediates firm adhesion of eosinophils to endothelial cells through VLA-4/VCAM-1 interaction. We found that preincubation of IL-4–stimulated HUVECs with anti-CD31 MoAb enhanced eosinophil adhesion to the IL-4–stimulated HUVECs (Figs 2 and 3) and that the endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs was prevented by anti–VCAM-1 MoAb and anti–VLA-4 MoAb (Fig 4), but not by anti–ICAM-1 MoAb, anti–LFA-1 MoAb, anti–P-selectin MoAb, or anti–E-selectin MoAb. We also found that CD31 stimulation of HUVECs increased the adhesive function of αvβ3 integrin to its ligand Arg-Gly-Asp (RGD) peptide (Fig 5), the binding of which reached a maximum at 10 minutes after the stimulation (Fig 6). Furthermore, we found that anti-αvβ3 integrin MoAb and GRGDSP peptide as well as soluble CD31 inhibited endothelial CD31-induced enhancement of eosinophil adhesion to IL-4–stimulated HUVECs (Figs 7 through 9). However, anti-αvβ3 integrin MoAb had no significant inhibitory effect on the eosinophil adhesion to IL-4–stimulated or unstimulated HUVECs without CD31 stimulation of HUVECs (Fig 2). Finally, CD31 stimulation of eosinophils increased the adhesive function of α4β1 integrin (VLA-4) to its ligand fibronectin (Fig 10) and their adhesion to IL-4–stimulated HUVECs in a VLA-4–dependent manner (Fig 11). Thus, it is indicated that the heterophilic αvβ3 integrin/CD31 interaction forms an important amplifier loop for β1 integrin-mediated adhesion of eosinophils to endothelial cells, but that the heterophilic interaction itself is not significantly involved in firm adhesion of eosinophils to endothelial cells.
αvβ3 integrin has been implicated in angiogenesis, in which αvβ3 integrin on the surface of endothelial cells transmits signals for the survival, migration, and differentiation of endothelial cells.47,48 It has recently been shown that αvβ3 integrin is a ligand for CD31 using adhesion assays to recombinant CD31.15 16 However, the functional importance of the heterophilic αvβ3 integrin/CD31 interaction between endothelial cells and leukocytes has not yet been elucidated. We provide the first evidence for the functional role of the heterophilic αvβ3 integrin/CD31 interaction that αvβ3 integrin, when activated, on endothelial cells is a signaling ligand for CD31 on leukocytes, but that the adhesive property of the heterophilic interaction is not significantly involved in firm adhesion of leukocytes to endothelial cells. The difference in binding of αvβ3 integrin to RGD peptide and CD31 on eosinophils may be due to the difference in the experimental conditions of the binding assays. That is, the binding of αvβ3 integrin on HUVECs to RGD peptide coated on the plates may be more sensitive to be detected than the binding of αvβ3 integrin on HUVECs to CD31 on eosinophils.
There has been the controversy over whether αvβ3 integrin can bind CD31. Sun et al49 reported that anti-αvβ3 integrin antibody failed to inhibit the binding of CD31 proteoliposomes to resting HUVECs. This inconsistency seems to be because CD31 can bind αvβ3 integrin on HUVECs with a high affinity only when αvβ3 integrin is activated (Figs 5 and 6). Furthermore, besides αvβ3 integrin, a 120-kD ligand50 and CD3851 have been reported as counter receptors for CD31 and L1 adhesion molecule has been reported as another ligand for αvβ3 integrin.52 Therefore, because a selective antagonist that specifically blocks αvβ3 integrin/CD31 interaction is not yet available, there is still a small possibility that other interactions that involve either αvβ3 integrin or CD31 but do not involve the specific interaction of αvβ3 with CD31 contribute to the amplification of VLA-4/VCAM-1 binding between eosinophils and endothelial cells.
We also demonstrate that CD31-mediated signaling activates αvβ3 integrin on endothelial cells. Previous studies showed that CD31 stimulation induced β1 and β2 integrin activation on a variety of leukocytes.10,21-23,42 Thus, our results indicate that CD31 also acts as a signaling molecule for integrin activation in nonhematopoietic cells. We have also found that, using specific kinase inhibitors, protein kinase C and PI3-kinase are involved in mediating the CD31-induced inside-out signaling for αvβ3 integrin activation on endothelial cells (Fig 12). In hematopoietic cells, 2 major pathways for integrin activation have been shown to be mediated by activation of protein kinase C and PI3-kinase.43-46 Protein kinase C may directly phosphorylate β integrin cytoplasmic domains, but it has been argued that no good correlation exists between the phosphorylated state and activation of integrin46; rather, it is likely that the immediate target of protein kinase C is some intracellular mediator acting upstream of the integrins. Recently, PI3-kinase has also been suggested to mediate integrin activation in hematopoietic cells, as indicated by the observations that the selective PI3-kinase inhibitor wortmannin blocks both β1 integrin activation and the thrombin-mediated activation of platelet integrin.44,45 53
We also show that CD31 stimulation activates α4β1 integrin (VLA-4) on eosinophils and thereby is importantly involved in the firm adhesion of eosinophils to endothelial cells through VLA-4/VCAM-1 interaction. It has been reported that CD31 stimulation induces β1 integrin activation on T cells10 and CD34+ hemopoietic progenitor cells21 and, to a lesser extent, β2 integrin activation on monocytes, neutrophils,22 and natural killer cells.23 For example, Tanaka et al10 showed that, using CD8+ T cells, cross-linking of CD31 with anti-CD31 MoAb increased β1 integrin-mediated adhesion to fibronectin and purified VCAM-1, indicating that CD31 can act as a signaling molecule for β1 integrin activation. However, the molecular mechanism of leukocyte CD31-mediated inside-out signaling for β1 integrin activation is still mostly unknown and remains to be elucidated.
Eosinophil infiltration into the tissue is a characteristic feature of allergic inflammation such as asthma.54,55 Recent studies have shown that the selective adhesion of eosinophils to vascular endothelial cells and its transendothelial migration are mediated by VCAM-1/VLA-4 interaction.38-41,56 VLA-4 is expressed on eosinophils but not on neutrophils.38-41 In addition, IL-4 selectively induces VCAM-1 but not ICAM-1 expression on endothelial cells34-36 and increases the adherence of eosinophils to endothelial cells.36 57 Therefore, our results provide a novel regulatory mechanism of VCAM-1/VLA-4 interaction that is controlled by endothelial αvβ3 integrin/eosinophil CD31 interaction.
In summary, we have shown that CD31-mediated inside-out signaling activates αvβ3 integrin on endothelial cells and that the increased heterophilic interaction between αvβ3 integrin on endothelial cells and CD31 on eosinophils induces β1 integrin-mediated adhesion of eosinophils to endothelial cells. These results indicate that the heterophilic αvβ3 integrin/CD31 interaction forms an important amplifier loop for β1 integrin-mediated adhesion of eosinophils to endothelial cells and thus is involved in the selective eosinophil recruitment in allergic inflammation.
Supported in part by grants from the Ministry of Education, Science and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Itsuo Iwamoto, MD, Department of Medicine II, Chiba University School of Medicine, 1-8-1 Inohana, Chiba City, Chiba 260, Japan; e-mail: iwamoto@intmed02.m.chiba-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal