Abstract
The molecular mechanisms that regulate membrane targeting/fusion during platelet granule secretion are not yet understood.N-ethylmaleimide-sensitive fusion protein (NSF), soluble NSF attachment proteins (SNAPs), and SNAREs (SNAP receptors) are elements of a conserved molecular machinery for membrane targeting/fusion that have been detected in platelets. We examined whether NSF, an ATPase that has been shown to play a critical role in membrane targeting/fusion in many cell types, is necessary for platelet granule secretion. Peptides that mimic NSF sequence motifs inhibited both -granule and dense-granule secretion in permeabilized human platelets. This inhibitory effect was sequence-specific, because neither proteinase K-digested peptides nor peptides containing similar amino acids in a scrambled sequence inhibited platelet secretion. The peptides that inhibited platelet granule secretion also inhibited the human recombinant -SNAP–stimulated ATPase activity of recombinant NSF. It was also found that anti-NSF antibodies, which inhibited recombinant -SNAP–stimulated ATPase activity of NSF, inhibited platelet granule secretion in permeabilized cells. The inhibition by anti-NSF antibodies was abolished by the addition of recombinant NSF. These data provide the first functional evidence that NSF plays an important role in platelet granule secretion.
DURING PLATELET GRANULE secretion, a large number of biologically active substances are released that have important roles in platelet activation, aggregation, and thrombus formation.1-3 Still, the mechanisms through which platelet activation is coupled to granule secretion are poorly understood. There is little known about the membrane targeting/fusion events related to platelet degranulation.
In many cells, N-ethylmaleimide–sensitive fusion protein (NSF),4 soluble NSF attachment proteins (SNAPs),5 and SNAREs (SNAP receptors) have been found to mediate intracellular membrane targeting/fusion events. According to the SNARE-hypothesis,6 vesicular (v-) and target (t-) membrane SNAREs form stoichiometric complexes that pair biological membranes in preparation for fusion. The v-SNARE synaptobrevin (also known as vesicle-associated membrane protein [VAMP]) and the t-SNAREs syntaxin and SNAP-25 form a stable ternary complex that docks the vesicle with its target membrane. This complex then binds SNAPs followed by NSF to form a 20S complex. In this model, the specificity of membrane targeting relies on specific pairing of SNAREs, whereas the highly conserved NSF, together with SNAPs, are the general elements of the machinery. NSF and SNAPs dissociate the stable v-/t-SNARE complexes using energy provided by the hydrolysis of ATP.7 8 The ATPase NSF has been shown to play a critical role in membrane targeting/fusion in many cells.
Recently, NSF, SNAPs, and SNAREs have been detected in platelets.9,10 Their presence in platelets suggests that membrane targeting/fusion events related to platelet degranulation are mediated by NSF and the SNARE machinery. However, the demonstration of these secretory molecules does not prove that platelet secretion requires an NSF-mediated mechanism. In addition to the numerous examples in which NSF was required in intracellular membrane targeting/fusion, NSF-independent mechanisms were also reported in yeast and mammals as well.11-14 Both NSF-dependent and NSF-independent, but SNARE-dependent, membrane targeting/fusion processes may coexist in the same cell type.14 Furthermore, it has been found recently that v- and t-SNAREs alone, when reconstituted into separate vesicles, are sufficient for assembling a fusion complex and for mediating the spontaneous fusion of the docked membranes.15 This argues that the SNAREs are both necessary and sufficient for membrane fusion and that NSF and the SNAPs are not part of the minimal fusion machinery. So, without functional evidence, the involvement of NSF in membrane targeting/fusion events associated with platelet granule secretion remains speculative.
The goal of this study was to determine whether the ATPase NSF plays a role in platelet granule exocytosis. We found that peptides that mimic NSF sequences and inhibit α-SNAP–stimulated ATPase activity of NSF in vitro inhibited both dense-granule and α-granule secretion in permeabilized human platelets. Anti-NSF antibodies, which inhibited α-SNAP–stimulated ATPase activity of NSF in vitro, also inhibited platelet granule secretion in permeabilized cells, and this inhibition could be abolished by the addition of recombinant NSF. These data provide the first functional evidence that NSF is important for platelet granule secretion.
MATERIALS AND METHODS
Materials.
Peptides acetylated at the NH2-terminus and amidated at the COOH-terminus were synthesized, purified to greater than 95% by high-performance liquid chromatography, and analyzed by Mass Spectral Analysis (MALDI/TOF) by Research Genetics Inc (Huntsville, AL) and Advanced ChemTech (Louisville, KY). β-Thromboglobulin enzyme-linked immunosorbent assay (ELISA) kit was from American Bioproducts (Parsippany, NJ). 5-Hydroxy [2-14C] tryptamine creatinine sulphate (50 μCi/mL at 56 mCi/mmol; 14C-serotonin) was from Amersham (Chicago, IL). Colorimetric lactate dehydrogenase (LDH) assay kit, saponin (catalogue no. S4521) and bovine thrombin were from Sigma Chemical Co (St Louis, MO). Escherichia coli XL-1 Blue was obtained from Stratagene (La Jolla, CA). PQE-30 vectors and Nickel-nitrilotriacetic acid-agarose (Ni-NTA-agarose) were from Qiagen Inc (Valencia, CA). Protein A Sepharose was from Pierce (Rockford, IL). Centrifugal concentrators were from Amicon (Beverly, MA). Double-stranded DNA sequencing kit was from US Biochemical (Cleveland, OH). Proteinase K was from Boehringer Mannheim Co (Indianapolis, IN). Enhanced chemiluminescence detection reagents were from Amersham (Piscataway, NJ).
Preparation of 14C-serotonin labelled platelet suspensions.
Human blood was drawn by venipuncture through 19-gauge needles from apparently healthy volunteers who had not taken any medication for at least 10 days. This protocol was approved by the Human Subject Committee at the Harvard School of Public Health. The blood (6 vol) was collected into 1 vol 85 mmol/L trisodium citrate, 65 mmol/L citric acid, and 110 mmol/L glucose, pH 4.5, and the sample was mixed gently by inversion. Platelet-rich plasma (PRP) was prepared by centrifugation. PRP was preincubated with 14C-serotonin (3 μL/mL PRP) for 60 minutes at 37°C. Platelets were pelleted by centrifugation at 1,500g for 10 minutes, the supernatant was completely removed, and the cells were resuspended in a modified Ca2+-buffering media16 containing 20 mmol/L PIPES, pH 7.4, 150 mmol/L potassium glutamate, 5 mmol/L glucose, 5 mmol/L ATP, 12.5 mmol/L magnesium diacetate, 2.5 mmol/L EDTA, 2.5 mmol/L EGTA, and 0.05% bovine serum albumin (BSA) (buffer A). The platelet count was adjusted to 1 × 109/mL and the sample was stored at 25°C and used within 3 hours.
Measurement of granule secretion in saponin-permeabilized platelets.
To 60 μL of buffer A, containing 10 to 16 μg/mL saponin and various additions, was added 20 μL platelet suspensions in 1.5-mL plastic tubes. Samples were incubated at 25°C for 10 minutes (experiments with peptides) or for 15 minutes (experiments with antibodies). Granule secretion was induced by increasing the free Ca2+-concentration to 10 μmol/L16 by adding 2.3 μL 100 mmol/L CaCl2 with or without 1.7 μL 50 U/mL thrombin. The samples were incubated for 5 minutes and centrifuged at 3,000g for 2.5 minutes, and 70 μL of supernatants was saved for scintillation counting of released 14C-serotonin and measurement of β-thromboglobulin and LDH. Under these experimental conditions, maximal granule secretion by 10 μmol/L free Ca2+ was typically 40% to 50% of total cell content both for 14C-serotonin and β-thromboglobulin. All sets of experiments contained these controls: samples containing 0.2% Triton X-100 for the measurement of total cellular 14C-serotonin, β-thromboglobulin, and LDH; samples with no CaCl2addition for the measurement of nonspecific leakage of the granule contents during the procedure. This nonspecific leakage was typically less than 10% to 15% of total secretion both for14C-serotonin and β-thromboglobulin. In agreement with earlier reports,17 the concentration of saponin required to produce the same degree of permeabilization estimated by LDH leakage had to be titrated for each individual experiment. For some experiments, peptides were pretreated with proteinase K at a peptide to enzyme ratio of 80 to 1 (wt/wt) at 37°C for 60 to 120 minutes.
Assay of stimulation of the ATPase activity of recombinant NSF by recombinant α-SNAP.
Preparation of recombinant proteins.
cDNA encoding mouse NSF, SKD2 clone in pYES2 vector, was generously provided by Dr Carol A. Vandenberg20 (University of California, Santa Barbara, CA). NSF cDNA was genetically engineered into the BamHI/Sal I site of pQE-30 expression vector, resulting in a construct comprising a His6 tag, the entire open reading frame, and the portion of the cDNA downstream of the stop codon. The NSF was expressed in XL-1 Blue Escherichia coli and purified as described by Whiteheart et al,8 except that gel filtration on a Superose 12 column was used in place of Superose 6 in the final purification step. Human α-SNAP was cloned from human platelet cDNA by the polymerase chain reaction using standard techniques. The primers corresponded to the 5’ and 3’ coding sequence and contained sites (BamHI andHindIII) that permitted ligation in frame to the pQE-30 vector. The sequence was verified by double-stranded DNA sequencing. Expression in XL-1 Blue E coli and purification of His6-α-SNAP was performed essentially as described by Whiteheart et al.7
Production and purification of anti-NSF antibodies.
Polyclonal rabbit anti-NSF antibodies were generated by immunizing a rabbit with purified His6-NSF as described by Morgan and Burgoyne.21 The IgG were purified from preimmune and immune rabbit sera by affinity chromatography on protein A Sepharose, dialyzed against Tris-buffered saline, pH 7.4, and concentrated to 12 mg/mL using Centricon-30 centrifugal concentrators.
RESULTS
Peptides that mimic NSF sequence motifs inhibit platelet granule secretion.
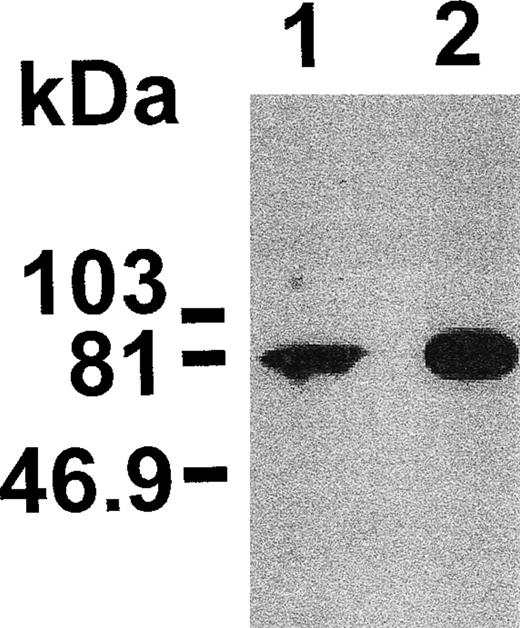
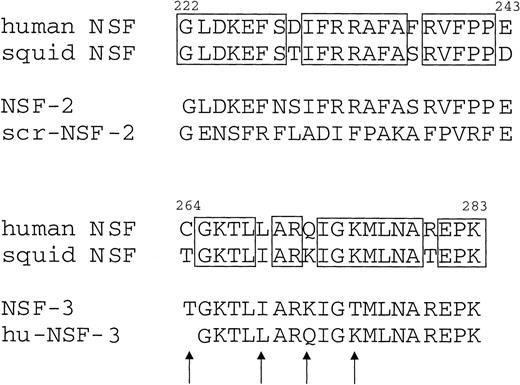
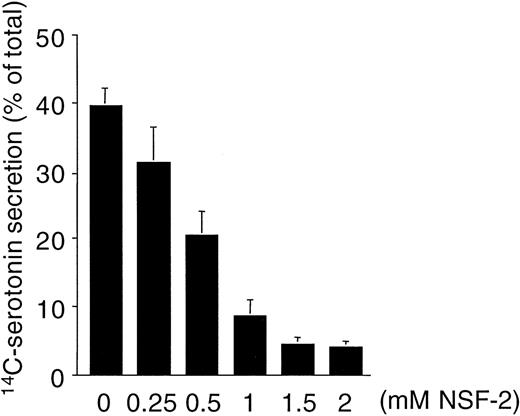
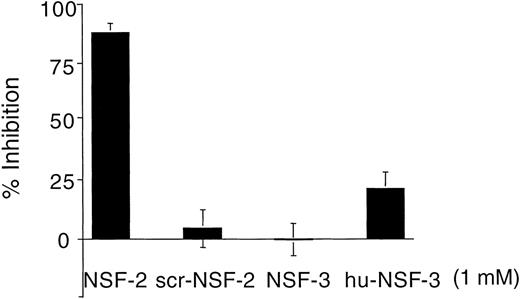
Figure 1 shows that NSF is present in human platelets. This prompted us to examine whether SNAP-triggered NSF activity is necessary for platelet secretion. In a recent report, Schweizer et al18 used peptides that correspond to potential sites of NSF-SNAP interaction to inhibit NSF function. Two of 6 peptides, NSF-2 and NF-3, inhibited neurotransmitter release by more than 50% when injected into the giant presynaptic terminal of squid.18 We used a similar approach to study whether NSF is necessary for platelet granule secretion. Although the small size of platelet makes microinjection difficult, substances can be introduced into the platelet cytosol by permeabilizing the cell membrane. Saponin-permeabilized human platelets were used to study the effect of various peptides on platelet granule secretion. The peptides used in this study as well as the corresponding sequence motifs in NSF are shown in Fig 2. The NSF-2 peptide inhibited platelet dense-granule secretion in a concentration-dependent manner (Fig 3). The inhibition reached a plateau at 1 to 1.5 mmol/L, a peptide concentration that was also effective in the squid model.18 To verify the specificity of this inhibitory effect, we used a scrambled peptide, scr-NSF-2 peptide, containing a similar amino acid composition to NSF-2, but with a different order. The scr-NSF-2 peptide had no effect on granule secretion (Fig 4), even when used in concentrations as high as 2 mmol/L (not shown), indicating that the inhibition with NSF-2 peptide was sequence-specific. Pretreatment of NSF-2 peptide with proteinase K, which digested the peptide, also abolished the inhibitory effect of NSF-2 (not shown), providing further evidence for the sequence specificity of inhibition by NSF-2. In contrast, NSF-3, which was equally effective as NSF-2 for inhibiting neurotransmission in the squid,18 did not inhibit dense-granule secretion in the human platelet (Fig 4). However, hu-NSF-3, a 19-mer peptide synthesized to mimic the human sequence for NSF-3, inhibited granule secretion in the human platelet (Fig 4) in a concentration-dependent manner (not shown).
Anti-NSF antibodies recognize NSF in human platelets. Platelet lysate (50 μg; lane 1) and recombinant NSF (50 ng; lane 2) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. The positions of prestained molecular mass markers are shown on the left.
Anti-NSF antibodies recognize NSF in human platelets. Platelet lysate (50 μg; lane 1) and recombinant NSF (50 ng; lane 2) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. The positions of prestained molecular mass markers are shown on the left.
Amino acid sequence of the peptides used in this study, as well as the corresponding sequence motifs in human and squid NSF. Residue numbers are indicated on the top of the human NSF sequences. Boxes indicate residues that are identical between the human and squid sequences. Arrows show amino acid differences between the NSF-3 and hu-NSF-3 peptides. The 19-mer hu-NSF-3 peptide is identical to the human NSF sequence from 265 to 283.
Amino acid sequence of the peptides used in this study, as well as the corresponding sequence motifs in human and squid NSF. Residue numbers are indicated on the top of the human NSF sequences. Boxes indicate residues that are identical between the human and squid sequences. Arrows show amino acid differences between the NSF-3 and hu-NSF-3 peptides. The 19-mer hu-NSF-3 peptide is identical to the human NSF sequence from 265 to 283.
Concentration-dependent inhibition of platelet dense-granule secretion by NSF-2 peptide in permeabilized human platelets. Platelets with 14C-serotonin–loaded dense granules were preincubated with saponin and various concentrations of NSF-2 peptides or with no peptide addition. Granule secretion was induced by increasing free Ca2+-concentration to 10 μmol/L. Secretion was measured by counting radioactivity in the cell supernatants (see Materials and Methods; mean ± SD, n = 2).
Concentration-dependent inhibition of platelet dense-granule secretion by NSF-2 peptide in permeabilized human platelets. Platelets with 14C-serotonin–loaded dense granules were preincubated with saponin and various concentrations of NSF-2 peptides or with no peptide addition. Granule secretion was induced by increasing free Ca2+-concentration to 10 μmol/L. Secretion was measured by counting radioactivity in the cell supernatants (see Materials and Methods; mean ± SD, n = 2).
Comparison of the effect of NSF-2, scr-NSF-2, NSF-3, and hu-NSF-3 peptides on platelet dense-granule secretion. Platelets with14C-serotonin–loaded dense granules were preincubated with saponin and different peptides (1 mmol/L) before granule secretion was induced by increasing free Ca2+ concentration to 10 μmol/L. Secretion was measured by counting radioactivity in the cell supernatants. The figure shows the percentage of inhibition of secretion by various peptides when granule secretion with no peptide additive is considered as 100% secretion (see Materials and Methods; mean ± SD, n = 4 to 8; platelet samples from 4 different donors).
Comparison of the effect of NSF-2, scr-NSF-2, NSF-3, and hu-NSF-3 peptides on platelet dense-granule secretion. Platelets with14C-serotonin–loaded dense granules were preincubated with saponin and different peptides (1 mmol/L) before granule secretion was induced by increasing free Ca2+ concentration to 10 μmol/L. Secretion was measured by counting radioactivity in the cell supernatants. The figure shows the percentage of inhibition of secretion by various peptides when granule secretion with no peptide additive is considered as 100% secretion (see Materials and Methods; mean ± SD, n = 4 to 8; platelet samples from 4 different donors).
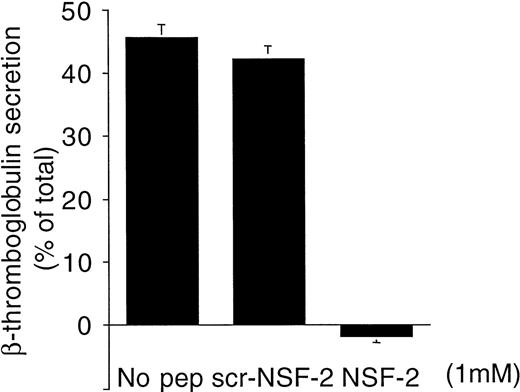
To examine the effect of these peptides on α-granule secretion, the release of β-thromboglobulin was measured by ELISA. NSF-2, which almost completely inhibited dense-granule secretion (Figs 3 and 4), practically abolished α-granule secretion as well, whereas scr-NSF-2 did not inhibit (Fig 5).
NSF-2 peptide inhibited -granule secretion in permeabilized human platelets. Platelets were preincubated with saponin and NSF-2 or scr-NSF-2 peptides or with no peptide. Granule secretion was induced by increasing free Ca2+-concentration to 10 μmol/L. -Granule secretion was monitored by ELISA measuring β-thromboglobulin in the cell supernatants (see Materials and Methods; mean ± SD, n = 2).
NSF-2 peptide inhibited -granule secretion in permeabilized human platelets. Platelets were preincubated with saponin and NSF-2 or scr-NSF-2 peptides or with no peptide. Granule secretion was induced by increasing free Ca2+-concentration to 10 μmol/L. -Granule secretion was monitored by ELISA measuring β-thromboglobulin in the cell supernatants (see Materials and Methods; mean ± SD, n = 2).
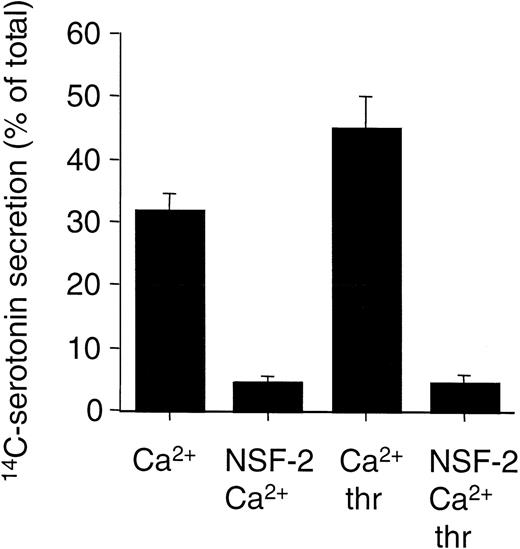
The previous data showed that peptides that mimic NSF sequence motifs inhibit platelet granule secretion elicited by Ca2+, which mimics the increased intracellular Ca2+ induced by cellular activation provoked by extracellular agonists. We examined whether the peptides could also inhibit agonist-induced granule secretion in platelet. This question is complicated by the need to permeabilize the platelets (in a Ca2+-buffering buffer system) to permit the entry of the peptides. However, CaCl2 (pCa = 5) and thrombin (1 U/mL) induced 30% to 40% more granule secretion in permeabilized cells compared with the effect of pCa = 5 alone (Fig 6). This indicated that the signaling pathways remained (at least partially) intact under the experimental conditions used in this study and made it possible to examine the effect of the peptides on agonist-induced granule secretion. Under these conditions, NSF-2 blocked secretion both induced by pCa = 5 alone or pCa = 5 and thrombin (Fig 6). This strongly suggests that these peptides inhibit both Ca2+-induced and thrombin-induced platelet granule secretion.
NSF-2 peptides inhibit both Ca2+-induced and agonist-induced platelet granule secretion. Platelets with14C-serotonin–loaded dense granules were preincubated with saponin and with or without NSF-2 peptides (1.5 mmol/L). Granule secretion was induced by increasing the free Ca2+-concentration to 10 μmol/L (Ca2+), with or without 1 U/mL thrombin (thr). Secretion was measured by counting radioactivity in the cell supernatants (see Materials and Methods; mean ± SD, n = 2; representative of 2 independent experiments with similar results).
NSF-2 peptides inhibit both Ca2+-induced and agonist-induced platelet granule secretion. Platelets with14C-serotonin–loaded dense granules were preincubated with saponin and with or without NSF-2 peptides (1.5 mmol/L). Granule secretion was induced by increasing the free Ca2+-concentration to 10 μmol/L (Ca2+), with or without 1 U/mL thrombin (thr). Secretion was measured by counting radioactivity in the cell supernatants (see Materials and Methods; mean ± SD, n = 2; representative of 2 independent experiments with similar results).
Peptides that inhibit platelet secretion also inhibit α-SNAP–stimulated ATPase activity of NSF.
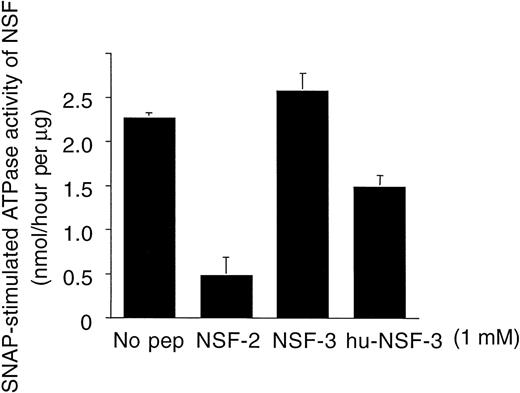
Experiments were performed to determine whether the effects of the NSF-2 and hu-NSF-3 peptides were related to their ability to interfere with SNAP-NSF interaction. In these assays, the effect of the peptides on the α-SNAP–stimulated ATPase activity of NSF was examined using recombinant proteins. It was found that both peptides inhibited human recombinant α-SNAP–stimulated ATPase activity of NSF (Fig 7). As seen with platelet secretion, NSF-2 was the most potent inhibitor, followed by hu-NSF3. NSF-3, which did not inhibit platelet granule secretion (Fig 4), also had no inhibitory effect on the SNAP-stimulated ATPase activity of NSF (Fig7).
Inhibition of human recombinant -SNAP–stimulated ATPase activity of recombinant NSF by peptides that mimic NSF sequence motifs. Peptide sequences are shown in Fig 2. Data are expressed as nanomoles of liberated phosphate per hour per microgram of NSF (see Materials and Methods; mean ± SD, n = 3).
Inhibition of human recombinant -SNAP–stimulated ATPase activity of recombinant NSF by peptides that mimic NSF sequence motifs. Peptide sequences are shown in Fig 2. Data are expressed as nanomoles of liberated phosphate per hour per microgram of NSF (see Materials and Methods; mean ± SD, n = 3).
Anti-NSF antibodies inhibit granule secretion in platelet.
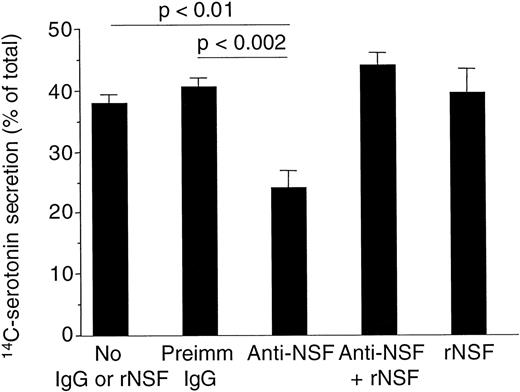
We used anti-NSF antibodies (see Fig 1) as an additional tool to verify the involvement of NSF in the process of platelet granule secretion. It was found that rabbit polyclonal anti-NSF antibodies inhibited α-SNAP–stimulated ATPase activity of NSF using recombinant proteins (not shown). These anti-NSF antibodies also significantly inhibited platelet granule secretion compared with samples with no antibodies or with preimmune rabbit IgG (Fig 8). The inhibition by anti-NSF antibodies was abolished by recombinant NSF, which itself did not affect granule secretion in these assays (Fig 8).
Anti-NSF antibodies inhibited platelet dense-granule secretion in permeabilized human platelets. Platelets with14C-serotonin-loaded dense granules were preincubated with saponin and 300 μg/mL rabbit IgG purified from preimmune serum (preimm. IgG), anti-NSF IgG from immune serum (anti-NSF) with or without 20 μg/mL recombinant NSF (rNSF). Granule secretion was induced by increasing free Ca2+-concentration to 10 μmol/L. Secretion was measured by counting radioactivity in the cell supernatants (see Materials and Methods; mean ± SD, n = 4) The P values were calculated using the paired Student’st-test.
Anti-NSF antibodies inhibited platelet dense-granule secretion in permeabilized human platelets. Platelets with14C-serotonin-loaded dense granules were preincubated with saponin and 300 μg/mL rabbit IgG purified from preimmune serum (preimm. IgG), anti-NSF IgG from immune serum (anti-NSF) with or without 20 μg/mL recombinant NSF (rNSF). Granule secretion was induced by increasing free Ca2+-concentration to 10 μmol/L. Secretion was measured by counting radioactivity in the cell supernatants (see Materials and Methods; mean ± SD, n = 4) The P values were calculated using the paired Student’st-test.
DISCUSSION
The accumulating evidence that the SNAREs are the core components of most intracellular membrane trafficking/fusion events suggests that SNAREs may play a critical role in the membrane targeting/fusion that occurs with platelet granule secretion. The assembly of v-/t-SNAREs appears to be under the kinetic control of various regulatory mechanisms, involving the members of Rab GTPase family and Sec1 family.21 NSF and SNAPs dissociate the stable v-/t-SNARE complexes using energy provided by the hydrolysis of ATP.22,23 Because of this, NSF and SNAPs may also have a role in controlling the kinetics of membrane targeting/fusion, acting either prefusion22,23 or postfusion.9,22However, because NSF-independent intracellular membrane targeting/fusion mechanisms exist,11-14 the involvement of NSF in a particular process, eg, in platelet granule secretion, cannot be assumed without functional evidence.
Consequently, we examined whether NSF plays a role in platelet granule secretion, a process in which membrane targeting/fusion is crucial. A saponin permeabilization assay was established to study the effects of various peptides and anti-NSF antibodies on platelet secretion. Saponin permeabilization has been successfully used to introduce molecules, up to the size of antibodies, into platelets.17 24-27 It was found that peptides that mimic human NSF sequence motifs inhibited granule secretion in permeabilized human platelets. In these studies, we have taken several steps to verify that the inhibitory effect was mediated through NSF. We first established that the inhibitory effects were sequence-specific because (1) digestion of the peptides by proteinase K abolished the inhibition; (2) peptides with a similar amino acid composition in a scrambled sequence did not inhibit; and (3) NSF-3, a 20-mer that differs from the corresponding human NSF sequence, did not inhibit, but an hu-NSF-3 peptide, which was identical with the human NSF sequence motif, did inhibit secretion. In addition, the inhibitory effects of the peptides on platelet secretion were directly related to their ability to block functional NSF-SNAP interactions in vitro: NSF-2 and hu-NSF-3, which inhibited platelet secretion, also inhibited human recombinant α-SNAP–stimulated ATPase activity of recombinant NSF. The NSF-3 peptide, which did not inhibit platelet secretion, did not inhibit ATPase activity. Finally, anti-NSF antibodies were used as an independent approach to verify the role of NSF in platelet secretion. Anti-NSF antibodies inhibited granule secretion in permeabilized platelets. The specificity of inhibition by anti-NSF antibodies was demonstrated by showing that (1) these antibodies recognize only 1 major band in platelet lysate and that this band comigrates with recombinant NSF; (2) they inhibited α-SNAP–stimulated ATPase activity of NSF with recombinant proteins; (3) recombinant NSF abolished the inhibitory effects of the anti-NSF antibodies both in vitro and in platelet secretion; and (4) preimmune IgG, purified from preimmune serum, did not inhibit. Taken together, these findings indicate that NSF is important for platelet secretion. The fact that dense-granule and α-granule secretion were almost completely inhibited by the NSF-2 peptide, the most potent inhibitor of NSF-ATPase activity in vitro, strongly suggests that platelet NSF plays a critical role in granule secretion that probably cannot be bypassed by other mechanisms.
Recent studies have indicated that platelets contain NSF, SNAPs, and a full complement of interacting SNARE machinery-related proteins.9,10 Indeed, during the review of our manuscript, Flaumenhaft et al28 published functional evidence for the involvement of SNARE proteins in Ca2+-induced α-granule secretion in streptolysin O-permeabilized platelets. Our study provides the first functional evidence for the involvement of NSF in platelet granule secretion and provides additional independent evidence for the hypothesis that the SNARE-machinery plays a pivotal role in regulated exocytosis in platelets.
ACKNOWLEDGMENT
The authors are grateful to Prof Carol A. Vandenberg for providing cDNA encoding mouse NSF. We thank Lin Liu, Michael L. Fitzgerald, Aiilyan Houng, and Brian Robinson for their help in cloning human α-SNAP and raising antibodies.
Supported in part by Grant No. HL-57314 from the National Institutes of Health and by a grant to Harvard School of Public Health from Bristol-Myers Squibb.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Guy L. Reed, MD, Cardiovascular Biology Laboratory, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal