Abstract
To define prognostic factors in infant acute lymphoblastic leukemia (ALL), the outcome of 106 infants (age ≤12 months) during 3 consecutive multicenter trials of the Berlin-Frankfurt-Münster group (ALL-BFM 83, 86, and 90) was retrospectively analyzed according to presenting features and early in vivo response to prednisone. The prednisone response was defined as the cytoreduction (number of blood blasts per microliter at day 8) to a 7-day prednisone prephase and 1 intrathecal dose of methotrexate on day 1. Prednisone good responder (PGR; <1,000 blasts/μL) received conventional therapy and prednisone poor responder (PPR; ≥1,000 blasts/μL) received intensified therapy. Infant ALL was characterized by a high incidence of a white blood cell count greater than 100 × 103/μL (57%), central nervous system leukemia (24%), lack of CD10 expression (59%), 11q23 rearrangement (49%) including the translocation t(4;11) (29%), and a comparatively high proportion of PPR (26%), which were all significantly associated with inferior outcome by univariate analysis. The estimated probability for an event-free survival at 6 years (pEFS) was by far better for PGR compared with PPR, who had a dismal prognosis despite intensified treatment (pEFS, 53% ± 6%v 15% ± 7%, P = .0001). Infant PGR, who were less than 6 months of age (n = 40), lacked CD10 expression (n = 43), and/or had an 11q23 rearrangement (n = 17) fared significantly better compared with corresponding PPR, as indicated by a pEFS of 44% ± 8%, 49% ± 8%, and 41% ± 12%, respectively. In multivariate analysis, PPR was the strongest adverse prognostic factor (relative risk, 3.3; 95% confidence interval, 1.9 to 5.8; P< .0001). Infants with PGR, comprising a major subgroup (74%) among infants, might successfully be treated with conventional therapy, whereas PPR require new therapeutic strategies, including early treatment intensification or bone marrow transplantation in first remission.
THE IDENTIFICATION OF prognostic factors and evolution of risk-adapted therapy for children with acute lymphoblastic leukemia (ALL) represents one of the success stories in modern clinical oncology. However, despite marked advances in therapy of childhood ALL,1 the probability of event-free survival (pEFS) for infants is approximately 35% only, irrespective of different treatment protocols.2-10 Early relapse rather than excessive toxic complications has been identified as the major factor responsible for the poor outcome in infant ALL. However, the young age of infants predisposes them to suffering from late effects of therapy, in particular to the development of neuropsychological toxicity.9,11,12 Therefore, the current debate centers around whether and which infants will need intensification of front-line therapy, including bone marrow transplantation (BMT) or other innovative treatment strategies.4,5,9 13-17
Because of the rarity of infants among children with ALL, the definition of independent parameters predicting outcome has been difficult. Age less than 6 months,2,4 white blood cell count (WBC) ≥50 × 103/μL,2,5pro-B-phenotype with lack of CD10 expression,5,15,18cytogenetic abnormalities of chromosome band 11q23,5 the translocation t(4;11),7,14,19 and most recently rearrangement of the mixed-lineage leukemia (MLL) gene at chromosome band 11q2313-15,20 21 have been related to the poor prognosis in infant ALL.
In trial ALL-BFM 83, the Berlin-Frankfurt-Münster (BFM) group has demonstrated the prognostic significance of blast cell reduction in peripheral blood to a 7-day prednisone prephase and 1 intrathecal dose of methotrexate on day 1 (prednisone response) as a parameter for early treatment response. Thus, a new independent risk factor called the prednisone poor response (PPR) was generated that identifies approximately 10% of all children with ALL with an pEFS of less than 50%.22 In subsequent ALL-BFM studies,8,23-25and confirmed by others,26 27 the prednisone response has emerged as the strongest predictor of outcome in childhood ALL.
To verify whether the prednisone response could be used as a reliable stratification factor in infant ALL, we retrospectively analyzed the outcome of 106 infants with ALL according to presenting features and initial response to prednisone treatment. Our results showed that the prednisone response was the strongest prognostic factor in infant ALL and might be used as a stratification factor in risk-adapted treatment protocols for infant ALL.
PATIENTS AND METHODS
Patient characteristics.
Between October 1, 1983 and March 31, 1995, 3,829 evaluable children with previously untreated ALL were enrolled onto 3 consecutive multicenter trials (ALL-BFM 83, 86, and 90). One hundred nineteen (3.1%) were infants ≤12 months of age, of whom 106 were evaluable for this study. Thirteen infants did not meet the study criteria for evaluation: acute myeloid leukemia, pre-existing Fanconi anemia (n = 2), death before start of treatment (n = 2), major protocol violation that was not enforced by the course of treatment or disease (n = 7), patient treated according to pilot protocol (n = 1), and patient treated in nonmember hospital (n = 1). This series included 47 cases previously reported.8 22
For all studies, informed consent from the guardians was obtained for each patient. The treatment protocols had been approved by the local ethical committee. The diagnosis of ALL and extramedullary disease was based on standard morphologic studies and cytochemical staining of leukemic cells as reported previously.8,22,23 Central nervous system (CNS) disease at diagnosis was defined by a WBC count of greater than 5 cells/μL with identifiable blasts in cerebral spinal fluid (CSF) or by a pathological mass detected by cranial computed tomography, with or without CSF pleocytosis. Blast cell immunophenotype,28 karyotype,29ploidy,30 and screening for the MLL fusion transcripts MLL/AF4, MLL/AF9, and MLL/ENL by reverse transcriptase-polymerase chain reaction (RT-PCR)31were determined as described previously.
Therapy stratification.
In all trials, patients were stratified into 1 of 3 treatment arms; there was no specific therapy branch for infants. In ALL-BFM 83, the leukemic cell burden as indicated by the BFM risk factor (RF = 0.2 log [blasts +1] + 0.06 × liver + 0.04 × spleen, with organ size in centimeters below costal margin)32 was used for stratification. Children with an RF less than 1.2 were assigned to the standard-risk group (SRG). Patients with an RF between ≥1.2 and <1.7 were stratified into the medium-risk group (MRG) or were stratified into the high-risk group (HRG) if they had an RF ≥1.7 or ≥5% marrow blasts on day 40. In addition, the prednisone response was prospectively evaluated for its prognostic significance, but not yet used for stratification. The number of leukemic blasts in blood was calculated on day 8 from the absolute leukocyte count and the percentage of blasts in a peripheral blood smear. All samples were centrally reviewed. The presence of more than 1,000 blasts/μL blood blasts on day 8 was defined as PPR.22 Retrospectively, all PPR in trial ALL-BFM 83 met the high-risk criteria used for this protocol and received high-risk therapy. In trials ALL-BFM 86 and 90, the prednisone response was used as an overriding stratification factor in combination with the RF. Assignment to SRG and MRG required a prednisone good response (PGR; <1,000/μL blood blasts on day 8). SRG was also defined by an RF less than 0.8, no CNS disease, no mediastinal mass, and non-T immunophenotype. Patients were classified at high risk if they had a PPR or ≥5% marrow blasts on day 33 or an acute undifferentiated leukemia. In addition, patients with a translocation t(9;22) were considered as high risk, regardless of their therapy response.8 23 In all trials, neither the translocation t(4;11) nor age less than 1 year was considered as a stratification factor.
Treatment.
Details of protocols ALL-BFM 83, 86, and 90 were reported previously.8,22 23 Steroid prephase, induction, reinduction, and intrathecal therapy were comparable for SRG and MRG infants within trials ALL-BFM 83 through 90. Infants who were less than 1 year of age at the intended time of cranial irradiation (CRT at 24 to 26 weeks after initial diagnosis of ALL) received neither preventive (12 Gy) nor curative (20/18 Gy) CRT. The major differences between all trials have been (1) the replacement of intermediate-dose methotrexate (ID-MTX; 500 mg/m2 4 times) in trial ALL-BFM 83 by high-dose methotrexate (HD-MTX; 5 g/m2 4 times) in trials ALL-BFM 86 and 90 during consolidation and (2) the introduction of different elements to high-risk treatment.
Statistical analysis.
EFS was calculated from the date of diagnosis to the last follow-up or to the first event (failure to achieve remission, early death, resistant leukemia, relapse, or death of any cause). Patients who failed to achieve a complete response were assigned to a failure time of 0. The Kaplan-Meier method was used to estimate survival rates with comparisons based on the 2-sided log-rank test.33 Standard errors were calculated using the formula of Greenwood.34Multivariate risk analysis to estimate the prognostic impact of prednisone response on pEFS was performed using Cox proportional hazards regression analysis.35 Differences in clinical and biological characteristics at time of diagnosis were analyzed by Fisher’s exact test or Mann-Whitney U-test. The data represent patient follow-up through July 31, 1998. The median observation time for all patients in complete remission (CR) was 5.5 years; and for the infants in the most recent trial ALL-BFM 90, the median observation time was 4.9 years (range, 2.6 to 7 years). Computations were performed using SAS (Statistical Analysis System Version 6.12; SAS Institute Inc, Cary, NC).
RESULTS
Table 1 depicts the presenting features of the evaluated infants. Distributions of patient characteristics were comparable between all trials, except for a higher proportion of PPR in trial ALL-BFM 86 (41%) compared with trials ALL-BFM 83 (15%) and 90 (20%). The 55 girls and 51 boys ranged in age from 5 days to 12 months (median, 5.8 months). WBC ranged from 1 to 1,290 × 103/μL (median, 120 × 103/μL). CNS disease was present in one fourth of the infants. The pro-B immunophenotype predominated with 50%, followed by the common-B and pre-B immunophenotype in 25% and 20% of the infants each. Fifty-nine percent of infants were CD10−, and 28% expressed 1 or more myeloid antigens in addition to lymphoid markers. Successful cytogenetic studies were performed on 59 infants (56%). One third had a normal karyotype. We observed 1 patient with hyperdiploidy (DNA index ≥1.16). Three infants presented with numerical aberrations (2 with trisomy 8 and 1 with trisomy 10); all were PPR. We found a high frequency of structural chromosomal aberrations (69%). One half of the infants presented with 11q23 rearrangements (n = 29). The translocation t(4;11)(q21;q23) was present in 17 patients and predominantly found in infants less than 6 months of age (81%). Twelve additional infants had other chromosomal aberrations with breakpoints in 11q23, 4 of whom had t(11;19), t(9;11), or 11q23 variants each. The 59 patients with adequate cytogenetic analysis were compared as to sex, age, WBC, RF, immunophenotype, prednisone response, and pEFS with the 47 infants for whom cytogenetics were not available. There were no significant differences both in the distribution of presenting clinical features and in pEFS (pEFS, 38% ± 7%v 50% ± 7%; P = .13).
Treatment Outcome According to Presenting Features in Infants With ALL (n = 106)
| Feature . | n (%) . | pEFS ± SE (%) . | P Value . |
|---|---|---|---|
| Sex | |||
| Female | 55 (52) | 46 ± 7 | .35 |
| Male | 51 (48) | 39 ± 7 | |
| Age (mo) | |||
| <6 | 50 (47) | 32 ± 6 | .009 |
| ≥6 ≤ 12 | 56 (53) | 56 ± 7 | |
| WBC (×103/μL) | |||
| <100 | 46 (43) | 52 ± 8 | .04 |
| ≥100 | 60 (57) | 37 ± 6 | |
| BFM RF | |||
| <1.7 | 87 (82) | 47 ± 6 | .06 |
| ≥1.7 | 19 (18) | 26 ± 10 | |
| CNS disease | |||
| Present | 25 (24) | 19 ± 8 | .0008 |
| Absent | 81 (76) | 51 ± 6 | |
| Cytogenetics* | |||
| Evaluable | 59 | 38 ± 7 | |
| Normal | 18 (31) | 56 ± 12 | |
| 11q23 rearranged | 29 (49) | 28 ± 8 | .03 |
| t(4;11)† | 17 | 18 ± 9 | .004 |
| Other abnormalities | 12 (20) | 42 ± 14 | |
| Not evaluable | 47 | 50 ± 7 | |
| Immunophenotype* | |||
| Evaluable | 104 | ||
| B-lineage | |||
| Pro-B-ALL | 52 (50) | 30 ± 7 | .005 |
| c-/pre-B ALL | 47 (45) | 56 ± 7 | |
| pro-T | 3 (3) | ||
| AUL | 2 (2) | ||
| CD 10 | |||
| CD10− | 62 (59) | 35 ± 6 | .02 |
| CD10+ | 42 (41) | 55 ± 8 | |
| Myeloid marker (≥1) | |||
| Present | 29 (28) | 45 ± 6 | .7 |
| Absent | 75 (72) | 40 ± 9 | |
| Prednisone response*,‡ | |||
| Evaluable | 105 | ||
| Good (<1,000/μL) | 78 (74) | 53 ± 6 | .0001 |
| Poor (≥1,000/μL) | 27 (26) | 15 ± 7 |
| Feature . | n (%) . | pEFS ± SE (%) . | P Value . |
|---|---|---|---|
| Sex | |||
| Female | 55 (52) | 46 ± 7 | .35 |
| Male | 51 (48) | 39 ± 7 | |
| Age (mo) | |||
| <6 | 50 (47) | 32 ± 6 | .009 |
| ≥6 ≤ 12 | 56 (53) | 56 ± 7 | |
| WBC (×103/μL) | |||
| <100 | 46 (43) | 52 ± 8 | .04 |
| ≥100 | 60 (57) | 37 ± 6 | |
| BFM RF | |||
| <1.7 | 87 (82) | 47 ± 6 | .06 |
| ≥1.7 | 19 (18) | 26 ± 10 | |
| CNS disease | |||
| Present | 25 (24) | 19 ± 8 | .0008 |
| Absent | 81 (76) | 51 ± 6 | |
| Cytogenetics* | |||
| Evaluable | 59 | 38 ± 7 | |
| Normal | 18 (31) | 56 ± 12 | |
| 11q23 rearranged | 29 (49) | 28 ± 8 | .03 |
| t(4;11)† | 17 | 18 ± 9 | .004 |
| Other abnormalities | 12 (20) | 42 ± 14 | |
| Not evaluable | 47 | 50 ± 7 | |
| Immunophenotype* | |||
| Evaluable | 104 | ||
| B-lineage | |||
| Pro-B-ALL | 52 (50) | 30 ± 7 | .005 |
| c-/pre-B ALL | 47 (45) | 56 ± 7 | |
| pro-T | 3 (3) | ||
| AUL | 2 (2) | ||
| CD 10 | |||
| CD10− | 62 (59) | 35 ± 6 | .02 |
| CD10+ | 42 (41) | 55 ± 8 | |
| Myeloid marker (≥1) | |||
| Present | 29 (28) | 45 ± 6 | .7 |
| Absent | 75 (72) | 40 ± 9 | |
| Prednisone response*,‡ | |||
| Evaluable | 105 | ||
| Good (<1,000/μL) | 78 (74) | 53 ± 6 | .0001 |
| Poor (≥1,000/μL) | 27 (26) | 15 ± 7 |
Percentage of evaluated infants.
Patients included in 11q23 rearranged.
One infant with WBC of 407,000/μL at diagnosis and a t(4;11) could not be evaluated for prednisone response due to exchange transfusion before day 8.
Univariate analysis for prognostic features associated with poor prognosis showed significance in descending order for PPR, CNS disease, the t(4;11), pro-B immunophenotype/lack of CD10 expression, age less than 6 months, 11q23 rearrangement, and WBC ≥100 × 103/μL. Neither coexpression of myeloid markers (Table 1) nor treatment protocol had a significant influence on prognosis. We noted no significant outcome difference for infants with respect to specific 11q23 rearrangements. Of the infants with the t(4;11)/MLL-AF4, 5 of 17 survived (3 in first CR; pEFS, 18% ± 9%). All survivors were PGR. Of the infants with 11q23 rearrangements other than the t(4;11)/MLL-AF4, 6 of 12 survived (5 in first CR; pEFS, 31% ± 14%; P= .17), of whom 5 were PGR. In respect to protocol treatment as a risk factor, we noted a tendency of steady improvement in pEFS for infants from trials ALL-BFM 83 to 90 (pEFS, 23% ± 12% [n = 13]v 37% ± 8% [n = 34] v 51% ± 7% [n = 59]; P = .27). A similar trend could be shown for PGR (pEFS, 27% ± 13% v 53% ± 11% v 60% ± 7%;P = .16), but the outcome for PPR remained constantly poor (pEFS, 0% v 14% ± 9% v 18% ± 12%;P = .99). However, none of the differences was statistically significant, neither in trend nor when comparing each trial separately.
In the most recent trial ALL-BFM 90, investigation of MLLfusion transcripts was performed on 24 of the 59 (41%) patients treated in this trial. MLL rearrangement for MLL/AF4,MLL/AF9, or MLL/ENL was found in 12 infants, with 6 being PGR and 6 PPR. Of the 6 infants with PGR and MLLrearrangement, 4 survived (3 in first CR and 1 in second CR). In contrast, of the 6 infants with PPR and MLL rearrangement, 2 survived (1 in first CR and 1 in second CR). Twelve infants presented with germline MLL configuration, all of whom had a PGR. In this group, 8 survived (6 in first CR and 2 in second CR).
The overall outcome at 6 years was worse for infants as compared with older children with ALL (pEFS, 43% ± 5% v 70% ± 2%;P = .0001). Infants with a PPR had a dismal prognosis compared with infants with a PGR (pEFS, 15% ± 7% v 53% ± 6%;P = .0001; Fig 1); the overall survival estimate (22% ± 8% v 65% ± 6%; P = .0001; see Table 3) differed slightly from pEFS only.
pEFS for infants with ALL according to prednisone response.
pEFS for infants with ALL according to prednisone response.
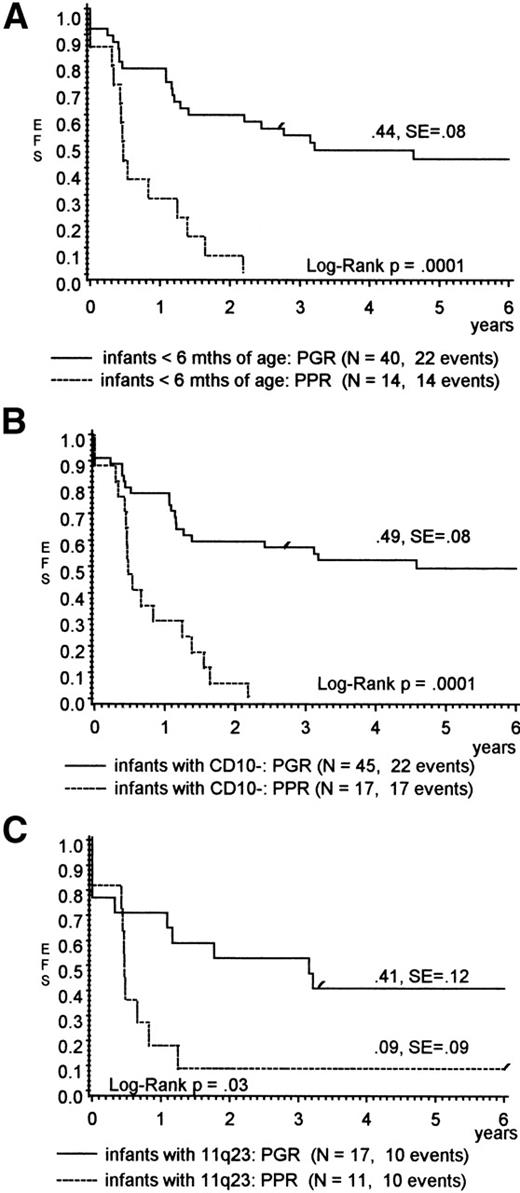
Table 2 shows the influence of prednisone response on other presenting features that had been assigned to a poor prognosis within the infant cohort by univariate analysis. Only a higher WBC (median, 250 × 103/μL for PPR v96 × 103/μL for PGR; P = .009) was significantly correlated with PPR. Infants who were less than 6 months of age, lacked CD10 expression, and/or had an 11q23 rearrangement, but demonstrated a PGR, had a favorable outcome (pEFS, 44%, 49%, and 41%, respectively), whereas almost all comparable PPR died (Fig 2A through C). For infants with the t(4;11), the pEFS results suggested a better outcome for PGR also, but were not significant (pEFS, 33% v 0%; P = .06). The predictive value of the prednisone response was statistically not significant for infants with CNS disease (Table 2), but the overall result for these children was poor in general.
Frequency (%) of Presenting Features and Outcome in Infants With ALL According to Prednisone Response (n = 105)
| Feature . | PGR (n = 78) . | PPR (n = 27) . | P Value . | ||
|---|---|---|---|---|---|
| % . | pEFS ± SE (%) . | % . | pEFS ± SE (%) . | ||
| Sex | |||||
| Female | 53 | 54 ± 13 | 48 | 23 ± 12 | .008 |
| Male | 47 | 52 ± 9 | 52 | 7 ± 7 | .0003 |
| Age (mo) | |||||
| <6 | 51 | 44 ± 8 | 52 | 0 ± 0 | .0001 |
| ≥6 ≤ 12 | 49 | 64 ± 8 | 48 | 31 ± 13 | .01 |
| WBC (×103/μL) | |||||
| <100 | 51 | 54 ± 9 | 22 | 33 ± 19 | .24 |
| ≥100 | 49 | 52 ± 8 | 78 | 10 ± 6 | .0002 |
| CNS disease | |||||
| Present | 22 | 28 ± 11 | 30 | 0 ± 0 | .17 |
| Absent | 78 | 60 ± 7 | 70 | 22 ± 10 | .0002 |
| Immunophenotype* | |||||
| CD10 | |||||
| Negative | 57 | 49 ± 8 | 63 | 0 ± 0 | .0001 |
| Positive | 43 | 59 ± 9 | 37 | 40 ± 15 | .14 |
| Myeloid marker | |||||
| Present | 28 | 45 ± 12 | 30 | 25 ± 15 | .16 |
| Absent | 72 | 57 ± 7 | 70 | 11 ± 7 | .0001 |
| Cytogenetics† | |||||
| Normal (n) | 42 (16) | 60 ± 13 | 10 (2) | 0 ± 0 | .02 |
| 11q23 rearranged (n) | 45 (17) | 41 ± 12 | 55 (11) | 9 ± 9 | .03 |
| t(4;11) (n)‡ | (9) | 33 ± 16 | (7) | 0 ± 0 | .06 |
| Other aberrations (n) | 13 (5) | 100 ± 0 | 35 (7) | 40 ± 22 | .22 |
| Feature . | PGR (n = 78) . | PPR (n = 27) . | P Value . | ||
|---|---|---|---|---|---|
| % . | pEFS ± SE (%) . | % . | pEFS ± SE (%) . | ||
| Sex | |||||
| Female | 53 | 54 ± 13 | 48 | 23 ± 12 | .008 |
| Male | 47 | 52 ± 9 | 52 | 7 ± 7 | .0003 |
| Age (mo) | |||||
| <6 | 51 | 44 ± 8 | 52 | 0 ± 0 | .0001 |
| ≥6 ≤ 12 | 49 | 64 ± 8 | 48 | 31 ± 13 | .01 |
| WBC (×103/μL) | |||||
| <100 | 51 | 54 ± 9 | 22 | 33 ± 19 | .24 |
| ≥100 | 49 | 52 ± 8 | 78 | 10 ± 6 | .0002 |
| CNS disease | |||||
| Present | 22 | 28 ± 11 | 30 | 0 ± 0 | .17 |
| Absent | 78 | 60 ± 7 | 70 | 22 ± 10 | .0002 |
| Immunophenotype* | |||||
| CD10 | |||||
| Negative | 57 | 49 ± 8 | 63 | 0 ± 0 | .0001 |
| Positive | 43 | 59 ± 9 | 37 | 40 ± 15 | .14 |
| Myeloid marker | |||||
| Present | 28 | 45 ± 12 | 30 | 25 ± 15 | .16 |
| Absent | 72 | 57 ± 7 | 70 | 11 ± 7 | .0001 |
| Cytogenetics† | |||||
| Normal (n) | 42 (16) | 60 ± 13 | 10 (2) | 0 ± 0 | .02 |
| 11q23 rearranged (n) | 45 (17) | 41 ± 12 | 55 (11) | 9 ± 9 | .03 |
| t(4;11) (n)‡ | (9) | 33 ± 16 | (7) | 0 ± 0 | .06 |
| Other aberrations (n) | 13 (5) | 100 ± 0 | 35 (7) | 40 ± 22 | .22 |
Percentage of evaluated infants (n = 104).
Percentage of evaluated infants (n = 58; PGR, n = 38; PPR, n = 20).
Patients included in 11q23 rearranged.
(A through C) pEFS for infants with ALL (A) less than 6 months of age, (B) lack of CD10 expression, or (C) presenting with 11q23 rearrangements according to prednisone response.
(A through C) pEFS for infants with ALL (A) less than 6 months of age, (B) lack of CD10 expression, or (C) presenting with 11q23 rearrangements according to prednisone response.
Table 3 summarizes the outcome including causes of death and relapse pattern according to prednisone response. Both groups achieved a similar remission induction rate (93% v96%). Regardless of prednisone response, the leading cause of failure was BM recurrence. For PPR, we noted isolated BM (74%) or CNS relapses (26%) only, whereas the relapse pattern in PGR was distributed to different sites and combinations. PPR was associated with early treatment failure. Almost two thirds (15 of 23) of all events took place during the first 6 months after initial diagnosis: 2 infants [t(4;11) present] did not achieve remission, 2 infants died in CR from septicemia and sudden death, and 11 of 18 relapses occurred within 6 months while being on intensive treatment (BM, 7; CNS, 4). In contrast, in the PGR group, one third (11 of 31) of all events took place within 6 months. Three infants, 2 of whom had a translocation t(4;11), did not achieve remission, and 8 additional patients relapsed while on intensive treatment.
Outcome for Infants With ALL According to Prednisone Response
| No Infants . | All Infants (105) . | PGR (78) . | PPR (27) . | |||
|---|---|---|---|---|---|---|
| n . | (%) . | n . | (%) . | n . | (%) . | |
| CR achieved | 100 | (95) | 75 | (96) | 25 | (93) |
| 1st CCR | 47 | (45) | 43 | (55) | 4 | (15) |
| pEFS ± SE (%) | 43 ± 5 | 53 ± 6 | 15 ± 7 | |||
| 6 yr-survival ± SE (%) | 48 ± 6 | 65 ± 6 | 22 ± 8 | |||
| Events | 58 | (55) | 35 | (45) | 23 | (85) |
| Induction failure | 5 | (5) | 3 | (4) | 2 | (7) |
| Death in CR | 4 | (4) | 13-150 | (1) | 33-151 | (11) |
| Relapse | 49 | (47) | 31 | (40) | 18 | (67) |
| Site of relapse | ||||||
| BM | 26 | 13 | 13 | |||
| CNS | 9 | 4 | 5 | |||
| Testes | 3 | 3 | — | |||
| BM/CNS | 10 | 10 | — | |||
| BM/testes | 1 | 1 | — | |||
| Second malignancy | 13-152 | 13-152 | — | |||
| No Infants . | All Infants (105) . | PGR (78) . | PPR (27) . | |||
|---|---|---|---|---|---|---|
| n . | (%) . | n . | (%) . | n . | (%) . | |
| CR achieved | 100 | (95) | 75 | (96) | 25 | (93) |
| 1st CCR | 47 | (45) | 43 | (55) | 4 | (15) |
| pEFS ± SE (%) | 43 ± 5 | 53 ± 6 | 15 ± 7 | |||
| 6 yr-survival ± SE (%) | 48 ± 6 | 65 ± 6 | 22 ± 8 | |||
| Events | 58 | (55) | 35 | (45) | 23 | (85) |
| Induction failure | 5 | (5) | 3 | (4) | 2 | (7) |
| Death in CR | 4 | (4) | 13-150 | (1) | 33-151 | (11) |
| Relapse | 49 | (47) | 31 | (40) | 18 | (67) |
| Site of relapse | ||||||
| BM | 26 | 13 | 13 | |||
| CNS | 9 | 4 | 5 | |||
| Testes | 3 | 3 | — | |||
| BM/CNS | 10 | 10 | — | |||
| BM/testes | 1 | 1 | — | |||
| Second malignancy | 13-152 | 13-152 | — | |||
Septicemia.
Treatment failure within the first 6 months (n = 2): septicemia, unknown cause (despite autopsy). Congestive heart failure after 1.2 years in CR.
Large-cell anaplastic lymphoma 4.5 years after diagnosis of ALL.
In this study, 9 infants developed an isolated CNS relapse. We noted a suggestive, but not significant association of isolated CNS relapse with PPR (5 of 9 infants) and CD10 expression (7 of 9 infants). Neither CNS disease at diagnosis of ALL, age less than 6 months, WBC ≥100 × 103/μL, nor treatment protocol including cranial irradiation were related to the risk of isolated CNS relapse.
In stepwise Cox regression analysis, PPR emerged as the most significant adverse prognostic factor, followed by age less than 6 months and CNS disease. Infants with PPR had a 3.3-fold increased risk for relapse compared with other infants (95% confidence interval, 1.9- to 5.8-fold; P < .0001; Table 4). The presence of 11q23 rearrangements including the t(4;11) did not have a significant effect on prognosis in the multivariate model, but cytogenetic data were available in one half of the patients only (Table4). Cox regression analysis for adverse features with data from infants for whom all data were available (n = 58) showed the same risk ratios, but, whereas PPR retained its statistical power (P = .002), age (P = .3) and CNS disease (P = .1) lost their statistical significance. Inclusion of protocol treatment as a risk factor showed no significance for treatment in the multivariate model.
Multivariate Analysis for Adverse Prognostic Features in Infants With ALL
| Feature4-150 (n = 105) . | Worse Category . | Relative Risk (95% CI) . | P Value . |
|---|---|---|---|
| Prednisone response | Poor | 3.3 (1.9-5.8) | <.0001 |
| Age | <6 mo | 1.9 (1.0-3.3) | .03 |
| CNS disease | Present | 1.8 (1.0-3.2) | .05 |
| 11q23 rearrangements4-151 | Present | 1.4 (0.6-2.9) | .41 |
| Feature4-150 (n = 105) . | Worse Category . | Relative Risk (95% CI) . | P Value . |
|---|---|---|---|
| Prednisone response | Poor | 3.3 (1.9-5.8) | <.0001 |
| Age | <6 mo | 1.9 (1.0-3.3) | .03 |
| CNS disease | Present | 1.8 (1.0-3.2) | .05 |
| 11q23 rearrangements4-151 | Present | 1.4 (0.6-2.9) | .41 |
Features achieving a multivariate significance level of P< .1 at the last step of regression analysis.
Multivariate analysis with data from patients for whom all data were available (evaluated n = 58).
DISCUSSION
In this large series of infants (n = 106) treated with effective risk-based ALL therapy, the prednisone response was the strongest prognostic parameter for outcome. A PGR identified three fourths of infants who achieved an EFS at 6 years of 53% with conventional therapy, whereas infants with PPR had an almost fatal outcome despite therapy intensification (pEFS, 15%; P = .0001). Of the other potential adverse prognostic factors studied, including WBC ≥100 × 103/μL, lack of CD10 expression, cytogenetic 11q23 rearrangements, the specific translocation t(4;11), and coexpression of myeloid markers, only age less than 6 months and CNS disease achieved marginal significance levels in the multivariate model. It should be noted that our patients were not treated uniformly. However, pEFS did not differ significantly for infants in trials ALL-BFM 83 versus 86 versus 90, neither for the overall cohort nor for PGR or PPR.
Regarding frequency and distribution of reported adverse prognostic features in infant ALL, we demonstrated similar results as other investigators in respect to age less than 6 months,4,10 WBC ≥100 × 103/μL,4,36 CNS disease,6,10,36 pro-B immunophenotype with lack of CD10 expression,6,10,37 lack of hyperdiploidy,7,38 and cytogenetic 11q23 rearrangements,4,5,7,10,13,14,20,21,39 including the t(4;11),4,7,10,13,39 the t(9;11),7,13,15,21 and the t(11;19).7,20 21
Age less than 6 months retained significance as a poor prognostic factor in the multivariate model, but a PPR seemed to have a major impact in this age group, as indicated by the fatal outcome of PPR compared with PGR (pEFS, 0% v 44%; P = .0001; Table 2 and Fig 2A). It is noteworthy that age seemed to influence the prognostic power of a t(4;11). During trials ALL-BFM 83 through 90, infants with a t(4;11) (n = 17) fared worse as compared with children (n = 17) who presented with the same translocation but were older than 1 year of age (pEFS, 18% ± 9% v 47% ± 12%; P = .06; Fig 3). Similar results from 32 children with ALL (pEFS, 17% ± 9%v 63% ± 19%; P = .04) were reported by Pui et al.40 These findings are interesting, because at the molecular level, children with a t(4;11) who were 1 to 9 years of age at diagnosis have been shown to display similar defects as infant cases.41 42
pEFS for children with ALL presenting with the t(4;11) according to age.
pEFS for children with ALL presenting with the t(4;11) according to age.
In contrast to other reports,9 10 we found a significant difference in the outcome of infants with or without CNS disease at diagnosis. However, an obvious explanation for this observation is missing. We noted neither an influence of the different protocols, in particular with regard to the dosage of MTX or CRT, nor a significant correlation of CNS disease with age less than 6 months, WBC ≥100 × 103/μL, lack of CD10 expression, 11q23 rearrangement, or PPR.
We confirmed the significant better outcome for infants with CD10+ compared with CD10−ALL.4,6,7,9,15,37 In contrast to other investigators, who demonstrated a significantly better pEFS in myeloid antigen-negative as compared with myeloid antigen-positive cases,37coexpression of myeloid antigens had no influence for prognosis of infants in these ALL-BFM trials.
Cytogenetic abnormalities involving chromosome band 11q23 have been correlated with a dismal prognosis in infant ALL,5particularly the t(4;11).5-7,14-16,19 In the present study, infants with 11q23 rearrangements (pEFS, 28% ± 8%) and/or the specific translocation t(4;11) (pEFS, 18% ± 9%) fared poorly as well. However, infants with 11q23 rearrangements, including those with the t(4;11), but a PGR had a relatively favorable outcome as indicated by an pEFS of 41% ± 12%, whereas almost all comparable PPR died (P = .03). Whereas 1 study suggested that infants with 11q23 partners other than the AF-4 gene may have a better outcome (P= .09),7 we found no significant difference (pEFS, 31 ± 14 v 18 ± 9; P = .17), which is in accordance with most other reports.15,17 43-45
Most recently, rearrangement of the MLL gene at chromosome band 11q23 has been associated with a dismal prognosis (pEFS, 5.3% to 28%) in infant ALL. The reported frequency of MLL rearrangement differed markedly between 50% and 81%,13-16,20,21 but has been 72% to 81% in the larger series.14,15,21 The present study lacked sufficient data regarding MLL status. Because of selection bias (screening for the specific fusion transcripts MLL/AF4, MLL/AF9, and MLL/ENL only), small sample size (n = 24), and the use of RT-PCR only, we were unable to draw firm conclusions regarding prognosis of MLL rearrangement and its association to prednisone response. However, we noted a correlation of germlineMLL with PGR, because all 12 infants with germline MLLwere PGR. Eight of them survived, of whom 6 were in first remission (pEFS, 58% ± 14%); 2 patients were in second CR after allogenic BMT, which is in accordance with reported results.14,15,20,21 Of the 12 infants with MLLrearrangement, 6 were long-term survivors (4 in first CR [3 PGR/1 PPR]; 2 remained in second CR [PPR/PGR]), which was comparable to the reported experiences.9,14,21 Therefore, half of the infants with a PGR who were evaluated for MLL rearrangements remained in first remission, irrespective of their MLL status (pEFS, 56% ± 12%). Various reports suggested that infants withMLL rearrangement may not represent a homogenous group.46,47 It remains to be evaluated whether theMLL gene rearrangement itself,13,41 the partner genes,21 or the fusion transcripts48 influence prognosis in infant ALL.
In all studies evaluating prognostic factors in infant ALL, patient numbers were relatively small and, in particular, 11q23/t(4;11)/MLL, CD10-/pro-B, and age less than 6 months were highly interrelated. Therefore, the identification of independent prognostic factors by multivariate analysis was impeded.14,15,17,20,21,49 Only 2 reports could demonstrate a significant prognostic value of MLL gene rearrangement13 and/or cytogenetic 11q23 rearrangements5 by multivariate analysis.
PPR did not correlate with other clinical or blast cell features evaluated except for high WBC. The association of PPR and high WBC seemed to be related to the definition of PPR itself. Because PPR is defined by an absolute blast cell count per microliter on day 8, the probability of being a poor responder is theoretically higher in patients with a high WBC at diagnosis. However, multivariate analysis showed that a WBC of ≥100 × 103/μL was not associated with prognosis and barely reached significance in univariate analysis. This finding might be in accordance with in vitro results from Ito et al,50 who found no significant correlation between the prednisolone concentration producing 50% cytotoxicity (LC50) and patient age, sex, WBC, presence of chromosomal translocations, ploidy, immunophenotype, and percentage of leukemic cells in S phase in BM samples of 28 children with B-lineage ALL.
Because antileukemic treatment itself is an important prognostic factor,51 the initial response to that applied therapy should be an important prognostic factor as well. Various reports have identified initial cytoreduction in blood8,22-25,27,52,53or BM36,54-58 as a powerful prognostic factor in childhood ALL, despite different treatment regimens or different time points of evaluation during induction. However, only 2 reports have evaluated infants in more detail. Miller et al56 found, in contrast to older children with ALL, no significance for percentage of lymphoblasts on day 14 BMs of infants, but neither provided detailed data nor included this finding in their discussion. Differences between their study and ours may reflect statistical variation resulting from small sample size, differences in treatment and outcome, or other factors not yet identified. In contrast, a retrospective study by the Children’s Cancer Study Group on the prognostic influence of age in childhood ALL stated that day-14 BM was the most significant predictor of disease-free survival for the infant group.36 Less infants had M1/M2 BM on day 14 as compared with older children with ALL (88% v 96%). However, because the frequency of M3 BMs was comparable (3% v 1%), it was not obvious from these data whether infants died from nonresponse or any other complications during early induction.
The outcome of infants with PPR and children older than 1 year of age with PPR treated during trials ALL-BFM 83 through 90 differed significantly (pEFS, 15% v 41%; P = .0003). Blast cells from infants presenting with PPR, 11q23 rearrangement, lack of CD10 expression, or other factors not yet identified may display different cellular pharmacodynamics that determine the sensitivity to antileukemic drugs. Kumagai et al59 showed that leukemic cells from infants with 11q23 abnormalities grew better on stromal cell layers in vitro, which was associated with a poor prognosis. Kersey et al60 recently demonstrated that MLL-AF4 leukemias were highly resistant to cell death that results from serum deprivation and had slower rates of proliferation and decreased cells in S phase compared with non–MLL-AF4 leukemias. Blast cells from infants with MLL rearrangements were shown to have a higher cell recovery rate when inoculated in SCID mice as compared with blast cells from other children with ALL,61 which correlated with a poor prognosis. In vitro studies showed that blasts from infants and blasts from children lacking CD10 expression were significantly more resistant to prednisolone, daunorubicin, and L-asparaginase than blast cells from other ALL children.62-64 Particularly, blast cells with 11q23 rearrangements are thought to arise in very primitive fetal hematopoietic precursors65 and may have a higher potential for different blast cell populations,66,67 clonal evolution,37,68 or lineage switching.67 In addition, MLL gene rearrangement is frequently observed in acute myeloblastic leukemia also.13 15 Thus, the prednisone response may represent 1 subclone of the blast cell population only and other subclones theoretically could be resistant or less responsive to standard ALL therapy.
A prospective international study currently in progress should further clarify the prognostic value of the prednisone reponse and blast cell markers such as 11q23 rearrangement, the specific translocations involving chromosome 11, and MLL rearrangement.
ACKNOWLEDGMENT
The authors thank U. Meyer and J. Regelsberger for data management, M. Zimmermann for statistical analysis, E. Odenwald for the expert cytology, the staff of the reference laboratories for excellent cooperation, and the nurses and physicians taking care of the treated infants.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael Dördelmann, MD, Medical School Hannover, Children’s Hospital, Department of Pediatric Hematology/Oncology, Carl Neuberg Str.1, 30625 Hannover, Germany; e-mail: bfm.studie@MH-Hannover.DE.