Abstract
Relapse of chronic myeloid leukemia (CML) in chronic phase after allogeneic stem cell transplantation (SCT) can be successfully treated by donor lymphocyte infusion (DLI). However, relapse of accelerated phase CML, blast crisis, or acute leukemia after allogeneic SCT are resistant to DLI in the majority of cases. In vitro-selected and expanded leukemia-reactive T-cell lines may be more effective in inducing an antileukemic response in vivo. To treat a patient with accelerated phase CML after allogeneic SCT, leukemia-reactive cytotoxic T-lymphocyte (CTL) lines were generated from her HLA-identical donor. Using a modification of a limiting dilution assay, T cells were isolated from the donor, selected based on their ability to inhibit the in vitro growth of CML progenitor cells, and subsequently expanded in vitro to generate CTL lines. Three CTL lines were generated that lysed the leukemic cells from the patient and inhibited the growth of leukemic progenitor cells. The CTL did not react with lymphocytes from donor or recipient and did not affect donor hematopoietic progenitor cells. The 3 leukemia-reactive CTL lines were infused at 5-week intervals at a cumulative dose of 3.2 × 109 CTL. Shortly after the third infusion, complete eradication of the leukemic cells was observed, as shown by cytogenetic analysis, fluorescence in situ hybridization, molecular analysis of BCR/ABL-mRNA, and chimerism studies. These results show that in vitro cultured leukemia-reactive CTL lines selected on their ability to inhibit the proliferation of leukemic progenitor cells in vitro can be successfully applied to treat accelerated phase CML after allogeneic SCT.
ALLOGENEIC STEM CELL transplantation (SCT) has been successfully applied in the treatment of hematological malignancies, including chronic myeloid leukemia (CML).1Depletion from an allogeneic stem cell graft of mature T lymphocytes, which mediate graft-versus-host disease (GVHD), results in an increased incidence of recurrence of leukemia after transplantation, especially for CML.2,3 Thus, the antileukemic effect of allogeneic SCT is not merely due to the cytoreductive treatment during the conditioning regimen before transplantation, but is also due to a graft-versus-leukemia (GVL) effect mediated by donor T cells.4,5 Patients with recurrence of leukemia after transplantation have been treated with donor lymphocyte infusions (DLI) from their stem cell donor. In patients with relapsed CML in chronic phase, 70% to 80% complete remissions have been reported after DLI.6-13 In CML accelerated phase, blast crisis, acute myeloid leukemia, and acute lymphoblastic leukemia, remission rates of only 10% to 30% have been reported.11-13 Despite a correlation between the occurrence of acute GVHD following DLI and the remission rate, disappearance of the leukemic cells can be observed in the absence of GVHD, suggesting that GVL and GVHD reactivity are not always mediated by the same effector cells.
The induction of tolerance to donor cells is the immunological basis for the successful treatment of relapsed leukemia after allogeneic SCT with DLI. Donor T cells recognizing the recipient-derived leukemic cells only and alloreactive donor T cells recognizing polymorphic antigens on all hematopoietic cells from the recipient may result in eradication of the leukemic cells without affecting donor hematopoiesis in the patient.14
Previously, we have shown that primary cytotoxic T lymphocyte (CTL) responses can be generated from donor lymphocytes against the leukemic cells from the patient in HLA-identical donor-recipient pairs.15,16 Using a newly developed assay, the progenitor cell inhibitor lymphocyte precursor (PCILp) frequency assay, we recently showed that T cells recognizing CML precursor cells may be responsible for the clinical response to DLI.17 We hypothesized that, if T-cell lines recognizing the CML precursor cells could be generated in vitro from peripheral blood of the stem cell donor, these leukemia-reactive CTL lines may be capable of eradicating the leukemic cells from the patient in vivo with a limited risk of GVHD.
In this report, we describe a patient with relapsed accelerated phase CML after allogeneic stem cell transplantation who was successfully treated with donor-derived leukemia-reactive CTL recognizing CML precursor cells.
MATERIALS AND METHODS
Case history.
Patient KG, a 43-year-old white female, was diagnosed as Ph+ CML in chronic phase in September 1991. Because no HLA-identical sibling was available, treatment was started with hydroxyurea and interferon-α-2b at 3 × 106 IU/d for 5 days per week.18 In 1992 and 1993, a hematological response, but no cytogenetic response was observed. In 1993, cytogenetic analysis showed 46XX, t(9;22), 7q−, −10, 12p+, −13, +2 marker chromosomes in 50% of the metaphases. Extended family typing showed an HLA-identical niece (HS) as a potential donor.19 The HLA-type of both donor and recipient was A1, A2, B7, B8, Cw7, DR15, DR17, DQ6, DQ2. Mixed lymphocyte cultures were negative in both directions. In 1994, allogeneic bone marrow transplantation was performed. The conditioning regimen consisted of Campath-1G intravenously (5 mg on days −8 through −3), cyclophosphamide at 60 mg/kg of body weight on days −6 and −5, and total body irradiation (6 Gy) on days −1 and 0. T-cell depletion of the graft was performed by incubation with Campath-1G (10 mg), as described.20 GVHD prophylaxis consisted of cyclosporin at 3 mg/kg/d. Normal engraftment was observed. At day 17, acute GVHD grade II of the skin developed that was treated with methylprednisolone. Cyclosporin toxicity included hypertension, fluid retention, and renal failure. Subsequent chronic GVHD of the skin required treatment with low-dose cyclosporin.
In 1995, a cytogenetic relapse was found with 46XX, t(2;20)(p12;q13), t(9;22)(q34;q11), t(8;15)(p10;q10) in all of 30 metaphases analyzed, followed by a hematological relapse. Platelet counts exceeded 1,000 × 109/L and required treatment with increasing doses of hydroxyurea. Discontinuation of the cyclosporin treatment did not result in a hematological or cytogenetic response. In November 1995, interferon-α-2b (3 × 106 IU/d) was initiated, but no improvement was observed. Cytogenetic analysis showed 46XX, t(2;20)(p12;q13), inv(2)(p12;q37), t(8;15)(p10;q10), t(9;22)(q34;q11) in all metaphases analyzed. Basophil counts were 5% to 10%. In April 1996, hydroxyurea treatment was discontinued and she received a DLI from her donor at a dose of 0.6 × 107 lymphocytes/kg of body weight. Eight weeks later, she developed acute GVHD of the skin that was treated by a short course of methylprednisolone (30 mg/d), whereas interferon treatment was stopped. Bone marrow cytogenetics showed 20% normal metaphases of 30 metaphases tested. In August 1996, 16 weeks after DLI, moderately severe chronic GVHD of the skin required treatment with cyclosporin and continuous treatment with low-dose corticosteroids. Karyotypic analysis of the bone marrow showed again 100% abnormal metaphases. Thrombocytosis (1,600 × 109/L) necessitated reinstallment of hydroxyurea therapy. Because reversal of the cytogenetic response was observed in the presence of chronic GVHD, dose-escalation of DLI was not applied.
In February, March, and April 1997, she was treated with 3 leukemia-reactive CTL lines at 5-week intervals at the doses of 0.2 × 109, 1.3 × 109, and 1.7 × 109 CTL, respectively. Side effects included chills 2 hours after the infusion and fever that resolved in 6 hours. In May 1997, she developed severe pancytopenia and pneumococcal pneumonia. While the chronic skin GVHD persisted, transient circumscript lesions were observed on her lower legs. Skin biopsies were compatible with erythema exsudativa multiforme. No acute GVHD was observed. A bone marrow biopsy showed almost complete aplasia, with scattered small foci of myeloid cells. Subsequently, a gradual increase in leukocytes and platelets was observed, resulting in normal white blood cell counts at day 105 after the first CTL infusion, with platelet counts of 30 × 109/L. Chimerism studies showed complete donor hematopoiesis. At days 140 and 200, bone marrow and peripheral blood cytogenetic analysis showed the absence of the Ph chromosome, which was confirmed by fluorescence in situ hybridization (FISH) on interphase cells using BCR- and ABL-specific probes and absence ofBCR/ABL mRNA transcripts using reverse transcriptase-polymerase chain reaction (RT-PCR). In October 1997, she was treated again for the persistant chronic GVHD of the skin with cyclosporin and corticosteroids. This resulted in a hypertensive crisis with renal failure, liver dysfunction, and signs of capillary leakage. After discontinuation of the cyclosporin, the bilirubin normalized and the oedema disappeared, but the renal insufficiency persisted with a creatinin clearance of 10 mL/min. One year after the treatment, she additionally developed angina pectoris. Two years after the treatment, the patient died of ischemic heart disease, being still in complete remission from the leukemia.
Patient and donor materials.
After informed consent was obtained, bone marrow and peripheral blood (PBL) were obtained from patient KG and her donor HS. After Ficoll Isopaque density cell separation, mononuclear cells (MNC) were harvested from the interphase and polymorphic mononuclear cells (PMN) were recovered from the pellet. MNC were cryopreserved under good manufacturing practice (GMP) conditions in Isove’s modified Dulbecco’s medium (IMDM; Biowhittaker, Verviers, Belgium) with 20 g/L clinical grade human albumin (Central Laboratory of the Blood Transfusion Service [CLB], Amsterdam, The Netherlands) and 10% dimethyl sulphoxide (DMSO). Thawed cells were washed twice and resuspended in IMDM containing 10% heat-inactivated heparin plasma from the bone marrow donor.
CML PCILp assay.17
Precursor cell frequency analysis of T lymphocytes recognizing the CML precursor cells was performed on PBL from the donor or the patient before and after treatment. MNC were plated into 96-well U-bottom plates using a pipetting robot (Biomek; Beckman, Mijdrecht, The Netherlands) at 2-fold dilutions from 40,000 cells/well down to 625 cells/well in 24 replicates per concentration. Each well was stimulated with 20,000 irradiated (25 Gy) leukemic cells from the patient obtained before transplantation or at relapse when 100% of the metaphases were of CML origin. Twenty-four wells contained irradiated stimulator cells only and were used as a reference. After 6 days of culture, 120 IU/mL of interleukin-2 (IL-2; Chiron, Amsterdam, The Netherlands) was added, and at day 9 all wells were restimulated with 10,000 irradiated leukemic MNC (CML-MNC) from the patient. Twice weekly, half of the medium was refreshed. After 21 days of culture, cells from each individual well were irradiated (15 Gy) and cocultured with 10,000 patient CML cells/well in medium consisting of IMDM with 15% donor plasma, 50 ng/mL stem cell factor (SCF; Amgen, Thousand Oaks, CA), 25 ng/mL IL-3 (Sandoz, Basel, Switzerland), 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Sandoz), 100 ng/mL granulocyte colony-stimulating factor (G-CSF; Amgen), 2 U/mL erythropoietin (EPO; Cilag Ag, Zug, Switzerland), 0.47 g/L human transferrin, and 5 × 10−5 mol/L β-mercaptoethanol. After 7 days of culture, 1 μCi3H-thymidine deoxyribose was added to each well; after another 6 hours of culture, 3H-thymidine incorporation was measured. Previous studies have shown that proliferation of the cells at day 7 reflected the progenitor cell growth of CD34+ CML cells.17 Inhibition of CML progenitor cell proliferation was calculated in comparison to the reference wells. An individual well was scored positive if the 3H-thymidine incorporation in that well was lower than the mean minus 3× SD of the reference wells. The percentage of growth inhibition of each well was determined by the following formula: (1 − [experimental CPM/mean CPM of reference wells]) × 100%. PCILp frequencies were calculated using a statistical computer program as described previously.21
Generation of leukemia-reactive CTL lines.
Using the computer-controlled pipetting robot in a biosafety cabinet placed in a clean room facility, CTL lines were generated under GMP conditions. MNC from donor HS were thawed, washed, resuspended in culture medium, and plated at a concentration of 20,000 cell/well in 10 round-bottomed 96-well microtiter plates. To each well, 20,000 irradiated (25 Gy) CML-MNC derived from the patient at relapse when 100% of the metaphases were leukemic were added. The cells were cultured in a sterile incubator at 37°C, 95% humidity, 5% CO2. After 6 days, IL-2 was added (120 IU/mL), and at day 9, each well was restimulated with 10,000 irradiated CML-MNC from the patient and the cells were expanded for 10 to 12 days. Growing wells were split using the pipetting robot. From each well, one fourth was used to analyze its potential to inhibit the CML progenitor cell growth as described in the PCILp assay, and the remaining three fourths of the cells from the wells were further expanded. From the positive wells containing PCIL, the cells were collected and pooled to a T-cell line. Using a 51Cr release assay, the T-cell line was analyzed for its capacity to lyse phytohemagglutinin-stimulated T cells (PHA-blasts) from the donor, leukemic cells from the patient, PHA blasts from the patient that were derived from her peripheral blood before transplantation, and stromal cells derived from bone marrow from the patient before the transplantation. Stromal cells were generated by culturing bone marrow MNC in the presence of 10% human serum. Two days before harvesting and testing, the cells were incubated with 300 U/mL of interferon-γ to upregulate major histocompatibility complex (MHC) expression. To determine whether CD4+ or CD8+ T cells were the main effector cells responsible for the cytolytic activity, blocking experiments were performed using antibodies against CD4, CD8, HLA-class I, or HLA-class II, as described previously.15 In addition, the CTL line was analyzed for its capacity to suppress the clonogenic erythroid (burst-forming unit-erythroid [BFU-E]) or myeloid (colony-forming unit–granulocyte-macrophage [CFU-GM]) precursor cell growth from the normal bone marrow from the donor or the CML bone marrow cells from the patient in a cell-mediated inhibition assay of hematopoietic progenitor cells in semisolid medium cultures as described previously.22 23
Molecular analysis of a clinical response.
Conventional cytogenetic analysis was performed on metaphases and FISH was performed to detect the Ph chromosome on interphase nuclei usingBCR- and ABL-specific probes.24 The presence of BCR/ABL mRNA transcripts was determined in PBL and bone marrow MNC from the patient by RT-PCR.24 Chimerism studies of MNC and/or PMN was performed after isolation of DNA from the cell fractions, followed by PCR using as primers oligonucleotides flanking the polymorphic regions of AFP and NGFβ,25 which were informative for cells derived from the donor HS and patient KG, respectively.
RESULTS
DLI.
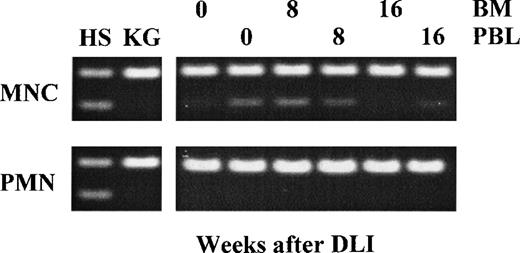
As determined by AFP polymorphism, before DLI, 5% of bone marrow MNC and 20% of PBL MNC were of donor origin, whereas all PMN were of recipient origin (Fig 1). Eight weeks after DLI, when GVHD was present, donor chimerism increased to approximately 20% in bone marrow MNC, but no significant increase in donor-derived PMN was observed. Sixteen weeks after DLI, these chimerism parameters had returned to pre-DLI values, as shown in Fig 1. The transient increase in donor MNC coincided with a transient increase of normal donor metaphases in the bone marrow to 20%.
Chimerism analysis in bone marrow (BM) or peripheral blood (PBL) from the patient after treatment with donor lymphocyte infusion (DLI). Cells of donor (HS) origin could be detected by AFP polymorphism (heterozygous 145 and 72 bp fragments). Temporary increase of donor cells was observed in MNC in BM at 8 weeks after DLI, but returned to pre-treatment levels at 16 weeks after DLI. PMN from the patient remained of patient origin.
Chimerism analysis in bone marrow (BM) or peripheral blood (PBL) from the patient after treatment with donor lymphocyte infusion (DLI). Cells of donor (HS) origin could be detected by AFP polymorphism (heterozygous 145 and 72 bp fragments). Temporary increase of donor cells was observed in MNC in BM at 8 weeks after DLI, but returned to pre-treatment levels at 16 weeks after DLI. PMN from the patient remained of patient origin.
Generation of CML reactive T-cell lines from donor HS.
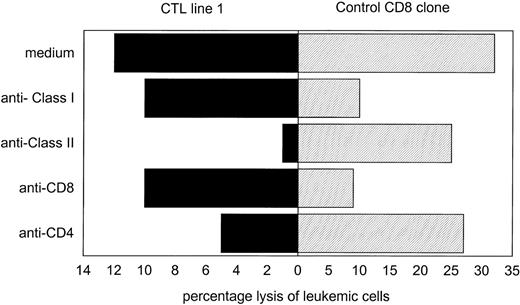
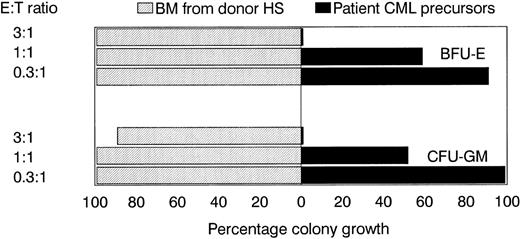
The PCILp frequency in PBL MNC from donor HS directed against CML cells from the patient was 26 per 106 MNC. From these data, we calculated that, if 20,000 donor MNC were plated per well, clonal expansion of T cells capable of inhibiting the CML precursor cells would be present in 40% to 50% of the wells. Three leukemia-reactive CTL lines were generated with 5-week intervals. The T-cell lines showed no bacterial or fungal contamination. For each CTL line, in a total of 960 wells, 20,000 donor MNC per well were stimulated with CML-MNC from the patient, of which 30% expressed CD34. Each individual positive well showing growth inhibition of 50% to 95% of the CML precursor cells in the PCILp assay was harvested from the microtiter plates. The numbers of positive wells harvested per line, the phenotypic analysis, and cytotoxic activity against CML-MNC and PHA blast or bone marrow stromal cells from patient KG as well as PHA blasts from donor HS are shown in Table 1. All CTL lines consisted of CD3+ TCRαβ T cells, with the majority of cells being CD4+. The CTL lines did not react with PHA blasts of patient origin and showed weak recognition of stromal cells derived from the bone marrow of the patient, showing a relative specificity for the patient leukemic cells. As shown in Fig2, the cytolytic activity against the leukemic cells could be inhibited by the addition of anti-CD4 but not anti-CD8 antibodies to the effector cells or by the addition of anti–HLA-DR, but not anti-HLA class I antibodies. Control experiments using an anti–HLA-A2 specific CTL clone showed that the leukemic cells were susceptable to HLA class I-restricted recognizion. The CTL lines showed specific inhibition of the CML hematopoietic progenitor cells from the patient, but did not affect normal donor hematopoietic progenitor cells, as shown in Fig 3.
Generation of 3 Leukemia-Reactive CTL Lines
| . | CTL Line . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| No. of positive wells harvested | 309 | 389 | 525 |
| No. of T cells harvested | 0.2 × 109 | 1.3 × 109 | 1.7 × 109 |
| Phenotype of CTL line | |||
| CD3 | 97% | 97% | 99% |
| CD4 | 92% | 82% | 86% |
| CD8 | 9% | 18% | 14% |
| TCRαβ | 97% | 96% | 99% |
| TCRγδ | <1% | 2% | <1% |
| Lysis* of CML-MNC | 30 | 24 | 18 |
| Lysis* of PHA-blast patient | 0 | 0 | 0 |
| Lysis* of BM stromal cells patient | 8 | NT | 10 |
| Lysis* of PHA-blast donor | 3 | 0 | 1 |
| . | CTL Line . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| No. of positive wells harvested | 309 | 389 | 525 |
| No. of T cells harvested | 0.2 × 109 | 1.3 × 109 | 1.7 × 109 |
| Phenotype of CTL line | |||
| CD3 | 97% | 97% | 99% |
| CD4 | 92% | 82% | 86% |
| CD8 | 9% | 18% | 14% |
| TCRαβ | 97% | 96% | 99% |
| TCRγδ | <1% | 2% | <1% |
| Lysis* of CML-MNC | 30 | 24 | 18 |
| Lysis* of PHA-blast patient | 0 | 0 | 0 |
| Lysis* of BM stromal cells patient | 8 | NT | 10 |
| Lysis* of PHA-blast donor | 3 | 0 | 1 |
Abbreviation: NT, not tested.
Percentage specific lysis in a 51Cr-release assay at a 10:1 effector:target ratio.
Recognition of leukemic cells by leukemia-reactive CTL or an anti–HLA-A2 CD8+ control CTL clone. CTL were preincubated with anti-CD4 or anti-CD8, or leukemic target cells were preincubated with anti-HLA class I or class II monoclonal antibodies for 30 minutes. The percentage of lysis was then measured in a51Cr-release assay at an effector:target ratio of 3:1. Recognition of leukemic cells by the leukemia-reactive CTL was blocked by anti-CD4 or anti-class II antibodies.
Recognition of leukemic cells by leukemia-reactive CTL or an anti–HLA-A2 CD8+ control CTL clone. CTL were preincubated with anti-CD4 or anti-CD8, or leukemic target cells were preincubated with anti-HLA class I or class II monoclonal antibodies for 30 minutes. The percentage of lysis was then measured in a51Cr-release assay at an effector:target ratio of 3:1. Recognition of leukemic cells by the leukemia-reactive CTL was blocked by anti-CD4 or anti-class II antibodies.
Suppression of leukemic but not normal donor BFU-E and CFU-GM growth by leukemia-reactive CTL line 3. CTL line 3 was irradiated (10 Gy) and incubated for 4 hours with bone marrow MNC from donor HS or patient KG at various effector:target ratios. After 4 hours, the cells were resuspended in methylcellulose as a single-cell suspension and cultured in the presence of multiple hematopoietic growth factors. Progenitor cell growth is expressed as the percentage of BFU-E or CFU-GM growth in the absence of CTL. All metaphases from MNC of patient KG were t(9;22) positive.
Suppression of leukemic but not normal donor BFU-E and CFU-GM growth by leukemia-reactive CTL line 3. CTL line 3 was irradiated (10 Gy) and incubated for 4 hours with bone marrow MNC from donor HS or patient KG at various effector:target ratios. After 4 hours, the cells were resuspended in methylcellulose as a single-cell suspension and cultured in the presence of multiple hematopoietic growth factors. Progenitor cell growth is expressed as the percentage of BFU-E or CFU-GM growth in the absence of CTL. All metaphases from MNC of patient KG were t(9;22) positive.
Treatment of patient KG with leukemia-reactive CTL lines.
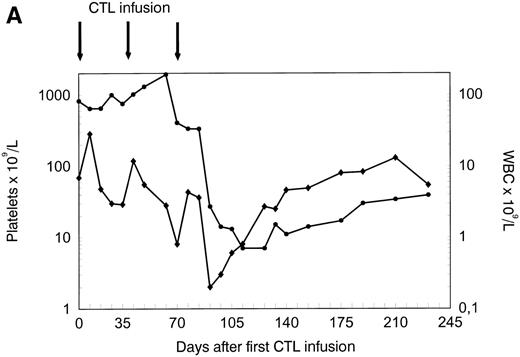
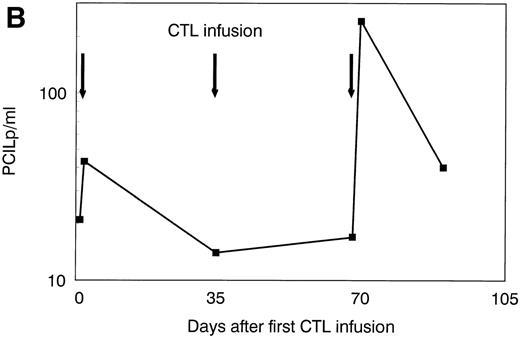
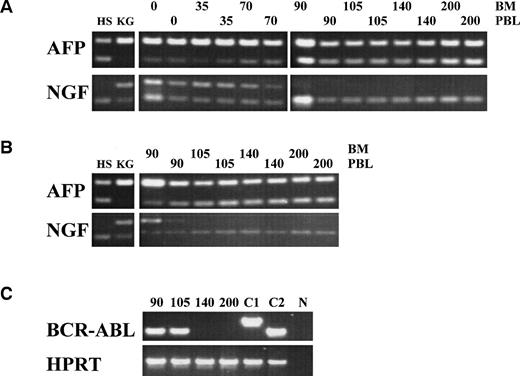
The patient was treated at days 0, 35, and 70 with CTL lines 1, 2, and 3, respectively. No clinical response was observed before infusion of CTL line 3 (day 70). A rapid hematologic response was observed between day 70 and day 90 (Fig 4A). Leukemia-reactive PCILp frequencies in peripheral blood of the patient measured before and 1 or 2 days after the infusion of the CTL lines showed a minor increase after the first infusion and a high increase of after the infusion of CTL line 3 (Fig 4B). Chimerism studies of bone marrow and PBL MNC and PMN as well as the detection of BCR/ABLtranscripts in bone marrow MNC are shown in Fig 5. Disappearance of the patient-specific polymorphic band was observed from the MNC fraction between days 70 and 105, as shown by NGF-β polymorphism (Fig 5A). In addition, no patient-derived PMN could be determined after day 90 in peripheral blood or bone marrow from the patient (Fig 5B). From day 105, all MNC and PMN from blood and bone marrow from the patient were found to be of donor origin. As shown in Fig 5C, BCR/ABLtranscripts could not be detected from day 140 after the infusion of CTL line 1 (detection limit, 1 per 104 cells). Similarly, at days 140 and 200, the Ph chromosome could no longer be detected by FISH and the karyotype normalized. These results illustrate the complete disappearance of leukemic cells and the reconstitution of full donor chimerism after treatment with the leukemia-reactive CTL lines.
Platelets, white blood cells (WBC), and PCILp before and after treatment with leukemia-reactive CTL lines 1, 2, and 3. (A) Between days 80 and 90, 10 to 20 days after the infusion of CTL line 3, a rapid decrease of (•) platelets and (⧫) WBC was observed, followed by a gradual recovery. (B) A strong increase of PCILp frequencies in PBL from the patient was observed between days 70 and 90.
Platelets, white blood cells (WBC), and PCILp before and after treatment with leukemia-reactive CTL lines 1, 2, and 3. (A) Between days 80 and 90, 10 to 20 days after the infusion of CTL line 3, a rapid decrease of (•) platelets and (⧫) WBC was observed, followed by a gradual recovery. (B) A strong increase of PCILp frequencies in PBL from the patient was observed between days 70 and 90.
Chimerism analysis and BCR/ABL minimal residual disease detection in bone marrow (BM) or peripheral blood (PBL) from the patient after treatment with leukemia-reactive CTL. Cells of donor (HS) origin could be detected by AFP polymorphism (heterozygous 145- and 72-bp fragments); cells of patient (KG) origin could be detected by NGF-β polymorphism (heterozygous for 100- and 50-bp fragments). (A) Between days 70 and 105 after the first CTL infusion, chimerism of MNC in BM and PBL completely converted to donor origin. (B) PMN from the patient were completely of donor origin from day 105 (5 weeks after the last CTL infusion). (C) Complete molecular remission was documented at day 140, 10 weeks after the last CTL infusion by disappearance of the patient b2a2 BCR/ABL transcript. C1, control b3a2 cDNA; C2, control b2a2 cDNA; N, negative control. RT-PCR of the HPRT gene served as an internal control for cDNA synthesis.
Chimerism analysis and BCR/ABL minimal residual disease detection in bone marrow (BM) or peripheral blood (PBL) from the patient after treatment with leukemia-reactive CTL. Cells of donor (HS) origin could be detected by AFP polymorphism (heterozygous 145- and 72-bp fragments); cells of patient (KG) origin could be detected by NGF-β polymorphism (heterozygous for 100- and 50-bp fragments). (A) Between days 70 and 105 after the first CTL infusion, chimerism of MNC in BM and PBL completely converted to donor origin. (B) PMN from the patient were completely of donor origin from day 105 (5 weeks after the last CTL infusion). (C) Complete molecular remission was documented at day 140, 10 weeks after the last CTL infusion by disappearance of the patient b2a2 BCR/ABL transcript. C1, control b3a2 cDNA; C2, control b2a2 cDNA; N, negative control. RT-PCR of the HPRT gene served as an internal control for cDNA synthesis.
DISCUSSION
We report here the first successful treatment with leukemia-reactive CTL lines showing a relative specificity for the leukemic cells in a patient with relapsed accelerated phase CML after allogeneic SCT. This patient had been treated with low-dose DLI 10 months before treatment with the leukemia-reactive CTL lines. After DLI, a temporary reduction of the leukemic cells was observed, but she developed acute GVHD followed by chronic GVHD of the skin, limiting further dose escalation of DLI. Several reports have shown that relapsed CML in chronic phase responds more frequently to DLI than CML in accelerated phase, blast crisis, or acute leukemia.6-13 One hypothetical explanation for this observation is that CML is a malignancy of hematopoietic precursor cells giving rise in chronic phase to malignant professional antigen-presenting cells such as dendritic cells that are capable of inducing strong T-cell responses.24,26 27 In contrast, the malignant cells present in accelerated phase CML, in blast crisis, or in acute leukemia may be more inappropriate antigen-presenting cells, which may lead to the induction of anergy rather than to a specific antileukemic T-cell response in vivo. We hypothesized that in vitro generation of leukemia-reactive CTL lines may bypass the induction phase of the antileukemic immune response in vivo.
In vitro cultured T-cell lines or clones that recognize viral antigens can be effective in suppressing Epstein-Barr virus (EBV)-associated lymphoproliferative disorders or cytomegalovirus (CMV) disease after allogeneic SCT without significant GVHD.28-32 Previously, we have shown that leukemia-reactive CTL lines can be generated with relative specificity for the leukemic cells.15,16 We have demonstrated that the generation of such leukemia-reactive CTL lines is more efficient using a modified limiting dilution assay.33 By pooling the T cells from individual wells that were inhibitory to CML precursor cells, CTL lines were generated showing cytolytic activity and growth-inhibitory activity of CML precursor cells from patient KG, but not of normal hematopoietic progenitor cells from the donor. In addition, no reactivity was found with donor or recipient PHA-stimulated lymphoblasts, whereas stromal cells derived from the leukemic bone marrow before transplantation were only weakly recognized, illustrating a relative specificity for the leukemic cells. Despite this relative specificity of the CTL lines for the leukemic cells, the antigen specificity of these CTL lines is unknown. Based on the relatively high PCILp frequency in donor peripheral blood, it is unlikely that these CTL recognized a BCR/ABL-specific peptide.34,35 Because many non-HLA molecules may be different between such HLA-matched, but not genotypically identical individuals, it is more likely that the majority of the CTL were directed against minor histocompatibility antigens (mHAg), with, at least in part, a restricted tissue distribution. Recently, several of such mHAg antigens restricted to the hematopoietic lineages have been characterized that could be recognized in the context of HLA class I molecules.36-38
Previously, we have shown that both CD4+ and CD8+ mHAg-specific CTL are capable of strong antigen specific growth inhibition of leukemic precursor cells.22,23,39,40 As shown by these specific inhibition of the recognition of leukemic cells by antibodies against CD4 and HLA class II, we demonstrated that the CD4+ cells, which were the majority of the cells present in the CTL lines, were responsible for most of the cytolytic activity. This does not exclude reactivity by the CD8+ cells as well, but these cells were present in the CTL lines at a frequency too low to allow sufficient inhibition by anti-CD8 and anti-class I antibodies. CD8+ T cells may require the presence of helper CD4+ T cells to exhibit their clinical effect. For an optimal effect, a direct cytolytic effect of the effector cells infused is probably not sufficient. It is likely that the infused cells have to proliferate and expand in vivo and survive for several weeks to months to exhibit their full clinical effect. Based on these previous findings, we hypothesized that leukemia-reactive CTL lines composed of both CD4+ and CD8+ T cells may be most efficient in exhibiting an antileukemic effect. The strong clinical response to the CTL lines was observed after infusion of the third CTL line. A total of 3.2 × 109 T cells was infused into the patient. We estimated the leukemic burden of the patient in bone marrow, spleen, and blood to be approximately 1 to 3 × 1012 cells. This would result in an initial effector target ratio of 1:1,000. Because the CML cells will be dividing in vivo, it is unlikely that, without in vivo expansion of the T cells, this treatment would have been effective. The presence of both CD4+ and CD8+ T cells in the lines have probably supported the in vivo proliferation.
Treatment of the accelerated phase CML with the leukemia-reactive CTL lines was effective in our patient. However, we cannot exclude that these cells have contributed to the persistence of the chronic GVHD that developed after DLI and was still present after the CTL treatment. We observed an increase in the skin GVHD around day 200 after the first CTL line infusion. This chronic GVHD was treated again with cyclosporin and prednisone. The immunosuppressive treatment 4 months after the clinical response did not result in a relapse of the leukemia, suggesting a sustained effect of the treatment of leukemia-reactive CTL.
In conclusion, treatment of relapsed chronic phase CML after allogeneic stem cell transplantation with DLI appears to be successful in most of the patients, without lethal GVHD in the majority of cases. Our results show that leukemia after allogeneic SCT can respond to treatment with leukemia-reactive CTL lines generated in vitro. These CTL lines may be more specific to the leukemic cells than to the GVHD target organs, leading to a relative specific antileukemic effect. However, because the treatment with leukemia-reactive CTL requires the presence of significant quantities of (cryopreserved) leukemic cells to be used as stimulator cells and a relatively complex infrastructure to generate such CTL lines under GMP conditions, the general applicability of this treatment may be limited at present. Future characterization of the target peptides that can be recognized by CTL on leukemic cells is essential. Adoptive immunotherapy of relapsed leukemia after allogeneic transplantation with in vitro-expanded CTL lines may significantly contribute to the curative potential of allogeneic stem cell transplantation for hematological malignancies.
ACKNOWLEDGMENT
The authors gratefully acknowledge Roel de Paus and Jacqueline Bergsma for their technical assistance, Dr S.L. Bhola for cytogenetic analysis, Dr Ronald van Soest for his help in preparing the figures, and Clary Wiarda for preparing the manuscript.
Supported by grants from the Dutch Cancer Society and the J.A. Cohen Institute for Radiopathology and Radiation Protection.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to J.H. Frederik Falkenburg, MD, PhD, Department of Hematology, C2-R, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail:falkenburg@hematology.azl.nl.