Abstract
All transretinoic acid (ATRA) followed by daunorubicin (DNR)-AraC chemotherapy (CT) has improved the outcome of acute promyelocytic leukemia (APL) by comparison to CT alone. In a randomized trial, (1) we compared 2 induction schedules (ATRA followed by CT [ATRA→CT] and ATRA plus CT [ATRA+CT, with CT added on day 3 of ATRA treatment]) and (2) we assessed the role of maintenance treatment. Four hundred thirteen patients ≤75 years of age and with newly diagnosed APL were included. Induction treatment was stratified on white blood cell (WBC) count and age: patients ≤65 years of age and with an initial WBC count of ≤5,000/μL (n = 208) were randomized between ATRA→CT and ATRA+CT (initially randomized patients); patients with a WBC count greater than (high WBC count group, n = 163) and patients 66 to 75 years of age with a WBC count greater than 5,000/μL (elderly group, n = 42) were not initially randomized and received ATRA+CT from day 1 and ATRA →CT, respectively. All patients achieving CR received 2 additional DNR-AraC courses (only 1 in patients 66 to 75 years of age) and were then randomized for maintenance between no treatment, intermittent ATRA (15 days every 3 months) for 2 years, continuous low-dose CT (6 mercaptopurine + methotrexate) for 2 years, or both, using a 2-by-2 factorial design. Overall, 381 (92%) of the patients achieved complete remission (CR), 31 (7%) suffered an early death, and only 1 patient had leukemic resistance. ATRA syndrome occurred in 64 patients (15%) and was fatal in 5 cases. The CR rate was similar in all induction treatment groups. Event-free survival (EFS) was significantly lower in the high WBC group (P = .0002) and close to significance in the elderly group (P = .086) as compared with initially randomized patients. Relapse at 2 years was estimated at 6% in the ATRA+CT group, versus 16% in the ATRA→CT group (P = .04, relative risk [RR] = .41). EFS at 2 years was estimated at 84% in the ATRA+CT group, versus 77% in the ATRA→CT group (P = .1, RR = .62). Two hundred eighty-nine patients were randomized for maintenance. The 2-year relapse rate was 11% in patients randomized to continuous maintenance CT and 27% in patients randomized to no CT (P = .0002) and 13% in patients randomized to intermittent ATRA and 25% in patients randomized to no ATRA (P= .02). An additive effect of continuous maintenance CT and intermittent ATRA was seen, and only 6 of the 74 patients who received both maintenance treatments had relapsed. Overall survival was improved in patients who received maintenance CT (P = .01), and there was a trend for better survival in patients who received maintenance ATRA (P = .22). Our findings strongly suggest that early addition of chemotherapy to ATRA and maintenance therapy combining continuous CT and intermittent ATRA can reduce the incidence of relapse in APL. This effect already translates into significantly better survival for maintenance treatment with continuous CT.
ACUTE PROMYELOCYTIC leukemia (APL) is a specific type of acute myeloid leukemia (AML) characterized by the morphology of blast cells1,2; by t(15;17) translocation,3 which fuses the PML gene on chromosome 15 to the retinoic acid receptor (RAR) α gene on chromosome 174,5; and by specific coagulopathy.6,7 Until recently, combination chemotherapy (CT) with anthracycline-cytosine arabinoside (Ara C) was the only treatment of APL, with complete remission (CR) in 65% to 80% of newly diagnosed cases.8-12 The remaining patients suffered early death, mainly from bleeding due to worsening of coagulopathy or resistance to CT. Fifty percent to 65% of the patients who achieved CR subsequently relapsed, and 30% to 40% survived at 2 years.8-12
All transretinoic acid (ATRA) can differentiate abnormal promyelocytes into mature granulocytes in APL in vitro and in vivo13-16and induced CR in 80% to 90% of newly diagnosed13,15-17and first-relapsing APL.18 ATRA rapidly improved coagulopathy without inducing aplasia. However, in 15% to 25% of the patients, it was associated with an ATRA syndrome that generally occurred with a rapid increase in white blood cells (WBC) and often had a fatal outcome.19-22 Furthermore, patients who achieved CR with ATRA and were maintained on ATRA alone or mild CT generally rapidly relapsed.16-18 Thus, we designed a strategy combining ATRA and intensive CT in newly diagnosed APL. CT was administered as consolidation or, in patients with rapidly increasing WBC counts, added to ATRA to prevent the ATRA syndrome.23 24
In a previous randomized study, we found that treatment with ATRA followed by CT gave better survival than CT alone in newly diagnosed APL, mainly by reducing the incidence of relapse,25,26 and these results were confirmed by a US intergroup randomized study.27 However, approximately 30% of the patients treated by ATRA followed by CT still relapsed, and new strategies were required to further reduce that number.
Results of the combination of ATRA and CT suggested that those 2 treatments had an additive or even synergistic effect in reducing the incidence of relapse in APL; whether this effect was optimal by using ATRA first, followed by CT, or by combining the 2 treatment modalities upfront was the first issue. Another issue was the possible role of maintenance treatment in APL. Pharmacological studies suggested that maintenance treatment with ATRA, using an intermittent rather than continuous administration, could reduce the relapse rate in APL.28 A similar benefit had also been suggested in nonrandomized studies for continuous low-dose maintenance CT.29 30
To address those issues, we performed a randomized study that (1) compared 2 induction schedules (ATRA followed by CT v ATRA plus CT) and (2) assessed the role of maintenance treatment with intermittent ATRA and continuous low-dose CT using a 2-by-2 factorial design.
PATIENTS AND METHODS
Eligibility Criteria
Between April 1, 1993 and December 31, 1996, 439 patients from 97 European centers (listed in the ) with newly diagnosed APL were included in this trial (APL93 trial), which had been approved by ethical committees in all participating countries.
Inclusion criteria were as follows: (1) diagnosis of APL, based on morphology criteria1 2; (2) age 75 years or less; and (3) written informed consent. Diagnosis had to be subsequently confirmed by presence of t(15;17) or PML-RARα gene rearrangement. In the absence of t(15;17) and if no analysis of the rearrangement could be made, review of initial marrow slides by an independent morphologist was mandatory.
Design
Objectives of the trial were to assess the optimal timing of ATRA treatment (before or with CT) and the role of maintenance therapy. Induction treatment was stratified by age and initial WBC count.
Patients ≤65 years of age with a WBC count less than 5,000/μL were randomized to receive the reference ATRA treatment of our previous trial (APL91 trial),25 ie, 45 mg/m2/d ATRA followed by CT (ATRA→CT group) or ATRA plus CT (ATRA+CT). In the ATRA→CT group, patients received 45 mg/m2/d ATRA orally until CR, with a maximum of 90 days. After CR achievement, they received a course of 60 mg/m2/d daunorubicin (DNR) for 3 days and 200 mg/m2/d AraC for 7 days (course I). However, course I was added to ATRA if the WBC count was increased to greater than 6,000/μL, 10,000/μL, or 15,000/μL by day 5, 10, and 15 of ATRA treatment, respectively, because, from our experience, patients were at risk of ATRA syndrome above those thresholds.20Patients randomized to the ATRA+CT group received the same combination of ATRA and CT, with course I of CT starting on day 3 of ATRA treatment. This 48-hour interval before onset of CT was based on our previous report, because it allowed correction of coagulopathy.20
Patients with a WBC count greater than 5,000/μL at presentation (irrespective of their age) and patients 66 to 75 years of age with a WBC count ≤5,000/μL were not randomized but received ATRA plus CT course I from day 1 (high WBC group) and the same schedule as in the ATRA→CT group (elderly group), respectively.
Treatment of coagulopathy during the induction phase was based on platelet support to maintain the platelet count at a level greater than 50 × 109/L until the disappearance of coagulopathy. The use of heparin, tranexamic acid, fresh frozen plasma, and fibrinogen transfusions was optional.
CR patients received 2 CT consolidation courses, including course II (identical to course I) and course III, consisting of 45 mg/m2/d DNR for 3 days and 1 g/m2 AraC every 12 hours for 4 days. The elderly group only received course II.
Three to 4 weeks after hematological recovery from this consolidation CT, patients who were still in CR were randomized both to receive or not receive intermittent ATRA (45 mg/m2/d, 15 days every 3 months) and to receive or not receive continuous CT with 6 mercaptopurine (90 mg/m2/d, orally) and methotrexate (15 mg/m2/wk, orally), according to a 2-by-2 factorial design stratified on the initial induction treatment group. The rationale for the schedule of ATRA maintenance was based on pharmacokinetic studies showing rapid decrease of peak serum levels of ATRA with its continuous use for more than 2 to 3 weeks, due to hypercatabolism of the drug.28 However, this metabolic change is reversible within a few weeks after discontinuation of the drug. Maintenance treatment was scheduled for 2 years. Randomizations for induction and maintenance were performed through a centralized telephone assignment procedure.
Endpoints
For induction treatment event-free survival (EFS), calculated from the date of randomization, was the major endpoint. Failures were defined as (1) failure to achieve CR, (2) relapse, and (3) death in CR. CR rate, relapse-free survival (calculated from CR achievement), and overall survival (calculated from the date of randomization) were secondary endpoints. Failures to achieve CR were classified as (1) early death (death during induction treatment with ATRA, without evidence of resistant leukemia) and (2) resistant leukemia (absence of CR after 90 days of treatment with ATRA, whether or not 1 CT course had been added). CR criteria included disappearance of abnormal promyelocytes on bone marrow aspirate normalization of coagulation and fibrinolysis parameters, a neutrophil count of greater than 1,500/μL, a platelet count greater than 100,000/μL, and no transfusion requirement.
For maintenance treatment, the time to relapse, which was calculated from the date of randomization for maintenance, was the major endpoint; survival and EFS, which were calculated for the date of this randomization, were secondary endpoints.
Sample Size
Our previous trial estimated the 1-year EFS at 75%, and we anticipated an improvement of 10% from ATRA+CT. Given a type I error of 5% and a type II error of 10%, the George and Desu method required 106 events, thus the inclusion of 251 patients with an annual accrual rate of 60 patients ≤65 years of age with a WBC count less than 5,000/μL. For maintenance treatment, similar assumptions about relapse required the inclusion of 361 patients given an overall annual accrual rate of 100 CR patients. Because a CR rate of 90% was expected, 400 patients had to be initially included.
Finally, the statistical strategy planned (1) a safety analysis to assess the CR rate at midaccrual, (2) a first interim analysis when the sample size would be obtained, and (3) a second interim analysis when the required number of events would be reached. Thus, we used the Pocock adaptation of the type I error (α’ = .03), given these 2 planned interim analyses.
Statistical Analysis
A safety analysis was performed at the reference date of March 1, 1995, when 186 patients had been included. The present analysis was performed at the reference date of January 1, 1998, in the 413 eligible patients included before January 1, 1997. This was the second interim analysis of the trial dealing with failure time endpoints. No patient was lost to follow-up. Analysis was made on an intention-to-treat basis in patients with confirmed diagnosis of APL. Censored criteria were analysed with the Kaplan-Meier estimate, the log-rank test, and Cox’s model.31-33 For each endpoint, treatment comparison was adjusted on a predetermined subset of prognostic parameters8-13 using Cox’s model: age, sex, WBC count, platelet count, absolute number of circulating blasts, M3subtype (classical M3v microgranular variant M3), and fibrinogen level. Continuous covariates were entered into native form after checking for log linear relationships. Cox’s model was used for testing interaction in the maintenance factorial design as well as to adjust for possible baseline confounders, including result of induction assignment. In the absence of interaction, it was decided to analyze separately each maintenance treatment, with the main analysis (adjusted or unadjusted for baseline covariates) being stratified on the other treatment using a Cox’s model. Relative risks were estimated with 5% confidence intervals (CI). All tests were 2-sided. The SAS software (SAS Institute, Cary, NC) was used.
RESULTS
Initial Characteristics of the Patient Population
In 413 of the 439 patients included, diagnosis of APL was confirmed by the presence of t(15;17) (n = 352), by the presence of PML-RARα transcript by reverse transcriptase-polymerase chain reaction (RT-PCR; n = 31), or by review of marrow slides (n = 30). The remaining 26 patients, in whom diagnosis of APL was not confirmed by cytogenetics, RT-PCR, or review of marrow, slides were excluded, according to the protocol. The median age of the 413 eligible patients was 46 years; 16 patients were children (≤15 years of age), 42 patients more than 65 years of age were included in the elderly group, 163 patients presenting with a WBC count greater than 5,000/μL were included in the high WBC group, and 208 patients were randomized between the ATRA→CT (109 patients) and the ATRA+CT (99 patients) groups. The ATRA→CT and ATRA+CT groups were well balanced for all pretreatment characteristics (Table 1).
Initial Characteristics of the Patients Included in APL 93 Trial According to Induction Treatment
| . | ATRA → CT Group . | ATRA + CT Group . | High WBC Group . | Elderly Group . |
|---|---|---|---|---|
| No. of patients | 109 | 99 | 163 | 42 |
| Male gender | 52 (48%) | 52 (50%) | 84 (52%) | 22 (52%) |
| Age (yrs) | 45 (2-64) | 43 (7-63) | 42 (1-73) | 69 (65-77) |
| WBC count (109/L) | 1.3 (0.3-4.7) | 1.4 (0.3-4.8) | 15.9 (5.1-97.6) | 1.25 (0.5-46) |
| Platelet count (109/L) | 38 (4-249) | 34 (3-215) | 24 (2-164) | 33.5 (4-252) |
| Circulating blasts (109/L) | 0.25 (0-4) | 0.28 (0-3.3) | 13.35 (0-96.6) | 0.23 (0-19.8) |
| Microgranular variant M3 | 7 (6%) | 5 (5%) | 40 (24%) | 2 (5%) |
| Fibrinogen level (g/L) | 1.9 (0.2-7) | 1.7 (0.3-6.5) | 1.4 (0-7.4) | 1.9 (0.4-5.4) |
| . | ATRA → CT Group . | ATRA + CT Group . | High WBC Group . | Elderly Group . |
|---|---|---|---|---|
| No. of patients | 109 | 99 | 163 | 42 |
| Male gender | 52 (48%) | 52 (50%) | 84 (52%) | 22 (52%) |
| Age (yrs) | 45 (2-64) | 43 (7-63) | 42 (1-73) | 69 (65-77) |
| WBC count (109/L) | 1.3 (0.3-4.7) | 1.4 (0.3-4.8) | 15.9 (5.1-97.6) | 1.25 (0.5-46) |
| Platelet count (109/L) | 38 (4-249) | 34 (3-215) | 24 (2-164) | 33.5 (4-252) |
| Circulating blasts (109/L) | 0.25 (0-4) | 0.28 (0-3.3) | 13.35 (0-96.6) | 0.23 (0-19.8) |
| Microgranular variant M3 | 7 (6%) | 5 (5%) | 40 (24%) | 2 (5%) |
| Fibrinogen level (g/L) | 1.9 (0.2-7) | 1.7 (0.3-6.5) | 1.4 (0-7.4) | 1.9 (0.4-5.4) |
Results of Induction Treatment
CR induction and early deaths.
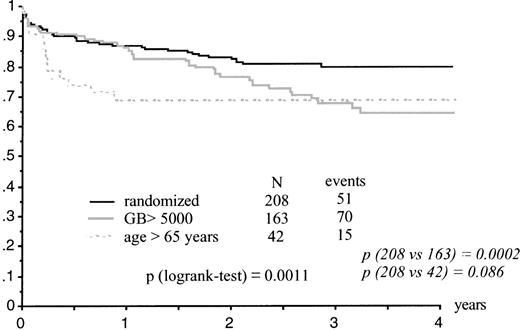
CR was obtained in 381 patients (92%; 95% exact CI, 90% to 95%), 31 (7%) patients suffered early death, and the remaining patient had resistant leukemia. The cause of death was sepsis in 13 cases, central nervous system (CNS) bleeding in 10 cases, ATRA syndrome in 5 cases, myocardial infarction in 1 case, liver failure in 1 case, and unknown in 1 patient. Prognostic factors for early death were older age (P = .006) and higher WBC count upon inclusion (P = .02). In the 4 patients in the elderly group who suffered early death, the cause of death was sepsis in 2 cases, ATRA syndrome in 1 case, and cardiac failure in 1 case.
In the ATRA→CT group, CT was added before CR achievement due to increasing WBC counts (>6,000/μL by day 5, >10,000/μL by day 10, or >15,000/μL by day 15 of ATRA treatment) in 60 of the 109 patients (55%).
ATRA syndrome.
In the absence of biological diagnostic criteria of ATRA syndrome, diagnosis of ATRA syndrome was made on clinical grounds by the association of at least 3 of the following signs, in the absence of other causes: fever, weight gain, respiratory distress, lung infiltrates, pleural or pericardial effusion, hypotension, and renal failure.19 Clinical signs of ATRA syndrome occurred in 64 (15%; 95% exact CI, 12% to 19%) patients. Sixty patients received CT and 58 also received intravenous dexamethasone. In 2 of them, with WBC counts of 186,000/μL and 38,000/μL upon admission, respectively, dyspnea, hypoxia, and pulmonary infiltrates were already present, before the onset of ATRA. Worsening of those signs occurred within hours on ATRA onset. In the other patients, signs developed after 1 to 35 days (median, 7 days) of ATRA treatment. They occurred later than day 14 of ATRA treatment in 21 (33%) patients. In 11 of those 21 patients, ATRA syndrome was observed upon recovery from the phase of aplasia that followed the addition of CT (indicated because of high WBC counts at diagnosis or during treatment). Fifty-five (86%) of the 64 patients achieved CR. Five died from ATRA syndrome, including 1 of the 5 patients in whom the addition of CT was not made and 1 of the 6 patients in whom the addition of dexamethasone was not made. The remaining 4 patients with moderate ATRA syndrome died from CNS bleeding (1 case), subsequent occurrence of sepsis (2 cases), and leukemic resistance (1 case), respectively. No pretreatment factor, including the WBC count, was significantly associated with the occurrence of the ATRA syndrome.
Results according to induction stratification.
Seventy-five of the 381 patients who achieved CR had relapsed, 29 had died in first CR, 18 of them from sepsis after a consolidation course, and 277 remained in first CR. The CR rate and incidence of ATRA syndrome did not significantly differ between randomized patients (ATRA →CT and ATRA+CT groups) and patients included in the high WBC count and elderly groups (Table 2).
APL 93 Trial Results According to Induction Treatment
| . | WBC <5,000/μL and Age ≤65 . | WBC >5,000/μL . | Age >65 and WBC <5,000/μL . | P Value (log rank) . | WBC <5,000/μL and Age ≤65 . | PValue (log rank) . | |
|---|---|---|---|---|---|---|---|
| ATRA → CT . | ATRA + CT . | ||||||
| n | 208 | 163 | 42 | 109 | 99 | ||
| CR rate | 94% | 90% | 90% | .72 | 95% | 94% | .79 |
| ATRA syndrome | 16% | 18% | 10% | .40 | 20% | 11% | .17 |
| Relapse at 2 yrs | 11% | 29% | 7% | .0001 | 16% | 6% | .04 |
| Death in CR | 6.7% | 5.5% | 19% | .010 | 7% | 6% | .71 |
| EFS at 2 yrs | 80% | 69% | 67% | .0009 | 77% | 84% | .10 |
| Survival at 2 yrs | 82% | 77% | 69% | .023 | 81% | 84% | .40 |
| . | WBC <5,000/μL and Age ≤65 . | WBC >5,000/μL . | Age >65 and WBC <5,000/μL . | P Value (log rank) . | WBC <5,000/μL and Age ≤65 . | PValue (log rank) . | |
|---|---|---|---|---|---|---|---|
| ATRA → CT . | ATRA + CT . | ||||||
| n | 208 | 163 | 42 | 109 | 99 | ||
| CR rate | 94% | 90% | 90% | .72 | 95% | 94% | .79 |
| ATRA syndrome | 16% | 18% | 10% | .40 | 20% | 11% | .17 |
| Relapse at 2 yrs | 11% | 29% | 7% | .0001 | 16% | 6% | .04 |
| Death in CR | 6.7% | 5.5% | 19% | .010 | 7% | 6% | .71 |
| EFS at 2 yrs | 80% | 69% | 67% | .0009 | 77% | 84% | .10 |
| Survival at 2 yrs | 82% | 77% | 69% | .023 | 81% | 84% | .40 |
Kaplan-Meier estimates of EFS were significantly lower in the high WBC group than in randomized patients (P = .0002) and close to significance in the elderly group (P = .086; Table 2 and Fig 1). Estimates of survival were significantly lower in the elderly group (P = .02) and in the high WBC group (P = .03). The high WBC group had significantly more and earlier relapses (P = .0001) than patients with WBC counts less than 5,000/μL.
Results of induction randomization in patients less than 65 years of age with a WBC count less than 5,000/μL.
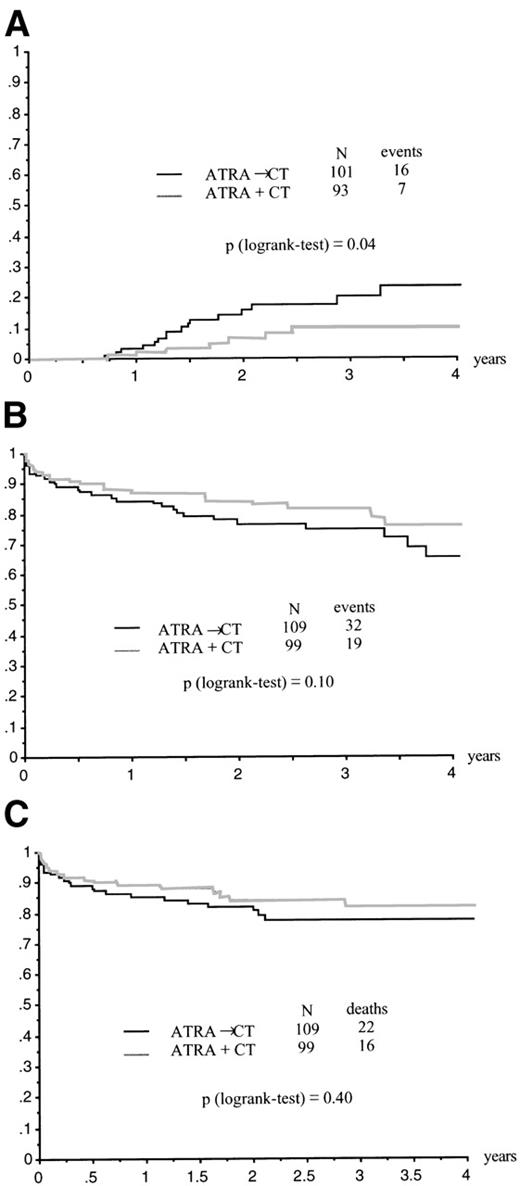
The CR rate was 95% (95% CI, 86% to 97%) in the ATRA→CT group and 94% (95% CI, 87% to 98%) in the ATRA+CT group (P= .79 by the Fisher’s test; Table 2). Relapse at 2 years was estimated at 6% ± 3% in the ATRA+CT group as compared with 16% ± 4% in the ATRA→CT group (P = .04 by the log-rank test; Fig 2A). Relative risk (RR) was estimated at .41, with a 95% CI of 0.17 to 0.99. Adjustment for age, sex, WBC count, platelet count, circulating blast count, microgranular variant M3, and fibrinogen slightly modify these estimates (RR = .38, 95% CI, 0.16 to 0.94; P = .04 by the Wald test). Kaplan-Meier estimate of EFS at 2 years was 84% ± 4% in the ATRA+CT group as compared with 77% ± 4% in the ATRA→CT group (P = .10 by the log-rank test; crude RR = .62 [95% CI, 0.35 to 1.10]; adjusted RR for baseline covariates = .62 [95% CI, 0 to 1.09; P = .1 by the Wald test; Fig 2B). Two-year survival was estimated at 84% ± 4% in the ATRA+CT group and 81% ± 4% in the ATRA→CT group (P = .40 by the log-rank test, RR = .76 [95% CI, 0.4 to 1.44]; adjusted RR for baseline covariates = .67 [95% CI, 0.35 to 1.29]; P = .23 by the Wald test; Fig 2C).
(A) Relapse according to initial randomization. (B) EFS according to initial randomization. (C) Overall survival according to initial randomization.
(A) Relapse according to initial randomization. (B) EFS according to initial randomization. (C) Overall survival according to initial randomization.
Maintenance
Of the 381 patients who had achieved CR, 348 had received full consolidation CT, were alive in CR, and could therefore be randomized for maintenance. Two hundred eighty-nine were randomized for maintenance. The remaining patients were not randomized because they had received an allograft (7 cases), had an unrelated disorder or were in poor medical condition (11 patients), had experienced side effects of ATRA during induction treatment (3 cases), or because of patient refusal (20 cases) or physician omission (18 cases).
Of the 289 patients randomized for maintenance, 73 were allocated to the absence of maintenance, 72 to intermittent ATRA, 70 to continuous CT, and 74 to the association of both treatments. One patient, who was included in the last group, refused to start treatment. No imbalances in pretreatment characteristics were seen between the treatment groups (Table 3). Overall, 56 relapses occurred before the reference date of January 1, 1998. Thirty-three patients had died, including 27 after relapse and 6 in first CR. Because no interaction between continuous CT and intermittent ATRA was found by the Cox’s model whatever the endpoint, we used the statistical strategy defined in the protocol, ie, separate analysis of each treatment, stratifying on the other maintenance treatment. Finally, to assess the influence of possible confounders measured at baseline, we adjusted analyses for baseline prognostic covariates as well as for the result of the induction assignment. Results are summarized in Table 4.
Initial Characteristics of Patients According to Randomization for Maintenance
| . | No ATRA . | ATRA . | No CT . | CT . |
|---|---|---|---|---|
| No. of patients | 143 | 146 | 145 | 144 |
| Male gender | 81 (57%) | 68 (47%) | 73 (51%) | 76 (52%) |
| Age (yrs) | 45 (13-74) | 44 (1-72) | 43 (1-72) | 45.5 (13-74) |
| WBC count (109/L) | 3 (0.4-97) | 2.9 (0.3-76.8) | 3.3 (0.4-76.8) | 2.7 (0.3-96.9) |
| Platelets (109/L) | 31 (3-249) | 31 (4-169) | 35 (4-249) | 25 (3-215) |
| Circulating blasts (109/L) | 1.3 (0-82.4) | 1.1 (0-72.2) | 1.5 (0-72.2) | 1.1 (0-82.4) |
| Microgranular M3 | 18 (13%) | 20 (14%) | 16 (11%) | 22 (16%) |
| Fibrinogen (g/L) | 1.6 (0.3-7) | 1.5 (0.2-5.6) | 1.5 (0.2-6.5) | 1.6 (0.2-7) |
| Induction assignment (ATRA → CT/ATRA + CT/high WBC group/elderly group) | 44/33/58/10 | 37/40/59/10 | 41/37/58/10 | 40/36/59/10 |
| . | No ATRA . | ATRA . | No CT . | CT . |
|---|---|---|---|---|
| No. of patients | 143 | 146 | 145 | 144 |
| Male gender | 81 (57%) | 68 (47%) | 73 (51%) | 76 (52%) |
| Age (yrs) | 45 (13-74) | 44 (1-72) | 43 (1-72) | 45.5 (13-74) |
| WBC count (109/L) | 3 (0.4-97) | 2.9 (0.3-76.8) | 3.3 (0.4-76.8) | 2.7 (0.3-96.9) |
| Platelets (109/L) | 31 (3-249) | 31 (4-169) | 35 (4-249) | 25 (3-215) |
| Circulating blasts (109/L) | 1.3 (0-82.4) | 1.1 (0-72.2) | 1.5 (0-72.2) | 1.1 (0-82.4) |
| Microgranular M3 | 18 (13%) | 20 (14%) | 16 (11%) | 22 (16%) |
| Fibrinogen (g/L) | 1.6 (0.3-7) | 1.5 (0.2-5.6) | 1.5 (0.2-6.5) | 1.6 (0.2-7) |
| Induction assignment (ATRA → CT/ATRA + CT/high WBC group/elderly group) | 44/33/58/10 | 37/40/59/10 | 41/37/58/10 | 40/36/59/10 |
Estimate of Relapse, EFS, and Survival According to Maintenance Treatment
| . | No ATRA (n = 143) . | ATRA (n = 146) . | No CT (n = 145) . | CT (n = 144) . |
|---|---|---|---|---|
| Relapse | ||||
| Events | 35 | 21 | 41 | 15 |
| 2-yr relapse | 25% | 13.5% | 27% | 11.5% |
| Log rank | .024 | .0002 | ||
| Stratified RR | .54 (.32-.92) | .33 (.18-.59) | ||
| P = .024 | P = .0003 | |||
| Stratified and adjusted4-150 RR | .62 (.36-1.10) | .31 (.17-.56) | ||
| P = .10 | P = .0002 | |||
| EFS | ||||
| Events | 38 | 24 | 44 | 18 |
| 2-yr survival | 18% | 13% | 23% | 8% |
| Log rank | .075 | .0006 | ||
| Stratified RR | .66 (.39-1.10) | .41 (.23-.71) | ||
| P = .10 | P = .0015 | |||
| Stratified and adjusted4-150 RR | .74 (.42-1.27) | .41 (.24-.71) | ||
| P = .27 | P = .0014 | |||
| Survival | ||||
| Deaths | 20 | 13 | 24 | 9 |
| 2-yr survival | 86% | 92% | 85% | 94% |
| Log rank | .19 | P = .008 | ||
| Stratified RR | .65 (.32-1.30) | .36 (.17-.78) | ||
| P = .22 | P = .0097 | |||
| Stratified and adjusted4-150 RR | .69 (.31-1.51) | .34 (.15-.79) | ||
| P = .35 | P = .0117 | |||
| . | No ATRA (n = 143) . | ATRA (n = 146) . | No CT (n = 145) . | CT (n = 144) . |
|---|---|---|---|---|
| Relapse | ||||
| Events | 35 | 21 | 41 | 15 |
| 2-yr relapse | 25% | 13.5% | 27% | 11.5% |
| Log rank | .024 | .0002 | ||
| Stratified RR | .54 (.32-.92) | .33 (.18-.59) | ||
| P = .024 | P = .0003 | |||
| Stratified and adjusted4-150 RR | .62 (.36-1.10) | .31 (.17-.56) | ||
| P = .10 | P = .0002 | |||
| EFS | ||||
| Events | 38 | 24 | 44 | 18 |
| 2-yr survival | 18% | 13% | 23% | 8% |
| Log rank | .075 | .0006 | ||
| Stratified RR | .66 (.39-1.10) | .41 (.23-.71) | ||
| P = .10 | P = .0015 | |||
| Stratified and adjusted4-150 RR | .74 (.42-1.27) | .41 (.24-.71) | ||
| P = .27 | P = .0014 | |||
| Survival | ||||
| Deaths | 20 | 13 | 24 | 9 |
| 2-yr survival | 86% | 92% | 85% | 94% |
| Log rank | .19 | P = .008 | ||
| Stratified RR | .65 (.32-1.30) | .36 (.17-.78) | ||
| P = .22 | P = .0097 | |||
| Stratified and adjusted4-150 RR | .69 (.31-1.51) | .34 (.15-.79) | ||
| P = .35 | P = .0117 | |||
Adjusted on induction assignment, age, gender, baseline WBC count, circulating blasts, platelet count, fibrinogen, and M3 subtype (classical M3v microganular M3).
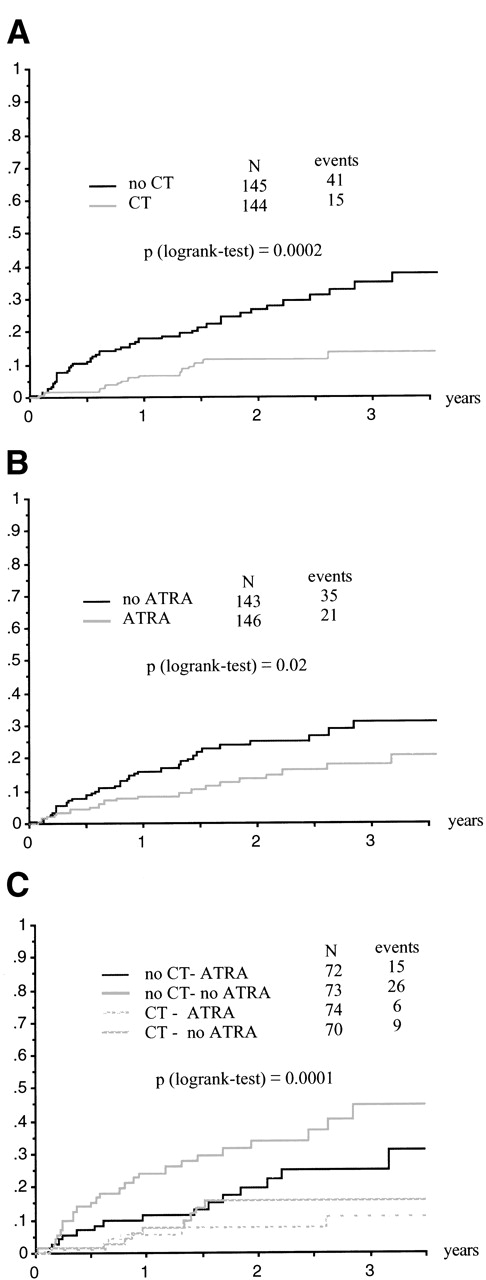
Forty-one and 15 relapses were recorded in patients without and with continuous CT (P = .0003, stratified Cox model). Thirty-five and 21 relapses were recorded in patients without and with intermittent ATRA (P = .024, stratified Cox model; Table 4 and Fig 3A and B). In patients randomized to receive both maintenance treatments, only 6 relapses occurred (Fig 3C), confirming the additive effect of these 2 maintenance treatments (2-year relapse rate estimated at 7.4%). These results were slighly modified when the comparison was adjusted on the predefined set of baseline characteristics (Table 4). Similar results were obtained with EFS (62 events; 56 relapses and 6 deaths in CR; Table 4). In patients randomized to receive both maintenance treatments, only 6 events occurred (2-year EFS estimated at 93% ± 3%). Overall survival was improved in patients who received maintenance CT (P = .01, RR = .36 [95% CI, 0.17 to 0.78]; stratified Cox model). A trend towards better survival was found in patients who received maintenance ATRA (P = .22, RR = .65; stratified Cox model). In patients receiving both maintenance treatments, only 4 deaths occurred (2-year survival estimated at 93%).
(A) Relapse according to maintenance CT. (B) Relapse according to maintenance ATRA. (C) Relapse according to maintenance treatment group.
(A) Relapse according to maintenance CT. (B) Relapse according to maintenance ATRA. (C) Relapse according to maintenance treatment group.
Patients in the high WBC count group seemed to benefit particularly from maintenance treatment, especially from the combination of ATRA and CT; indeed, 15 of the 28 (54%) patients of that induction group who were randomized to no maintenance relapsed, as compared with 11 of the 30 (37%) patients randomized to intermittent ATRA alone, 7 of the 29 (24%) patients randomized to continuous CT alone, and only 3 of the 29 (10%) randomized to both maintenance treatments (P = .003). Differences in the maintenance treatment groups were less important in patients initially included in the ATRA→CT group: 6 of 22 (23%), 2 of 18 (11%), 1 of 21 (5%), and 2 of 19 (10%) relapses after no maintenance, ATRA alone, CT alone, and both, respectively (P = .13). Likewise, differences in the maintenance treatment groups were less important in patients initially included in the ATRA+CT group: 4 of 17 (23%), 1 of 20 (5%), 1 of 16 (6%), and 0 of 20 (0%) relapses after no maintenance, ATRA alone, CT alone, and both, respectively (P = .06); numbers of patients were too small in the elderly group for adequate comparisons.
Side effects of ATRA treatment during maintenance included liver function test abnormalities in 9% of the patients maintained with ATRA alone and 32% of the patients maintained with ATRA plus CT. One patient had severe myositis during maintenance, which was attributed to ATRA. No ATRA syndrome was recorded during maintenance. Liver function tests abnormalities were found in 36% of the patients who received CT alone for maintenance. Maintenance CT was also associated with lung pneumocystis infection in 2 cases. Cytopenias were usual after CT, often requiring dose reduction or transient discontinuation. Overall, maintenance ATRA and maintenance CT were prematurely stopped in 7 and 16 of the patients, respectively. In the 74 patients allocated to combined maintenance treatment, 3 had early discontinuation of CT and 6 had early discontinuation of both ATRA and CT. None of the 6 deaths in CR that occurred during maintenance treatment could be attributed to the drugs used for maintenance.
DISCUSSION
Results of the present trial showed that, even when a large number of centers are involved, CR rates greater than 90% can be achieved in newly diagnosed APL by adding CT to ATRA from the onset of treatment or when WBC counts rapidly increase. This confirmed the 91%, 95%, and 89% CR rates obtained with the combination of ATRA and CT in our previous APL 91 trial, in the Italian experience, and in the Japanese experience, respectively.25,34,35 The US intergroup study found a CR rate of only 70% in newly diagnosed APL treated with ATRA followed by CT.27 However, in the subset of patients who fulfilled all diagnostic criteria and whose response was fully evaluated, the CR rate ranged between 80% and 85%. Because the CR rates obtained with CT alone rarely exceeded 75% to 80%, it appears that combining ATRA and CT, in addition to lowering the incidence of relapse, can also somewhat increase the CR rate in APL. Improved CR rates result in part from the almost absence of cases of resistant leukemia with ATRA combined with CT. They also probably result from a low incidence of fatal cases of ATRA syndrome and from reduction of the early death rate in APL presenting with high WBC counts. Indeed, in the present report, the CR rate was 90% in the high WBC group (and 89% in patients with WBC counts >10,000/μL). By comparison, CR rates ranging between 50% and 70% have been reported in patients with WBC counts greater than 10,000/μL treated with CT alone.7-12 25
ATRA syndrome, the major side effect of ATRA treatment, occurred in 15% of our patients. A high peak value of circulating WBC and basal expression of CD13 on blast surface were predictive of the ATRA syndrome in the New York experience,36 but, in our trial, no predictive factors of the ATRA syndrome, including pretreatment WBC count, were found.37 The absence of higher incidence of ATRA syndrome that we observed in patients with high WBC counts could have resulted from the systematic addition of CT to ATRA in those patients. Addition of CT to ATRA in patients with high WBC counts at diagnosis or during ATRA treatment in APL and administration of high dose dexamethasone at the earliest symptom also appear to have reduced the incidence of fatal ATRA syndrome to 1.2% in the present trial and to 1% to 1.5% in other large published experiences.34 35
Although the combination of ATRA and CT has improved the outcome of APL, some patients still relapse. In the present trial, we tested several approaches to reduce the occurrence of relapse, including the comparison between ATRA→CT and ATRA+CT, and the role of maintenance therapy by intermittent ATRA, continuous low-dose CT, or both.
Reduction of the occurrence of relapses in APL with the combination of ATRA and CT over CT alone, which was observed in our previous APL 91 trial, suggested an additive or even synergistic effect of both treatment modalities. Here, in patients presenting with a WBC count less than 5,000/μL, ATRA+CT gave a significantly lower incidence of relapse and, with borderline significance, better EFS than ATRA→CT. These findings therefore strongly suggest than the additive or synergistic effect between ATRA and CT is optimal when both treatments are administered simultaneously in APL patients presenting with low WBC counts. Simultaneous administration of ATRA and CT is also recommended, from our experience, in patients with high WBC counts to reduce the risk of severe ATRA syndrome. A third possible association between ATRA and CT, ie, CT followed by ATRA, was also tested by the US intergroup study.27 These investigators found a similar incidence of relapse after ATRA followed by CT and CT followed by ATRA. However, administration of ATRA upfront is generally preferred in APL, because it allows for better control of coagulopathy.
Two nonrandomized studies had suggested that maintenance with continuous low-dose CT using 6 mercaptopurine and methotrexate and using a POMP regimen, respectively, could decrease the relapse rate in APL.29 30 In the present trial, we confirmed in a randomized design that maintenance with continuous low-dose 6 mercaptopurine and methotrexate significantly reduced the incidence of relapse and significantly improved EFS and survival. These findings strongly support a role for maintenance CT in APL, even in patients who have previously received intensive consolidation CT.
We also observed that intermittent maintenance with ATRA significantly reduced the incidence of relapse. The US intergroup study had shown a beneficial role for continuous maintenance with ATRA. In patients who had received ATRA followed by CT, 10 of the 46 cases who received continuous maintenance with ATRA relapsed, as compared with 21 of the 54 cases who received no maintenance.27 Furthermore, continuous maintenance with ATRA was significantly associated with improved survival. Prolonged use of ATRA rapidly leads to a sharp decrease of its serum levels, due to hypercatabolism of the drug.38 However, this metabolic pathway is reversible a few weeks after ATRA discontinuation, and high serum levels of ATRA can then be obtained by new drug intake.28 Thus, intermittent maintenance with ATRA, as used in the present study, could prove more efficient than continuous maintenance with ATRA in reducing the incidence of relapse. However, relapses that occur in patients who receive or have recently received ATRA have been found to be highly resistant to this drug.22 38 Thus, long-term follow-up will be required to determine if the beneficial effect of maintenance with ATRA on the relapse rate we observed in this study clearly translates into survival benefit, although there was already a trend for improved survival in patients who received this maintenance.
Finally, continuous low-dose CT and intermittent ATRA had an additive effect in this study, and the 2-year relapse rate was estimated at only 7.4% in patients who received both maintenance treatments. Maintenance treatment and especially the combination of intermittent ATRA and continuous CT seemed to benefit particularly patients with high WBC counts.
In conclusion, our results confirm that high CR rates (>90%) can be obtained in a large multicenter study in APL by combining ATRA and CT. They strongly suggest that early addition of CT to ATRA and maintenance combining continuous CT and intermittent ATRA can reduce the incidence of relapse. Whether all of these effects translate into better survival will only be determined by longer follow-up.
Dr P. Fenaux and Dr L. Degos served as cochairmen. Dr C. Chastang and S. Chevret-Chastang (Department of Biostatistics, Hopital St Louis, Paris, France) served as biostatiscians. The following clinical departments participated in APL93 trial.
French APL group.
S. Castaigne, H. Dombret (Paris), R. Zittoun (Paris), E. Archimbaud (Lyon), P. Travade (Clermont Ferrand), C. Gardin (Clichy), A. Guerci (Nancy), P. Fenaux (Lille), A.M. Stoppa (Marseille), F. Dreyfus (Paris), F. Stamatoulas (Rouen), F. Rigal-Huguet (Toulouse), H. Guy (Dijon), J.J. Sotto (Grenoble), F. Maloisel (Strasbourg), J. Reiffers, J. M. Boiren (Pessac), A. Gardembas (Angers), D. Bordessoule (Limoges), N. Fegueux (Montpellier), F. Lefrerè (Paris), T. Lamy (Rennes), M. Hayat (Villejuif), E. Deconinck (Besancon), E. Guyotat (St Etienne), M. Martin (Annecy), E. Cony-Makhoul (Bordeaux), J.P. Abgrall (Brest), O. Reman (Caen), B. Desablens (Amiens), J.L. Harousseau (Nantes), Y. Bastion (Lyon), J.P. Pollet (Valenciennes), J. Pulik (Argenteuil), M. Lepeu (Avignon), M. Renoux (Bayonne), P. Morel (Lens), P. Henon (Mulhouse), N. Gratecos (Nice), P. Colombat (Tours), D. Machover (Villejuif), A. Dor (Antibes), P. Casassus (Bobigny), J. Donadio (Castelnou), B. Salles (Chalon), B. Legros (Clermont Ferrand), P. Audhuy (Colmar), A. Dutel (Compiègne), N. Philippe (Lyon), B. Benothman (Meaux), C. Christian (Metz), C. Margueritte (Montpellier), F. Witz (Nancy), A. Pesce (Nice), A. Baruchel (Paris), L. Sutton (Paris), C. Quetin (Pointe à Pitre), B. Pignon (Reims), E. Vilmer (Paris), E. Bourquard (StBrieuc), J.P. Marolleau (Paris), P. Robert (Toulouse), B. Despax (Toulouse), G. Nedellec, P. Auzanneau (Paris), M. Janvier (St Cloud).
Spanish AML group.
O. Rayon (Oviedo), M. Sanz (Valencia), J. San Miguel (Salamanca), J. Montagud (Valencia), E. Condé (Santander), P. Javier de la Serna (Madrid), G. Martin (Valencia), M. Perez Encinas (Santiago), J.P. Torres Carrete (Juan Canalejo), J. Zuazu (Barcelone), J. Odriozola (Madrid), E. Gomez-Sanz (Madrid), L. Palomera (Zaragoza), L. Villegas (Almeria), A. Deben (Juan Canalejo), P. Besalduch (Palma de Mallorca).
Cooperative AML study group, Germany.
H. Link (Hannover), A. Ganser (Frankfurt), E. Wandt (Nurnberg), A. Breitenbach (Stuttgart), B. Brennscheidt (Freiburg), D. Herrmann (Ulm), H. Soucek (Dresden), H. Strobel (Erlangen)
SAKK Swiss AML group.
K. Geiser (Berne), M. Fey (Berne), U. Hess (St. Gallen).
Belgian groups.
J.L. Michaux (Bruxelles), A. Bosly (Yvoir), E. Meeus (Anvers), A. Boulet (Mons).
Dutch groups.
P. Daenen (Groningen), P. Muus (Nijmegen).
Submitted December 7, 1998; accepted April 14, 1999.
Supported by the Programme Hospitalier de Recherche Clinique, the Centre Hospitalier Universitaire (CHU) of Lille, and by the Association pour la Recherche contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Pierre Fenaux, MD, Service des Maladies du Sang, CHU, 1 place de Verdun, 59037 Lille, France.
Deceased.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal