Therapeutic resistance is a major obstacle in the treatment of acute myeloid leukemia (AML). Such resistance has been associated with rapid drug efflux mediated by the multidrug resistance gene 1 (MDR1; encoding P-glycoprotein) and more recently with expression of other novel proteins conferring multidrug resistance such as MRP1 (multidrug resistance–associated protein 1) and LRP (lung resistance protein). To determine the frequency and clinical significance of MDR1, MRP1, and LRP in younger AML patients, we developed multiparameter flow cytometric assays to quantify expression of these proteins in pretreatment leukemic blasts from 352 newly diagnosed AML patients (median age, 44 years) registered to a single clinical trial (SWOG 8600). Protein expression was further correlated with functional efflux by leukemic blasts [assessed using two substrates: Di(OC)2and Rhodamine 123] and with the ability of MDR-reversing agents to inhibit efflux in vitro. MDR1/P-glycoprotein expression, which was highly correlated with cyclosporine-inhibited efflux, was noted in only 35% of these younger AML patients, distinctly lower than the frequency of 71% we previously reported in AML in the elderly (Blood89:3323, 1997). Interestingly, MDR1 expression and functional drug efflux increased with patient age, from a frequency of only 17% in patients less than 35 years old to 39% in patients aged 50 years (P = .010). In contrast, MRP1 was expressed in only 10% of cases and decreased with patient age (P = .024). LRP was detected in 43% of cases and increased significantly with increasing white blood cell counts (P = .0015). LRP was also marginally associated with favorable cytogenetics (P = .012) and French-American-British (FAB) AML FAB subtypes (P = .013), being particularly frequent in M4/M5 cases. Only MDR1/P-glycoprotein expression and cyclosporine-inhibited efflux were significantly associated with complete remission (CR) rate (PMDR1 = .012; Pefflux = .039) and resistant disease (RD; PMDR1= .0007; Pefflux = .0092). No such correlations were observed for MRP1 (PCR = .93;PRD = .55) or LRP (PCR = .50; PRD = .53). None of these parameters were associated with overall or relapse-free survival. Unexpectedly, a distinct and nonoverlapping phenotype was detected in 18% of these cases: cyclosporine-resistant efflux not associated with MDR1, MRP1, or LRP expression, implying the existence of other as yet undefined efflux mechanisms in AML. In summary, MDR1 is less frequent in younger AML patients, which may in part explain their better response to therapy. Neither MRP1 nor LRP are significant predictors of outcome in this patient group. Thus, inclusion of MDR1-modulators alone may benefit younger AML patients with MDR1(+) disease.
RESISTANCE TO CHEMOTHERAPY is one of the major obstacles to the effective treatment of patients with acute myeloid leukemia (AML).1,2 While nearly 80% of younger AML patients may initially achieve a complete remission with current therapeutic regimens, the majority of these patients relapse with resistant disease; even with aggressive therapy, the overall 5-year survival rate is only 35%.1-3 One of the best-characterized drug-resistance mechanisms in AML is active drug extrusion mediated by the multidrug resistance protein 1 (known as MDR1 or P-glycoprotein). MDR1-mediated drug resistance is of particular interest as MDR1 confers cross-resistance to a wide variety of structurally unrelated antineoplastic drugs used in the therapy of AML. In addition, MDR1 modulators such as verapamil, cyclosporine A (CsA), and its nonimmunosuppressive analogue PSC833 can reverse MDR1-mediated resistance in vitro and in vivo. Large clinical trials have been developed to assess the benefits of incorporation of first- or second-generation MDR1 modulators (such as PSC833) into AML therapy.4-7 While several trials are ongoing, encouraging results have already emerged from one recently completed randomized trial that incorporated CsA in the treatment of patients with high-risk AML.7 This trial found that both overall survival (OS) and relapse-free survival (RFS) were significantly improved among patients whose treatment included CsA. Three-year OS was 22% among CsA-treated patients compared with 6% among the control group, while 3-year RFS was also significantly improved at 43% compared with 10%.7
MDR1 is frequently expressed in AML and is associated with lower complete remission (CR) rates and, in some studies, shorter overall survivals for patients treated with standard therapeutic regimens.8-14 Our recent studies focusing on elderly AML patients (with a median age of 68 years) found that MDR1 was expressed in greater than 70% of cases; in these patients, the CR rate was significantly and independently associated with MDR1 expression, secondary AML, and unfavorable cytogenetics. CR rate ranged from 81% in patients with MDR1(−) de novo AML with favorable or intermediate cytogenetics, to 13% in patients with MDR1(+) secondary AML and unfavorable cytogenetics. Resistant disease was likewise associated independently with MDR1 and unfavorable cytogenetics.8 Thus, MDR1 was a key biologic factor in explaining the poor clinical response of these elderly patients to induction chemotherapy.
Although multidrug resistance mediated by MDR1 appears to be of biologic and clinical importance in AML, it is now apparent that other novel proteins may be associated with a multidrug resistant (MDR) phenotype. One such protein, the multidrug resistance–associated protein MRP1, is distantly related to MDR1, and like MDR1, lowers intracellular drug accumulation by promoting drug efflux.15Although the exact mechanism of MRP1-mediated drug transport is not yet fully understood, recent studies indicate that MRP functions as a transporter of glutathione S-conjugates.16-18 Very recent studies have also reported the existence of multiple MRP1 homologs, and their relative roles in conferring resistance are as yet undefined.19 Another protein associated with an MDR phenotype is the lung resistance protein LRP. LRP was recently shown to be a component of the major vault protein complex located in the cytoplasm and at the nuclear pore.20 As nuclear pore complexes mediate the bidirectional transport of substances between the nucleus and cytoplasm, it has been hypothesized that LRP may confer resistance by altering drug transport between the cytoplasm and the nucleus. While the biologic and clinical significance of these alternative MDR-associated proteins has not been extensively studied in AML, preliminary studies on limited numbers of patients have reported conflicting results.21-28
To determine the frequency of the expression and the biologic and clinical significance of MDR1 in younger AML patients and to contrast these results with our prior studies in elderly patients,8we undertook a retrospective study of a large cohort of previously untreated AML patients registered to a single Southwest Oncology Group therapeutic trial (SWOG 8600).29 In addition, the availability of this cohort gave us the opportunity to assess the biologic and clinical significance of MRP1 and LRP in a large number of uniformly treated AML patients. The results of these studies were correlated with pretreatment clinical parameters, cytogenetics, and therapeutic outcome.
MATERIALS AND METHODS
Patients.
All biologic samples were obtained at initial diagnosis before therapy from patients registered to a previously reported single Southwest Oncology Group study, SWOG study 8600: a randomized investigation of high-dose versus standard-dose cytosine arabinoside (Ara-C) with daunorubicin in patients with previously untreated AML.29In this study, patients with previously untreated AML were randomized on a 2-to-1 basis to daunomycin 45 mg/m2 for 3 days and standard-dose Ara-C versus daunomycin and high-dose Ara-C. Those patients on the standard-dose arm who achieved CR after 1 or 2 cycles of therapy were again randomized to consolidation therapy with either daunomycin and standard-dose Ara-C versus daunomycin and high-dose Ara-C. Patients who achieved CR on the high-dose induction were assigned to consolidation with daunomycin and high-dose Ara-C. Thus, this study asked, in a randomized fashion, whether standard-dose or high-dose Ara-C was superior for induction, and among those induced with standard-dose Ara-C, whether standard-dose or high-dose Ara-C was superior for consolidation. This study included patients with an antecedent hematologic disorder and treatment-related AML as well as de novo AML. However, information about such patient histories was not collected. After a brief preliminary trial for patients 65 years or older, the study was limited to those less than 65 years old at diagnosis. The high dose of Ara-C was initially defined as 2 g/m2/d for patients ≥50 and 3 g/m2/d for age <50. However, after 2 years the Data Monitoring Committee for the study reduced the dose to 2 g/m2/d for age <50. The standard dose of Ara-C was 200 mg/m2 for all ages throughout the study. The diagnosis of AML was confirmed by central histopathologic review by the SWOG Leukemia Pathology Committee using standard French-American-British (FAB) criteria as modified by SWOG.30 In cases of AML FAB M0, the myeloid phenotype of the blasts was confirmed by immunophenotyping performed at the SWOG Myeloid Leukemia Repository at the University of New Mexico.
Biologic studies.
Blasts from pretreatment bone marrow or peripheral blood samples were enriched upon sample receipt by density gradient separation, and an initial immunophenotyping panel performed to confirm the myeloid lineage of the leukemia. In cases where extra cells remained, these were aliquotted and cryopreserved in 90% fetal calf serum and 10% dimethyl sulfoxide (DMSO) at −135°C. The current studies analyzing MDR1, LRP, and MRP1 expression, as well as functional efflux, were subsequently performed on these cryopreserved samples after thawing. Our previous studies have reported successful assessment of MDR1 and functional dye/drug efflux on such appropriately cryopreserved samples.31 In addition, we have shown that simultaneous assessment of efflux in contaminating T cells in the leukemic specimens serves as an additional quality control of sample integrity and viability.
Expression of the drug resistance proteins MDR1, MRP1, and LRP.
MDR1 expression by leukemic blasts was measured using 2 MDR1-specific antibodies, MRK16 (Kamiya Biotech, Thousand Oaks, CA) and MM4.17 (courtesy of D. Cohen, Novartis, East Hanover, NJ) in three-color flow cytometric assays where blasts were costained with MRK16/MM4.17, the hematopoietic stem/progenitor cell antigen CD34, and the pan-myeloid antigen CD33, as previously described.31MRK16/MM4.17 staining was detected in a 2-step approach using first biotin-labeled anti-mouse antibody (1 μg/106 cells; Becton Dickinson, San Jose, CA) to detect MRK16/MM4.17, and then RED613 (Becton Dickinson) to detect the biotin label. This approach allows accurate analysis of MRK16/MM4.17 staining in a phenotypically gated myeloid blast population and correlation of MDR1 protein, CD34, and CD33 expression. The biotin-avidin detection system augments the signal obtained with dim staining with MRK16/MM4.17. The method is identical to that we have previously described with the exception of the use of a lower concentration of second-step biotin anti-mouse antibody in this protocol to decrease nonspecific staining of leukemic blasts.31 Appropriately matched isotype controls (same IgG subclass at the same protein concentration as the antibody tested) were used in all assays. The MDR1(+) DOX cell lines and MDR 1(−) 8226/S parental line (kindly provided by W.S. Dalton, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL) were used as controls in the standardization of these assays.32
For detection of the intracellular epitopes of MRP1 and LRP, leukemic blasts were fixed in 3.7% formaldehyde for 10 minutes at room temperature and were then permeabilized in a 50% acetone/50% Hanks’ balanced salt solution (HBSS) solution for 5 minutes at 4°C. The cells were then washed twice in cold HBSS, pelleted, and incubated with the primary antibody (0.5 μg/μL for LRP56, 1.0 μg/μL for MRPm6) for 20 minutes at 4°C. The LRP56 antibody was used to detect LRP protein, while MRPm6 was used to detect MRP1 (both from Kamiya Biotech). After staining, the cells were washed and labeled with an fluorescein isothiocyanate (FITC)-labeled second-step antibody. The LRP-expressing cell line MR20 and a sensitive parental control (courtesy of W.S. Dalton) and the MRP1-expressing cell lines SW-1573/S (MRP) (courtesy of G.J.R. Zaman, Netherlands Cancer Institute, Amsterdam, The Netherlands) and HL60/Adr (courtesy of M.S. Center, Kansas State University, Manhatten, KS) and sensitive parental controls were used to establish and standardize the flow cytometric procedures.33-35
Functional efflux.
To assess functional drug efflux and correlate efflux with MDR1 expression, the abilities of leukemic blasts to efflux the fluorescent dyes Di(OC)2 and Rhodamine 123 (Rh123) were measured in single-color flow cytometric assays, as described.31 The fluorescent dye Di(OC)2 is an MDR1 substrate; however, unlike other MDR1 substrates it does not appear to be transported by MRP1 and may thus be a more specific substrate than other drugs/dyes for assessment of MDR1-mediated transport.36 In contrast, Rh123 appears to be transported both by MDR1 as well as by MRP1, although, as observed by us and described by others, MRP1-mediated transport takes place more slowly.37 38
Briefly for efflux assays with both dyes, leukemic blasts were incubated in media containing Di(OC)2 or Rh123 for 45 minutes at 37°C. The blasts were then washed, and an aliquot of cells put on ice for measurement of baseline dye uptake. The residual blasts were resuspended in fresh dye-free media with or without the MDR1-modulator CsA (2,500 ng/mL; Sandoz Pharmaceuticals, Basel, Switzerland) and incubated for 105 minutes at 37°C in a shaking water bath to allow efflux. Cells were then resuspended in fresh 4°C media and placed on ice for immediate flow cytometric analysis. The MDR1(+) DOX cell lines and MDR1(−) 8226/S parental line (kindly provided by W.S. Dalton) were used for the standardization of this procedure.32
The efflux assays described above were optimized for detection of functional MDR1 activity. MRP1-mediated functional efflux is slower, and thus may not be readily detected in assays designed to detect MDR1 activity. Therefore, MRP1-mediated efflux was further examined in 10 selected MRP1(+) cases with sufficient cells remaining, and in 4 MRP1(−) controls by examining efflux of Rh123, Di(OC)2, and daunomycin (6.0 μmol/L) with or without CsA using longer efflux time periods (3 hours, 90 minutes, and 2 hours, respectively) as well as by examining the ability of leukemic blasts to efflux these dyes after glutathione depletion with DL-buthionine (S,R) sulfoximine (BSO).16 Because MRP1-mediated Rh123 efflux is dependent on the presence of intracellular glutathione, cellular depletion of glutathione by incubation with BSO will inhibit such efflux. Briefly for these latter experiments, leukemic blasts were incubated for 20 hours at 37°C in media with or without BSO (25 μmol). After BSO incubation, the cells were washed and then incubated in media with Rh123, Di(OC)2, or daunomycin, with or without BSO for 1 hour. The blasts were then washed and allowed to efflux the fluorescent dye in the presence of BSO for 3 hours, 90 minutes, and 2 hours, respectively.16
Measurement of protein expression and efflux.
Analyses were performed on a FACScan flow cytometer using Lysis II software (Becton Dickinson). Expression of CD34 was reported as positive, weakly positive, or negative by comparing staining of leukemic blasts with CD34 to an isotype control.39;40 In addition, for the positive cases, it was noted whether the entire blast population or only a subpopulation stained with CD34. Expression of the drug resistance markers MDR1, MRP1, and LRP on gated leukemic blasts compared with control cells was measured using a modification of the Kolmogorov-Smirnov (KS) statistic, denoted D, which measures the difference between two distribution functions and generates a value ranging from 0 to 1.0.41 The method was modified by ascribing a negative value to cases where the median fluorescence intensity of the control cells was brighter than that of antibody-stained cells. Because the KS statistic sensitively identifies even small differences in fluorescence, it is useful in detection of low-level antigen expression as occurs frequently with MDR1 expression in primary patient samples.31,42 MRK16/MM4.17 staining intensity was categorized for descriptive purposes as follows: bright (D ≥ 0.30), moderate (0.20 ≤ D < 0.30), dim (0.15 ≤ D < 0.20), and negative (D < 0.15); however, correlations with clinical outcome were largely performed using the D value as a continuous variable. The D value cut-points for MDR1 expression in this study were slightly higher than those used in our previous study on elderly patients with AML.31 We used higher D values in this study because we have found that the lower concentration of biotin anti-mouse antibody used allows better discrimination between MDR1(+) and MDR1(−) cases. A direct comparison between the two techniques was made on 10 patient samples with varying levels of MDR1 expression, with very similar results. In addition, the distribution of D values obtained using the original technique for the previously reported 189 AML patients over age 558 was almost identical to that obtained with the new method for 333 similar patients in a more recent clinical trial (SWOG-9333, results not shown).
Staining with LRP 56 was considered positive with a D value ≥0.25, while MRP1 staining was considered positive with a D value ≤0.20. These D value cut-points were derived based on observations of the staining of MRP1- and LRP-positive cell lines and on the higher level of nonspecific background fluorescence sometimes found using these intracellular markers.
Di(OC)2 and Rh123 efflux were assessed by analyzing cellular fluorescence of gated leukemic blasts after baseline dye uptake, and after efflux in the presence/absence of CsA. Differences in cellular fluorescence were analyzed using the KS statistic. For CsA inhibited efflux, the difference between cellular fluorescence after efflux in the presence or absence of CsA was measured; dim efflux was defined as 0.20 ≤ D < 0.25, moderate efflux as 0.25 ≤ D < 0.40 and strong efflux as D ≥ 0.40.
Cytogenetic analysis.
Cytogenetic studies on pretreatment bone marrow or unstimulated blood samples were performed using standard G-banding with trypsin-Giemsa or trypsin-Wright’s staining in SWOG-approved cytogenetics laboratories. ICSN 1995 guidelines were followed for clonal definition and description of individual and numerical karyotypic anomalies.43 Karyotypes were considered normal diploid if no clonal abnormalities were detected in a minimum of 20 metaphases examined and if 2 cell-processing methods were used. Each karyotype was independently reviewed by at least 3 members of the SWOG Cytogenetics Committee.
Statistical methods.
Demographic and clinical data for patients on this study were collected with quality-control review according to standard procedures of the SWOG. Expression of MDR1, MRP1, LRP, and efflux were represented as quantitative variables using the KS statistic D, and categorized for descriptive purposes as described above. Correlation among multidrug resistance and efflux variables was estimated using Spearman’s rank order correlation coefficient (rS). Unweighted least squares and logistic regression (LR) analyses were performed to identify variables predictive of MDR1, MRP1, or LRP expression or efflux.44,45 Standard criteria were used to define CR and relapse.29 Patient response was coded as CR, partial response (PR), stable disease, early death (death before any assessment of response), and not adequately assessed. OS was measured from randomization until death from any cause, with observation censored for patients last known alive. According to the protocol, patients were not to be transplanted in first CR. Thus, data regarding bone marrow transplantation (BMT) were not collected for study 8600, and consequently survival was not censored at BMT. RFS was measured from establishment of CR until relapse or death from any cause, with observation censored for patients last known alive without report of relapse. Distributions of OS and RFS were estimated by the method of Kaplan and Meier.46 Analyses of prognostic factors for treatment outcomes were based on LR models for CR and proportional hazards (PH) regression models for OS and RFS.45 47 These included both simple regression models to examine the separate effects of individual prognostic factors, and multiple regression models to examine the joint effects of multiple factors. The potential prognostic factors available for this study are shown in Tables 1 and2. Statistical significance is represented by two-tailed P values. Analyses were based on clinical and biologic data available March 11, 1997.
Clinical and Pathologic Characteristics of 352 AML Cases on Study 8600
| . | Median . | Range . |
|---|---|---|
| Age (yr) | 44 | 17-69 |
| Marrow blasts (%) | 78 | 0-99 |
| WBC count (×109/L) | 37.4 | 0.7-416 |
| Peripheral blasts (%) | 51 | 0-99 |
| Peripheral blasts (×109/L) | 12.3 | 0-378 |
| Hemoglobin (g/dL) | 9.3 | 4.5-15.0 |
| Platelets (×109/L) | 52 | 3-700 |
| No. | Percent | |
| Female | 145 | 41 |
| Male | 207 | 59 |
| FAB (central review) | ||
| M1 | 72 | 20 |
| M2 | 145 | 41 |
| M3 | 30 | 9 |
| M4 | 54 | 15 |
| M5 | 31 | 9 |
| M6 | 2 | 1 |
| M7 | 3 | 1 |
| M0 | 7 | 2 |
| M0/M5 | 1 | 0.3 |
| Myeloid, NOS | 7 | 2 |
| Treatment arm | ||
| Ara-C (200 mg/m2) + DNR | 245 | 70 |
| Ara-C (2.0 g/m2) + DNR | 90 | 26 |
| Ara-C (3.0 g/m2) + DNR | 17 | 5 |
| . | Median . | Range . |
|---|---|---|
| Age (yr) | 44 | 17-69 |
| Marrow blasts (%) | 78 | 0-99 |
| WBC count (×109/L) | 37.4 | 0.7-416 |
| Peripheral blasts (%) | 51 | 0-99 |
| Peripheral blasts (×109/L) | 12.3 | 0-378 |
| Hemoglobin (g/dL) | 9.3 | 4.5-15.0 |
| Platelets (×109/L) | 52 | 3-700 |
| No. | Percent | |
| Female | 145 | 41 |
| Male | 207 | 59 |
| FAB (central review) | ||
| M1 | 72 | 20 |
| M2 | 145 | 41 |
| M3 | 30 | 9 |
| M4 | 54 | 15 |
| M5 | 31 | 9 |
| M6 | 2 | 1 |
| M7 | 3 | 1 |
| M0 | 7 | 2 |
| M0/M5 | 1 | 0.3 |
| Myeloid, NOS | 7 | 2 |
| Treatment arm | ||
| Ara-C (200 mg/m2) + DNR | 245 | 70 |
| Ara-C (2.0 g/m2) + DNR | 90 | 26 |
| Ara-C (3.0 g/m2) + DNR | 17 | 5 |
Frequency of MDR1, LRP, MRP1, Functional Efflux, and CD34 Expression Among 352 Previously Untreated AML Patients Under Age 70
| Parameter . | Intensity . | No. . | Percent . |
|---|---|---|---|
| MDR1 (MRK16) | Bright (D ≥ 0.30) | 64 | 18 |
| Moderate (0.20 ≤ D < 0.30) | 35 | 10 | |
| Dim (0.15 ≤ D < 0.20) | 24 | 7 | |
| Negative (D < 0.15) | 228 | 65 | |
| MDR1 (MM4.17) | Bright (D ≥ 0.30) | 89 | 25 |
| Moderate (0.20 ≤ D < 0.30) | 37 | 11 | |
| Dim (0.15 ≤ D < 0.20) | 25 | 7 | |
| Negative (D < 0.15) | 200 | 57 | |
| LRP56 | Positive (D ≥ 0.25) | 138 | 43 |
| Negative (D < 0.25) | 180 | 57 | |
| MRP1 | Positive (D ≥ 0.20) | 32 | 10 |
| Negative (D < 0.20) | 289 | 90 | |
| CsA inhibited efflux | Bright (D ≥ 0.40) | 87 | 28 |
| (DiOC2) | Moderate (0.25 ≤ D < 0.40) | 43 | 14 |
| Dim (0.20 ≤ D < 0.25) | 12 | 4 | |
| Negative (D < 0.20) | 172 | 55 | |
| CsA inhibited efflux | Bright (D ≥ 0.40) | 70 | 22 |
| (Rh123) | Moderate (0.25 ≤ D < 0.40) | 39 | 12 |
| Dim (0.20 ≤ D < 0.25) | 12 | 4 | |
| Negative (D < 0.20) | 197 | 62 | |
| CD34 | Positive (all blasts) | 134 | 38 |
| Positive (blast subset) | 70 | 20 | |
| Negative | 147 | 42 |
| Parameter . | Intensity . | No. . | Percent . |
|---|---|---|---|
| MDR1 (MRK16) | Bright (D ≥ 0.30) | 64 | 18 |
| Moderate (0.20 ≤ D < 0.30) | 35 | 10 | |
| Dim (0.15 ≤ D < 0.20) | 24 | 7 | |
| Negative (D < 0.15) | 228 | 65 | |
| MDR1 (MM4.17) | Bright (D ≥ 0.30) | 89 | 25 |
| Moderate (0.20 ≤ D < 0.30) | 37 | 11 | |
| Dim (0.15 ≤ D < 0.20) | 25 | 7 | |
| Negative (D < 0.15) | 200 | 57 | |
| LRP56 | Positive (D ≥ 0.25) | 138 | 43 |
| Negative (D < 0.25) | 180 | 57 | |
| MRP1 | Positive (D ≥ 0.20) | 32 | 10 |
| Negative (D < 0.20) | 289 | 90 | |
| CsA inhibited efflux | Bright (D ≥ 0.40) | 87 | 28 |
| (DiOC2) | Moderate (0.25 ≤ D < 0.40) | 43 | 14 |
| Dim (0.20 ≤ D < 0.25) | 12 | 4 | |
| Negative (D < 0.20) | 172 | 55 | |
| CsA inhibited efflux | Bright (D ≥ 0.40) | 70 | 22 |
| (Rh123) | Moderate (0.25 ≤ D < 0.40) | 39 | 12 |
| Dim (0.20 ≤ D < 0.25) | 12 | 4 | |
| Negative (D < 0.20) | 197 | 62 | |
| CD34 | Positive (all blasts) | 134 | 38 |
| Positive (blast subset) | 70 | 20 | |
| Negative | 147 | 42 |
RESULTS
Patient characteristics.
SWOG study 8600 accrued 863 patients from November 1986 through December 1991 (Table 1). The SWOG Leukemia Repository had sufficient cryopreserved pretreatment material to retrospectively study one or more of the following: CD34, MDR1, MRP1, LRP expression, and functional efflux in 387 of these 863 cases. Because specimen submission was not initially a mandatory eligibility requirement on this clinical trial, 275 (71%) of the 387 patients with specimens entered the study after 1988, compared with 497 (58%) of the entire 863 patients. Submission of specimens to SWOG-approved laboratories for cytogenetic analysis during this time period was also not required and was limited to 109 patients, of whom 86 (79%) entered the study after 1988. Of the 387 cases with cells available, 35 were subsequently excluded because the diagnosis of AML was not confirmed by the SWOG Leukemia Pathology Committee either because of inadequate materials (25 cases), or because morphologic and immunophenotypic data were diagnostic of a different entity (acute lymphoblastic leukemia, myelodysplasia, myelofibrosis). The remaining 352 cases are the subject of this study. These 352 cases came from patients registered at 81 SWOG-affiliated centers.
The patients in this final cohort had a median age of 44 years (range, 17 to 69 years) and included 145 women (41%) and 207 men (59%; see Table 1). Treatment outcomes in the 352 patients available for study did not differ significantly from those of the remaining 421 patients with confirmed diagnoses of AML on study 8600. The CR rates of the included and excluded patients were 53% and 55%, respectively (P = .56). The estimated hazard ratios (included relative to excluded) were 1.11 (95% confidence interval [CI], 0.95 to 1.30), and 1.12 (CI, 0.91 to 1.39) for OS and RFS, respectively. Thus, there was no evidence that the patients selected for this study differed from the total group with respect to clinical outcome.
The entire morphologic spectrum of AML was represented in this patient group (Table 1). The majority (217 of 352; 62%) of cases classified according to the SWOG-modified FAB criteria30 had either FAB M1 or M2 morphology. Thirty patients (9%) had acute promyelocytic leukemia (FAB M3) while the remainder had predominantly AML M4 or M5 (85 patients; 24%).
Drug resistance and efflux phenotypes of AML cases.
Using the MDR1-specific antibody MRK16 and sensitive multicolor flow cytometric assays, MDR1 proteins were detected on the leukemic blasts of 123 of 351 cases (35%; CI, 30% to 40%; Table 2; Fig 1). These MDR1+ cases included 64 (18%; CI, 14% to 22%) with very bright staining (D ≥ 0.30) and 35 cases (10%; CI, 7% to 13%) with moderate staining (0.20 ≤ D < 0.3; Table 2). Very similar results were obtained using the MRK16 and MM4.17 antibodies (rs = .84; Table 3). Of the 351 patients tested with MM4.17, 126 (36%; CI, 31% to 41%) showed bright or moderate staining, and another 25 (7%; CI, 4% to 10%) showed dim staining. When we examined MDR1 and CD34 among the different the Ara-C dose groups, we found no indication that the distributions of MDR or CD34 variables differed among these groups.
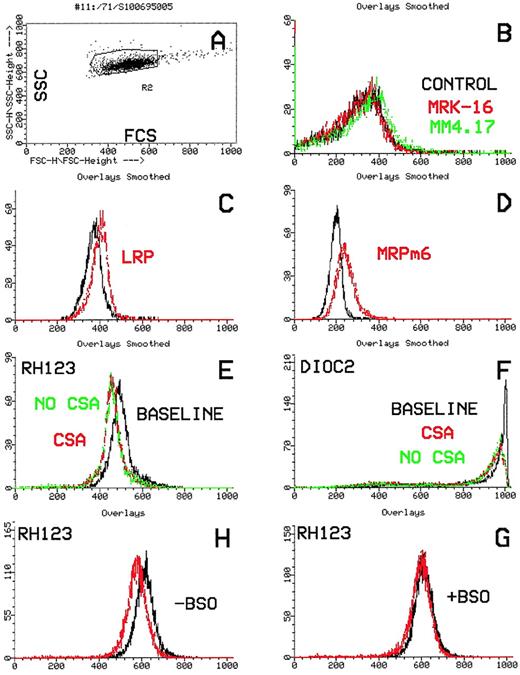
Flow cytometric histograms of a typical MDR1(+)/efflux(+) AML case. (A) Dot-plot showing laser light characteristics of leukemic blasts (forward scatter, x-axis; side scatter, y-axis). (B) Leukemic blasts stained with the MDR1-specific antibodies, MRK16 (red) and MM4.17 (green) compared with the control (black). The leukemic blasts stain positively with both antibodies. (C and D) Leukemic blasts stained with the monoclonal antibodies LRP and MRPm6 (red) compared with the control (black). The blasts are positive for LRP and negative for MRP1. (E and F) Flow cytometric histograms showing functional efflux of Rh123 and Di(OC)2 by leukemic blasts. Increasing cellular fluorescence (x-axis) plotted against cell number (y-axis). Functional efflux in the absence of the MDR1 inhibitor, CsA (green), is compared with efflux in the presence of CsA (red) and with the baseline (black). There is significant efflux of both Di(OC)2 and Rh123, which is blocked by CsA.
Flow cytometric histograms of a typical MDR1(+)/efflux(+) AML case. (A) Dot-plot showing laser light characteristics of leukemic blasts (forward scatter, x-axis; side scatter, y-axis). (B) Leukemic blasts stained with the MDR1-specific antibodies, MRK16 (red) and MM4.17 (green) compared with the control (black). The leukemic blasts stain positively with both antibodies. (C and D) Leukemic blasts stained with the monoclonal antibodies LRP and MRPm6 (red) compared with the control (black). The blasts are positive for LRP and negative for MRP1. (E and F) Flow cytometric histograms showing functional efflux of Rh123 and Di(OC)2 by leukemic blasts. Increasing cellular fluorescence (x-axis) plotted against cell number (y-axis). Functional efflux in the absence of the MDR1 inhibitor, CsA (green), is compared with efflux in the presence of CsA (red) and with the baseline (black). There is significant efflux of both Di(OC)2 and Rh123, which is blocked by CsA.
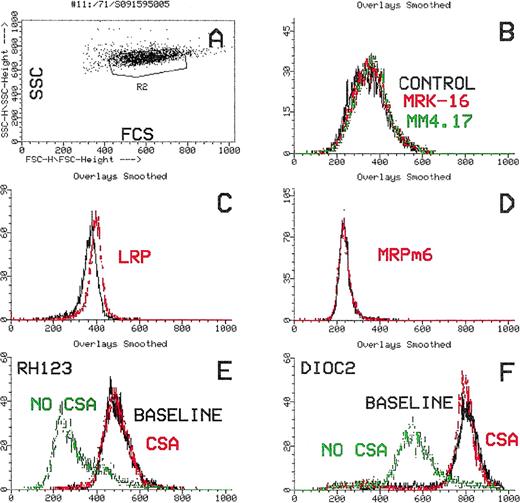
Flow cytometric histograms of an MRP1(+) AML case. (A) Dot-plot showing laser-light characteristics of leukemic blasts (forward scatter, x-axis; side scatter, y-axis). (B) Leukemic blasts stained with the MDR1-specific antibodies, MRK16 (red) and MM4.17 (green), compared with the control (black). The leukemic blasts are negative for MDR1. (C and D) Leukemic blasts stained with the monoclonal antibodies LRP and MRPm6 (red) compared to the control. The blasts are LRP(+) and MRP1(+). (E and F) Flow cytometric histograms showing functional efflux of Rh123 and Di(OC)2 by leukemic blasts. Increasing cellular fluorescence (x-axis) plotted against cell number (y-axis). Functional efflux in the absence of the MDR1 inhibitor, CsA (green), is compared with efflux in the presence of CsA (red) and with the baseline (black). There is efflux of Rh123 that is not inhibited by CsA (E). No Di(OC)2 efflux is shown (F). (G and H). Rh123 efflux after incubation of leukemic blasts for 18 hours in the absence (G) or presence of BSO (H). Cellular fluorescence after efflux (red) compared with baseline (black). Rh123 efflux is detected (G) that is inhibited by BSO incubation.
Flow cytometric histograms of an MRP1(+) AML case. (A) Dot-plot showing laser-light characteristics of leukemic blasts (forward scatter, x-axis; side scatter, y-axis). (B) Leukemic blasts stained with the MDR1-specific antibodies, MRK16 (red) and MM4.17 (green), compared with the control (black). The leukemic blasts are negative for MDR1. (C and D) Leukemic blasts stained with the monoclonal antibodies LRP and MRPm6 (red) compared to the control. The blasts are LRP(+) and MRP1(+). (E and F) Flow cytometric histograms showing functional efflux of Rh123 and Di(OC)2 by leukemic blasts. Increasing cellular fluorescence (x-axis) plotted against cell number (y-axis). Functional efflux in the absence of the MDR1 inhibitor, CsA (green), is compared with efflux in the presence of CsA (red) and with the baseline (black). There is efflux of Rh123 that is not inhibited by CsA (E). No Di(OC)2 efflux is shown (F). (G and H). Rh123 efflux after incubation of leukemic blasts for 18 hours in the absence (G) or presence of BSO (H). Cellular fluorescence after efflux (red) compared with baseline (black). Rh123 efflux is detected (G) that is inhibited by BSO incubation.
Spearman Rank Correlation Coefficients of Multidrug Resistance and Efflux Variables
| . | MDR1 . | Other Multidrug Resistance Proteins . | CsA-Inhibited Efflux . | ||
|---|---|---|---|---|---|
| MM4.17 . | LRP56 . | MRP1 . | DiOC2 . | Rh123 . | |
| MDR1 | |||||
| MRK16 | .84 (<.0001) | .08 (.16) | −.02 (.72) | .55 (<.0001) | .58 (<.0001) |
| MM4.17 | .10 (.083) | −.05 (.41) | .60 (<.0001) | .59 (<.0001) | |
| Other multidrug resistance proteins | |||||
| LRP56 | −.02 (.68) | .01 (.88) | .04 (.49) | ||
| MRP1 | −.05 (.41) | −.03 (.59) | |||
| CsA-inhibited efflux | |||||
| Di(OC)2 | .88 (<.0001) | ||||
| . | MDR1 . | Other Multidrug Resistance Proteins . | CsA-Inhibited Efflux . | ||
|---|---|---|---|---|---|
| MM4.17 . | LRP56 . | MRP1 . | DiOC2 . | Rh123 . | |
| MDR1 | |||||
| MRK16 | .84 (<.0001) | .08 (.16) | −.02 (.72) | .55 (<.0001) | .58 (<.0001) |
| MM4.17 | .10 (.083) | −.05 (.41) | .60 (<.0001) | .59 (<.0001) | |
| Other multidrug resistance proteins | |||||
| LRP56 | −.02 (.68) | .01 (.88) | .04 (.49) | ||
| MRP1 | −.05 (.41) | −.03 (.59) | |||
| CsA-inhibited efflux | |||||
| Di(OC)2 | .88 (<.0001) | ||||
Correlation coefficients are based on protein expression or efflux as measured by Kolmogorov-Smirnov D values. Two-tailed P values are shown in parentheses. Numbers of patients range from 295 to 351 depending on availability of data.
The lung resistance protein LRP was expressed very frequently in selectively gated leukemic blasts using the antibody LRP56 on permeabilized leukemic cells. Of 318 cases studied with LRP, 138 (43%; CI, 38% to 49%) were positive. In contrast, MRP1 was detected in only 32 of 321 cases (10%; CI, 7% to 13%) using the specific MRPm6 antibody (Table 2; Fig 2).
In samples from individual patients, the pattern of expression of the various MDR-associated proteins was frequently very complex. All possible combinations of MDR-associated proteins were identified and no specific correlations of one or more of these proteins were found. For example, of the 313 patients who could be studied for expression of all 3 MDR-associated proteins, 47 of 91 (52%) of patients with moderate or bright MRK16 expression were LRP+, as were 89 of 222 (40%) of patients with no or dim MRK16 staining. Similarly, MRP1 expression was found as frequently among MRK16 moderate/bright cases (9 of 91; 10%) as among MRK16 negative/dim cases (23 of 222; 10%). Figure 1 illustrates a typical MDR1+ case where the blasts are LRP as well as MDR1-positive, but are negative for MRP1.
Functional efflux was evaluable for 89% of patients using DiOC2, and in 90% using Rh123. Functional efflux inhibited by the MDR1-reversing agent cyclosporine (CsA) was detected at a similar frequency using these two efflux substrates and the two measurements were highly correlated (rs = .88; Table 3). Di(OC)2 efflux was detected in 130 of 314 AML cases (41%; CI, 36% to 47%), including 87 cases with bright efflux (28%; CI, 23% to 33%; Table 2), while Rh123 efflux was found in 109 of 318 cases (34%; CI, 29% to 39%; Table 2). Interestingly, we also detected distinct extrusion of Rh123 which was not inhibited by CsA in 56 of 318 cases tested (18%); these cases appeared distinct from those with MDR1-mediated, CSA-inhibited efflux. Our preliminary studies of this group of patients have found no correlation with MRP1 or LRP expression. We are currently characterizing this novel phenotype in greater detail.
Correlations among the expression of MDR1, LRP, MRP1, and efflux are summarized in Table 3. MDR1 expression as measured with MRK16 and MM4.17 staining was highly positively correlated with CsA-inhibited efflux of both Rh123 and Di(OC)2, withrs ranging from .55 to .60 (Table 3, Fig 1). In contrast, LRP and MRP1 were not significantly with each other or with MDR1 expression or efflux, with rs ranging from −.05 to .10. Focusing on the MRP1(+) samples, we developed functional efflux assays optimized to detect MRP1 activity by prolonging the time over which efflux was assessed and by using DL-buthionine (S,R)-sulfoximine (BSO) to deplete intracellular glutathione stores and thereby inhibit MRP function (see Materials and Methods). Ten MRP1(+) patient samples with sufficient cryopreserved material available, and 4 MRP1(−) controls were tested. RH123 efflux was shown in all 10 MRP1(+) samples, with efflux intensity ranging from very low (3 cases; 0.15 < D < 0.20) to high (2 cases; D > 0.40). In 9 of 10 cases, BSO-induced glutathione depletion resulted in an inhibition of Rh123 efflux (Fig 2). In contrast among the controls, 2 MDR1(+) cases showed efflux not inhibited by BSO, while 2 MDR1(−) cases were efflux(−).
Despite the strong correlation of MDR1 and CsA-inhibited efflux, discrepant cases were identified as we have previously reported in other patient cohorts.31 For example, among 313 patients with both MDR1 (MRK16) and CsA-inhibited Di(OC)2 efflux measured, 12 (4%) were MRK16 moderate/bright but efflux negative/dim, and 46 (15%) were MRK16 negative/dim but efflux moderate/bright. Figure 3 illustrates one such case where the blasts showed strong CsA-inhibited efflux, although none of the MDR-associated proteins were expressed; we presume that efflux in such cases is mediated by novel mechanisms.
Flow cytometric histograms of an MDR1(−)/LRP(−)/MRP1(−)/Efflux(+) AML case. (A) Dot plot showing laser-light characteristics of leukemic blasts (forward scatter, x-axis; side scatter, y-axis). (B) Leukemic blasts stained with the MDR1-specific antibodies, MRK16 (red) and MM4.17 (green), compared with the control (black). The leukemic blasts are negative for MDR1. (C and D) Leukemic blasts stained with the monoclonal antibodies LRP and MRPm6 (red) compared with the control (black). The blasts are negative for LRP and MRP1. (E and F) Flow cytometric histograms showing functional efflux of Rh123 and Di(OC)2 by leukemic blasts. Increasing cellular fluorescence (x-axis) plotted against cell number (y-axis). Functional efflux in the absence of the MDR1 inhibitor, CsA (green), is compared with efflux in the presence of CsA (red) and with the baseline (black). There is significant efflux of both Di(OC)2 and Rh123 that is blocked by CsA.
Flow cytometric histograms of an MDR1(−)/LRP(−)/MRP1(−)/Efflux(+) AML case. (A) Dot plot showing laser-light characteristics of leukemic blasts (forward scatter, x-axis; side scatter, y-axis). (B) Leukemic blasts stained with the MDR1-specific antibodies, MRK16 (red) and MM4.17 (green), compared with the control (black). The leukemic blasts are negative for MDR1. (C and D) Leukemic blasts stained with the monoclonal antibodies LRP and MRPm6 (red) compared with the control (black). The blasts are negative for LRP and MRP1. (E and F) Flow cytometric histograms showing functional efflux of Rh123 and Di(OC)2 by leukemic blasts. Increasing cellular fluorescence (x-axis) plotted against cell number (y-axis). Functional efflux in the absence of the MDR1 inhibitor, CsA (green), is compared with efflux in the presence of CsA (red) and with the baseline (black). There is significant efflux of both Di(OC)2 and Rh123 that is blocked by CsA.
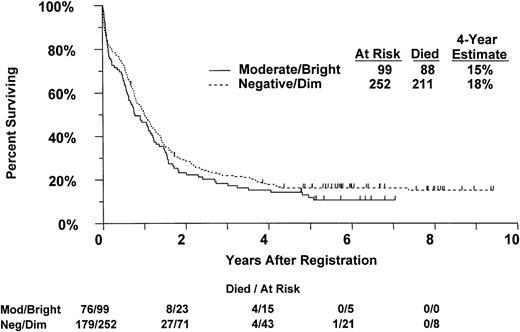
Correlation of overall survival by MDR1 expression as measured with MRK16 staining in 351 previously untreated AML patients.
Correlation of overall survival by MDR1 expression as measured with MRK16 staining in 351 previously untreated AML patients.
Correlation of drug-resistant phenotype with clinical, morphologic, and biologic disease factors.
Expression of MDR1 and CsA-inhibited efflux varied with age, FAB subtype, and CD34 expression. There was a progressive increase of MDR1 expression with age in this patient cohort (P = .010 for MRK16). Moderate or bright MDR1 expression was seen in 19 of 109 (17%) patients under age 35, in 33 of 121 (27%) ages 35 to 49, and in 47 of 121 (39%) over 50 years old. A similar pattern was seen for CsA-inhibited Di(OC)2 efflux (P = .0056). Significant heterogeneity of MDR1 expression was observed among the FAB categories (with M0, M6, M7, M0/M5, and other AML pooled into a single category) (P = .0001). As shown in Table 4, moderate/bright MDR1 expression was relatively more frequent in M1 and M2 cases (81 of 217, 37%) and less frequent in M3, M4, and M5 (11 of 115; 10%). A similar association was seen for CsA-inhibited Di(OC)2 efflux (P = .0001; Table 4). As we have previously reported in elderly patients,8 MDR1 expression was highly associated with expression of the stem and progenitor cell antigen CD34 (P < .0001). Only 12 of 147 (8%) patients with no or weak CD34 had moderate/bright MDR1 expression, compared to 17 of 70 (24%) cases with strong CD34 expression in a subpopulation of blasts, and 70 of 134 (52%) with uniform strong CD34 expression by the blasts. A similar association was seen between CD34 expression and CsA-inhibited Di(OC)2 efflux (P < .0001). There were no significant associations of MDR1 or efflux with any of the other variables in Table 1. Essentially the same results were obtained using CsA-inhibited Rh123 efflux or MM4.17 staining.
Correlation of MDR1 Expression, Functional Efflux, and LRP Expression With FAB Subtype
| FAB . | MDR1 Expression (MRK16) . | Efflux (Di(OC)2) . | LRP Expression (LRP56) . | MRP1 Expression (MRPm6) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Bright/Moderate (D ≥ 0.20) . | % . | Patients . | Positive (D ≥ 0.20) . | % . | Patients . | Positive (D ≥ 0.25) . | % . | Patients . | Positive (D ≥ 0.20) . | % . | |
| M1 | 72 | 29 | 40 | 67 | 37 | 55 | 69 | 30 | 43 | 69 | 10 | 15 |
| M2 | 145 | 52 | 36 | 132 | 73 | 55 | 132 | 54 | 41 | 135 | 11 | 8 |
| M3 | 30 | 2 | 7 | 25 | 1 | 4 | 25 | 7 | 28 | 27 | 3 | 11 |
| M4 | 54 | 8 | 15 | 46 | 17 | 37 | 49 | 27 | 55 | 48 | 4 | 8 |
| M5 | 31 | 1 | 3 | 27 | 4 | 15 | 27 | 17 | 63 | 27 | 2 | 7 |
| Other | 19 | 7 | 37 | 17 | 19 | 59 | 16 | 3 | 19 | 15 | 2 | 13 |
| FAB . | MDR1 Expression (MRK16) . | Efflux (Di(OC)2) . | LRP Expression (LRP56) . | MRP1 Expression (MRPm6) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Bright/Moderate (D ≥ 0.20) . | % . | Patients . | Positive (D ≥ 0.20) . | % . | Patients . | Positive (D ≥ 0.25) . | % . | Patients . | Positive (D ≥ 0.20) . | % . | |
| M1 | 72 | 29 | 40 | 67 | 37 | 55 | 69 | 30 | 43 | 69 | 10 | 15 |
| M2 | 145 | 52 | 36 | 132 | 73 | 55 | 132 | 54 | 41 | 135 | 11 | 8 |
| M3 | 30 | 2 | 7 | 25 | 1 | 4 | 25 | 7 | 28 | 27 | 3 | 11 |
| M4 | 54 | 8 | 15 | 46 | 17 | 37 | 49 | 27 | 55 | 48 | 4 | 8 |
| M5 | 31 | 1 | 3 | 27 | 4 | 15 | 27 | 17 | 63 | 27 | 2 | 7 |
| Other | 19 | 7 | 37 | 17 | 19 | 59 | 16 | 3 | 19 | 15 | 2 | 13 |
The patterns of associations of LRP and MRP1 with clinical and morphologic parameters were quite distinct from MDR1. LRP expression increased significantly with increasing marrow blast percentage (P = .0008) and white blood cells (WBCs) (P = .0015). For example, LRP positivity increased from 28 of 105 (27%) patients with WBC count < 20 × 109/L to 40 of 76 (53%) and 70 of 137 (51%) patients with WBC counts of 20 to 49.9 × 109/L, and ≥50.0 × 109/L, respectively. LRP expression did not vary significantly with age (P = .49) or CD34 expression (P= .34). There was marginally significant heterogeneity of LRP expression among FAB subtypes (P = .013). As shown in Table 4, LRP expression was highest in FAB M4 and M5 subtypes (44 of 76, 58%), intermediate in M1 and M2 (84 of 201, 42%), and lowest in M3 (7 of 25, 28%). In contrast to MDR1, MPR1 was marginally more frequently seen in younger AML patients, demonstrating a marginally significant decreasing trend with increasing age (P = .024). MRP1 was detected in 10 of 101 (10%) of patients under age 35 and in 14 of 112 (13%) of those ages 35 to 39, but in only 8 of 108 (7%) of those over 50 years old. MRP1 was not significantly correlated with any of the remaining variables in Table 1.
Cytogenetic analysis.
Because of the lack of submission requirements in the early stages of this clinical trial, cytogenetic studies performed and reviewed in approved SWOG cytogenetic laboratories were available on only 96 of the 352 cases in this study. Fifteen cytogenetic studies were deemed inadequate after review by the SWOG Cytogenetics Committee, largely due to the examination of insufficient metaphases. Of the 81 acceptable cases, 34 (42%) had normal karyotypes. Abnormalities associated with de novo AML were found in 22 cases (27%), including 11 cases (14%) with t(8;21), 4 cases (5%) with inv(16)/t(16;16), 5 cases (6%) with t(15;17), and 2 (2%) with t(6;9). In addition, abnormalities of 11q were quite frequent, being present in 10 cases (12%). In contrast, abnormalities associated with myelodysplasia and secondary AML were less frequently detected in this patient group, with only 9 cases (11%) showing −5/5q−, −7/7q−, or +8, including 1 patient with abnormalities of both 5 and 7, and another with t(8;21) and −7/7q−.
When the 81 patients with cytogenetic data were grouped into favorable, intermediate, and unfavorable prognostic categories according to our previously published scheme,8 20 (25%) were classified as favorable, 35 (43%) as intermediate, 23 (28%) as unfavorable, and 3 (4%) were not classifiable.8 48-53 This cytogenetic categorization, treated as an ordinal response variable, was analyzed in relation to the MDR phenotype, efflux, and the variables in Table 1using multiple logistic regression analysis. Only 2 variables were even marginally significantly associated with cytogenetic category: marrow blast percentage (P = .0007) and LRP expression (P = .012). LRP expression was associated with more favorable cytogenetics; among the 72 patients with both LRP and cytogenetic data, 11 of 18 (61%) with favorable cytogenetics were LRP+, compared to 16 of 32 (50%) and 10 of 22 (45%) of those with intermediate or unfavorable cytogenetics, respectively. Of the other factors considered, neither MRP1 (P = .37), nor CsA-inhibited Di(OC)2 efflux (P = .41) were significantly related to cytogenetic category. There was a marginal, nonsignificant trend between poor-prognosis cytogenetics and MDR1 expression (P = .091). Nine of 23 (39%) with unfavorable cytogenetics had moderate or bright MDR1 expression, compared to 7 of 35 (20%) or 4 of 20 (20%) with intermediate or favorable cytogenetics, respectively. However, because of the limited number of cases with cytogenetic data, these results do not constitute strong evidence against the possibility that MDR and/or efflux are associated with cytogenetic characteristics. Interestingly, among the 8 MRP+ cases with cytogenetic data, 4 had normal cytogenetic studies, which confers intermediate risk, and the other 4 had favorable abnormalities [3 with t(8;21) and 1 with inv(16)]. One additional MRP+ patient with a nonevaluable pretreatment cytogenetic study was inferred to have t(15;17) and 4 additional clonal aberrations when subsequent relapse studies were examined. The cytogenetic risk status of this patient is uncertain, because t(15;17) is favorable while having 5+ clonal abnormalities is unfavorable. Among the 4 patients with inv(16), 1 was MDR1+, 1 was MRP1+, and 3 were LRP+.
Correlation of clinical, morphologic, and biologic disease factors with CR rate.
Of the 352 patients, 185 (53%; CI, 47% to 58%) achieved a CR, including 139 (39%) after 1 induction course.29 Of the 213 patients who did not achieve CR after 1 induction, 98 received a second induction course on protocol and 46 (47%) of these 98 achieved CR. Thirty-one patients (9%) were classified as early deaths (all after a single induction attempt), and another 41 (12%) were not adequately assessed for response (including 1 who refused protocol therapy and 2 who received 2 induction attempts). The remaining 95, who had resistant disease, are discussed further below. Induction therapy with standard-dose Ara-C was given to 245 (70%) of the patients, while 107 (30%) received high-dose Ara-C (see Materials and Methods; Patients). There was no significant difference in CR rate between these 2 groups, with 127 (52%) of patients treated with standard-dose Ara-C, and 58 (54%) of those treated with high-dose Ara-C achieving CR. Although the CR rate was slightly lower for patients over age 50 (57 of 121, 47%) compared with younger patients (128 of 231, 55%), the CR rate did not vary significantly with age in simple logistic regression analysis (P = .18).29 CR rate also decreased slightly but not significantly with increasing WBC counts in these patients (P = .12 and .18 without and with adjustment for the effect of FAB, respectively).
In simple logistic regression analyses, the CR rate was marginally significantly associated with MDR1 expression (P = .012) and with efflux (P = .039), with the CR rate decreasing as MDR1 expression or efflux increased (Table 5). These effects were largely caused by poor outcomes of patients with extremely high levels of MDR1 or efflux. For example, none of the 9 patients with extremely high MDR1 expression (MRK16 D ≥ 0.70) achieved CR; there was less variation of the CR rate over lower levels of MDR1 expression. The CR rate was not significantly associated with expression of LRP (P = .50) or MRP1 (P = .93; Table 5).
Treatment Outcomes by Expression of Multidrug Resistance Proteins MDR1, LRP, and MRP, CsA-Inhibited Functional Efflux, and CD34 Among 352 AML Patients
| . | No. of Patients . | Complete Remission (CR) . | Resistant Disease (RD) . | OS . | RFS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % CR . | 95% CI (%) . | P . | % RD . | 95% CI (%) . | P . | OS at 4 yr (%) . | 95% CI (%) . | P . | RFS at 4 yr (%) . | 95% CI (%) . | P . | ||
| MDR1 (MRK16) | |||||||||||||
| Bright | 64 | 48 | 36-61 | .0125-150 | 36 | 24-48 | .00075-150 | 20 | 10-30 | .47 | 23 | 8-37 | .775-150 |
| Moderate | 35 | 46 | 29-62 | 37 | 21-53 | 6 | 0-13 | 6 | 0-18 | ||||
| Dim | 24 | 63 | 43-82 | 25 | 8-42 | 17 | 3-30 | 20 | 0-40 | ||||
| Negative | 228 | 54 | 47-60 | 23 | 18-29 | 18 | 13-23 | 17 | 10-24 | ||||
| MDR1 (MM4.17) | |||||||||||||
| Bright | 89 | 44 | 34-54 | .0395-150 | 36 | 26-46 | .00925-150 | 17 | 9-25 | .47 | 23 | 10-36 | .865-150 |
| Moderate | 37 | 65 | 49-80 | 22 | 8-35 | 11 | 2-20 | 8 | 0-19 | ||||
| Dim | 25 | 44 | 25-63 | 40 | 21-59 | 28 | 10-46 | 36 | 8-65 | ||||
| Negative | 200 | 56 | 49-62 | 23 | 17-28 | 17 | 12-22 | 15 | 8-22 | ||||
| LRP56 | |||||||||||||
| Positive | 138 | 50 | 42-58 | .505-150 | 25 | 18-33 | .535-150 | 17 | 11-23 | .53 | 21 | 11-30 | .925-150 |
| Negative | 180 | 53 | 46-61 | 29 | 22-36 | 16 | 11-22 | 15 | 8-23 | ||||
| MRP1 | |||||||||||||
| Positive | 32 | 56 | 39-73 | .935-150 | 19 | 5-32 | .555-150 | 13 | 2-23 | .58 | 11 | 0-26 | .435-150 |
| Negative | 289 | 52 | 46-58 | 28 | 23-34 | 17 | 13-21 | 17 | 11-23 | ||||
| CsA-inhibited Di(OC)2efflux | |||||||||||||
| Bright | 87 | 39 | 29-49 | .0395-150 | 39 | 29-49 | 13 | 6-20 | .455-150 | 18 | 5-30 | .935-150 | |
| Moderate | 43 | 67 | 53-81 | 23 | 11-36 | 23 | 11-36 | 28 | 11-44 | ||||
| Dim | 12 | 50 | 22-78 | 50 | 22-78 | 8 | 0-24 | 17 | 0-46 | ||||
| Negative | 172 | 55 | 47-62 | 22 | 15-28 | 16 | 10-21 | 12 | 5-18 | ||||
| CsA-inhibited Rh123 efflux | |||||||||||||
| Bright | 70 | 37 | 26-48 | .0285-150 | 41 | 30-53 | .00365-150 | 13 | 5-21 | .36 | 23 | 7-39 | .835-150 |
| Moderate | 39 | 62 | 46-77 | 26 | 12-39 | 15 | 4-27 | 13 | 0-26 | ||||
| Dim | 12 | 58 | 30-86 | 25 | 1-49 | 33 | 7-60 | 43 | 6-80 | ||||
| Negative | 197 | 55 | 48-62 | 23 | 16-30 | 16 | 11-21 | 15 | 8-22 | ||||
| CD34 | |||||||||||||
| Pos (total) | 134 | 51 | 43-60 | .925-151 | 33 | 25-41 | .165-151 | 18 | 11-24 | .795-151 | 19 | 9-28 | .945-151 |
| Pos (sub) | 70 | 54 | 43-66 | 24 | 14-34 | 16 | 7-24 | 21 | 8-34 | ||||
| Weak/Neg | 147 | 53 | 45-61 | 23 | 16-30 | 17 | 11-23 | 14 | 7-22 | ||||
| . | No. of Patients . | Complete Remission (CR) . | Resistant Disease (RD) . | OS . | RFS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % CR . | 95% CI (%) . | P . | % RD . | 95% CI (%) . | P . | OS at 4 yr (%) . | 95% CI (%) . | P . | RFS at 4 yr (%) . | 95% CI (%) . | P . | ||
| MDR1 (MRK16) | |||||||||||||
| Bright | 64 | 48 | 36-61 | .0125-150 | 36 | 24-48 | .00075-150 | 20 | 10-30 | .47 | 23 | 8-37 | .775-150 |
| Moderate | 35 | 46 | 29-62 | 37 | 21-53 | 6 | 0-13 | 6 | 0-18 | ||||
| Dim | 24 | 63 | 43-82 | 25 | 8-42 | 17 | 3-30 | 20 | 0-40 | ||||
| Negative | 228 | 54 | 47-60 | 23 | 18-29 | 18 | 13-23 | 17 | 10-24 | ||||
| MDR1 (MM4.17) | |||||||||||||
| Bright | 89 | 44 | 34-54 | .0395-150 | 36 | 26-46 | .00925-150 | 17 | 9-25 | .47 | 23 | 10-36 | .865-150 |
| Moderate | 37 | 65 | 49-80 | 22 | 8-35 | 11 | 2-20 | 8 | 0-19 | ||||
| Dim | 25 | 44 | 25-63 | 40 | 21-59 | 28 | 10-46 | 36 | 8-65 | ||||
| Negative | 200 | 56 | 49-62 | 23 | 17-28 | 17 | 12-22 | 15 | 8-22 | ||||
| LRP56 | |||||||||||||
| Positive | 138 | 50 | 42-58 | .505-150 | 25 | 18-33 | .535-150 | 17 | 11-23 | .53 | 21 | 11-30 | .925-150 |
| Negative | 180 | 53 | 46-61 | 29 | 22-36 | 16 | 11-22 | 15 | 8-23 | ||||
| MRP1 | |||||||||||||
| Positive | 32 | 56 | 39-73 | .935-150 | 19 | 5-32 | .555-150 | 13 | 2-23 | .58 | 11 | 0-26 | .435-150 |
| Negative | 289 | 52 | 46-58 | 28 | 23-34 | 17 | 13-21 | 17 | 11-23 | ||||
| CsA-inhibited Di(OC)2efflux | |||||||||||||
| Bright | 87 | 39 | 29-49 | .0395-150 | 39 | 29-49 | 13 | 6-20 | .455-150 | 18 | 5-30 | .935-150 | |
| Moderate | 43 | 67 | 53-81 | 23 | 11-36 | 23 | 11-36 | 28 | 11-44 | ||||
| Dim | 12 | 50 | 22-78 | 50 | 22-78 | 8 | 0-24 | 17 | 0-46 | ||||
| Negative | 172 | 55 | 47-62 | 22 | 15-28 | 16 | 10-21 | 12 | 5-18 | ||||
| CsA-inhibited Rh123 efflux | |||||||||||||
| Bright | 70 | 37 | 26-48 | .0285-150 | 41 | 30-53 | .00365-150 | 13 | 5-21 | .36 | 23 | 7-39 | .835-150 |
| Moderate | 39 | 62 | 46-77 | 26 | 12-39 | 15 | 4-27 | 13 | 0-26 | ||||
| Dim | 12 | 58 | 30-86 | 25 | 1-49 | 33 | 7-60 | 43 | 6-80 | ||||
| Negative | 197 | 55 | 48-62 | 23 | 16-30 | 16 | 11-21 | 15 | 8-22 | ||||
| CD34 | |||||||||||||
| Pos (total) | 134 | 51 | 43-60 | .925-151 | 33 | 25-41 | .165-151 | 18 | 11-24 | .795-151 | 19 | 9-28 | .945-151 |
| Pos (sub) | 70 | 54 | 43-66 | 24 | 14-34 | 16 | 7-24 | 21 | 8-34 | ||||
| Weak/Neg | 147 | 53 | 45-61 | 23 | 16-30 | 17 | 11-23 | 14 | 7-22 | ||||
Two-tailed P value from simple logistic (CR, RD) or proportional hazards (OS, RFS) regression analysis with multidrug resistance or efflux treated as a quantitative variable.
P value for heterogeneity among the 3 indicated CD34 groups based on simple logistic (CR, RD) or proportional hazards (OS, RFS) regression analysis.
Because Ara-C is not transported by MDR1, and high doses of Ara-C could theoretically overcome MDR1-mediated drug resistance, the consequence of Ara-C dose on the effects of MDR1 expression on CR rate was also examined (Table 6). We first asked whether the distribution of MDR1 expression was similar in the conventional-dose and high-dose Ara-C groups and found that it was. We next asked whether the effect of MDR1 expression on CR rates differed in the two groups. There was no significant interaction between Ara-C dose (treated as a dichotomous variable) and MDR1 expression treated as a quantitative (continuous) variable (P = .67) on CR rate (Table 6). Thus, in the context of this clinical trial and our retrospective analysis, MDR1(+) patients were no more likely to achieve CR when treated with high-dose versus standard-dose Ara-C. This interaction remained nonsignificant after adjustment for the effects of FAB subtype (P = .74). Thus, there was no evidence that high-dose Ara-C had an effect on the influence of MDR1 expression on CR rate or resistant disease in the context of this clinical trial. Similar analyses using CsA-inhibited Di(OC)2 efflux rather than MDR1 (MRK16) expression gave similar results, ie, no significant interactions with the effects of Ara-C dose were observed (results not shown).
Response to Treatment by Ara-C Induction Dose and Expression of Multidrug Resistance Protein MDR1 Among 352 AML Patients
| . | Patients Randomized to SDAC . | Patients Randomized to HDAC . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients . | % CR . | 95% CI (%) . | % RD . | 95% CI (%) . | No. of Patients . | % CR . | 95% CI (%) . | % RD . | 95% CI (%) . | |
| MDR1 (MRK16) | ||||||||||
| Bright | 43 | 49 | 34-64 | 35 | 21-49 | 21 | 48 | 26-69 | 38 | 17-59 |
| Moderate | 26 | 46 | 27-65 | 42 | 23-61 | 9 | 44 | 14-79 | 22 | 3-60 |
| Dim | 16 | 69 | 41-85 | 25 | 7-52 | 8 | 50 | 16-84 | 25 | 3-65 |
| Negative | 160 | 52 | 44-60 | 25 | 18-32 | 68 | 59 | 47-71 | 19 | 10-28 |
| . | Patients Randomized to SDAC . | Patients Randomized to HDAC . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients . | % CR . | 95% CI (%) . | % RD . | 95% CI (%) . | No. of Patients . | % CR . | 95% CI (%) . | % RD . | 95% CI (%) . | |
| MDR1 (MRK16) | ||||||||||
| Bright | 43 | 49 | 34-64 | 35 | 21-49 | 21 | 48 | 26-69 | 38 | 17-59 |
| Moderate | 26 | 46 | 27-65 | 42 | 23-61 | 9 | 44 | 14-79 | 22 | 3-60 |
| Dim | 16 | 69 | 41-85 | 25 | 7-52 | 8 | 50 | 16-84 | 25 | 3-65 |
| Negative | 160 | 52 | 44-60 | 25 | 18-32 | 68 | 59 | 47-71 | 19 | 10-28 |
Multiple logistic regression analysis identified FAB subtype as the only clearly significant prognostic factor for CR (P = .0018). In particular, the CR rate tended to be relatively low for M1 and M3 (39 of 102, 38%), intermediate for M5 (15 of 31, 48%), and high for M2 and M4 (124 of 199, 62%; Table 7). As noted above, MDR1 expression and CsA-inhibited Di(OC)2efflux were also associated with M1 and M2 FAB subtypes. Therefore, the associations of MDR1, efflux, and other factors on CR rate were examined after adjustment for FAB subtype and were found to be essentially unchanged. For example, as shown in Table 7, the trend of lower CR rates in patients with MDR1 expression of functional efflux was generally consistent across FAB categories. Consequently adjusting the analyses of CR rates for the effect of FAB subtype had little impact on the effects of MDR, efflux, or CD34 [P values adjusted for FAB were P = .014 for MDR1 expression, .031 for CsA-inhibited Di(OC)2 efflux, .44 and .81 for LRP and MRP1 expression, and .88 for CD34; Table 7]. This suggests that the effects of MDR1 expression and CsA-inhibited efflux on response are independent of the effect of FAB type. Multiple logistic regression analysis showed no evidence for an additive effect on CR rate between expression of the different drug resistance markers, such as between MDR1 and LRP expression.
Correlation of FAB Subtype, MDR1 Expression, and Functional Efflux With Complete Remission Rate Among 352 Untreated AML Patients
| . | All Patients . | MDR1 (MRK16) Expression . | Efflux (DiOC2) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive (D ≥ 0.20) . | Negative/Dim (D < 0.20) . | Positive (D ≥ 0.20) . | Negative (D < 0.20) . | |||||||
| FAB | Patients | % CR | Patients | % CR | Patients | % CR | Patients | % CR | Patients | % CR |
| M1 | 72 | 38 | 29 | 31 | 43 | 42 | 37 | 24 | 30 | 47 |
| M2 | 145 | 63 | 52 | 56 | 93 | 68 | 73 | 58 | 59 | 69 |
| M3 | 30 | 40 | 2 | 100 | 28 | 36 | 1 | 0 | 24 | 46 |
| M4 | 54 | 59 | 8 | 50 | 46 | 61 | 17 | 71 | 29 | 52 |
| M5 | 31 | 48 | 1 | 100 | 30 | 47 | 4 | 75 | 23 | 43 |
| Other | 20 | 35 | 7 | 29 | 12 | 42 | 10 | 30 | 7 | 43 |
| Total | 352 | 53 | 99 | 47 | 252 | 55 | 142 | 49 | 172 | 55 |
| . | All Patients . | MDR1 (MRK16) Expression . | Efflux (DiOC2) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive (D ≥ 0.20) . | Negative/Dim (D < 0.20) . | Positive (D ≥ 0.20) . | Negative (D < 0.20) . | |||||||
| FAB | Patients | % CR | Patients | % CR | Patients | % CR | Patients | % CR | Patients | % CR |
| M1 | 72 | 38 | 29 | 31 | 43 | 42 | 37 | 24 | 30 | 47 |
| M2 | 145 | 63 | 52 | 56 | 93 | 68 | 73 | 58 | 59 | 69 |
| M3 | 30 | 40 | 2 | 100 | 28 | 36 | 1 | 0 | 24 | 46 |
| M4 | 54 | 59 | 8 | 50 | 46 | 61 | 17 | 71 | 29 | 52 |
| M5 | 31 | 48 | 1 | 100 | 30 | 47 | 4 | 75 | 23 | 43 |
| Other | 20 | 35 | 7 | 29 | 12 | 42 | 10 | 30 | 7 | 43 |
| Total | 352 | 53 | 99 | 47 | 252 | 55 | 142 | 49 | 172 | 55 |
The CR rate did not vary significantly among the 3 cytogenetic categories (P = .49). However, because only 78 (22%) of the 352 cases were classified cytogenetically, the small number of patients analyzed precludes this as strong evidence against an effect of cytogenetics on CR.
Correlation with resistant disease.
Ninety-five (27%) of the 352 patients had disease that was resistant to induction therapy. This includes 27 who achieved only partial response (including 10 after 2 induction attempts) and 68 with stable disease (40 after 2 induction attempts). Simple logistic regression analyses of resistant disease were generally complementary to the corresponding analyses of CR. The resistant disease rate increased with increasing MDR1 (detected by MRK16) expression (P = .0007) and CsA-inhibited Di(OC)2 efflux (P = .0092), but not with expression of LRP (P = .53) or MRP1 (P = .55), or CD34 (P = .16; Table 5). Very similar results were obtained when patients with partial response were excluded from the resistant disease group (data not shown). Resistant disease was also correlated with FAB subtype (P = .0097), being more frequent in M1 (30 of 72 or 42%) compared with all others (65 of 280 or 23%). As noted above, M1 was also associated with greater MDR1 expression and CsA-inhibited Di(OC)2 efflux. However, even after adjusting for the effects of FAB in multiple logistic regression analysis, resistant disease was still correlated to MDR1/MRK16 expression (P = .0069), although interestingly not to CsA-inhibited Di(OC)2 efflux (P = .104). When the effect of high Ara-C dose on resistant disease was examined, there was no significant interaction between Ara-C dose and MDR1 expression and resistant disease (P = .97 without adjustment for FAB, .95 after adjustment for FAB). The RD rate increased slightly but not significantly with increasing WBC count (P = .70 and .93 without and with adjustment for the effect of FAB, respectively).
Correlation with OS and RFS.
Three hundred of the 352 patients have died and the remaining 52 have been in follow-up a median 6.0 years (range, 8 months to 9.4 years). The estimated median survival was 11.7 months (95% CI, 9.4 to 13.5 months). OS was significantly associated with 3 factors in multiple proportional hazards regression analysis; OS decreased significantly with increasing patient age (P < .0001), increasing peripheral blood blast count (P = .0077), and varied significantly among FAB categories (P < .0001). After adjusting for these factors there was no association between OS and MDR1 (P = .59), MRP1 (P = .81), LRP (P = .53), CsA-inhibited efflux (P = .23), or CD34 expression (P = .93; Table 5). The estimated distributions of OS are shown by MDR1 expression in Fig 1. There was no significant interaction between the effects of Ara-C dose and MDR1 expression in the analysis of OS, either without (P = .90) or with (P = .63) adjustment for the effects of age, blast count, and FAB category. OS decreased slightly with increasing WBC count; this was marginally significant in the simple proportional hazards regression analysis (P = .028), but not after adjusting for the effects of age, peripheral blast count, and FAB (P = .38).
Of the 185 patients who achieved CR, 133 have relapsed and another 21 died without report of relapse. The estimated median RFS was 8.5 months (95% CI, 7.2 to 9.7 months). Only 1 clearly significant prognostic factor was identified in multivariate proportional hazards regression analysis of RFS: RFS decreased significantly with increasing age (P = .0009). After adjusting for the effect of age, there was no evidence that any of the MDR markers had any effect on RFS (MDR1:P = .75; MRP1: P = .25; LRP: P = .68; CsA-inhibited efflux: P = .79; CD34: P = .83). There was no significant interaction between the effects of Ara-C dose and MDR1 expression in the analysis of RFS, either without (P = .32) or with (P = .44) adjustment for the effect of age. RFS did not vary significantly in relation to WBC count (P = .95 and .93 without or with adjustment for the effect of FAB, respectively).
Among the 78 patients with cytogenetic data, there was marginally significant heterogeneity of OS among the 3 groups (P = .038). This was largely due to poor survival in the unfavorable category: the estimated hazard ratios (using the favorable group as the baseline) were 0.94 (95% CI, 0.51 to 1.74) for intermediate and 2.01 (95% CI, 1.03 to 3.92) for unfavorable.
DISCUSSION
This biologic correlative study of 352 relatively young adult AML patients treated on a single clinical trial provides further confirmatory data that expression of MDR1/P-glycoprotein is associated with a lower rate of complete response to induction chemotherapy and with a higher rate of resistant disease. In contrast, our studies found no evidence that expression of the alternative MDR-associated proteins MRP1 or LRP were associated with a worse outcome in AML.
The significant association observed between MDR1 expression and functional efflux and both CR rate and resistant disease in this younger group of AML patients compliments and extends our previous studies in older AML patients8 and confirms the findings of several other groups.8-14,25,54 This consistent association now observed between MDR1 expression and a poorer clinical outcome in AML supports the idea that MDR1 is an important biologic factor in AML, and that therapy with MDR1 modulators may indeed ultimately benefit patients with MDR1(+) disease. However, compared with our studies in older AML patients, we noted interesting differences in this younger cohort. Only 35% of these younger patients were MDR1(+) and 41% were efflux(+). These data stand in contrast to our studies in older patients where 71% of cases were MDR1(+) and 58% showed functional drug efflux.8 However, it is interesting that in this study of younger patients, the frequency of MDR expression increased by decade, from a frequency of only 17% in patients less than 35 years of age to a frequency of 39% in patients 50 years of age. In addition, MDR1 expression was not as strongly associated with clinical response among the 352 younger patients as it was among the older patient group. In the younger patients, the CR rate for patients with moderate to strong MDR1 expression was 47% in comparison to 55% in those with weak or no MDR1 expression, compared with CR rates of 34% and 59% among the same MDR groups in our study of elderly patients. Moreover among these younger patients, the reduction in the CR rate was largely confined to those with extremely high MDR1 expression while the older patients showed a more consistent trend of decreasing CR rate with increasing MDR1. This trial included patients randomized to receive either high-dose or standard-dose Ara-C as part of the induction regimen. Because Ara-C is not transported by MDR1, we would have expected that the effects of MDR1 expression would be abrogated in those patients treated with high-dose Ara-C, and that CR rate would thus be similar in this group regardless of MDR1 expression. Rather surprisingly, this was not the case. MDR1 expression influenced achievement of CR, both among patients treated with high-dose and standard-dose Ara-C. The reason for this result is not clear, but suggests that possibly other resistance mechanisms associated with MDR1 may also play a role in AML in these patients.
Similar to our previous study on elderly AML, we found no significant association of OS or RFS with MDR1 expression. This is in contrast to the findings of other groups who have found an association between MDR1 expression and OS. The reasons for these differences are unclear. The extensive use of high-dose Ara-C in this study could have blunted any effect of MDR1 on survival. Alternatively, other age-related mechanisms may play a part in relapse in these patients. Among these younger AML patients, both OS and RFS decreased significantly with increasing age. Our current studies failed to identify any resistance mechanism that would explain this increasingly worse outcome with advancing age. In fact, our studies appear to rule out 2 possible alternatives, LRP and MRP1, because neither of these MDR-associated proteins was significantly associated with CR rate or OS or RFS in this study.
In this study, and in contrast to our previous work in elderly AML, the morphologic categorization of a patient’s leukemia according to the FAB criteria was significantly associated with CR rate and OS. The reasons for this are not entirely clear, although there are some hints that this association may occur because FAB subtype is associated with distinct biologic AML subsets. For example, FAB subtypes M1 and M3 were associated with the worst CR rate; FAB M1 in this study was associated with MDR1 expression while AML M3 is already a well-defined biologic entity associated with an underlying t(15;17) and a distinct clinical course. Thus, the poor prognosis of FAB M1 cases may be explained partly by MDR1 expression. The lower CR rate in M3 patients may be, in part, explained by the use of a relatively low dose of daunomycin in this trial conducted in the pre-ATRA era.28 Interestingly, as reported by others, the M3 AML cases in this study showed a very low frequency (7%) of MDR1 expression.55 This may in part explain the steep dose-response curve to daunomycin found in this FAB subtype.56 The results of our current trials and those of others where both cytogenetics and MDR phenotype are being analyzed will be helpful in better delineating the underlying biologic mechanisms that may be associated with poor response in certain FAB groups.
Although the alternative MDR-associated protein LRP was expressed in 43% of our AML cases, we found no correlation between LRP expression and clinical outcome. In contrast to MDR1 (which is more frequently associated with unfavorable cytogenetics,8 the FAB AML M1 subtype, and increasing age), LRP expression was associated with more favorable cytogenetic abnormalities and was more frequently expressed in AML cases with a more differentiated morphology (FAB AML M4 or M5). The 43% frequency of LRP expression that we observed is quite similar to the range of 35% to 48% reported by other investigators studying smaller numbers of AML patients at diagnosis and relapse.21,22,57 Using similar flow cytometric methodology to our own, Legrand et al28 recently found no correlation between LRP expression and clinical outcome in a series of 53 AML patients. In contrast, using immunohistochemical techniques (IHC), both List et al21 and Filipits et al22 found that LRP expression was associated with a poorer clinical response in AML. Whether the discrepant findings in these clinical studies arise due to differences in methodology to detect LRP (flow cytometry vIHC), differences in the types of patients studied (initial diagnosisv relapse) or through correlations of LRP with other factors is presently unclear. The use of different detection methods certainly may play a role. Although detection of LRP expression using IHC and flow cytometry is highly correlated in cell lines, there is only a weak correlation between the 2 methods when tested on primary leukemia samples.58 A second explanation for these discrepant findings may be related to the correlation of LRP expression with a variety of other possible prognostic factors in AML. Both our study and that of Filipits et al found that LRP expression increases significantly with increasing WBC count and blast cell count, 2 factors associated with poor prognosis in some AML studies. However, because WBC count is not uniformly found to be prognostically significant, and in fact in both these studies was not correlated with outcome, this cannot be the only explanation. A second possibility may be differences in the association of LRP with cytogenetic factors in different studies. In our study, LRP expression was marginally associated with favorable cytogenetics, while other studies have found either no association or a trend between LRP expression and less favorable karyotype. The reason for these differences is currently unclear.21 26
Of interest in this study was the identification of a distinct group of AML patients (18% of the total cases studied in this report) who demonstrated CSA-resistant efflux that was not correlated with MDR1, MRP1, or LRP expression. The mechanism of efflux in these cases is unclear, but may be related to expression of other newly described members of the adenosine triphosphate (ATP)-binding cassette (ABC) family of transporters.59-62 We are currently investigating the mechanism of efflux in these patients and the effects of other MDR-reversing agents. Clearly, further studies of the biologic and clinical significance of other new ABC transporters in AML samples are highly warranted.
Our study is the largest to our knowledge to examine the frequency of expression and clinical significance of MRP1 in AML samples. The low frequency of 10% MRP1 expression in these previously untreated AML cases is quite similar to that of smaller studies on untreated AML patients, where the frequencies of MRP1 expression have ranged from fewer than 10% to 30%.23-25,27,63-65 Our study on 352 patients found no relationship between MRP1 expression and clinical response, corroborating the largest clinical study reported to date on 80 de novo AML patients.23 Other studies on smaller numbers of heterogeneous AML patients subtypes using different methodologies have shown conflicting results.24-27 Interestingly, in the study of Legrand et al,28 MRP1 protein expression was not significantly associated with clinical response while calcein-AM efflux (attributed to, but not necessarily specific for, MRP1) was correlated with clinical response. These studies and our own point to the need to develop highly sensitive and specific assays to distinguish efflux mediated by different MDR-conferring proteins in future clinical correlative studies.
In summary, our current study again confirms the clinical relevance of MDR1 expression and functional CsA-inhibited drug efflux in AML. Because over a third of the younger AML patients that we have reported had MDR1(+) disease, incorporation of MDR1 modulators may benefit a significant fraction of these patients. In contrast, the clinical relevance of both LRP and MRP1 expression are less clear and our studies imply they may lack clinical significance in AML. Further clinical correlative studies incorporating measurements of MDR protein expression and novel functional assays on large numbers of uniformly treated AML patients may help us reach a better understanding of the role of these and other novel alternative drug-resistance mechanisms in AML.
Supported by Department of Health and Human Services (DHHS) National Institutes of Health (NIH) grants to the SWOG Leukemia Biology and Cytogenetics Programs (NIH U10 CA 32102) and the State of New Mexico Dedicated Health Research Fund. C.P.L is supported in part by a Translational Research Award from the Leukemia Society of America. M.L.S is supported in part by CA33572 and CA30206 [Hem/BMT grant].
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Southwest Oncology Group Operations Office, 14980 Omicron Dr, San Antonio, TX 78245-3218.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal