Members of the Bcl-2 gene family have been implicated in the regulation of cell death induced by cytostatic drugs. In some malignancies such as B-cell lymphoma, there is evidence that high expression of Bcl-2 is an independent negative prognostic marker and the overexpression of Bcl-2 has been shown to confer resistance to cytotoxic drugs by preventing drug-induced apoptosis. This function of Bcl-2 can be antagonized by apoptosis-promoting members of the Bcl-2 family. We previously showed that overexpression of Bax restores the chemosensitivity of Bax-deficient breast cancer cell lines. Therefore, we investigated whether the death-promoting Bcl-2 homologue Bik/Nbk can enhance cytostatic drug-induced apoptosis. As a model, we used the T-cell leukemia H9 (CD3+ and CD4+CD8−), which is resistant to corticosteroid-induced cell death and does not express endogenous Bik/Nbk. Sensitivity for drug-induced apoptosis was increased 10- to 39-fold in cells transfected with the full-length coding sequence of Bik/Nbk. In addition, apoptosis induced via CD95/Fas or heat shock was increased to a similar extent. These data show that Bik/Nbk, which, unlike Bax, carries only a BH3 but no BH1 or BH2 domain may be a target to enhance chemosensitivity. The complete suppression of tumor growth in a severe combined immunodeficient mouse xenotransplant model suggests that, in analogy to Bax, Bik/Nbk may function as a tumor suppressor gene.
MEMBERS OF THE BCL-2 FAMILY are key regulators of apoptosis and their deregulation has been implicated in the development of malignancy1 and the resistance of tumor cells to cytostatic drug-induced cell death.2,3 Previously, we have shown that the proapoptotic gene Bax, if overexpressed in breast cancer cells, which lack endogenous Bax,4 blocks the ability of these cells to form tumors in severe combined immunodeficient (SCID) mice.5 In addition, Bax confers to these cells increased drug sensitivity.2 In metastatic colorectal, cancer, we recently described that the lack of Bax is a negative prognostic factor, especially in those patients carrying a wild-type p53 gene.6
In this report, we have examined the effect of another member of the Bcl-2 family on drug sensitivity in the mature T-ALL (acute lymphoblastic/lymphocytic leukemia) cell line H9 (CD3+ and CD4+CD8−). Bik/Nbk7,8 is, like Bax9 and Bak,10 a BH3 containing member of the Bcl-2 family and is expressed only in a restricted subset of human tissues, as we show in the present work. This suggests that Bik/Nbk may play a role in the tissue specific regulation of apoptosis. To investigate whether Bik/Nbk enhanced the sensitivity to apoptosis of H9 cells, we generated stable transfectants expressing Bik/Nbk. (The H9 parental cell line does not express Bik/Nbk). Interestingly, the H9 T-lymphoma cells are refractory to corticosteroid-induced cell death, which appears to be a negative prognostic factor in childhood11,12 and possibly as well in adult13acute lymphoblastic leukemia.
The transfectants showed an increased sensitivity to Fas-triggered death and apoptosis after exposure to anticancer drugs or heat shock. Moreover, their ability to form tumors in vivo was completely suppressed.
These experiments have, therefore, helped us to understand the role of Bik/Nbk in the control of apoptosis and determine that Bik/Nbk preferentially enhances cell death induced on DNA damage or Fas-triggering. Therefore, this “minimal” death module, which, unlike other Bcl-2 homologues, expresses only the BH3 Bcl-2-homology domain, may be employed not only to further investigate the mechanisms of apoptosis induction but may also provide a target to enhance chemosensitivity of cancer cells.
MATERIALS AND METHODS
Cell culture and transfection.
All cells were maintained in 1640 RPMI (Seromed-Biochrom, Hamburg, Germany), 10% heat-inactivated fetal calf serum (GIBCO-BRL, Karlsruhe, Germany), 2 mmol/L L-Glutamine (GIBCO-BRL, Karlsruhe, Germany), and penicilline-streptomycin (Seromed-Biochrom, Hamburg, Germany) as described.14
Bik/Nbk cDNA was cloned as a 1 kilobase (kb) Bgl II fragment into the mammalian expression vector pCIN4, a derivative of pCIN1/pIRESneo15 (Clontech Lab, San Diego, CA). This plasmid (30 μg) was transfected into H9 cells (CD3+, CD4+CD8−) by electroporation with a Bio-Rad electroporator at 960 μF/250V. Stable clones were generated by limiting dilution into 96-well flat-bottomed plates with selection in normal medium containing 1 mg/mL G418-sulphate (GIBCO-BRL). Colonies were diluted into larger well and flasks and expanded for analysis. pCIN4 mock transfectants (H9 3D8) were generated in parallel and displayed the same apoptotic behavior as the parental line.
Western blot analysis.
Cells in log phase were harvested and lysed in sodium dodecyl sulfate (SDS) sample buffer containing 10% β-mercaptoethanol, then boiled for 5 minutes and sonicated (4 × 5-second cycles at 10 μm amplitude in an MSE soniprep 150 sonicator). Proteins were separated on Tris-glycine 4% to 20% gradient acrylamide gel and blotted onto Hybond enhanced chemoluminescence (ECL) nitrocellulose membrane (Amersham, Braunschweig, Germany). Immunodetection was performed at 4°C by using a polyclonal goat anti-Nbk antibody (Santa-Cruz, Santa Cruz, CA) at 1 μg/mL and visualized by using the ECL detection system (Amersham), according to the manufacturer’s instructions. Glucocorticoid receptor (GR) protein was detected as described above by the use of a polyclonal rabbit antiserum (diluted 1:1,000; Santa-Cruz), which recognized the N-terminus of both GR-alpha (95 kD) and GR-beta (90 kD).
Northern blot analysis.
Human multiple tissue Northern blots (Clontech) were hybridized with a32P-α-dCTP (Amersham) random labeled 423bpBamH1-Sma1 fragment of Bik/Nbk in 50% formamide buffer at 43°C for 24 hours, followed by washes in 0.1% SDS 0.1 × standard salt concentration (SSC) buffer at 50°C. The blots were exposed for 10 days with intensifying screens at −70°C.
RNA preparation, polymerase chain reaction (PCR), and Southern blot analysis.
Total RNA was purified from 5 × 106 cells by using the RNAzolB method.16 RNA (4 μg) was used for first strand cDNA synthesis with the GeneAmp RNA PCR kit (Perkin Elmer, Weiterstadt, Germany), according to the manufacturer’s instructions. PCR was performed by using a vector specific T7 forward primer: 5′-TAA-TAC-GAC-TCA-CTA-TAG-GG-3′ and a Bik/Nbk specific reverse primer: 5′-TTC-CAA-AGA-ATC-GAA-GTC-CT-3′ in PCR buffer containing 20 pmol of each primer, 1.5 mmol/L MgCl2, 200 mmol/L dNTPs, and 2.5 units Taq polymerase. Cycling conditions were 30 cycles (94°C, 30 seconds; 60.5°C, 1 minute; 72°C, 1 minute). The PCR fragments were analyzed on a 1.2% gel and identified as Bik/Nbk by Southern blotting onto Hybond N+ membrane (Amersham) and high stringency hybridization using a 32P-γ-ATP labeled oligonucleotide corresponding to the 5′ terminus of Bik/Nbk (4 to 28 bp inclusive).
Measurement of apoptosis.
After induction of apoptosis by cytotoxic drugs, anti-APO-1 IgG3, anti-CD3, dexamethasone, or heat shock for 24 to 72 hours, the cells were pelleted, washed with ice-cold phosphate-buffered saline in U-form 96-well plates and gently resuspended in 300 μL hypotonic fluorochrome solution (propidium iodide 50 μg/mL, 0.1 mol/L sodium citrate plus 0.1% Triton ×100) as previously described.14,17 After overnight incubation at 4°C in the dark, the propidium-iodide content of the individual nuclei was measured on a FACSort (Becton Dickinson, Heidelberg, Germany). Cell debris was excluded by raising the forward-scatter threshold adequately. Apoptotic nuclei displayed a decreased DNA content below the G1 peak, paralleled by an increase of the side scatter. In addition, apoptosis was measured in the dexamethasone-induced cultures by assessing the decrease of cell size (forward light scatter signal, FSC) and the increase of membrane permeability (uptake of propidium iodide, 1 μg/mL) during late stage apoptosis as described.18
Animal experiments.
Animals were kept and treated in accordance with German animal protection laws. C.B-17 scid/scid mice (SCID mice) were from our own breeding colony. The mice were kept in isolators under gnotobiotic conditions. Food and water were autoclaved. The mice were not subjected to antibiotic drug treatment. No mouse pathogens were detected. For serology, sterile sentinel mice were added to the colony. These mice were serologically negative for Sendai, PVM, MVM, Reo3, MHV (Corona), Theiler’s GD VII, Polyoma, K-Adeno, and m.-Adeno virus. Leakiness was determined by measuring mouse Igmol/L and mouse IgG, and mice expressing serum titers above 50 μg/mL IgM or IgG were excluded from the study. H9 lymphoma cells were injected s.c. into the inguinal region (107 per animal). Tumor diameters were measured with calipers in two dimensions and the tumor volume was calculated as described.19
RESULTS
Resistance to cytostatic drug-induced apoptosis is the major obstacle for the cure of lymphoid malignancies. In this regard, the resistance to corticosteroid-induced death appears to be a prominent negative clinical prognostic factor in acute lymphoblastic leukemia.11,12 In this line, bcl-2 and bcl-xL have been shown to prevent cytostatic drug-induced cell death.3Therefore, we were interested in testing whether the expression of the death promoting Bcl-2 homologue Bik/Nbk may enhance sensitivity for drug-induced apoptosis in the T-lymphoma cell line H9, which is refractory to corticosteroid-triggered cell death.
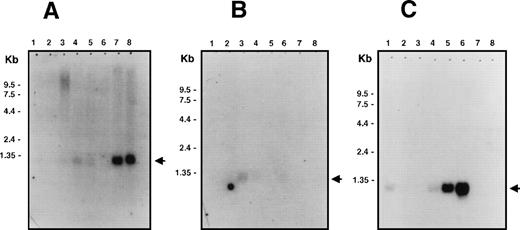
Northern blot analysis showed that Bik/Nbk has a restricted tissue distribution with expression being detected mainly in epithelial cells (Fig 1). High levels of expression are seen in the kidneys and pancreas (Fig 1A) with lower levels in placenta, lungs, liver (Fig 1A), prostate, and testis (Fig 1B). No hybridization was seen in the heart, brain, skeletal muscle, spleen, thymus, ovary, small intestine, colon, and peripheral blood leukocytes (Fig 1A and B). The cell lines Raji (EBV-positive type III Burkitt lymphoma) and SW480 (colon adenocarcinoma) had the highest levels of mRNA expression with lower levels in HL-60 and MOLT-4 cells (Fig 1C). Thus, in contrast to the lack of Bik/Nbk expression in nonmalignant lymphoid or colon tissue, Bik/Nbk mRNA appears to be expressed ectopically in some tumor-derived cell lines.
Multiple tissue Northern blot analysis for Bik/Nbk expression. PolyA+ RNA (2 μg) were loaded per lane. Arrows indicate the position of the 1.1 kb Bik/Nbk transcript. (A) Multiple human tissue Northern blot analysis. Lane 1, heart; 2, brain; 3, placenta; 4, lung; 5, liver; 6, skeletal muscle; 7, kidney; 8, pancreas. (B) Lane 1, spleen; 2, thymus; 3, prostate; 4, testis; 5, ovary; 6, small intestine; 7, colon; 8, peripheral blood leukocytes. (C) Human cell lines Northern blot analysis. Lane 1, promyelocytic leukemia HL60; 2, cervix carcinoma HeLa S3; 3, erythroid leukemia K562; 4, T-cell leukemia MOLT4; 5, type III Burkitt lymphoma Raji; 6, colorectal adenocarcinoma SW480; 7, lung carcinoma A549; 8, melanoma G361.
Multiple tissue Northern blot analysis for Bik/Nbk expression. PolyA+ RNA (2 μg) were loaded per lane. Arrows indicate the position of the 1.1 kb Bik/Nbk transcript. (A) Multiple human tissue Northern blot analysis. Lane 1, heart; 2, brain; 3, placenta; 4, lung; 5, liver; 6, skeletal muscle; 7, kidney; 8, pancreas. (B) Lane 1, spleen; 2, thymus; 3, prostate; 4, testis; 5, ovary; 6, small intestine; 7, colon; 8, peripheral blood leukocytes. (C) Human cell lines Northern blot analysis. Lane 1, promyelocytic leukemia HL60; 2, cervix carcinoma HeLa S3; 3, erythroid leukemia K562; 4, T-cell leukemia MOLT4; 5, type III Burkitt lymphoma Raji; 6, colorectal adenocarcinoma SW480; 7, lung carcinoma A549; 8, melanoma G361.
With regard to the above expression pattern where Bik/Nbk was detected preferentially in the epithelial but not the lymphoid compartment (disregarding the ectopic expression in Raji cells), a lymphoid cell system appeared suitable for the functional analysis of Bik/Nbk. The ability of Bik/Nbk to enhance sensitivity for drug-induced apoptosis was, therefore, assessed in the corticosteroid-resistant H9 T-ALL cell line, which does not express detectable levels of endogenous Bik/Nbk as shown by Western blot analysis (Fig 2B).
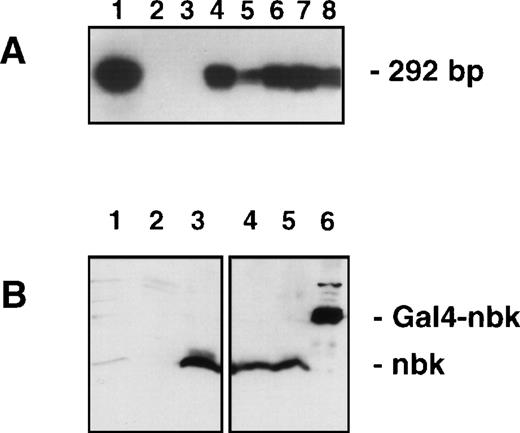
Analysis for Bik/Nbk expression in transfected H9 T-lymphoma cells. (A) RT-PCR and Southern blot: The exogenous mRNA transcript was detected by RT-PCR with primers designed to vector specific forward sequence and Bik/Nbk specific reverse sequence, yielding a 292-bp fragment. DNA was electrophoresed, Southern blotted and hybridized by the use of a third, radiolabeled oligonucleotide specific for the 5′ terminus of Bik/Nbk. Positive and negative controls were pCIN.Nbk plasmid (lane 1) and water (lane 2). Lane 3, H9 3D8 control transfected cells; lane 4, clone no. 2; lane 5, clone no. 7; lane 6, clone no. 10; lane 7, clone no. 13; lane 8, clone no. 16. (B) Western blot analysis for Bik/Nbk expression: Protein extracts from SW480 colon carcinoma cells (lane 1), H9 3D8 control cells (lane 2) or Bik/Nbk transfected clones no. 2 (lanes 3 and 4) and 10 (lane 5) were separated by SDS-polyacrylamide gel electrophoresis and Western blot analysis with a goat antiserum against Bik/Nbk. Yeast expressed Bik/Nbk Gal4-Nbk fusion protein served as a positive control (lane 6). Bands were visualized by means of ECL.
Analysis for Bik/Nbk expression in transfected H9 T-lymphoma cells. (A) RT-PCR and Southern blot: The exogenous mRNA transcript was detected by RT-PCR with primers designed to vector specific forward sequence and Bik/Nbk specific reverse sequence, yielding a 292-bp fragment. DNA was electrophoresed, Southern blotted and hybridized by the use of a third, radiolabeled oligonucleotide specific for the 5′ terminus of Bik/Nbk. Positive and negative controls were pCIN.Nbk plasmid (lane 1) and water (lane 2). Lane 3, H9 3D8 control transfected cells; lane 4, clone no. 2; lane 5, clone no. 7; lane 6, clone no. 10; lane 7, clone no. 13; lane 8, clone no. 16. (B) Western blot analysis for Bik/Nbk expression: Protein extracts from SW480 colon carcinoma cells (lane 1), H9 3D8 control cells (lane 2) or Bik/Nbk transfected clones no. 2 (lanes 3 and 4) and 10 (lane 5) were separated by SDS-polyacrylamide gel electrophoresis and Western blot analysis with a goat antiserum against Bik/Nbk. Yeast expressed Bik/Nbk Gal4-Nbk fusion protein served as a positive control (lane 6). Bands were visualized by means of ECL.
Therefore, the full-length cDNA of Bik/Nbk was cloned into the pCIN4 vector (pCIN4.Nbk) and stably overexpressed in the H9 cells. Clones selected for resistance to G418 and isolated by limiting dilution were screened for transgene expression by reverse transcription (RT)-PCR followed by Southern blot analysis. In Fig 2A, no exogenous Bik/Nbk is detected in the mock transfectants (H9 3D8), whereas a signal is detected in the pCIN4.Nbk transfectants. The clones no. 2, 10, and 16 showed a strong Bik/Nbk hybridization signal and were subjected to further functional assays as described below.
Protein expression was determined by Western blot analysis. In the H9 3D8 control cells no Bik/Nbk could be detected (Fig 2B). In contrast, the clones nos. 2 and 10 show overexpression of the 22.5 kD Bik/Nbk protein. A Gal4-Nbk fusion protein expressed in yeast served as a positive control. However, the colon adenocarcinoma cell line SW480, which shows strong expression of Bik/Nbk RNA (Fig 1C), showed only weak, but detectable Bik/Nbk endogenous protein expression (Fig 2B).
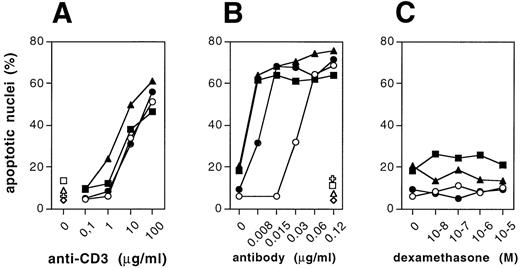
To test for the effect of Bik/Nbk on apoptosis sensitivity, the transfectants were exposed to the cytostatic drugs etoposide, epirubicin, and taxol. After a 72-hour culture, apoptosis was assessed on the single cell level by flow cytometric measurement of the nuclear DNA content. All three clones showed an increased susceptibility for drug-induced apoptosis. Sensitivity for all three drugs was strongly enhanced as compared with the control cells (Fig 3A through C). Comparison of the ED50 concentrations for apoptosis induction shows a 10.1- to 39.3-fold sensitization for drug-induced apoptosis in the Bik/Nbk transfectants as compared with the H9 mock transfectants (Table1).
Sensitization of H9 cells for drug-induced cell death. Cells were exposed to cytostatic drugs for 72 hours. Apoptosis was determined on the single cell level by measuring the DNA content of individual nuclei by flow cytometry. Hypodiploid nuclei were considered as apoptotic. (A) Epirubicin, (B) etoposide, (C) taxol. Cell culture in the presence of the drugs (). Medium controls (only in [A]) ().
Sensitization of H9 cells for drug-induced cell death. Cells were exposed to cytostatic drugs for 72 hours. Apoptosis was determined on the single cell level by measuring the DNA content of individual nuclei by flow cytometry. Hypodiploid nuclei were considered as apoptotic. (A) Epirubicin, (B) etoposide, (C) taxol. Cell culture in the presence of the drugs (). Medium controls (only in [A]) ().
ED50 Concentrations for Drug-Induced Apoptosis
| Cell Type . | ED50 Concentration ± SD . | ||
|---|---|---|---|
| Epirubicin (ng/mL) . | Etoposide (ng/mL) . | Paclitaxel (ng/mL) . | |
| H9 3D8 mock | 51.3 ± 2.3 | 710.8 ± 37.4 | 7.4 ± 1.2 |
| nbk clone no. 2 | 4.39 ± 0.92 | 52.1 ± 5.5 | 0.73 ± 0.9 |
| (11.7) | (13.6) | (10.1) | |
| nbk clone no. 10 | 4.1 ± 1.3 | 18.1 ± 2.3 | 0.35 ± 0.8 |
| (12.5) | (39.3) | (21.14) | |
| nbk clone no. 16 | 4.2 ± 0.3 | 38.9 ± 7.3 | 0.41 ± 0.8 |
| (12.2) | (18.3) | (18) | |
| Cell Type . | ED50 Concentration ± SD . | ||
|---|---|---|---|
| Epirubicin (ng/mL) . | Etoposide (ng/mL) . | Paclitaxel (ng/mL) . | |
| H9 3D8 mock | 51.3 ± 2.3 | 710.8 ± 37.4 | 7.4 ± 1.2 |
| nbk clone no. 2 | 4.39 ± 0.92 | 52.1 ± 5.5 | 0.73 ± 0.9 |
| (11.7) | (13.6) | (10.1) | |
| nbk clone no. 10 | 4.1 ± 1.3 | 18.1 ± 2.3 | 0.35 ± 0.8 |
| (12.5) | (39.3) | (21.14) | |
| nbk clone no. 16 | 4.2 ± 0.3 | 38.9 ± 7.3 | 0.41 ± 0.8 |
| (12.2) | (18.3) | (18) | |
Cells were exposed to cytostatic drugs for 72 hours. Apoptosis was determined on the single cell level by measuring the DNA content of individual nuclei by flow cytometry. Numbers in brackets indicate the factor of sensitization as compared with the control cells. Data represent the mean of triplicates ± SD.
In T cells, the CD95 death receptor and its ligand have been implicated in the control of apoptosis.14,17,20,21 In addition, drug-induced apoptosis was suggested to depend, in part, on activation of the Fas ligand and subsequent CD95/Fas ligation. Therefore, we assessed the effect of Bik/Nbk on CD95 triggered death of the H9 T cells. CD95/Fas-triggered death was augmented in all three clones (Fig4B), whereas activation-induced death on crosslinking of the CD3ε chain of the T-cell receptor was only marginally enhanced (Fig 4A). The CD95 or CD3 ε-chain receptor densities in the transfectants were measured by flow cytometry and showed no significant difference (data not shown). As in the case of drug-mediated apoptosis, the Bik/Nbk transfectants showed an increased apoptosis susceptibility on ligation of the CD95/Fas death receptor by agonistic anti-CD95 mab (anti-APO-1 IgG319) as compared with cultures exposed to an isotype matched control mab (FII23c19).
Sensitization of H9 cells for cell death induced by Fas/CD95, CD3− crosslinking, or dexamethasone. (A) Activation-induced cell death on CD3 cross-linking by immobilized anti-CD3 monoclonal antibody (MoAb) (clone OKT3). Plates were coated with OKT3 (coating concentration 0.1 to 100 μg/mL) as described.14,17 (B) Induction of cell death by CD95/Fas triggering by (soluble) anti-CD95 MoAb (clone anti-APO-1 IgG3). (C) Cell death induction by dexamethasone, which was added to the cultures at concentrations from 10−8 mol/L to 10−5mol/L. Cell death was determined on the single-cell level by measuring the DNA content of individual nuclei by flow cytometry. Data represent the mean of triplicates ± SD. (A through C) H9 control cells (○), Bik/Nbk clone no. 2 (◍), Bik/Nbk clone no. 10 (▩), Bik/Nbk clone no. 16 (▴). Medium control in (A) or cultures incubated with FII23c isotype-matched control antibody (0.12 μg/mL19) in (B): H9 control cells (◊), Bik/Nbk clone no. 2 (✙), Bik/Nbk clone no. 10 (□), Bik/Nbk clone no. 16 (▵).
Sensitization of H9 cells for cell death induced by Fas/CD95, CD3− crosslinking, or dexamethasone. (A) Activation-induced cell death on CD3 cross-linking by immobilized anti-CD3 monoclonal antibody (MoAb) (clone OKT3). Plates were coated with OKT3 (coating concentration 0.1 to 100 μg/mL) as described.14,17 (B) Induction of cell death by CD95/Fas triggering by (soluble) anti-CD95 MoAb (clone anti-APO-1 IgG3). (C) Cell death induction by dexamethasone, which was added to the cultures at concentrations from 10−8 mol/L to 10−5mol/L. Cell death was determined on the single-cell level by measuring the DNA content of individual nuclei by flow cytometry. Data represent the mean of triplicates ± SD. (A through C) H9 control cells (○), Bik/Nbk clone no. 2 (◍), Bik/Nbk clone no. 10 (▩), Bik/Nbk clone no. 16 (▴). Medium control in (A) or cultures incubated with FII23c isotype-matched control antibody (0.12 μg/mL19) in (B): H9 control cells (◊), Bik/Nbk clone no. 2 (✙), Bik/Nbk clone no. 10 (□), Bik/Nbk clone no. 16 (▵).
In contrast to the effect seen with the DNA damaging agents and taxol or heat shock (see below), we observed no increase in cell death susceptibility to dexamethasone (Fig 4C). Addition of dexamethasone in concentrations ranging from 10−8 to 10−5mol/L did not elevate the death rate above percentages seen in the medium controls of the H9 3D8 control cells or the Bik/Nbk transfectants. To address the question of whether corticosteroid occurs in the absence of endonuclease activation in the H9 cells, we performed a flow cytometric analysis of H9 cells in which we assessed cell size and cell membrane permeability of H9 cells during apoptosis induction as additional parameters. Apoptotic cells are known to shrink and this leads to a decrease of the forward light scatter (FSC) as measured by flow cytometry. In addition, late stage apoptotic cells show an increased membrane permeability, which was determined by addition of propidium iodide (PI). Thus, cells with a decrease in FSC and an increased uptake of PI can be considered as apoptotic.18 In comparison and in clear contrast to anti-CD3–induced H9 cells, neither H9 3D8 cells nor Bik/Nbk H9 transfectants showed such signs of apoptosis after induction with dexamethasone, thereby showing that dexamethasone does not induce apoptosis in these cells (not shown). We were also unable to observe induction of a DNA ladder after induction with dexamethasone, unlike CD3 or CD95/Fas triggering which induce activation of genomic DNA fragmentation.22
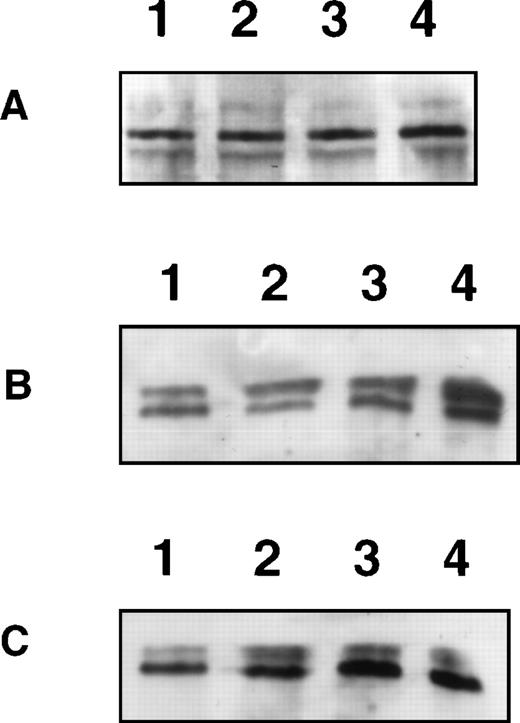
To further exclude that dexamethasone does not induce apoptosis in the H9 T cells because of defects in GR expression and function we performed a Western blot analysis for GR expression in H9 3D8 and the Bik/Nbk transfectants (Fig 5A). There was no difference in GR-alpha (95 kD) or GR-beta (90 kD) expression levels. In addition, the GR expression is known to be under control of steroid responsive elements in T cells.23 Thus, exposure of H9 T cells to dexamethasone led to an induction of GR expression in both the H9 3D8 control cells and the Bik/Nbk transfectants (Fig 5B and C). Additional evidence for the presence of a functional GR receptor and signaling pathway come from the observation that glucocorticoids induce promoter activation in H9 T cells transfected with retroviral long terminal repeat promoter constructs containing GR-response elements.24 Therefore, the GR receptor and signaling pathway appear to be intact in the H9 T cells and the Bik/Nbk clones.
Expression and induction of the GR in H9 cells. (A) Western blot analysis for GR- (95 kD, upper band) and GR-β (90 kD, lower band) expression. Lane 1, H9 3D8 cells; lane 2, H9 Bik/Nbk clone no. 16, lane 3, clone no. 10; lane 4, clone no. 2. (B) Western blot analysis for induction of GR expression by dexamethasone in H9 3D8 cells. Lane 1, medium control; lane 2, 24-hour culture in the presence of dexamethasone (10−6 mol/L); lane 3, 48 hours; lane 4, 72-hour induction. (C) Induction of GR expression by dexamethasone in H9 Bik/Nbk clone no. 16. Lane 1, medium control; lane 2, 24-hour culture in the presence of dexamethasone (10−6 mol/L); lane 3, 48 hours; lane 4, 72 hours.
Expression and induction of the GR in H9 cells. (A) Western blot analysis for GR- (95 kD, upper band) and GR-β (90 kD, lower band) expression. Lane 1, H9 3D8 cells; lane 2, H9 Bik/Nbk clone no. 16, lane 3, clone no. 10; lane 4, clone no. 2. (B) Western blot analysis for induction of GR expression by dexamethasone in H9 3D8 cells. Lane 1, medium control; lane 2, 24-hour culture in the presence of dexamethasone (10−6 mol/L); lane 3, 48 hours; lane 4, 72-hour induction. (C) Induction of GR expression by dexamethasone in H9 Bik/Nbk clone no. 16. Lane 1, medium control; lane 2, 24-hour culture in the presence of dexamethasone (10−6 mol/L); lane 3, 48 hours; lane 4, 72 hours.
Thus, Bik/Nbk could promote sensitivity toward drug-induced apoptosis and CD95/Fas-mediated death, but could not render the steroid refractory H9 cells sensitive for steroid-induced apoptosis.
To assess the effect of Bik/Nbk on another physiologic apoptotic stimulus, and in addition to the death induced by cytostatic drugs, we investigated the response to heat shock (Table2). The cells were incubated for 4 hours at temperatures ranging from 37°C to 45°C. After heat shock, the cells were cultured for a further 24 to 48 hours at 37°C. Cell death by apoptosis was induced at temperatures of 39°C and above. Apoptosis was detectable in the H9 3D8 mock transfectants after 24 hours (Table2) and reached a maximum after 48 hours (Table 2). No further increase was seen after 72 to 96 hours (not shown). At higher temperatures, the death rate increased but cell death was necrotic as evidenced by uptake of trypan blue (data not shown) and decreased DNA fragmentation as compared with the lower temperatures. Overexpression of Bik/Nbk increased the response for heat shock triggered apoptosis. Whereas, 15% of the H9 control cells underwent apoptosis after 48 hours, apoptosis induction was observed in 28% to 38% of the Bik/Nbk transfectants (Table 2).
Heat–Shock-Induced Apoptosis
| Hours . | Temperature . | H9 3D8 . | Clone No. 2 . | Clone No. 10 . | Clone No. 16 . |
|---|---|---|---|---|---|
| 24 | 37 | 2.4 ± 0.5 | 2.1 ± 2.6 | 3.3 ± 2.7 | 2.8 ± 3.2 |
| 24 | 41 | 10.9 ± 1.1 | 16.9 ± 1.2 | 23.1 ± 3.1 | 18.2 ± 2.1 |
| 48 | 37 | 2.4 ± 0.9 | 2.1 ± 3.4 | 3.3 ± 3.3 | 2.8 ± 3.7 |
| 48 | 41 | 15 ± 0.6 | 28 ± 4.2 | 38.7 ± 3.5 | 32.5 ± 3.9 |
| Hours . | Temperature . | H9 3D8 . | Clone No. 2 . | Clone No. 10 . | Clone No. 16 . |
|---|---|---|---|---|---|
| 24 | 37 | 2.4 ± 0.5 | 2.1 ± 2.6 | 3.3 ± 2.7 | 2.8 ± 3.2 |
| 24 | 41 | 10.9 ± 1.1 | 16.9 ± 1.2 | 23.1 ± 3.1 | 18.2 ± 2.1 |
| 48 | 37 | 2.4 ± 0.9 | 2.1 ± 3.4 | 3.3 ± 3.3 | 2.8 ± 3.7 |
| 48 | 41 | 15 ± 0.6 | 28 ± 4.2 | 38.7 ± 3.5 | 32.5 ± 3.9 |
Cells were heated for 4 hours from 37°C to 45°C. Subsequently, the cells were cultured for 24 to 48 hours at 37°C until apoptosis was determined on the single cell level by measuring the DNA content of individual nuclei by flow cytometry. Data represent the mean of triplicates ± SD.
In previous experiments, we observed a decrease of tumorigenicity of breast cancer cells when transfectants overexpressing the apoptosis promoter Bax were xenotransplanted into SCID mice. Therefore, we tested whether the ectopic overexpression of Bik/Nbk decreases tumorigenicity of H9 T lymphoma cells in such a xenotransplantation model in SCID mice. Cells (107) were injected subcutaneously (s.c.) into the inguinal region. Tumor growth was observed starting at week 6 after transplantation (Table 3). The H9 3D8 mock transfected cells grew to large local s.c. tumors. Some mice developed macroscopic lymph node dissemination. Hind limb paralysis was observed as previously encountered in the case of Nalm-6 pre–B-ALL xenotransplantation.25 Mice were, therefore, sacrificed 9 weeks after transplantation. In contrast, no tumors developed in the mice transplanted with Bik/Nbk transfectants (Table 3). These mice remained tumor free for a further 3 months after the mice transplanted with H9 control cells had to be sacrificed.
Suppression of Tumor Growth by Bik/Nbk
| Animal . | Tumor Volume ± SD (cm3) . | Tumor Take Rate . |
|---|---|---|
| H9 3D8 | 2.49 ± 0.27 | 7/7 |
| Clone no. 2 | 0 | 0/6 |
| Clone no. 10 | 0 | 0/6 |
| Clone no. 16 | 0 | 0/6 |
| Animal . | Tumor Volume ± SD (cm3) . | Tumor Take Rate . |
|---|---|---|
| H9 3D8 | 2.49 ± 0.27 | 7/7 |
| Clone no. 2 | 0 | 0/6 |
| Clone no. 10 | 0 | 0/6 |
| Clone no. 16 | 0 | 0/6 |
SCID mice were injected s.c. with 107cells at t = 0. H9 3D8: n = 7 animals, transfectants: n = 6. Tumor growth was measured at 9 weeks after xenotransplantation in two dimensions with calipers and tumor volumes were calculated as described.19
DISCUSSION
Members of the Bcl-2 family are key regulators of apoptosis. Overexpression of Bcl-2 in the B-cell compartment of transgenic mice leads to B-cell hyperplasia. Subsequent dysregulation of genes such as c-myc can lead to the development of B-cell lymphoma.26 The ability of Bcl-2 to prevent apoptosis is antagonized by the proapoptotic members of the Bcl-2 family.9,27 Tissue hyperplasia and tumor promotion can be achieved by the inactivation of such proapoptotic genes as demonstrated by the phenotype of Bax knock out mice28 and transgenic Bax k.o. mice carrying a truncated SV40 large T antigen.29 The mechanism of action of the proapoptotic genes is nevertheless unclear, although recent evidence has implicated the APAF-1 gene, a homologue of theCaenorhabditis elegans ced-4 gene.30In this model, Bcl-xL, caspase-9, and APAF-1 form a ternary complex.31,32 The role of Bax in this complex could be to compete for binding to the caspase activating protein APAF-1 and effect cell death. Additional data show that Bcl-2 prevents mitochondrial permeability shift transition33 and that Bax may directly activate mitochondria resulting in the induction of the mitochondrial permeability transition and the release of cytochrome C.34
In previous experiments, we observed that breast cancer cells have a defect in Bax expression.4 The reconstitution of Bax in the breast cancer cells restored apoptosis sensitivity for serum starvation or Fas-triggered death.5 Additional experiments showed that Bax, as well, increased the sensitivity of the breast cancer cells to apoptosis induced by exposure to DNA damaging agents such as anthracyclin drugs.2 In metastatic colorectal cancer, we recently described that the lack of Bax is a negative prognostic factor, especially in those patients carrying a wild-type p53 gene.6 In contrast to the broad expression of Bax and Bak, the expression of Bik/Nbk seems to be restricted to epithelial tissues. Given the proapoptotic properties, Bik/Nbk could be involved in tissue specific maintenance of homeostasis in these adult tissues as suggested for Bcl-2 or Bax.35 In this context, it was surprising to find that some of the cell lines tested showed constitutive overexpression of Bik/Nbk. In the case of Raji, this overexpression shows a good correlation with a high sensitivity for Fas-triggered apoptosis.14 Mutations in the Bik/Nbk gene like in the case of Bax,36 p53,37 or deregulated activation of the Bik/Nbk gene by EBV-encoded factors (in the case of Raji), may also be the cause for the ectopic expression. Nevertheless, we cannot exclude that this overexpression is paralleled by defects in the downstream death signaling cascade and concomittant deregulation of the upstream death effector Bik/Nbk. In this line, the SW480 cells, which show elevated Bik/Nbk mRNA, express only low levels of endogenous Bik/Nbk protein.
H9 T cells do not express detectable levels of endogenous Bik/Nbk and are resistant to glucocorticoid-induced apoptosis. Therefore, we investigated in this model whether the overexpression of Bik/Nbk in stably transfected cells increased the sensitivity to cytostatic drug-induced apoptosis and rendered them sensitive to steroid-induced cell death. This question is of interest in light of data that suggest that primary resistance to steroid-induced cell death is an important negative prognostic factor that may predict treatment failure in acute lymphocytic leukaemia.11 12
In H9 cells stably transfected with Bik/Nbk, we observed sensitization of the cells to cytostatic drug-induced cell death as compared with the parental line and the mock transfectants. This increase of drug sensitivity amounted to a 10- to 39-fold reduction in the ED50 for the topoisomerase inhibitors epirubicin and etoposide as well as for the microtubule disrupting drug paclitaxel (taxol). These results show and confirm that the induction of apoptosis by these agents in H9 cells is controlled, in part, by a Bcl-2-dependent pathway, which is enhanced by the overexpression of Bik/Nbk. In contrast, we were unable to reverse the steroid resistance in these cells. Previous reports showed that Bcl-2 prevents steroid-induced death in normal and malignant lymphoid cells.38,39 The glucocorticoid receptor expression levels and signaling both appear to be functional in the H9 cells and the transfectants. Thus, the resistance to dexamethasone-induced apoptosis may be because of a defective response upstream of Bcl-2, Bax, or Bik/Nbk. Nevertheless, recent evidence suggests that Bcl-2 and Bax or Bik/Nbk independently regulate cell death.1 40 In this regard, it is unclear whether Bik/Nbk (and Bax) regulate a corticosteroid-independent pathway of apoptosis as opposed to the antagonization of corticosteroid-triggered apoptosis by Bcl-2.
In a xenotransplantation SCID mouse model, Bik/Nbk overexpressing cells were unable to form tumors. The data obtained from these experiments are in line with our previous observations that reconstitution of Bax expression in xenotransplanted breast cancer cells decreases tumor formation in SCID mice. The clonogenic potential of H9 T cells can, thus, be abrogated by the overexpression of Bik/Nbk. This is in line with the decreased clonogenicity (data not shown) and elevated spontaneous (background) apoptosis of the Bik/Nbk transfectants in vitro. In this context, it would be interesting to examine whether Bik/Nbk expression is lost or downregulated during tumorigenesis as we observed for Bax in breast cancer4,5 and high-grade metastatic colorectal cancer.6
In the Bik/Nbk transfectants, we observed an increase in sensitivity to CD95 triggered cell death. Thus, Bik/Nbk not only sensitizes cells to drug-induced apoptosis, but also to CD95/Fas-triggered apoptosis, ie, one of the major physiologic programmed cell death pathways in lymphoid cells. In malignant disease, recent observations have suggested that drug-induced cell death by a variety of compounds, including antimetabolites, leads to induced expression of CD95/Fas and FasL.41 The Bik/Nbk data are consistent with this model and suggest that Bik/Nbk acts on a pathway that sensitizes the cells to apoptosis that is common to both stimuli. The Bik/Nbk enhancement of cell death under these circumstances is also in line with results that suggest CD95/Fas triggered death can be inhibited by overexpression of Bcl-2 and can be enhanced by Bax expression.5
The effect of Bik/Nbk overexpression was examined in another physiologic model of cell death. We observed an enhancement of cell killing after heat shock, in contrast to treatment with anti-CD3 and dexamethasone in which killing was unaffected. This was surprising in the case of the anti-CD3–mediated death, because this is considered to be mediated by the CD95/Fas ligand. Nevertheless, there is evidence that additional, Fas-independent pathways participate in the activation-induced cell death on CD3-crosslinking, which could be Fas-independent, such as other members of the death ligand and receptor superfamilies, eg, tumor necrosis factor receptor-mediated signals.42 In addition we were not able, as previously shown,14,17,20 to completely abrogate CD3-triggered apoptosis by addition of high-affinity blocking anti-CD95/Fas antibodies19 to the culture. These and many other findings from other groups show that the activation-induced cell death of T cells is not mediated exclusively by the CD95/Fas receptor/ligand interaction. This is also in line with the fact that antigen receptor-triggered apoptosis in B cells is clearly independent from CD95/Fas,14 which further corroborates the fact that additional, CD95/Fas-independent signals participate in antigen receptor-mediated cell death in lymphoid cells.
Thus, Bik/Nbk differentially sensitized the T-lymphoma cells to a variety of apoptotic stimuli. This might be related to structural properties of Bik/Nbk.
The Bcl-2 family protein Bik/Nbk7,8 contains only one of the signature domains of the Bcl-2-family, the BH3 domain. The BH3 domain is conserved both in the proapoptotic and the antiapoptotic Bcl-2 family proteins. The BH3 domain of the proapoptotic proteins may serve a dual function. It appears to be essential for their cell death activity and for mediating homodimerization with antiapoptosis proteins.8,43,44 Because BH3-alone containing proapoptotic proteins (Bik/Nbk, Bid, Hrk, Bad, Mtd) share only the BH3 domain in common, it has been discussed whether the BH3 domain is a death effector module and is postulated to elicit its cell death activity by inactivating the antiapoptotic proteins through heterodimerization. This is supported by the observation that the BH3 domain is an apoptosis effector in a cell-free system.44 However, mutational analysis of the BH3 domain in Bik40 and a novel proapoptotic Bcl-2 homologue, Mtd,45 suggests that heterodimerization via the BH3 domain with survival proteins alone is insufficient to explain their cell death inducing activity. Nevertheless, Bik/Nbk may also function as a naturally dominant negative antagonist whose role in the cell is to bind to and inactivate antiapoptotic genes, eg, Bcl-2 and Bcl-xL, like in the case of Bad. Given the above described capability of members of the Bcl-2 family to induce apoptosis even in the absence of a functional BH3 domain we would favor the direct induction of the mitochondrial apoptotic signaling cascade and subsequent activation of caspases. Such a view of a direct action of Bik/Nbk, independent from dimerization to Bcl-2 or Bcl-xL, is supported by data where the BH3 domain in Bax or Bak was deleted.27 46 This prevents homodimerization of Bax but the BH3-deleted mutants retain their capability to enhance apoptosis.
The Bik/Nbk data presented here establish that Bik/Nbk differentially controls the cellular apoptotic response, depending on the type of induction stimulus. In our previous work, we showed that overexpression of Bax may enhance chemosensitivity.2 Recent data show that such an effect of Bax may be caused by the direct activation of the mitochondrial death cascade.34 Such an effect appears to be independent from the interaction with Bcl-2/Bcl-x via the BH1/BH2 domains, which would be in line with our findings that the proapoptotic Bcl-2 homologue Bik/Nbk, which lacks the BH1 and BH2 domain, enhances drug and Fas-mediated apoptosis. Taken together, these data support the role of Bik/Nbk as a direct death effector not only in propagation of cell death on DNA damage, but also for CD95/Fas crosslinking. Such a differential control of different apoptotic pathways could be mediated by functional domains apart from the BH3 signature domain.
Finally, the fact that Bik/Nbk can sensitize cells to drug-induced apoptosis suggests that upregulation of such tissue-specific dominant negative Bcl-2–like protein might yield a therapeutic strategy to overcome drug resistance and tumors refractory to cytotoxic therapies.
ACKNOWLEDGMENT
The authors thank Clarissa von Haefen for expert technical assistance and P.H. Krammer (German Cancer Research Center, Heidelberg) for the generous gift of anti-CD95/Fas MoAb clone anti-APO-1 IgG3 and B. Champion and S. Rees (Glaxo Wellcome, Stevenage) for providing H9 cells and the pCIN4 vector, respectively.
P.T.D. and K.-T.P. equally contributed to this work and share first authorship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter T. Daniel, MD, Department of Hematology, Oncology and Tumor Immunology, Charité—Campus Berlin-Buch, Humboldt-University, Lindenberger Weg 80, 13125 Berlin-Buch, Germany; e-mail: pdaniel@mdc-berlin.de.

![Fig. 3. Sensitization of H9 cells for drug-induced cell death. Cells were exposed to cytostatic drugs for 72 hours. Apoptosis was determined on the single cell level by measuring the DNA content of individual nuclei by flow cytometry. Hypodiploid nuclei were considered as apoptotic. (A) Epirubicin, (B) etoposide, (C) taxol. Cell culture in the presence of the drugs (). Medium controls (only in [A]) ().](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/3/10.1182_blood.v94.3.1100.415a16_1100_1107/5/m_blod41516003x.jpeg?Expires=1769312511&Signature=n9KryHpVFIuOYlQSyjdA2zcPCh~2YfsDP55nty826vYfEvLH3rNzH6cr3lmbUP4aodaXopvvF5MqCdO8PdxhDpG0~1hzQVIKDUtDClEBrNpv5xnbbrSNW1lQbGXVVF4kABWaDlZwMrXMQK2Ll7xspeWHAiFp1757CAj4ouAbZuYkCIsqVYYoeY1jmiVc7n2sqMMWnxB593U40NqxYOm5AX1Xd8Z-q0S1a~PhKsClK5MnACRd1M7sUyUUVotDfGO1qnh5RcX4tGv~qK99S0anmF49fWIpIryXD6qB9FyTylorKLzAMU-o0bzpCtJ2QiIQeCDZVFhobI-TeVyNwikaOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal