Interleukin-12 (IL-12) is a potentially critical factor in the immune response against human immunodeficiency virus (HIV) because it is important for regulating proliferation and interferon-γ (IFN-γ) production by T cells and natural killer (NK) cells, antigen presentation and accessory cell function by macrophages and dendritic cells, and cytolytic activities of cytotoxic T-lymphocyte cells and NK cells, which are all functions known to be dysfunctional in patients with acquired immune deficiency syndrome. Peripheral blood mononuclear cells (PBMC) from HIV-infected patients have been previously shown to be deficient in the ability to produce IL-12 in response to the bacterial pathogen Staphylococcus aureus Cowan. In this study, impaired IL-12 production in cells from PBMC of HIV-infected patients compared with healthy donors was observed across a broad panel of stimuli derived from infectious pathogens with or without priming with cytokines such as IFN-γ and IL-4, which amplify the IL-12 induction signal. Analysis of p40 and p35 mRNA accumulation showed that reductions in both subunits contribute to the lower IL-12 secretion of cells from HIV-infected individuals. PBMC from HIV-infected donors also failed to upregulate the IL-12 receptor β2 chain (IL-12Rβ2) in response to mitogenic stimuli. The expression of the IL-12Rβ2 gene could, however, be restored by in vitro exposure to rIL-12. Thus, it is possible that a primary IL-12 defect may lead to secondary deficiencies in expression of the genes for IL-12Rβ2 and IFN-γ, thus amplifying immune deficiency during HIV infection.
INTERLEUKIN-12 (IL-12) is primarily an antigen-presenting cell (APC) derived cytokine, most commonly produced by monocytes/macrophages but also by dendritic cells, neutrophils, and other phagocytic or APC-like cells.1,2 The IL-12 molecule is composed of two subunits, p40 and p35, which combine to form the p70 heterodimer, and although both are required for biological activity, the two components are differentially regulated. Typically, free p40 monomer is produced in great excess to p70 by approximately 10-fold to 100-fold.3 However, it is not apparent whether free p40 can exert biological activity, although p40 homodimers have been shown to be antagonistic to IL-12 activity in murine systems.4,5Messenger RNA expression of p40 is difficult to detect from cultures of unstimulated monocytes but transcriptional activity occurs within 1 hour after stimulation with bacterial products such as lipopolysaccharide (LPS).6 The light chain, p35, is not released in monomeric form and must be secreted from the same cell as p40 to form the p70 heterodimer. Low levels of p35 mRNA are detectable constitutively and expression is upregulated by the same stimuli that induce p40 and with similar kinetics to p40.6
We have previously examined peripheral blood mononuclear cells (PBMC) from human immunodeficiency virus (HIV)-infected individuals forStaphylococcus aureus–induced IL-12–producing potential and found a marked deficiency in the production of both p40 and p70 compared with control PBMC.7 This impairment was selective for IL-12 production as no significant variation in the secretion of tumor necrosis factor-α (TNF-α), IL-1β, or IL-10 could be found between the two groups of subjects. It was especially of interest that IL-10 levels were equivalent as this belied a potential mechanistic role for IL-10, a notable IL-12 antagonist, in the reduced capacity to produce IL-12 during HIV infection.8 Subsequently, we found that a 24 hour priming period with IL-4 or IL-13 before the S aureus stimulus nearly amplified IL-12 production to levels set by primed cultures from healthy controls, indicating that the IL-12 impairment was largely recoverable.9 The authors as well as other investigators have demonstrated that cells from HIV-infected patients can respond to exogenous IL-12 and that several cellular immune functions, which are often depressed during progressive HIV infection such as T-cell proliferation, T cell and natural killer (NK) cell production of IL-2 and interferon-γ (IFN-γ), and cytotoxic T lymphocyte (CTL) and NK lytic activities, can be partially restored.10 11
Because IL-12 is intimately involved in the promotion and regulation of many forms of antiviral immune responses, we attempted to more closely examine and characterize this deficiency of IL-12 production during HIV infection. We report here that the HIV-mediated dysregulation of IL-12 production is not confined to S aureus stimulation but is evident across a broad panel of stimuli. This impairment can be observed at the level of mRNA expression of both p40 andp35 genes. Furthermore, our data suggest that the underproduction of IFN-γ commonly observed in HIV-infected cells is potentially the result of a deficiency in the expression of high-affinity IL-12R on the cell surface, which, in turn, may stem from the original IL-12 defect.
MATERIALS AND METHODS
Patients.
HIV-1–seropositive adults at various stages of the disease were enrolled in this study. HIV-1 serology was confirmed by using commercially available assays. All patients were repeatedly tested positive for HIV-1 antibodies by enzyme-linked immunosorbent assay and confirmed by Western blot analysis. The HIV+ patient pool consisted of 22 individuals, 12 men, 10 women, with an average age of 38 and an age range of 21 to 62. When segregated into groups according to CD4 T-cell count/μL, the group counts were less than 200 CD4 T/μL: 6; 200-500 CD4 T/μL: 7; greater than 500 CD4 T/μL: 6; unknown: 3. Information on drug therapy for this population was limited; when divided into nucleoside analog drugs/protease inhibitor numbers, the patient pool consisted of 2/0: 2; 3/0: 1; 1/1: 1; 2/1: 4; 0/0: 4; unknown: 10. Several of the patients had opportunistic infections of some kind: Pneumocystis cariniipneumonia (PCP): 5; Candida albicans: 5; Herpes: 2; Kaposi’s sarcoma: 2; sinusitis: 2; tuberculosis: 1. HIV-1 mRNA load data were available for only a small fraction of the patient blood samples. Healthy HIV-1–seronegative donors were included as control subjects and were similar in regards to age range and gender makeup. Informed consent was obtained from all participants in the study.
Cell separation.
All reagents used in this study were selected for low levels of endotoxin contamination by the Limulus amoebocyte assay. PBMC were separated by Ficoll-Paque density gradient centrifugation (Pharmacia Biotech AB, Uppsala, Sweden), resuspended in RPMI-1640 complete medium (GIBCO-BRL, Grand Island, NY), and supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), L-glutamine, and antibiotics.
Culture conditions and reagents.
To induce IL-12 protein synthesis, PBMC (2 × 106cells/mL) from HIV-seronegative and HIV-infected individuals were cultured for 20 to 24 hours in the presence of the following stimuli: medium alone, 1:10,000 S aureus Cowan strain Pansorbin cells (Calbiochem, San Diego, CA), 1 μg/mL LPS (Sigma, St. Louis, MO), 10 μg/mL Lipoteichoic acid (LTA) (Sigma), or 1:200 OK432-5KE streptococcal preparation (Chugai Pharm, Tokyo, Japan). In the instances in which cultures were primed with IL-4 (10 ng/mL; Genzyme, Cambridge, MA) or IFN-γ (100 ng/mL; Endogen, Woburn, MA), cells were cultured in the presence of the cytokine for 16 to 24 hours before stimulation. C albicans strains CA-6 and PCA-2 were kindly provided by Dr Simonetta Mocci (DNAX, Palo Alto, CA) and were grown to stationary phase at 37°C without CO2 on Sabouraud Dextrose Agar plates (Becton Dickinson, Cockeysville, MD), rinsed from the plates with 1× phosphate-buffered saline (PBS) applied with syringes, washed, counted, and incubated at 65°C for 1 hour for heat inactivation. Yeast cells were cultured at a 1:2 yeast cell:PBMC ratio for 24 hours. Stimulations used to induce IFN-γ production were 10 ng/mL O-tetradecanoylphorbol 13-acetate (Sigma) + 1 μg/mL ionomycin (Sigma), 5 μg/mL phytohemagglutinin (PHA) (Sigma), 20 U/mL IL-2, 2 ng/mL IL-12 (Genetics Institute, Andover, MA), and 1:1,000 dilutions of OKT3 (anti-CD3) and CK248 (anti-CD28) antibodies for 48 hours. In some cases, OKT3 was prebound to 96-well round-bottomed plates at 5 μg/mL in carbonate/bicarbonate buffer. IFN-α (Roche, Milano, Italy) was used at 1,000 U/mL. Cell-free supernatants were then harvested, aliquoted, and stored at −80°C until assayed for p40, p70, and IFN-γ by radioimmunoassay as described.3
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR).
PBMC from patients and healthy individuals were cultured as described above for the generation of supernatants except for a stimulation period with LPS/S aureus/LTA/OK432 of 4 hours instead of 24 hours. Total RNA was extracted via the Ultraspec isolation system (Biotecx, Houston, TX), purified by isopropanol precipitation and ethanol washes, and quantitated by spectrophotometry. RT reactions were performed with 3 μg RNA from each sample with 5× first strand buffer, DTT, and Superscript II RT from GIBCO-BRL and RNase inhibitor from Boehringer-Mannheim (Indianapolis, IN) at 37°C/90 minutes and 95°C/10 minutes in a PTC-100 thermal cycler (MJ Research, Watertown, MA). PCR reactions for p40, p35, TNF-α, IFN-γ, IL-12R β1 and β2 chains, and hypoxanthine-phosphoribosyl transferase (HPRT) were performed with 5 μL of a 100-μL volume containing cDNA reverse transcribed from 3 μg RNA. Other components of the PCR reaction were 10× PCR buffer, Advantage cDNA polymerase (Clontech, Palo Alto, CA), 2.5 mmol/L dNTPs (Boehringer-Mannheim), and 200 nmol/L 5′ and 3′ primers. DNA sequences for the primers were: 5′ p40: CCAAGAACTTGCAGCTGAAG; 3′ p40: TGGGTCTATTCCGTTGTGTC; 5′ p35: GATGAGCTGATGCAGGCC; 3′ p35: CAAACCTCCCTGGAGCGAA; 5′ TNF-α; GAGTGATCGGCCCCCAGAGG; 3′ TNF-α: TGCGGCTGATGGTGTGGGTG; 5′ IFN-γ: AGTTATATCTTGGCTTTTCA; 3′ IFN-γ: ACCGAATAATTAGTCAGCTT; 5′ IL-12Rβ1: ACAGAGAAGTCTCCTGAGGT; 3′ IL-12Rβ1: GGACAGCATGTGGAGCTGTA; 5′ IL-12Rβ2: AGAGCGCGACACGTGCGG; 3′ IL-12Rβ2: GGCTGTTCTGGAGCAACAC; 5′ HPRT: CCTGCTGGATTACATCAAAGCACTG; 3′ HPRT: TCCAACACTTCGTGGGGTCCT. Thermal cycler conditions were 95°C/5 minutes, Tm/1 minute, 72°C/1 minute for 1 cycle, and 95°C/40 seconds, Tm/40 seconds, 72°C/40 seconds for 35 cycles (except HPRT: 40 cycles). These conditions were found to be subsaturating for all PCR products. Ten microliters of each sample were electrophoresed through a 1.5% agarose/TAE gel with ethidium bromide and scanned via a FluorImager (Molecular Dynamics, Sunnyvale, CA). PCR products were verified by confirming the known base-pair sequence length. Bands were assigned densitometric values by the ImageQuaNT program (Molecular Dynamics), and these values were normalized to HPRT values.
Competitor constructs for the IL-12Rβ1 and IL-12Rβ2 PCR assays were constructed by PCR mutagenesis techniques. Briefly, the IL-12Rβ1 and IL-12Rβ2 PCR assays were designed to yield a 500-bp PCR product with a restriction site at the center of the sequence (EcoRI for IL-12Rβ1, BamHI for IL-12Rβ2), which when cut would yield two 250-bp fragments. Overlapping primers were designed which amplified the 500-bp fragment but with a 1-nucleotide substitution mutation in the restriction site, thus destroying it. This mutant 500-bp sequence was ligated into the vector pCR2.1 (InVitrogen, Carlsbad, CA) and used to transform XL-1 Blue Supercompetent cells (Stratagene, La Jolla, CA) on X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) plates. Clones with the mutant sequences were selected, grown up via miniprep, and quantitated against λH3 marker. Consequently, a titration of three known concentrations of IL-12Rβ1 or IL-12Rβ2 competitor (1.372, 0.457, and 0.152 fg, selected from earlier trials) were amplified in a PCR reaction with 5 μL of sample cDNA under the conditions described above. EcoRI and BamHI restriction digests were performed on 10 μL of the PCR product for 1 hour at 37°C followed by gel electrophoresis. Uncut competitor bands migrated to 500 bp and cut wild-type sample bands traveled to 250 bp. Quantitative values were assigned to the samples via comparison to the competitor values by proportional calculations.
Statistics.
Statistical significance between groups of data was calculated via the generation of P values via an unpaired student’sT test.
RESULTS
IL-12 secretion is deficient in PBMC from HIV-infected patients over a panel of various stimuli.
We first expanded our initial finding of a defect in IL-12 production by PBMC from HIV-infected individuals in response to S aureus7 by measuring the capacity for IL-12 synthesis in response to a panel of stimuli. These different stimulating regimens have been previously characterized in regards to their IL-12-inducing function and represent several levels of potency. Similar to fixedS aureus, OK432 is also a preparation of killed gram-positive streptococcal bacteria. LPS and LTA are cell-wall components of gram-negative and gram-positive bacteria, respectively. When used by themselves, these agents are moderate inducers of p40 protein production (102 to 103 pg/mL) and poor inducers of p70 (100 to 102 pg/mL). However, a 16-hour priming regimen of IFN-γ converts stimulation with LPS or LTA to potent induction of both p40 (104 to 105 pg/mL) and p70 (103 to 104 pg/mL). Table1 shows a consistent reduction in the capacity for p40 production by cells from HIV+ patients compared with uninfected controls across the entire panel of stimuli. This disparity reaches statistical significance in the cases of stimulation with S aureus, OK432, and LTA (preceded by priming with IFN-γ), which are all examples of moderate p40 inducers. It appears that extremely potent stimulating conditions, such as IFN-γ/LPS, can maximize the p40 production pathway such that HIV+ monocytes begin to approach the p40 production capacity of uninfected cells. On the other hand, agents such as LPS and LTA provide a much poorer stimulus for p40, which may mask the disparity between the groups observed with more potent agents. However, it is important to note that the trend across the entire panel consistently showed an impairment in p40 production from HIV-infected cultures.
Induction of IL-12 p40 and p70 by a Panel of Stimuli Is Impaired in PBMC From HIV-Infected Patients Compared With Healthy Controls
| Conditions . | HIV− . | HIV+ . | P Value . | ||||
|---|---|---|---|---|---|---|---|
| Mean . | SEM . | n . | Mean . | SEM . | n . | ||
| p40 | |||||||
| Medium | 138 | 73 | 15 | 52 | 12 | 20 | .126 |
| S aureus | 10,044 | 2,232 | 15 | 3,384 | 864 | 20 | .002 |
| LPS | 1,962 | 1,312 | 15 | 352 | 38 | 21 | .098 |
| IFN-γ/LPS | 124,510 | 15,416 | 15 | 93,624 | 14,326 | 15 | .163 |
| OK432 | 14,464 | 6,610 | 15 | 4,820 | 774 | 21 | .049 |
| LTA | 589 | 203 | 7 | 179 | 50 | 6 | .096 |
| IFN-γ/LTA | 45,788 | 11,400 | 7 | 7,348 | 1,372 | 6 | .010 |
| p70 | |||||||
| Medium | 11 | 2 | 14 | 10 | 2 | 21 | .945 |
| S aureus | 53 | 11 | 14 | 10 | 2 | 21 | <.0001 |
| LPS | 24 | 5 | 14 | 15 | 3 | 21 | .074 |
| IFN-γ/LPS | 14,610 | 2,882 | 14 | 9,484 | 1,755 | 18 | .116 |
| OK432 | 156 | 111 | 14 | 37 | 11 | 21 | .157 |
| LTA | 19 | 3 | 7 | 15 | 2 | 6 | .258 |
| IFN-γ/LTA | 3,136 | 1,006 | 7 | 689 | 216 | 6 | .050 |
| Conditions . | HIV− . | HIV+ . | P Value . | ||||
|---|---|---|---|---|---|---|---|
| Mean . | SEM . | n . | Mean . | SEM . | n . | ||
| p40 | |||||||
| Medium | 138 | 73 | 15 | 52 | 12 | 20 | .126 |
| S aureus | 10,044 | 2,232 | 15 | 3,384 | 864 | 20 | .002 |
| LPS | 1,962 | 1,312 | 15 | 352 | 38 | 21 | .098 |
| IFN-γ/LPS | 124,510 | 15,416 | 15 | 93,624 | 14,326 | 15 | .163 |
| OK432 | 14,464 | 6,610 | 15 | 4,820 | 774 | 21 | .049 |
| LTA | 589 | 203 | 7 | 179 | 50 | 6 | .096 |
| IFN-γ/LTA | 45,788 | 11,400 | 7 | 7,348 | 1,372 | 6 | .010 |
| p70 | |||||||
| Medium | 11 | 2 | 14 | 10 | 2 | 21 | .945 |
| S aureus | 53 | 11 | 14 | 10 | 2 | 21 | <.0001 |
| LPS | 24 | 5 | 14 | 15 | 3 | 21 | .074 |
| IFN-γ/LPS | 14,610 | 2,882 | 14 | 9,484 | 1,755 | 18 | .116 |
| OK432 | 156 | 111 | 14 | 37 | 11 | 21 | .157 |
| LTA | 19 | 3 | 7 | 15 | 2 | 6 | .258 |
| IFN-γ/LTA | 3,136 | 1,006 | 7 | 689 | 216 | 6 | .050 |
PBMC from controls and patients were incubated at 2 × 106/mL for 16 h ± IFN-γ followed by 24 hours of stimulation with S aureus, LPS, OK432, LTA, or medium alone. Cell-free supernatants were harvested and assayed for IL-12 p40 and p70 via RIA. P values were calculated via an unpaired student’st-test and compared with the control group versus the patient group in each category of stimulation.
The secretion of the p70 heterodimer largely parallels the pattern of expression described above for the p40 monomer. Again, the general trend is a reduced ability of HIV+ monocytes to produce p70 compared with uninfected cells (Table 1). Moderately potent types of stimuli such as S aureus and IFN-γ/LTA show significant p70 deficits for HIV-infected subjects, whereas extreme or weakly inducing stimuli also indicate a reduction in p70 but less markedly. Because the p70 heterodimer is produced at much lower concentrations than p40, it is expected that poor stimuli such as LPS or LTA would not be sufficient to clearly show the p70 dysregulation. Additionally, no correlation between CD4 T-cell counts of the patients and IL-12 response was found, indicating that the IL-12 deficiency is induced early in infection and is maintained throughout disease progression, which is a finding we reported previously.7
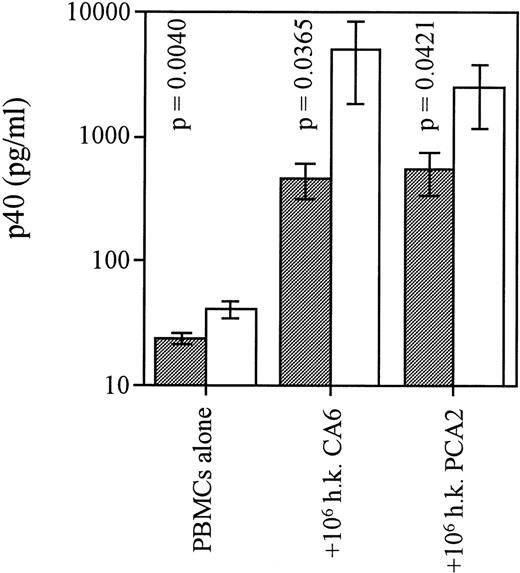
We further explored the deficiency in IL-12 production with regards to stimulation with an opportunistic agent that infects patients with acquired immune deficiency syndrome, C albicans. We used two separate strains of C albicans: CA6, a virulent strain, lethal in CDF1 mice, which induces a Th2 response in these animals, and PCA2, a lab-adapted strain that prompts a healing Th1 response in those mice.12 Both live and heat-killed preparations of C albicans have been used to stimulate cytokine production from murine cells. We found that heat-killed C albicans induced moderate levels of p40 release from cultures of uninfected PBMC (Fig1). In contrast, cells from HIV+ patients reacted poorly to this stimulation in regards to p40 production, confirming a widespread IL-12 production deficiency. None of the C albicans preparations induced detectable p70 levels in PBMC cultures (data not shown).
PBMC from HIV-infected individuals are deficient in the p40 response to C albicans. Freshly harvested yeast cells of strain CA-6 or PCA-2 were washed twice in 1 × PBS and cultured for 24 hours with 2 × 106/mL PBMC from control subjects or patients. Tests were conducted to find optimal culture conditions for the use of yeast cells: heat-inactivation at 65°C for 1 hour (heat killed) before addition to culture at a 1:2 yeast cell:PBMC ratio was found to be a more effective IL-12–stimulus than addition of live C albicans. Cell-free supernatants were assayed for p40 content via RIA. n = 16 for HIV+ group and n = 7 for HIV−group. Bars represent means of each cohort; P values were calculated via an unpaired student’s t-test and compared HIV+ (░) and HIV− (□) groups for each condition; error bars indicate SEM.
PBMC from HIV-infected individuals are deficient in the p40 response to C albicans. Freshly harvested yeast cells of strain CA-6 or PCA-2 were washed twice in 1 × PBS and cultured for 24 hours with 2 × 106/mL PBMC from control subjects or patients. Tests were conducted to find optimal culture conditions for the use of yeast cells: heat-inactivation at 65°C for 1 hour (heat killed) before addition to culture at a 1:2 yeast cell:PBMC ratio was found to be a more effective IL-12–stimulus than addition of live C albicans. Cell-free supernatants were assayed for p40 content via RIA. n = 16 for HIV+ group and n = 7 for HIV−group. Bars represent means of each cohort; P values were calculated via an unpaired student’s t-test and compared HIV+ (░) and HIV− (□) groups for each condition; error bars indicate SEM.
IL-12 mRNA expression in PBMC from HIV-infected patients is dysfunctionally regulated across a panel of stimuli.
To further define at what level the defect in IL-12 production by PBMC from HIV-infected patients was taking place, we examined the expression of p40 and p35 mRNA in HIV+ patients versus controls after stimulation with several stimuli. Similarly to our evaluation of IL-12 protein secretion, we find that the induction of mRNA expression of both IL-12 subunits as measured by RT-PCR is reduced in cell cultures from HIV-infected individuals (Table 2). Densitometric values were derived from ethidium bromide–stained bands, normalized to HPRT values, and reported as the fold-increase in expression of each stimulatory condition compared with the unstimulated control. Reduced accumulation of both p40 and p35 transcripts is consistently observed across the panel of stimuli, except in cases of extremely potent stimuli, such as IFN-γ/LPS. We observed that this reduction of IL-12 mRNA levels appeared to be specific for that cytokine, as TNF-α expression was equivalent between the two cohorts (data not shown). A more substantial reduction in p40 mRNA expression than in p35 mRNA expression was generally observed in the HIV+ group, which agreed with the more significant discrepancy observed for p40 protein production (Table 1) compared with p70 protein.
Induction of IL-12 mRNA by a Multistimulus Panel Is Impaired in Cells From HIV-Infected Subjects
| Conditions . | p40 . | p35 . | ||
|---|---|---|---|---|
| HIV− . | HIV+ . | HIV− . | HIV+ . | |
| −/− | 1 | 1 | 1 | 1 |
| −/S aureus | 112.3 | 49.1 | 5.7 | 3.2 |
| −/LPS | 72.2 | 7.4 | 2.6 | 1.7 |
| IFN-γ/LPS | 96.9 | 98.5 | 6.3 | 4.5 |
| −/OK432 | 117.9 | 75.6 | 5.1 | 2.6 |
| −/LTA | 90.7 | 27.1 | 1.9 | 1.2 |
| IFN-γ/LTA | 183.5 | 48.0 | 9.9 | 6 |
| IL-4/S aureus | 125.6 | 44.1 | 7.9 | 3.7 |
| Conditions . | p40 . | p35 . | ||
|---|---|---|---|---|
| HIV− . | HIV+ . | HIV− . | HIV+ . | |
| −/− | 1 | 1 | 1 | 1 |
| −/S aureus | 112.3 | 49.1 | 5.7 | 3.2 |
| −/LPS | 72.2 | 7.4 | 2.6 | 1.7 |
| IFN-γ/LPS | 96.9 | 98.5 | 6.3 | 4.5 |
| −/OK432 | 117.9 | 75.6 | 5.1 | 2.6 |
| −/LTA | 90.7 | 27.1 | 1.9 | 1.2 |
| IFN-γ/LTA | 183.5 | 48.0 | 9.9 | 6 |
| IL-4/S aureus | 125.6 | 44.1 | 7.9 | 3.7 |
PBMCs were isolated from 13 HIV+ patients and 10 control subjects. PBMCs (2.5 × 106) from each donor were stimulated in1 mL culture medium for 16 h ± IFN-γ or IL-4 followed by 4 hours of stimulation with S aureus Cowan (SAC), LPS, OK432, LTA, or medium alone. The same 2 mL Ultraspec was used for all subjects within a condition, thus each PCR band represents pooled RNA from 2.5 × 106 cells from each of the 13 or 10 individuals of the group. RT-PCR was performed for the detection of p40, p35, TNF-α, and HPRT message. Densitometric values in arbitrary units were assigned to the ethidium bromide-stained bands by the ImageQuaNT program and these were normalized to HPRT values. Fold-increase values quantitate the increase of p40 and p35 mRNA expression after stimulation compared with unstimulated cells (−/−).
IFN-γ production is depressed in HIV+patients.
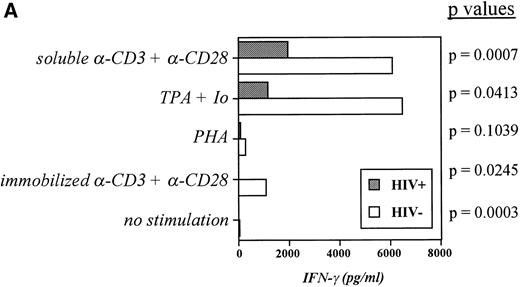
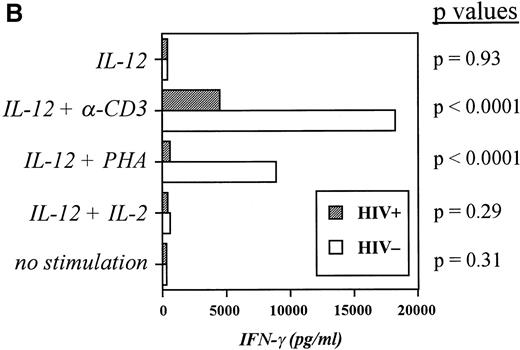
Although some reports have shown the synthesis of IFN-γ to be markedly diminished in HIV+ cells from peripheral blood and in clones derived from blood cells,13-15 controversy still exists as to whether such a diminishment is truly indicative of negative regulation of IFN-γ during infection with HIV. To ascertain whether an impairment in IFN-γ production as well as in IL-12 production is observable in our cultures, PBMC from both groups of subjects were stimulated over 48 hours with a variety of T-cell mitogens, and IFN-γ production was recorded. Figure2A indicates that IFN-γ production is indeed depressed in cultures from infected individuals and that this depression is significant in all cases with the exception of PHA stimulation. Furthermore, the disparity in IFN-γ production between the two groups became more pronounced when the cells were stimulated in the presence of IL-12, which boosted IFN-γ production by control cultures considerably but induced much less IFN-γ from infected cultures when stimulated with PHA or anti-CD3 (Fig 2B). This deficiency in the response to IL-12 led us to examine the expression of IL-12R in the two subject groups.
Induction of IFN-γ by a panel of T-cell mitogenic stimuli is impaired in HIV-infected PBMC compared with controls. (A) PBMC from controls and patients were incubated at 2 × 106/mL for 48 hours with medium alone, -CD3 (soluble or platebound) + -CD28, TPA + ionomycin, or PHA. Cell-free supernatants were harvested and assayed for IFN-γ. The Pvalues comparing the control group versus patient group in each category of stimulation are listed and were calculated via an unpaired student’s t-test. Data are derived from 10 patients and 10 controls. (B) PBMC from controls and patients were cultured for 48 hours with medium, -CD3 (soluble), PHA, or IL-2 in the presence or absence of IL-12. Cell-free supernatants were harvested and assayed for IFN-γ. The P values comparing the control group versus patient group in each category of stimulation are listed and were calculated via an unpaired student’s t-test. Data are from individual donors (n = 21 for HIV+ and n = 17 for HIV−).
Induction of IFN-γ by a panel of T-cell mitogenic stimuli is impaired in HIV-infected PBMC compared with controls. (A) PBMC from controls and patients were incubated at 2 × 106/mL for 48 hours with medium alone, -CD3 (soluble or platebound) + -CD28, TPA + ionomycin, or PHA. Cell-free supernatants were harvested and assayed for IFN-γ. The Pvalues comparing the control group versus patient group in each category of stimulation are listed and were calculated via an unpaired student’s t-test. Data are derived from 10 patients and 10 controls. (B) PBMC from controls and patients were cultured for 48 hours with medium, -CD3 (soluble), PHA, or IL-2 in the presence or absence of IL-12. Cell-free supernatants were harvested and assayed for IFN-γ. The P values comparing the control group versus patient group in each category of stimulation are listed and were calculated via an unpaired student’s t-test. Data are from individual donors (n = 21 for HIV+ and n = 17 for HIV−).
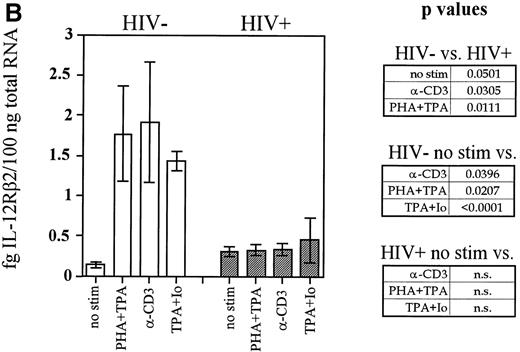
Expression of IL-12Rβ2 is defective in HIV-infected individuals.
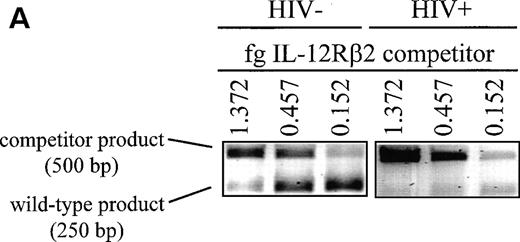
Another gene whose expression may be dependent on adequate levels of IL-12 is that of IL-12Rβ2. Recent reports suggest that although the IL-12Rβ1 subunit is constitutively expressed on the cell surface and is only modestly regulated, IL-12Rβ2 is not expressed on naive T cells or NK cells and must be upregulated by signals that may be at least partially provided by IL-12 itself.16 To investigate the expression of IL-12Rβ2 in HIV-infected subjects, we conducted competitive RT-PCR assays on mitogen-stimulated PBMC cultures that were stimulated with T-cell mitogens for 20 hours. PCR was conducted on cDNA samples from these cultures in competition with known concentrations of a competitor construct. This construct has a sequence virtually identical with that of the wild-type fragment with one nucleotide substitution that inactivates a naturally occurring BamHI site. The close identity of the sequences ensures that they are amplified with the same efficiency during the PCR reaction. The wild type and competitor PCR products can be then distinguished by conductingBamHI restriction digests, which will cut the wild-type and not the competitor fragments followed by electrophoresis to separate them on an agarose gel. A representative example of competitive IL-12Rβ2 RT-PCR performed on one control and one patient is shown in Fig3A. In this way we were able to derive data that accurately reflect the quantity of IL-12Rβ2 mRNA expressed in our cultures. Cumulative data from the patient and control groups are shown in Fig 3B. T-cell mitogens are able to induce on average 3-fold to 5-fold higher quantities of IL-12Rβ2 message in cells from the uninfected group compared with HIV-infected cells. In contrast, cells from patients showed little or no ability to enhance IL-12Rβ2 expression above the minimal constitutive levels observed in unstimulated cells. IL-12Rβ1 expression, on the other hand, was not significantly different between the two groups of subjects, nor was its expression markedly enhanced by mitogen stimulation in either group (data not shown), indicating that the cultures were not demonstrating reduced IL-12Rβ2 expression simply through widespread cellular apoptosis. Thus, expression of IL-12Rβ2, but not IL-12Rβ1, is markedly diminished in HIV-infected individuals.
Induction of IL-12Rβ2 mRNA is depressed in cells from HIV-infected individuals. PBMC from nine HIV+ patients and six healthy controls were cultured at 2 × 106/mL for 20 hours with PHA + TPA, anti-CD3 (OKT3), TPA + ionomycin (Io), or medium alone. RNA was extracted and competitive RT-PCR for IL-12Rβ2 was performed as described in Materials and Methods. (A) Representative samples of PHA + TPA-stimulated samples from each cohort are shown to demonstrate competitive IL-12Rβ2 RT-PCR. Near-equivalent competition is observed at 0.457 fg IL-12Rβ2 for the control sample and at 0.152 fg for the patient sample. (B) Densitometric values in arbitrary units were assigned by the ImageQuaNT program to the ethidium bromide-stained bands of wild-type and competitor fragments, which most closely approached equivalent competition. IL-12Rβ2 quantities were computed through the following formula: Unknown Sample Quantity = (ImageQuaNT Sample Value)(Quantity of Competitor)/(ImageQuaNT Competitor Value). Data represent the means of values derived from nine patients and six controls (except patients stimulated with TPA + ionomycin, wheren = 2). The P values comparing patient versus control group IL-12Rβ2 expression and unstimulated (no stim) versus stimulated groups are listed and were calculated via an unpaired student’s t-test. n.s., not significant.
Induction of IL-12Rβ2 mRNA is depressed in cells from HIV-infected individuals. PBMC from nine HIV+ patients and six healthy controls were cultured at 2 × 106/mL for 20 hours with PHA + TPA, anti-CD3 (OKT3), TPA + ionomycin (Io), or medium alone. RNA was extracted and competitive RT-PCR for IL-12Rβ2 was performed as described in Materials and Methods. (A) Representative samples of PHA + TPA-stimulated samples from each cohort are shown to demonstrate competitive IL-12Rβ2 RT-PCR. Near-equivalent competition is observed at 0.457 fg IL-12Rβ2 for the control sample and at 0.152 fg for the patient sample. (B) Densitometric values in arbitrary units were assigned by the ImageQuaNT program to the ethidium bromide-stained bands of wild-type and competitor fragments, which most closely approached equivalent competition. IL-12Rβ2 quantities were computed through the following formula: Unknown Sample Quantity = (ImageQuaNT Sample Value)(Quantity of Competitor)/(ImageQuaNT Competitor Value). Data represent the means of values derived from nine patients and six controls (except patients stimulated with TPA + ionomycin, wheren = 2). The P values comparing patient versus control group IL-12Rβ2 expression and unstimulated (no stim) versus stimulated groups are listed and were calculated via an unpaired student’s t-test. n.s., not significant.
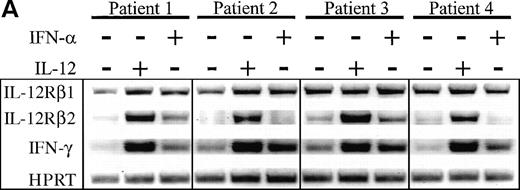
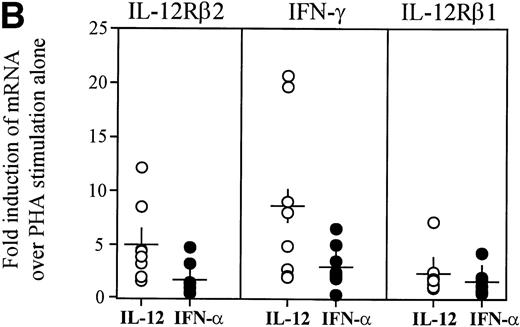
IL-12 can restore the impaired expression of IL-12Rβ2.
As previous reports have indicated a dependence on the presence of IL-12 for optimal IL-12Rβ2 expression,17 we determined whether exposure of HIV+ PBMC to IL-12 could restore IL-12Rβ2 message to levels observed in normal individuals. We had previously determined that a 72-hour exposure to IL-12 or IFN-α (also reported to have IL-12Rβ2-enhancing properties17), could markedly enhance IL-12Rβ2 mRNA accumulation in PHA-stimulated PBMC from healthy individuals, although IL-12 was consistently more potent than IFN-α in this respect (data not shown). Thus, PBMC from patients were similarly stimulated with PHA in the presence of IL-12 or IFN-α for 72 hours then examined for IL-12Rβ1 and IL-12Rβ2 as well as IFN-γ expression. As evidenced in Fig 4, IL-12 was particularly effective at the marked enhancement of IL-12Rβ2 expression to levels consistent with those observed in uninfected subjects (data not shown). On the other hand, IFN-α showed a modest capacity to induce IL-12Rβ2, which was somewhat donor dependent. The lesser ability of IFN-α to enhance IL-12Rβ2 compared with IL-12 was not related to an inability of HIV+ cells to respond to IFN-α, because we observed similar disparities between the effects of these two cytokines with uninfected subjects (data not shown). IFN-γ expression was enhanced by IL-12 or IFN-α stimulation of HIV+ PBMC in similar fashion to IL-12Rβ2, although levels of IL-12Rβ1 message were modulated very little compared with control cultures. These data demonstrate that the presence of IL-12 can restore deficient IFN-γ production, at least in part, by enhancing the proportion of high-affinity IL-12Rs on the cell surface, thus allowing for downstream events to occur.
Enhancement of IL-12Rβ2 and IFN-γ transcript expression in HIV-infected PBMC by IL-12 and IFN-. PBMC from eight patients were cultured at 2 × 106/mL for 72 hours with 2 μg/mL PHA ± 2 ng/mL IL-12 or 1,000 U/mL IFN-. (A) RNA was extracted and RT-PCR was performed for the detection of IL-12Rβ1, IL-12Rβ2, IFN-γ, and HPRT mRNA. Representative results from 4 of 8 donors are shown. (B) Densitometric values were assigned to bands by ImageQuaNT, normalized to HPRT values, and displayed as fold induction of message levels compared with PHA stimulation alone. Data points are from individual donors (n = 8) and the mean is represented by (+).
Enhancement of IL-12Rβ2 and IFN-γ transcript expression in HIV-infected PBMC by IL-12 and IFN-. PBMC from eight patients were cultured at 2 × 106/mL for 72 hours with 2 μg/mL PHA ± 2 ng/mL IL-12 or 1,000 U/mL IFN-. (A) RNA was extracted and RT-PCR was performed for the detection of IL-12Rβ1, IL-12Rβ2, IFN-γ, and HPRT mRNA. Representative results from 4 of 8 donors are shown. (B) Densitometric values were assigned to bands by ImageQuaNT, normalized to HPRT values, and displayed as fold induction of message levels compared with PHA stimulation alone. Data points are from individual donors (n = 8) and the mean is represented by (+).
DISCUSSION
This study confirms and extends our previous observation that IL-12 production as induced by S aureus stimulation is handicapped in cells from HIV-infected individuals.7 Here, we show that this defect is evident regardless of the type of stimulating agent used. Depressed IL-12 production is shown in the form of reductions in the amounts of p40 and p70 protein synthesized by HIV-infected cells as well as in the levels of p40 and p35 mRNA they express. We also find deficits in the expression of two downstream genes in the IL-12-initiated cascade of events: IL-12Rβ2 and IFN-γ. Whether these reductions are the result of abnormally low levels of IL-12 present in the HIV-infected environment or whether there are separate but specific HIV-directed mechanisms for those reductions is presently unclear. Because both CD4 and CD8 T cells can respond to IL-12, it is a possibility that the depressed IL-12Rβ2 expression in HIV-infected cells may be related to the differing proportions of CD4:CD8 T cells between patients and controls. However, the relative expression patterns of IL-12Rβ2 on CD4 versus CD8 T cells is not yet characterized.
Our first investigation of the IL-12 defect, which examined only S aureus as a stimulus for IL-12 production, found approximately a 10-fold difference in p40 production between the control and the infected groups and a 5-fold disparity in p70 levels.7 In the present study, we report higher means of IL-12 p40/p70 release by cultures from HIV-infected individuals, which render the differential production of IL-12 between controls and patients in this study less dramatic than previously shown. One explanation for this discrepancy could rely on the different therapy protocols received by the HIV-infected cohort examined in this study. Initially, our studies drew on a pool of patients receiving one or two nucleoside-base inhibitors such as zidovudine or lamivudine. Currently, the cohort is composed of patients receiving much more aggressive therapies, some for several years, which routinely include two or three nucleoside analogs as well as a protease inhibitor such as nevirapine, nelfinavir, indinavir, or ritonavir. Treatment with nucleoside analogs and protease inhibitors has been shown to partially restore certain cellular functions such as HIV-specific proliferation,18,19 and the difference in IFN-γ production by T cells between patients and controls has been observed to be partially ameliorated in correlation with the number of anti-HIV drugs taken by the infected individual.20Therefore, it is possible that the drug regimens to which our patient pool is subjected are responsible for narrowing the IL-12 production gap between subject groups. However, despite these drug treatments, a consistent and unmistakable reduced capacity to produce optimal levels of IL-12 under most stimulatory conditions remains.
Some data from other laboratories are in agreement with our findings. Reductions in p35 and p40 mRNA in HIV+ cultures in response to S aureus have been shown by Chougnet et al,21however, no other stimuli were examined in that case. An IL-12–deficient response by patients to S aureus and not to LPS was observed in another study,22 although the different experimental protocol involving whole blood cultures may have influenced the capacity for IL-12 production. Harrison and Levitz23 investigated IFN-γ priming with S aureusstimulation of HIV+ PBMC and derived data on p70 protein levels similar to that generated in the present study with IFN-γ + LPS as a stimulus. However, unlike the present study, these investigations did not analyze the IL-12 production defect by using a broad panel of stimuli while simultaneously examining different parameters of IL-12 expression including p40 and p70 protein release and p40 and p35 mRNA accumulation. Another study performed by Harrison and Levitz24 confirmed the deficient IL-12 production for PBMC of HIV-infected patients after S aureus stimulation, as we had previously shown,7 but, in contrast to the data presented here, found no such deficiency after stimulation with C albicans. However, in their report the authors examined only p40 mRNA expression via RT-PCR without presenting protein data for C albicans–stimulated cultures.24 In our study, we observed that C albicans yeast cells were only moderate inducers of p40 from uninfected cells and did not support detectable p70 production. Therefore, it may be difficult to show the IL-12 impairment at the level of mRNA when using a stimulus of such mild potency. Furthermore, differences in assay sensitivity may account for some inconsistencies between the two reports, because Harrison and Levitz24 additionally failed to detect a p40 mRNA deficiency after S aureus stimulation, as we did, and only reported a reduction in p70 protein levels. Finally, our study was conducted with considerably larger cohorts of subjects, thus better managing variability between individuals within both subject groups.
IL-12 has been well characterized as an indispensable agent for the optimal production of IFN-γ by T cells and NK cells25 and as a recent report17 also indicates, it may play a role in the induction and/or enhancement of the β2 chain of its own receptor. Surface expression of IL-12Rβ2 is required for high-affinity IL-12 binding and for mediation of numerous IL-12 activities, including T-cell proliferation, IFN-γ production by T cells and NK cells, and cytolytic activity,14,26,27 all of which have been observed to be depressed during progressive HIV infection.28,29Although there is some controversy that surrounds the issue of impaired IFN-γ production during HIV infection,30,31 our data clearly demonstrate a reduced IFN-γ response by HIV+PBMC, and other reports that present contrasting data may do so by examining RNA levels from unstimulated cells that may not reflect a defect in the IFN-γ response to stimulation.30 More evidence for an inability of IL-12 to optimally signal in HIV-infected cells comes from a recent report, which noted that expression of the transcriptional activators STAT5 and STAT1 are selectively decreased in T cells that are infected in vitro or are derived from HIV+patients.32 Both of these STATs, as well as STAT4, are known to be activated after stimulation through the IL-12R.33 The danger represented by this lack of a proper IL-12-signaling pathway in HIV-infected individuals is underlined by studies of some individuals who were found to harbor deficient copies of IL-12Rβ1 because of inherited mutation and consequently were immunodeficient and suffered from chronic disseminated mycobacterial infections.34 35
The stimulatory agents used in this study were those we previously characterized for their IL-12–inducing properties,3,36 and most are preparations of or components of gram-positive or -negative bacteria. S aureus and OK432 are heat killed and pickled preparations of S aureus and Streptococcus pyogenes gram-positive bacilli, whereas LPS and LTA are purified cell-wall components of gram-negative and gram-positive bacteria, respectively. LPS and LTA are known to signal through binding to CD14 on the surface of B cells or monocytes,37 while the signaling mechanism of S aureus is also largely dependent on CD14 expression.38 Although IFN-γ and IL-4, used as priming agents in our studies, signal through differing mechanisms, these agents are not directly responsible for activation of p40 or p35 gene transcription, as priming with IFN-γ or IL-4 alone without subsequent stimulation has no effect on p40/p35 message levels.9 39 Thus, it appears that signaling through CD14 may be a common mechanism of the panel of stimuli examined in this study and, thus, a possible target for HIV-mediated dysfunction.
It does not seem likely that HIV acts to negatively regulate CD14 surface expression on monocytes, as the percentage of CD14+monocytes from infected subjects has actually been observed to increase compared with healthy controls.40 In addition, the mechanism of bacterial endotoxin signaling through CD14 is poorly defined. It is known that association with LPS-binding protein transfers LPS to interact with CD14, followed by internalization of LPS and ultimately the provocation of NFκB translocation to the nucleus, perhaps through a tyrosine kinase cascade.41,42 It is possible that infection with HIV may interfere at some point in this sequence of events to hinder the initiation of p40/p35 transcription, perhaps by preventing the activation of NFκB, which has been shown to positively promote human p40 transcription.43
The reduced IFN-γ and IL-12Rβ2 levels, which we observe during HIV infection, may be secondary effects derived from a primary HIV-influenced dysregulation of IL-12. Although different stimuli were used to induce IL-12 and IFN-γ production, the T-cell mitogenic stimuli used for IFN-γ production have been previously observed to be at least partially dependent on IL-12 (and IL-12R) to activate IFN-γ (G.T. unpublished observations). Thus, it follows that administration of exogenous IL-12 should restore those downstream deficiencies in IL-12Rβ2 and IFN-γ, which we proved here for IL-12Rβ2 and which has been shown previously for IFN-γ. The addition of exogenous IFN-α, on the other hand, appears to provide a more modest signal for IL-12Rβ2 enhancement, and a consistent increase in IL-12Rβ2 expression is not evident with cells from every subject studied. Earlier reports from our laboratory and others have clearly indicated that cells from most infected subjects can respond to IL-12 administration vigorously.10 11 Although addition of IL-12 to 48-hour cultures of HIV+ PBMCs did not appreciably enhance IFN-γ secretion (Fig 2B), we observed that at least 72 hours of exposure to IL-12 were necessary for restoration of both IFN-γ and IL-12Rβ2 mRNA expression, indicating a longer period of culture would eventually result in increases in IFN-γ protein production.
This study indicates that the capacity to express high-affinity IL-12R in response to T-cell mitogenic stimuli is severely diminished in HIV+ patients. However, we show that IL-12 itself can circumscribe this impairment by directly enhancing the expression of IL-12Rβ2, thus allowing high-affinity IL-12R responsiveness and eventual IFN-γ production. The mechanism by which IL-12 signals on cells lacking high-affinity IL-12Rs is unclear. It is known that IL-12 can bind to a low affinity receptor consisting of IL-12Rβ1 alone27 and perhaps can at least partially signal through it or through an unidentified associated chain to upregulate IL-12Rβ2 expression. On the other hand, IL-12 may be able to signal on HIV+ cells after sufficient rounds of PHA-instigated expansion of a proportionally small IL-12Rβ2+ subset have occurred. Nonetheless, these findings lend further support for a potentially vital role for IL-12 or an IL-12-agonist as part of a multidrug therapy. The restoration of the cellular-based HIV-specific immune response by IL-12 in combination with a direct attack on the virus itself by protease inhibitors and other drugs might be effective at reducing viral load simultaneously with establishing long-lasting anti-HIV immunity.
ACKNOWLEDGMENT
We are grateful to each of the donor participants for donating blood as well as to D. Davis, D. McGhee, A. Holloway, R. Anthony, B. Gallagher, B. McManus, M. Smerkanich, S. Dix-Lassiter, J. Schull, and the Board and Staff of Philadelphia FIGHT.
Supported in part by the Public Health Service (PHS) Grants No. AI34412, CA10815, CA20833, CA32898, AI34758, by the Adult Clinical Trial Group (ACTG), by funds from Advanced Technology Laboratories, by AIDS funds from the Commonwealth of Pennsylvania, and by the W.W. Smith Charitable Trust (J.C.). J.D.M. is supported by the National Institutes of Health (NIH) postdoctoral fellowship AI09627. These studies were also, in part, supported by the Philadelphia Foundation and Mrs. M. Stengel Miller’s support of the HIV-1 Partnership Program for Basic Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Giorgio Trinchieri, MD, The Wistar Institute, 3601 Spruce St, Philadelphia, PA 19104; e-mail:trinchieri@wista.wistar.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal