Abstract
Retinoic acid receptor (RAR) is the target of several chromosomal translocations associated with acute promyelocytic leukemias (APLs). These rearrangements fuse RAR to different partner genes creating the chimeric proteins: PML-RAR, PLZF-RAR, and NPM-RAR. Although the vast majority of APLs respond to retinoic acid therapy, those associated with PLZF-RAR are resistant. We have used retroviruses to express PML-RAR, PLZF-RAR, NPM-RAR, RAR403 (a dominant negative mutant of RAR), and wild-type RAR in murine bone marrow progenitors and found that all of these constructs blocked differentiation and led to the immortalization of myeloid progenitors. This cellular transformation is specific to an alteration of the RAR pathway because overexpression of RARβ, RARγ, or RXR did not result in similar growth perturbations. Pharmacological doses of RA induced differentiation and inhibited proliferation of cells transformed with either of the APL fusion genes, including PLZF-RAR, whereas physiological retinoic acid concentrations were sufficient to reverse the phenotype of cells transformed with wild-type RAR. The cellular responses to retinoic acid were accompanied by a sharp decrease in the amount of the RAR-fusion proteins expressed in the cells. Our findings suggest that the oncogenicity of RAR-fusion proteins results from their nature to behave as unliganded RAR in the presence of physiological concentrations of retinoic acid.
ACUTE PROMYELOCYTIC leukemias (APLs) are characterized by an early block in myeloid maturation and are associated with chromosomal translocations that disrupt the retinoic acid receptor α (RARα) gene located on chromosome 17 (q21). RARα is a member of the retinoic acid receptor (RAR) family that comprises two other isotypes, RARβ and RARγ. These receptors share marked structural similarity in their ligand and DNA binding domains but display a distinct tissue-specific pattern of expression1,2 and control the expression of different subsets of target genes.3 When dimerized with a retinoid X receptor (RXR), RARs bind to specific promoter sequences of downstream genes and activate their transcription in the presence of alltrans retinoic acid (RA) or 9-cis RA. In the absence of ligand, RAR/RXR heterodimers interact with nuclear receptor corepressors (termed SMRT and N-CoR) that recruit histone deacetylases which induce chromatin modifications and repression of transcription.4 RA has a broad range of effects during embryogenesis as well as on adult tissues and, in the case of the hematopoietic compartment, RARα appears to be the principal mediator of RA’s activity. For example, a mutation in RARα was shown to block the granulocytic differentiation of the HL-60 promyelocytic cell line in response to RA,5 and the expression of a dominant negative mutant, RARα403, in murine bone marrow cells leads to the immortalization of a multipotent hematopoietic progenitor.6
The vast majority of APLs are associated with a t(15;17) chromosomal translocation that fuses RARα to the PML (promyelocytic leukemia) gene. These leukemias are characterized by their sensitivity to RA therapy. PML is a ubiquitous phosphoprotein whose expression is induced by interferons and acts as a growth suppressor in several cell lines.7,8 It is concentrated within poorly understood, discrete, nuclear compartments called nuclear bodies or PODs (PML oncogenic domains) that are disrupted in APLs associated with the t(15;17) translocation. The chimeric protein PML-RARα retains the DNA-binding and ligand-binding domains of RARα and the amino-terminal portion of PML. Transfection studies have shown that PML-RARα can antagonize the transactivation function of wild-type RARα on RA-inducible promoters.9-11 Moreover, PML-RARα can heterodimerize with RXRs and PML, and this could lead to an interference with the normal function of these proteins as well.
Three variant chromosomal translocations have been identified in APLs that result in the fusion of RARα with PLZF, NPM, or NuMA.12-14 All of the different fusion proteins retain the same portion of RARα. The PLZF (promyelocytic leukemia zinc finger) gene located on band 11q23 encodes a protein that binds to specific DNA sequences and represses transcription by interacting with the corepressor SMRT or N-CoR.11,15-17 PLZF shares some common features with PML: it can inhibit proliferation of cell lines18 and it localizes in nuclear structures similar to PML nuclear bodies.19 Unlike all other APLs, those associated with the PLZF-RARα fusion protein respond poorly to RA therapy. The two other RARα fusion partners that have been cloned in APLs present little similarities with PML or PLZF; NPM (nucleophosmin) is a ubiquitously expressed RNA-binding nucleolar phosphoprotein and NuMA is a nuclear mitotic apparatus protein. The only apparent common denominators among these four partners are their nuclear localization and their ability to participate in protein-protein interactions.
The causal role of PML-RARα and PLZF-RARα in the pathogenesis of APLs has been demonstrated in several transgenic mouse models.16,20-22 However, these in vivo studies are impractical to analyze a large series of mutants, and most of the work performed to address the molecular mechanisms of cellular transformation by PML-RARα and PLZF-RARα has relied upon the U937 promonocytic cell line that can be induced to differentiate in culture. In this cellular system, both fusion genes are capable of blocking differentiation, but whereas the antidifferentiative effect of PML-RARα is abolished in the presence of pharmacological doses of RA, the action of PLZF-RARα is resistant to RA.23,24 Analyses of mutants of PML-RARα in U937 cells have provided important insights into the structure-function relationships of this oncogene.25 26 However, the U937 cell line that requires vitamin D3 and transforming growth factor β to differentiate is a somewhat artificial system and the abnormal growth of these immortalized cells may interfere with the biologic activities of the RARα-fusion proteins. This raises the question of the relevance of such a cellular model to elucidate the oncogenic processes that take place in APLs. To meet the need of a more physiological system, we have expressed RARα-fusion genes in normal murine bone marrow cells using retroviral vectors and characterized the effect of these genes on the differentiation and the proliferation of myeloid progenitors in the presence or absence or RA. We have compared PML-RARα, PLZF-RARα, NPM-RARα, the dominant negative mutant RARα403 (a truncated version of RARα that is interrupted in the C-terminal ligand-binding domain), and wild-type RARα in murine hematopoietic progenitors. We provide here the first experimental demonstration of the transforming properties of the NPM-RARα fusion protein in primary cells. Our findings also point to the central role of RARα deregulation in the pathogenesis of APLs and support the hypothesis that the main contribution of the PML, PLZF, and NPM partner genes may be to abolish the sensitivity of the resulting fusion proteins to physiological concentrations of RA.
MATERIALS AND METHODS
Design and production of retroviral constructs.
All cDNAs were cloned in the poly-linker upstream of the PGK-neo cassette of the MSCVneoEB retroviral vector.27 The 1.7-kbEcoRI fragment encoding human RARα was derived from the pSG5-RARα vector. The RARα403 mutant was generated by inserting a universal termination Xba I linker (Biolabs, Beverly, MA) between the Sma I sites in the human RARα cDNA. The 3087 nucleotide (nt) EcoRI-Sca I PML-RARα fragment was derived from the pSG5-PML-RARαL vector.9 A 2,980-ntEcoRI fragment encoding PLZF-RARα was generated from the pGEM vector. The integrity of the first 700 nt of the PLZF-RAR coding sequence was confirmed by Dye Terminator (PE Applied Biosystems, Foster City, CA) sequencing reactions performed and processed on an ABI 377 automated sequencer (PE Applied Biosystems) using an oligo upstream of the cloning site in the MSCV vector and PLZF oligos (5′-cctcttccaccgcaatag-3′; 5′-gcctccgtgtcattgtcg-3′; and 5′-ggaagtccaaagtatagtgttgac-3′). The NPM-RARα construct was based on the NPMl-RARα cDNA encoded by a 2.0-kbBamHI fragment derived from a pSG5 vector.13 In pilot studies, we compared the transforming properties of NPMs-RARα and NPMl-RARα, short and long form, respectively,13 but found that these were very similar, so we only included the NPMl-RARα form in our subsequent work, which is referred to as NPM-RARα in this report. A partially digested EcoRI-BamHI 1.4-kb fragment encoding human RARβ was derived from pCOD20 plasmid.28 A 1.9-kbEcoRI fragment encoding murine RARγ was derived from a pSG5 vector. A 1.7-kb EcoRI fragment encoding human RXRα was derived from a PTZ18 plasmid. The MIE vector was engineered by exchanging the NFGR cDNA with the EGFP (enhanced green fluorescent protein) cDNA (Clontech, Palo Alto, CA) in the MIN vector.29 Retroviral supernatants were produced from transiently transfected Bosc23 ecotropic packaging cells,30as described.31
Infection of primary myeloid progenitors and methylcellulose colony-forming assays.
Enrichment of primitive progenitors from bone marrow of 5-fluorouracil–treated BA.1 mice by magnetic bead depletion was performed as described.29 The panel of lineage antibodies was directed against the following antigens: CD5, CD8a, CD11b (Mac-1), Gr-1, and B220 (Pharmingen Inc, San Diego, CA). Methods used to transduce the progenitors and methylcellulose culture conditions were described previously.31 Between 500 and 15,000 cells were plated per dish, depending on the viral vector used (a higher number of cells transduced with PML-RARα were seeded to compensate for the lower titer generated with that construct). Where indicated, RA (1 μmol/L, 0.1 μmol/L, 10 nmol/L, or 1 nmol/L; Sigma, St Louis, MO) and/or trichostatin A (TSA; 20 ng/mL; Wako Bioproducts, Richmond, VA) were added. We chose to use TSA at a concentration of 20 ng/mL, because we found that higher doses were toxic to the cell lines immortalized by the different RARα fusion genes. All methylcellulose cultures were set up in triplicates, and each data point corresponds to the mean number of colonies scored after 7 days of culture.
Establishment of immortalized cell lines in liquid culture.
Cells from third passage methylcellulose dishes containing 50 to 300 colonies were harvested and seeded in RPMI 1640 medium containing 20% horse serum plus supplements (50 U/mL penicillin G, 50 μg/mL streptomycin, 2 mmol/L L-glutamine, and 0.05 mmol/L 2-mercaptoethanol) in the presence of recombinant murine interleukin-3 (IL-3; 10 ng/mL; R&D Systems, Minneapolis, MN) and stem cell factor (100 ng/mL; Systemix, Palo Alto, CA). Nonadherent cells were split into fresh media approximately once per week and were maintained for more than 12 months.
Assessment of myeloid differentiation.
For morphological analysis, cells in liquid culture were spun in a cytocentrifuge onto glass slides and stained with Wright-Giemsa. Immunophenotypic analysis of cells harvested on day 7 of methylcellulose culture in the presence of G418 was performed by staining with phycoerythrin (PE)-conjugated anti–Gr-1 and fluoresceine isothyocyanate (FITC)-conjugated anti–Mac-1 antibodies (Pharmingen Inc), as described.29 Cells were labeled with isotype controls IgG2b-PE and IgG2b-FITC to set the quadrants delimiting the positive populations.
Western blot analysis of protein expression.
Whole cell extracts were made from G418-resistant primary methylcellulose colonies pooled after 8 to 10 days of culture or from immortalized cells grown in suspension. Protein quantification was performed using the BCA reagent (Pierce, Rockford, IL). Protein samples (20 μg per lane) were separated on 4% to 12% polyacrylamide precast gels (Novex, San Diego, CA). After electrophoretic transfer, the nitrocellulose membranes were blocked with 10% skimmed milk and incubated with rabbit polyclonal serum raised against human RARα, RARβ, RARγ, or RXRα (Santa Cruz Biotechnologies, Santa Cruz, CA). Proteins were detected with horseradish peroxidase-conjugated protein A (Amersham, Arlington Heights, IL) using a standard chemiluminescence Western blotting protocol (ECL system; Amersham).
RESULTS
Expression of RARα fusion genes in primary murine myeloid progenitors transduced with retroviral vectors.
To determine the transforming properties of APL-derived RARα fusion genes, we cloned PML-RARα, PLZF-RARα, and NPM-RARα cDNAs in the MSCV (murine stem cell virus) retroviral vector27 under the control of the viral long terminal repeat (LTR) and upstream of an internal phospho glycerate kinase (PGK)-neomycine cassette. High-titer retroviral supernatants were generated by transient transfection of the Bosc23 packaging cells.30 This method of viral production does not require the selection of stably transfected packaging cells and therefore minimizes the risk of generating retroviral vectors with acquired mutations. This is crucial when the encoded genes have toxic properties, as is the case for PML-RARα.32-34 As a positive control, we transduced cells with the dominant negative mutant RARα403,35 which can immortalize primary murine bone marrow cells.6 We also compared in our assay the overexpression of wild-type RARα, RARβ, RARγ, and RXRα. With the exception of PML-RARα, the recombinant retroviral supernatants generated with the different transgenes were similar in their efficiency to transduce hematopoietic progenitors. The percentage of G418-resistant colonies was in the range of 10% to 15%, with the PML-RARα encoding vector, whereas it reached 70% to 100% with all of the other vectors. The inferior titer of the PML-RARα vector is most likely due to the toxic properties of PML-RARα that may hinder the growth of most of the PML-RARα–transduced colony-forming cells.
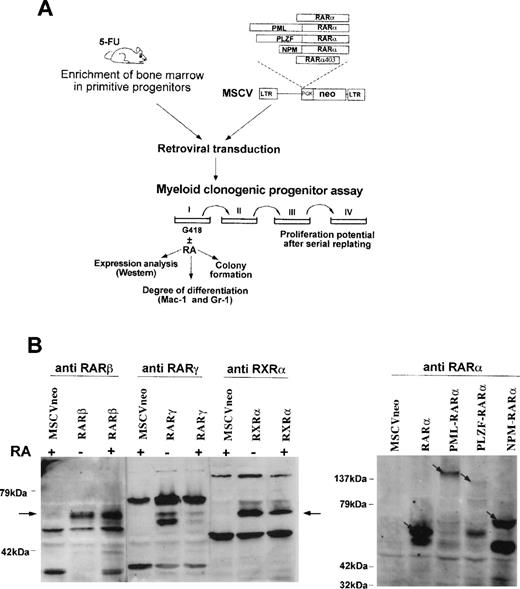
To study the biological effects of these chimeric genes in primary murine hematopoietic cells, we used a myeloid transformation assay similar to that which we developed to dissect the transforming properties of another leukemic fusion gene, HRX-ENL.29 31The experimental strategy is shown in Fig1A. In brief, bone marrow cells from mice treated with 5-fluorouracil and further enriched in primitive progenitors by depletion of cells expressing markers of terminal differentiation were transduced with the recombinant retroviral supernatants and seeded in methylcellulose myeloid cultures in the presence of G418. The primary colonies were scored after 7 days of growth, and the cells from pooled colonies were harvested and seeded into secondary methylcellulose cultures. The cultures were serially replated in a total of four successive rounds and the proliferative potential of the transduced cells was assessed by scoring the number of colonies formed at each passage. To determine how the transduced genes affected myeloid maturation, we analyzed the expression of the differentiation markers Mac-1 and Gr-1 on G418-selected cells harvested from the primary colonies using flow cytometry.
Experimental strategy and protein expression in primary hematopoietic progenitors.(A) Scheme of the experimental approach used to study the effect of RAR-fusion genes on the proliferation and myeloid differentiation of the target cells and to evaluate the responsiveness of the transformed cells to retinoic acid. (B) Western blot analysis of cells transduced with the different vectors after 8 days of culture in methylcellulose (20 μg of protein was loaded per lane). When indicated, the cells were grown in the presence of RA (1 μmol/L). Arrows point to exogenously expressed proteins detected with the specific antihuman retinoid receptor antibodies indicated above each panel.
Experimental strategy and protein expression in primary hematopoietic progenitors.(A) Scheme of the experimental approach used to study the effect of RAR-fusion genes on the proliferation and myeloid differentiation of the target cells and to evaluate the responsiveness of the transformed cells to retinoic acid. (B) Western blot analysis of cells transduced with the different vectors after 8 days of culture in methylcellulose (20 μg of protein was loaded per lane). When indicated, the cells were grown in the presence of RA (1 μmol/L). Arrows point to exogenously expressed proteins detected with the specific antihuman retinoid receptor antibodies indicated above each panel.
The expression of the appropriate proteins was shown by Western analysis performed on cells from pooled G418-resistant colonies (see Fig 1B). The lack of antibodies directed against epitopes conserved in the RARα403 mutant precluded protein expression analysis of cells transduced with this construct. We noted that cells transduced with the RARα or the NPM-RARα constructs expressed consistently much higher levels of the transgene than cells transduced with the PML-RARα or the PLZF-RARα fusion genes. Because this cannot solely be accounted for by a difference in viral titer, it must also reflect a toxicity of PML-RARα and PLZF-RARα or variations in expression at the RNA and/or protein level.
RARα and RARα mutant genes affect the differentiation and proliferation of myeloid progenitors.
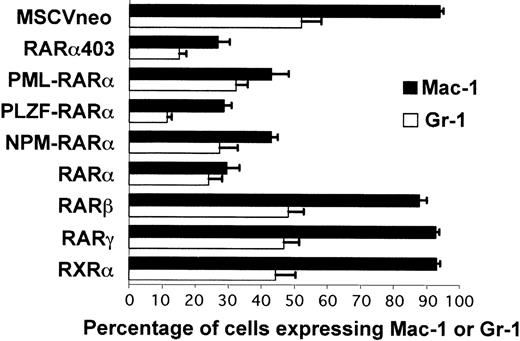
The methylcellulose cultures were performed in the presence of IL-3, IL-6, stem cell factor, and granulocyte-macrophage colony-stimulating factor (GM-CSF); a combination of cytokines that stimulates myeloid cell growth and differentiation resulting in the formation of granulocytic and/or macrophagic colonies. Under these conditions, cells that are initially negative for the expression of lineage markers will mature and generate colonies primarily composed of differentiated cells. Thus, after 7 days of culture, more than 95% of the cells mock-transduced (not shown) or infected with the MSCVneo control vector expressed Mac-1 and/or Gr-1. We observed that this myeloid maturation was greatly impaired when progenitors were transduced with vectors encoding RARα or either of the RARα mutants (Fig 2). The block in differentiation was most striking in cells transduced with PLZF-RARα, RARα403, or RARα, with less than 30% of the cells expressing Mac-1 or Gr-1. PML-RARα and NPM-RARα had a milder effect, with an average of 40% to 50% mature myeloid cells present in the culture. This impairment of differentiation was not observed in cells transduced with RARβ, RARγ, or RXRα, which were indistinguishable from the control MSCVneo-infected cells.
RAR and RAR403 and RAR-fusion genes inhibit myeloid differentiation of primary progenitors. The bars represent the percentages of cells transduced with the different vectors that express Mac-1 and Gr-1 after 7 days of methylcellulose cultures. Each value is the average of 5 to 7 independent transduction experiments (±SEM).
RAR and RAR403 and RAR-fusion genes inhibit myeloid differentiation of primary progenitors. The bars represent the percentages of cells transduced with the different vectors that express Mac-1 and Gr-1 after 7 days of methylcellulose cultures. Each value is the average of 5 to 7 independent transduction experiments (±SEM).
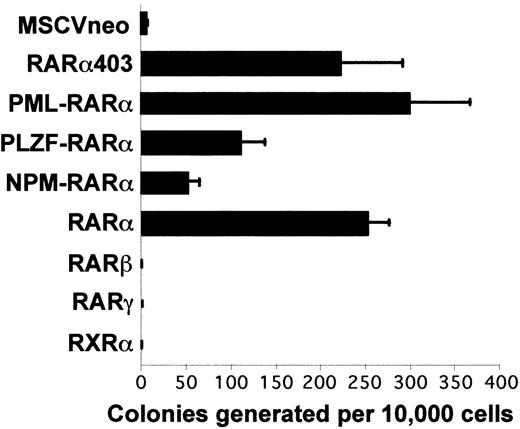
To compare the effect of the different transgenes on proliferation, we measured the colony-forming ability of the transduced cells along serial passages in methylcellulose culture. The cloning efficiency observed upon tertiary plating is represented in Fig 3. As previously observed, cells transduced with the control MSCVneo vector had limited replating ability and very few colonies of more than 100 cells were generated in tertiary methylcellulose cultures.29 The progenitors transduced with RARβ, RARγ, and RXRα similarly exhausted their proliferative potential upon serial replating. In sharp contrast, retroviral transfer of RARα or the RARα mutants enabled the cells to remain highly proliferative. The progenitors transduced with RARα, RARα403, or PML-RARα had the highest cloning efficiency, generating 200 to 300 colonies per 10,000 seeded cells; progenitors transduced with PLZF-RARα had a significantly lower plating efficiency, generating about 130 colonies per 10,000 cells, and NPM-RARα-transduced progenitors formed only about 50 colonies per 10,000 cells. Furthermore, the colonies generated with NPM-RARα transduced progenitors were smaller than those obtained with the other constructs (data not shown). This sustained replatability in semisolid culture correlated with an unlimited growth potential in vitro, because the cells transduced with RARα or either of the RARα mutant genes could be transferred from pooled third passage methylcellulose colonies into suspension cultures and maintained for more than 1 year in liquid media supplemented with IL-3 and stem cell factor.
RAR and RAR403 and RAR-fusion genes induce the proliferation in vitro of primary myeloid progenitors. Each bar represents the number of colonies per 10,000 input cells in a third passage of methylcellulose culture (mean of 4 to 9 independent experiments ± SEM)
RAR and RAR403 and RAR-fusion genes induce the proliferation in vitro of primary myeloid progenitors. Each bar represents the number of colonies per 10,000 input cells in a third passage of methylcellulose culture (mean of 4 to 9 independent experiments ± SEM)
Effects of RA on cells transformed by RARα or RARα mutants.
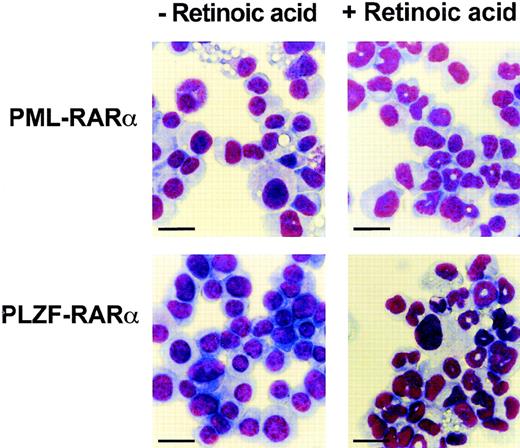
We first studied the effect of RA on the growth and differentiation of the immortalized cell lines established with RARα or the various RARα mutants. In all of the cell lines, RA treatment (1 μmol/L) strongly inhibited cellular growth (data not shown). This was accompanied by a loss of viability of the cells expressing RARα, RARα403 and NPM-RARα, and in the cells transduced with PML-RARα or PLZF-RARα, a clear induction of granulocytic differentiation was observed (Fig 4).
Cells immortalized by PML-RAR or PLZF-RAR undergo granulocytic maturation in response to RA. Wright Giemsa stain of cytospin preparations made from cells grown in the absence (left) or in the presence (right) of RA (1 μmol/L) for 5 days. Bar = 20 μm.
Cells immortalized by PML-RAR or PLZF-RAR undergo granulocytic maturation in response to RA. Wright Giemsa stain of cytospin preparations made from cells grown in the absence (left) or in the presence (right) of RA (1 μmol/L) for 5 days. Bar = 20 μm.
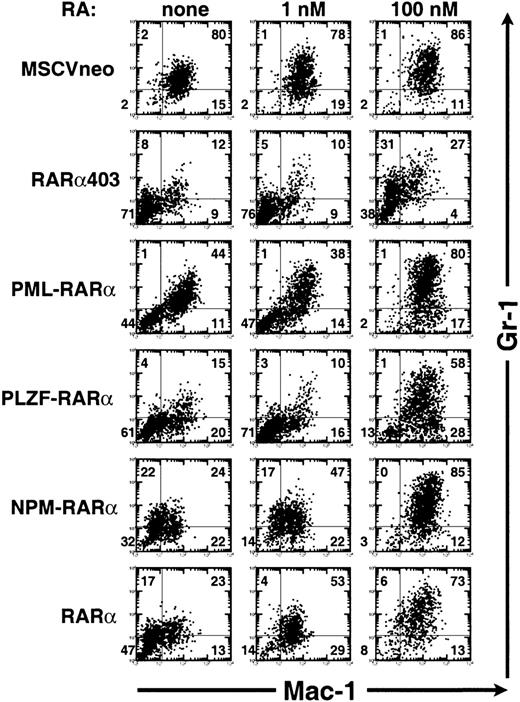
To further characterize the effect of RA on myeloid cell growth and differentiation, we studied its effects on freshly transduced primary progenitors in methylcellulose culture. After retroviral transduction, lineage-depleted bone marrow cells were split in G418-containing methylcellulose cultures in the absence or presence of RA (1 or 100 nmol/L). The effect of RA on myeloid differentiation was assessed by analyzing the profile of Mac-1 and Gr-1 expression in cells harvested from the primary colonies. The results of a representative experiment are shown in Fig 5. RA treatment of cells transduced with the control MSCVneo vector did not significantly alter the percentage of cells expressing Mac-1/Gr-1, although an increase in the intensity of Gr-1 expression was noted. Similar results were found in cells expressing RARβ, RARγ, or RXRα (data not shown). RARα403-transduced cells exposed to RA at 100 nmol/L concentration underwent a moderate degree of differentiation, with more than 50% of the cells expressing Gr-1. On the other hand, RA-treatment (100 nmol/L) of the cells transduced with RARα, PML-RARα, PLZF-RARα, or NPM-RARα induced an almost complete differentiative response accompanied by the expression of Mac-1 on more than 85% of the cells and an upregulation of Gr-1. Remarkably, the maturation of the PLZF-RARα–expressing cells induced by 100 nmol/L RA was comparable to that seen in cells expressing the other RARα-fusion genes, and in the presence of 1 μmol/L RA, the Mac-1/Gr-1 profile of the PLZF-RARα-cells was indistinguishable from that of the cells expressing the other fusion genes (data not shown). At a dose of 1 nmol/L of RA, a concentration within the physiological range, the cells expressing RARα403 or either of the fusion proteins remained blocked at an immature myeloid stage, whereas the differentiation block of the RARα-transformed cells was essentially abolished (compare 47% of Mac-1−/Gr-1− cells v 14% of Mac-1−/Gr-1− cells in the presence of 1 nmol/L RA on Fig 5).
RA triggers the myeloid maturation of progenitors transformed by RAR, PML-RAR, PLZF-RAR, or NPM-RAR. Analysis of the expression of Mac-1 (horizontal axis) and Gr-1 (vertical axis) by flow cytometry in cells harvested after 7 days of methylcellulose culture in the absence of exogenous RA (left column) or in the presence of 1 nmol/L RA (middle column) or 100 nmol/L RA (right column). These are the results of a representative analysis; similar findings were obtained in three independent transductions experiments.
RA triggers the myeloid maturation of progenitors transformed by RAR, PML-RAR, PLZF-RAR, or NPM-RAR. Analysis of the expression of Mac-1 (horizontal axis) and Gr-1 (vertical axis) by flow cytometry in cells harvested after 7 days of methylcellulose culture in the absence of exogenous RA (left column) or in the presence of 1 nmol/L RA (middle column) or 100 nmol/L RA (right column). These are the results of a representative analysis; similar findings were obtained in three independent transductions experiments.
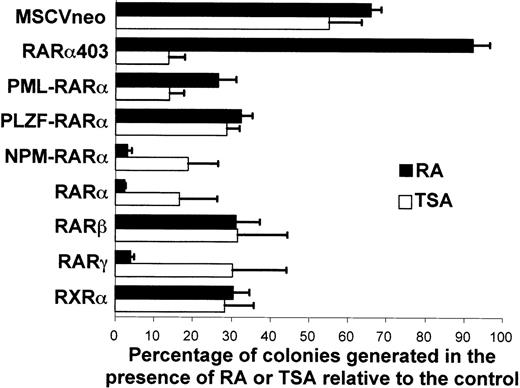
The effect of RA on the proliferation of the myeloid progenitors transduced with the different vectors was determined by scoring the number of primary colonies formed in the presence of RA (1 μmol/L) relative to the number of colonies formed in its absence (Fig 6). The clonogenicity of the cells transduced with the empty MSCVneo vector was substantially diminished by RA, with a 35% decrease in the number of colonies formed. The negative effect of RA on cellular proliferation was much more pronounced in cells transduced with RARα or either of the RARα-fusion genes, because the inhibition in the colony formation potential reached approximately 70% (PML-RARα and PLZF-RARα) or more than 95% (RARα and NPM-RARα) in the presence of RA. On the other hand, the proliferative potential of the cells transduced with RARα403 was not significantly affected by the addition of RA as the reduction in plating efficiency was less than 5%. This indicates that RARα403 actually had protective properties against the antigrowth effect of RA in myeloid progenitors. The overexpression of RARβ, RARγ, or RXRα increased the cells’ sensitivity to the antiproliferative effect of RA to comparable degrees as did RARα or the RARα-fusion mutants. Similar results were obtained in the presence of 100 nmol/L of RA (data not shown). In the presence of physiological concentrations of RA (1 nmol/L), no significant reduction in the number of colonies was observed in culture of cells transduced by either RARα403 or the RARα-fusion genes. On the other hand, cells overexpressing wild-type RARα generated on average twofold less colonies in the presence of 1 nmol/L RA relative to the colonies formed without exogenous RA (data not shown).
Effect of RA and TSA on the clonal proliferation of primary myeloid progenitors transduced with RAR, RAR403, RARβ, RARγ, RXR, or RAR-fusion genes. The number of primary colonies formed in the presence of RA (▪) or TSA (□) is represented as a percentage of the numbers of colonies formed in the absence of both RA or TSA (mean of 3 experiments ± SEM).
Effect of RA and TSA on the clonal proliferation of primary myeloid progenitors transduced with RAR, RAR403, RARβ, RARγ, RXR, or RAR-fusion genes. The number of primary colonies formed in the presence of RA (▪) or TSA (□) is represented as a percentage of the numbers of colonies formed in the absence of both RA or TSA (mean of 3 experiments ± SEM).
Our finding that RA reverses the transforming effects of PLZF-RARα in bone marrow cells appears to be in contradiction to previous reports on the lack of response of U937 cells or transgenic mice expressing PLZF-RARα.16,24 To rule out the possibility that this discrepancy may be the result of mutations in our cDNA, we sequenced a region of the MSCV-PLZF-RARα vector that encompasses the POZ domain of PLZF (see Materials and Methods) that mediates interaction with corepressors17 and confirmed that all of the nucleotides were conserved.
Reports that inhibitors of histone deacetylases, such as TSA, could enhance the differentiative effect of RA in cells transformed by PML-RARα or PLZF-RARα by relieving the repressive effect of the fusion proteins on transcription11,16,26 prompted us to explore the effect of TSA in our assay. After retroviral infection, the cells were seeded in methylcellulose media with or without the addition of TSA with or without RA, and the cellular proliferation and differentiation were monitored. TSA alone inhibited the proliferation of all of the cells, regardless of the nature of the genes encoded by the MSCV vector (Fig 6), and resulted in a reduction of colony formation of approximately twofold. However, the antigrowth effect on cells transduced with RARα or either of the RARα-fusion genes was more pronounced (between 70% and 90% reduction in colony formation). The combined treatment of TSA and RA (at either 1 μmol/L or 100 nmol/L) further reduced the cloning potential of the cells (data not shown). To determine if TSA induced the differentiation of the cells transduced with the different constructs, we monitored the effect of TSA on the expression of Mac-1 and Gr-1 but found that TSA did not induce myeloid maturation or enhance the differentiative effect of RA (data not shown). Because inhibitors of histone deacetylases have been reported to enhance expression of integrated retroviral sequences,36 we were concerned that this phenomenon may be occurring and contributing to the lack of differentiative activity of TSA in our system. To test this, we transduced myeloid progenitors with a MSCV vector encoding the green fluorescent protein (MIE vector, see Materials and Methods) and examined by flow cytometry how the expression of this marker was affected by TSA. With this sensitive technique, we did not detect any upregulation of the transduced gene (data not shown), making it unlikely that variations in retroviral expression mask the effects of TSA on cellular differentiation per se.
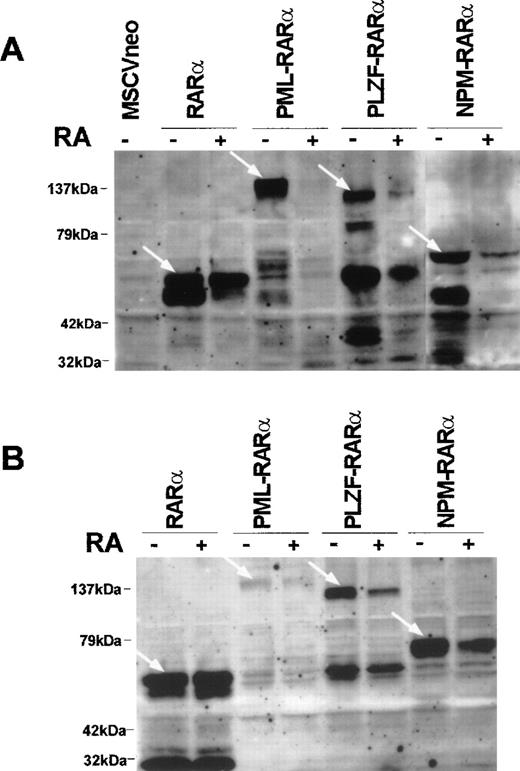
To determine whether the reversal of the transformed phenotype induced by RA (ie, the reduction in the proliferative potential and the induction of myeloid differentiation) was associated with a variation in the expression of RARα or of the RARα-fusion proteins, we performed Western blot analyses on the transduced cells grown in the absence or presence of RA. As depicted in Fig 7A, the levels of PLZF-RARα, PML-RARα, and NPM-RARα proteins were reduced in cells grown in methylcellulose containing RA (1 μmol/L) compared with cells cultured without exogenous RA. However, in cells overexpressing wild-type RARα, no overt alteration in protein level was induced by RA exposure. We also examined the effect of RA on the protein-expression levels of RARβ, RARγ, and RXRα and found that the level of RARγ was reduced in cells treated with RA, whereas the levels of RARβ or RXRα remained constant (see Fig 1B). The reduction of the RARα-fusion gene products observed in the transduced population could result from a selective antigrowth effect of RA towards the cells expressing higher amounts of the transgene. To explore this possibility, we examined the effect of RA on the stable cell lines immortalized with either RARα or the RARα fusion mutants, which can be assumed to express uniform levels of the transgene as these cell lines were found to be monoclonal based on their proviral integration pattern (data not shown). We analyzed the protein levels in the cell lines 40 hours after supplementing the suspension cultures with 1 μmol/L RA and found the same pattern as that displayed by freshly transduced bone marrow (Fig 7B). Altogether, our results show that the inhibition of growth and the induction of myeloid differentiation observed in primary progenitors transformed with RARα-fusion genes, in response to RA treatment, are accompanied by a reduction in the expression levels of the RARα-fusion proteins.
RA induces the degradation of PML-RAR, PLZF-RAR, and NPM-RAR. (A) Western blot analysis of progenitors cultured in methylcellulose for 8 days in the absence or presence of RA (1 μmol/L) after transduction with RAR (15 μg of protein per lane), PML-RAR (30 μg of protein per lane), PLZF-RAR (40 μg of protein per lane), or NPM-RAR (20 μg of protein per lane). (B) Western blot analysis of cell lines established with RAR or RAR-fusion gene treated or not treated with RA (1 μmol/L) for 40 hours (20 μg of protein per lane). The transduced proteins are designated by the white arrows.
RA induces the degradation of PML-RAR, PLZF-RAR, and NPM-RAR. (A) Western blot analysis of progenitors cultured in methylcellulose for 8 days in the absence or presence of RA (1 μmol/L) after transduction with RAR (15 μg of protein per lane), PML-RAR (30 μg of protein per lane), PLZF-RAR (40 μg of protein per lane), or NPM-RAR (20 μg of protein per lane). (B) Western blot analysis of cell lines established with RAR or RAR-fusion gene treated or not treated with RA (1 μmol/L) for 40 hours (20 μg of protein per lane). The transduced proteins are designated by the white arrows.
DISCUSSION
Most of the studies performed to unravel the oncogenic processes involved in APL have relied on stable clones derived from U937 cells transfected with PML-RARα or PLZF-RARα.23,24,26 This cellular tool has been exploited to conduct structure-function analyses with a series of deletion mutants of PML-RARα and has yielded important information. However, studies in a biologically more relevant system are required before conclusions can be drawn on the mechanisms at play in the pathogenesis of APLs. Recently, several laboratories have generated PML-RARα transgenic mice that develop a myeloproliferative disease that progresses to acute myeloid leukemia reactive to RA therapy.20-22 These works have provided formal evidence of the decisive role of PML-RARα in the development of APLs; however, only a minority of mice developed the disease and generally after a long latency, indicating that other events were necessary for full transformation in these models. The experimental approach we present here offers several advantages over those systems. Because we are working with primary bone marrow cells that have not been exposed to other transforming agents, we are able to study the effect of the introduced genes not only on the differentiation, but also on the proliferation potential ex vivo of the transduced progenitors. Furthermore, the target cells are analyzed immediately after transduction, making this system a one-step transformation assay in which the occurrence of other confounding events is very unlikely. Finally, our assay lends itself to structure-function analyses in which series of mutants can be compared with respect to their effects on myeloid differentiation, cellular proliferation, and sensitivity to RA as well as to other potential therapeutic agents.
The aim of our study was to analyze the proliferation and differentiation potential of primary myeloid progenitors expressing RAR-mutants or wild-type RAR and RXR proteins. The major finding in the present work is that overexpression of wild-type RARα can alter myeloid cell growth and differentiation in vitro. This somewhat surprising result has been reported previously. Onodera et al37 showed that transduction of RARα in murine bone marrow blocked maturation at the promyelocyte stage in vitro, and transfection studies performed in U937 cells also showed that RARα could partially impair the differentiation of this cell line.25 The novelty of our study resides in the important finding that this effect of wild-type RARα is abolished in the presence of physiological concentrations of RA. This indicates that this phenomenon is dependent on the experimental conditions used, implying that, in contrast to RARα mutants, overexpression of wild-type RARα in vivo would unlikely be associated with abnormal myeloid growth. But it also sheds light on its molecular basis, suggesting that the transforming potential of RARα coincides with its function as a transcriptional repressor. Interestingly, a recent study has shown that, in media depleted of T3 hormone, overexpression of wild-type thyroid hormone receptor (c-ErbA/TRα) promotes proliferation and arrests differentiation of primary avian erythroblasts similarly to that observed with the oncogene v-ErbA.38 Thus, it appears that a common mechanism for oncogenic transformation by altered nuclear receptors may be to mimic the activity of their normal counterpart in the nonliganded state.
Our observation that, unlike RARα, overexpression of RARβ or RARγ did not affect differentiation or proliferation in our assay reinforces the view that these events are predominantly regulated by target genes of RARα in myeloid cells. However, this repartition is not absolute, because overexpression of RARγ conferred a high sensitivity to the antiproliferative effect of RA (Fig 6), which was corroborated by the detection of a smaller amount of the RARγ protein in the population of transduced cells selected in the presence of RA (Fig 1B).
The fact that RARα and RARα403 are as potent as RARα-fusion proteins in blocking differentiation and immortalizing primary myeloid progenitors argues that the pathogenesis of APLs resides primarily in an alteration of the RARα signalling pathway and not from disruption of the normal function of the fusion partners. In particular, the absence of a significant proliferative advantage of the cells expressing PML-RARα or PLZF-RARα compared with cells overexpressing RARα (see Fig 3) undermines the hypothesis that a suppression of the antigrowth effects of PML or PLZF by a dominant negative effect of the fusion mutants contributes to the expansion of myeloid progenitors. The diversity among the four fusion partners of RARα cloned in APLs was a first indication that a repression in their normal function resulting from the haplo-insufficiency in their gene copy and/or a dominant negative effect of the fusion protein played little, if any, role in leukemogenesis. The recent finding that PML may be a mediator of RA signalling also suggests that a deregulation of the RA signalling pathway is critical in the genesis of APLs.39
Several mechanisms have been proposed to explain how RARα-fusion proteins disrupt the RA signalling pathway. The chimeras may titrate out RXR molecules and interfere with RAR/RXR signalling (this could also affect other pathways requiring the participation of RXR such as the one mediated by the vitamin D3 receptor/RXR heterodimer); the fusion proteins may bind to the promoter of RA-responsive genes and behave like constitutive repressors; the target gene specificity of the fusion proteins may be altered and result in the aberrant transactivation of proto-oncogenes40; the fusion genes may sequester proteins of the N-CoR or SMRT corepressor complexes that are also required for other inhibitors of cell growth that function through repression of transcription such as the MAD/MAX complex, the retinoblastoma protein, PLZF, or other yet to be discovered growth suppressors. Our study provides elements that help sort out which of these molecular scenarios are more likely involved in the genesis of APLs. The fact that, unlike RARα, overexpression of RARβ and RARγ does not transform the cells argues against the possibility that the titration of RXR molecules is the key event in transformation, because RARβ and RARγ should heterodimerize with RXR with similar affinity as RARα. For the same reason, it seems improbable that squelching of corepressors by RARα/RXR or RARα mutant/RXR heterodimer is essential, although we cannot rule out that, in myeloid progenitors, RARα/RXR dimers interact with corepressors in a distinct manner compared with RARβ/RXR or RARγ/RXR dimers.41Transformation by RARα also indicates that leukemogenesis does not require the transactivation of aberrant target genes by the fusion proteins. Our results are compatible with the hypothesis that, like cells overexpressing RARα in the quasi-absence of RA, the transformation of cells expressing RARα-fusion proteins results from a transcriptional repression of RARα target genes. However, definite confirmation of this hypothesis would require additional study, such as using our assay to test mutant fusion proteins that can no longer bind to DNA or to corepressors.
The behavior of PML-RARα, PLZF-RARα, and RARα403 as constitutive transcriptional repressors of RA-target genes in the presence of physiologic concentrations of RA has been documented and has been attributed to the persistent binding of the mutant proteins to transcriptional corepressors.11,15,16,26,42 However, these studies showed that, unlike what was observed with PML-RARα, the repressing activity of PLZF-RARα persisted in the presence of pharmacological concentrations of RA. This was paralleled by the lack of differentiative response of PLZF-RARα–transformed U937 or transgenic mouse bone marrow cells to RA stimulation16,24; simultaneous RA and TSA treatment was necessary to trigger differentiation of these cells. In contrast, we found that PLZF-RARα–expressing cells were as responsive to the differentiative and antigrowth effects of RA as were cells expressing PML-RARα. This was observed both on stable PLZF-RAR cell lines in liquid culture (Fig4) and on freshly transduced progenitors in methylcellulose (Fig 5). It is possible that this discrepancy between our findings and previous reports is due to disparities between experimental models. However, our approach is similar to the transgenic model in that both are based on murine hematopoietic precursors and RA did have some therapeutic activity in the PLZF-RARα mice when used at high dosage.16 It is noteworthy, nevertheless, that the leukemias developing in the PLZF-RARα transgenic mice have a more mature phenotype (they express high levels of Mac-1 and Gr-1) compared with our PLZF-RARα–transduced primary cells (judging by the profiles of Mac-1 and Gr-1 expression, Figs 2 and 5). This difference in the degree of myeloid maturation may account for distinct sensitivities to the differentiative effect of RA similar to what has been described for normal murine hematopoietic progenitors.43 44Alternatively, the combination of growth factors used in our cultures may provide a costimulatory boost to the differentiative effect of RA strong enough to override the block exerted by PLZF-RARα.
The RA-sensitivity of PLZF-RARα transformed cells in our assay may also appear in contradiction with the poor responsiveness of PLZF-RARα APLs to RA therapy. However, in these leukemias, the reciprocal RARα-PLZF fusion product, which has been shown to have oncogenic properties (C.L. and J. Licht, unpublished data), is always expressed and may account for the lack of therapeutic effect of RA. Furthermore, complete remission has been reported in a case of PLZF-RARα APL treated with a combination of RA and granulocyte-colony-stimulating factor, indicating that this type of leukemia is not intrinsically resistant to RA, provided that the proper costimulus is administered.45
In addition to reducing the transcriptional repression exerted by RARα-fusion proteins, the therapeutic activity of RA has been proposed to result from its ability to induce the degradation of the chimeric proteins in the leukemic cells. This has been documented in NB4 cells that express PML-RARα,46-48 and the induction of the catabolism of the fusion protein was shown to be mediated through the proteasome pathway.49 The degradation of the PLZF-RARα protein in response to RA treatment has also been reported.50 It is not clear whether the degradation of RARα-fusion proteins plays a causal role in the differentiative response to RA; however, it is unlikely that this phenomenon is secondary to myeloid differentiation, because it can also be observed in transfected fibroblasts treated with RA.50 The finding that another effective therapeutic agent in the treatment of APLs, arsenic trioxide, also triggers the catabolism of PML-RARα further supports the hypothesis that this phenomenon could underlie the therapeutic effect of RA in APLs.46 48 Our protein expression analyses support the view that a reduction in the levels of the RARα-fusion proteins contributes to the therapeutic effect of RA and show that this applies to the NPM-RARα chimera as well. This mechanism could in fact play a major role in the RA-induced differentiation of the cells transformed by PLZF-RARα, because biochemical and transcriptional studies have suggested that, because of the association of corepressors through the PLZF moiety of the chimera, this mutant’s ability to repress transcription is insensitive to RA.
The novel experimental system we have developed has allowed us to demonstrate that, in addition to blocking differentiation, RARα-fusion genes stimulate cellular proliferation. The RA-sensitive transforming effect of wild-type RARα suggests that the balance between transcriptional repression and transcriptional activation of RARα-target genes plays a key role in APL genesis. This cellular model will be of great utility to further dissect the mechanisms underlying APLs; it can be exploited to test RARα mutants that can no longer bind to DNA or to transcriptional cofactors and to identify target genes critical for cellular transformation.
ACKNOWLEDGMENT
The authors gratefully acknowledge M. Cleary for critical comments on this work, A. Dejean for initiating this project and providing molecular clones, and B. Ford and T. Austin for careful review of the manuscript. We are also indebted to P. Chambon and P.G. Pelicci for antibodies and cDNAs and to A. Gorvad for sequence analysis.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Catherine Lavau, PhD, Systemix Inc, Palo Alto, CA 94304; e-mail: catherine.lavau@pharma.novartis.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal