Abstract
Aberrant proliferation, differentiation, and/or migration of progenitors observed in various hematological malignancies may be caused by defects in expression and/or function of integrins. In this study, we have developed a new fluorescent beads adhesion assay that facilitates flow cytometric investigation of lymphocyte function-associated antigen 1 (LFA-1)– and very late activation antigen-4 (VLA-4)–mediated functional adhesion in B-lineage acute lymphoblastic leukemia (ALL) of both the CD10− and CD10+ (leukemic) cell population within one blood or bone marrow sample. Surprisingly, of the 20 B-lineage ALL patients investigated, 17 contained a leukemic cell population with LFA-1– and/or VLA-4–mediated adhesion defects. Five patients contained CD10+ cells that did not exhibit any LFA-1–mediated adhesion due to the lack of LFA-1 surface expression. The CD10+ cells from 10 ALL patients expressed LFA-1 that could not be activated by the phorbol ester phorbol 12-myristate 13-acetate (PMA), whereas the CD10− cells expressed a functional LFA-1. Seven patients contained CD10+ cells that expressed a PMA-unresponsive form of VLA-4. The PMA unresponsiveness of the integrins LFA-1 and VLA-4 expressed by the CD10+ cells may be due to mutations in the integrins itself, in protein kinases, or in other intracellular molecules involved in integrin adhesion. These data clearly demonstrate the importance of investigating integrin function in addition to integrin surface expression. The strikingly high frequency (85%) of adhesion defects in ALL could suggest a causal relationship between integrin-mediated adhesion and B-lineage ALL.
STEADY-STATE HEMATOPOIESIS occurs in the bone marrow (BM) microenvironment in which hematopoietic progenitor cells adhere selectively to stromal cells and extracellular matrix (ECM).1-3 In these BM niches, the proliferation, differentiation, and maturation of the progenitors is tightly regulated by growth factors, chemokines,4,5 and cell adhesion molecules that are expressed by progenitors as well as stromal cells.6-8 Cell adhesion molecules not only regulate the physical association of the progenitors with the BM microenvironment, but the binding of their counterreceptors also generate intracellular signals that could directly affect growth and maturation of the hematopoietic progenitors.9-12 Integrins, particularly those that belong to the β1-integrin (CD29)13,14 and β2-integrin families (CD18),15 16 represent an important group of cell adhesion molecules expressed by hematopoietic progenitors.
Among the β1-integrins expressed by hematopoietic cells, the very late activation antigen-4 (VLA-4; CD49d/CD29) and VLA-5 (CD49e/CD29) have been extensively studied.7,17,18 Both integrins bind to fibronectin, a major component of the ECM.19,20 VLA-4 is also able to bind to the vascular cell adhesion molecule 1 (VCAM-1), which is expressed by stromal cells.21 An important β2-integrin expressed by progenitor cells is the lymphocyte function-associated antigen 1 (LFA-1; CD11a/CD18).7,18,22LFA-1 mediates adhesion through binding of the intercellular adhesion molecule-1 (ICAM-1),23 ICAM-2,24and ICAM-3.25-27 Both ICAM-1 and ICAM-3 have been found on early hematopoietic cells,28 whereas stromal cells express ICAM-1 as well as ICAM-2 (R. Torensma, personal communications, January 1998).
The adhesive properties of hematopoietic cells are not only regulated by the expression levels of integrins and their ligands, but are also dependent on the activation of integrins by intracellular signaling, so-called inside-out signaling.29-31 Only activated β1- or β2-integrin molecules can interact with their ligands, demonstrating that integrin-mediated adhesion is a strictly regulated event. LFA-1 can be activated through intracellular signaling events triggered by a number of leukocyte surface receptors, such as the T-cell receptor, CD3, and CD19,30,32,33 or by the phorbol ester phorbol 12-myristate 13-acetate (PMA), which activates the protein kinase C cascade. Furthermore, integrin-mediated adhesion can also be stimulated by certain anti–β1-integrin or anti–β2-integrin antibodies that induce and stabilize active conformations of these integrins.34-38
The involvement of integrins in adhesion of hematopoietic progenitor cells to BM stroma has been assessed in several functional studies. Infusion of blocking anti–VLA-4 antibodies in primates causes mobilization of hematopoietic progenitors into the bloodstream.39 The interaction of VLA-4 with VCAM-1 mediates the binding of both primitive and committed progenitors to stromal cells,18,40 and antibodies to VLA-4 inhibit lymphopoiesis, myelopoiesis, and erythropoiesis in vitro.40-42 These studies implicate a critical role for VLA-4 in regulating the in vivo migration and trafficking of hematopoietic progenitors by providing interaction with the BM stromal cells.
In contrast to the role of the β1-integrins, little is understood about the role of the β2-integrin LFA-1 in hematopoiesis. Infusion of primates with blocking anti–LFA-1 antibody did not result in any mobilization of the progenitors into the bloodstream.39However, interleukin-8 (IL-8) and IL-1 mobilization of hematopoietic progenitor cells in mice could be inhibited by a single injection of anti–LFA-1 blocking antibodies,43 suggesting a role for LFA-1 in the localization of early hematopoietic cells in the BM microenvironment. LFA-1 may be a distinct regulator for growth and maturation of progenitor cells, because β2-integrins are involved in the formation of progenitor cell aggregates and can be activated by CD34-induced intracellular signals.44,45 Furthermore, the expression of LFA-1 on progenitor cells is highly variable and appears to be related to the maturation state of the progenitors.22 46
Interactions between hematopoietic progenitors and components of the BM microenvironment play a pivotal role in progenitor proliferation, differentiation, and migration, and adhesion molecules such as VLA-4, VLA-5, and LFA-1 are critical regulators of these interactions. Changes in expression and activation states of these adhesion molecules are likely to reflect diverse stages of hematopoiesis. Therefore, aberrant progenitor proliferation, maturation, and/or homing in various hematological malignancies may be correlated to defects in expression and/or function of cell adhesion molecules. The expression of various adhesion molecules in hematological malignancies has been extensively investigated, whereas functional studies on these molecules are very limited.47-50 In this study, we have analyzed both LFA-1– and VLA-4–mediated adhesion in B-lymphocyte acute lymphoblastic leukemia (ALL). ALL is a clonal hematopoietic disorder characterized by cell maturation arrest and accumulation of malignant lymphoblasts in marrow, lymphatic, and nonlymphatic tissues, and in most cases lymphoblasts migrate from the marrow into the peripheral blood. Aberrant ALL progenitor proliferation, differentiation, and homing could be correlated to altered adhesion properties of the ALL blasts. Even though the exact mechanisms of adhesion of ALL cells to BM stroma are not clear, integrins have been recognized to mediate those cellular interactions that are important in ALL biology.48 50-52
CD10 (common ALL antigen [CALLA]) is routinely used in the immunophenotyping of acute lymphatic leukemias as a marker for both cALL and pre-B-ALL. The CD10 antigen is also expressed on the cell surface of pre-pre-B, pre-B, and early-B cells during normal B-cell differentiation. Because healthy BM contains only a low percentage of these CD10+ cells, CD10 is an excellent marker to identify the leukemic (CD10+) cell population within cALL or pre-B-ALL. To study the integrin-mediated adhesion in ALL, we have developed a novel adhesion assay that allows rapid analysis of LFA-1– and VLA-4–mediated adhesion of large numbers of samples by flow cytometry. By using dual-color fluorescence analysis with CD10 as a marker, we were able to measure the adhesion of both the CD10− and CD10+ cell population within one BM or blood sample. The results demonstrate that, even though the leukemic blasts from most ALL patients express normal levels of LFA-1 and VLA-4, these integrins can not be activated by intracellular signalling and thus are defective on ALL blasts.
MATERIALS AND METHODS
Cells.
Samples were obtained from 20 untreated B-lineage ALL patients 16 to 64 years of age at the time of initial diagnosis. Diagnosis of B-lineage ALL was based on routine morphological/cytochemical evaluation according to the standard French-American-British criteria as well as by immunophenotyping using a panel of well-characterized monoclonal antibodies (MoAbs). Mononuclear cell fractions were isolated from BM or peripheral blood samples by Ficoll-Hypaque density gradient centrifugation. All patients were from the same hospital center (University Hospital Nijmegen, Nijmegen, The Netherlands). The B-lineage ALL subclassification for common ALL and pre-B-ALL used in this study has been described elsewhere.53 Briefly, the leukemic lymphoblast population in common ALL and pre-B-ALL are positive for both CD10 and CD19, whereas the more differentiated pre-B-ALL also express cytoplasmic Igμ. The cALL and pre-B-ALL patients chosen here are also positive for CD34, except for patients no. 13, 15, 18, and 19. Patients no. 1 through 15 have been diagnosed as cALL, and patients no. 16 through 20 have been diagnosed as pre-B-ALL. All samples were obtained from BM, except for those from patients no. 14 and 20, which were obtained from peripheral blood.
Isolation of CD34+ cells from healthy donors (donors d1 through d4).
CD34+ cells from 4 healthy BM donors were isolated as follows. CD34+ cells were rosetted with anti-CD34 MoAb-coated magnetic beads (Dynal, Oslo, Norway) for 60 minutes at 4°C with gentle rotation. CD34+ cells were collected magnetically and subsequently released from the beads with DETACHaBEAD (Dynal, Oslo, Norway). Isolated cells were free from beads and their purity exceeded 95% as determined with flow cytometry.54
MoAbs.
The anti-β2 chain MoAb KIM185 and the anti-β1 chain MoAb TS2/16 were used to activate LFA-155 and VLA-4,37respectively. The anti-αL MoAb NKI-L15 and the anti-α4 MoAb HP2/1 were used to specifically block the adhesion of LFA-116 and VLA-4,56 respectively. CD10, the cALL antigen (CALLA)-MoAb clone B-E3, was obtained from Immuno Quality Products (Groningen, The Netherlands).
Plate adhesion assay.
Cell adhesion to both ICAM-1 and VCAM-1 was performed as follows. A 96-well flat-bottom plate (MaxiSorp; Nunc, Roskilde, Denmark) was precoated with 50 μL goat-antihuman Fc-specific F(ab′)2 (4 μg/mL; Jackson Immuno Research Laboratories, Inc, West Grove, PA) for 1 hour at 37°C and blocked with 1% bovine serum albumin (BSA) in Tris-sodium buffer (20 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L CaCl2, 2 mmol/L MgCl2) for 30 minutes at 37°C. The plate was coated with 500 ng/mL ICAM-1 Fc or VCAM-1 Fc protein overnight at 4°C. ICAM-1-Fc or VCAM-1-Fc consist of the extracellular part of both proteins fused to a human IgG1 Fc fragment. ICAM-1-Fc was produced in Chinese Hamster Ovary K1 cells cotransfected with the ICAM-1-IgG1Fc26 (20 μg) and pEE14 (5 μg) vector similar to how it is described for soluble CD4.57 The ICAM-1-Fc concentration in the supernatant was determined by an IgG1 enzyme-linked immunosorbent assay (ELISA), and the supernatant was used without further purification. Purified VCAM-1-Fc was kindly provided by Dr Roy Lobb (Biogen, Cambridge, MA).58 Cells (20,000 to 40,000/well) were labeled in phosphate-buffered saline (PBS) with Calcein-AM (25 μg/107 cells/mL; Molecular Probes, Eugene, OR) for 30 minutes at 37°C. Labeled cells were washed and preincubated for 15 minutes at room temperature (RT) with different stimuli (100 nmol/L PMA [Calbiochem, La Jolla, CA], 5 μg/mL activating MoAbs, and/or 10 μg/mL blocking MoAbs). Cells were allowed to adhere for 30 minutes at 37°C. Nonadherent cells were removed by three washes with warm Tris-sodium-BSA buffer (20 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L CaCl2, 2 mmol/L MgCl2, 0.5% BSA [wt/vol]). The adherent cells were lysed with 100 μL lysis buffer (50 mmol/L Tris, 0.1% Triton X-100), and the fluorescence was quantified using the Cytofluor II (Perseptive Biosystems, Framingham, MA). Results are expressed as the mean percentage of cells binding from triplicate wells. Values are depicted as integrin-specific adhesion, ie, cell adhesion percentage minus cell adhesion percentage in the presence of an integrin-blocking MoAb.
Ligand coating of fluorescent microspheres.
Carboxylate-modified TransFluorSpheres (488/645 nm, 1.0 μm; Molecular Probes) were coated with adhesion ligands as follows. Streptavidin was covalently coupled to the TransFluorSpheres as described by the manufacturer. Briefly, 20 μL streptavidin (5 mg/mL in 50 mmol/L MES-buffer) was added to 50 μL TransFluorSpheres. Thirty microliters of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC; 1.33 mg/mL) was added and the mixture was incubated at RT for 2 hours. The reaction was stopped by the addition of glycin to a final concentration of 100 mmol/L. The streptavidin-coated beads were washed three times with PBS (50 mmol/L phosphate, 0.9% NaCl, pH 7.4) and resuspended in 150 μL PBS, 0.5% BSA (wt/vol). This suspension remains stable for 2 months if stored at 4°C. The streptavidin-coated beads (15 μL) were incubated with biotinylated goat-antihuman anti-Fc Fab2fragments (6 μg/mL) in 0.5 mL PBS, 0.5% BSA for 2 hours at 37°C. The beads were washed once with PBS, 0.5% BSA and incubated with human IgG1 Fc fused ligands (ICAM-1 Fc, VCAM-1 Fc; 250 ng/mL) in 0.5 mL overnight at 4°C. The ligand-coated beads were washed, resuspended in 100 μL PBS, 0.5% BSA, and stored at 4°C.
Fluorescent beads adhesion assay.
For cell adhesion to ICAM-1 and VCAM-1, cells were resuspended in Tris-sodium-BSA (20 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L CaCl2, 2 mmol/L MgCl2, 0.5% BSA [wt/vol]; 5 × 106 cells/mL). Fifty thousand cells were preincubated with or without LFA-1– or VLA-4–blocking MoAb (20 μg/mL) for 10 minutes at RT in a 96-well V-shaped–bottom plate. The ligand-coated beads (20 beads/cell) and different integrin stimuli (100 nmol/L PMA; LFA-1– or VLA-4–activating MoAbs: KIM185 and TS2/16 [10 μg/mL]) were added and the suspension was incubated for 30 minutes at 37°C. The cells were washed with the Tris-sodium-BSA buffer and incubated for 10 minutes at RT with fluorescein isothiocyanate (FITC)-conjugated anti-CD10 antibody. After washing, the cells were resuspended in 100 μL Tris-sodium-BSA buffer. LFA-1– or VLA-4–mediated adhesion of the CD10+ and CD10− cells was measured by flow cytometry using the FACScan (Becton Dickinson & Co, Oxnard, CA). Values are depicted as integrin-specific adhesion, ie, cell adhesion percentage minus cell adhesion percentage in the presence of a specific anti-integrin blocking MoAb.
RESULTS
Fluorescent beads adhesion assay.
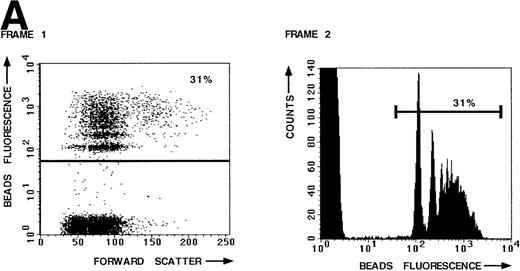
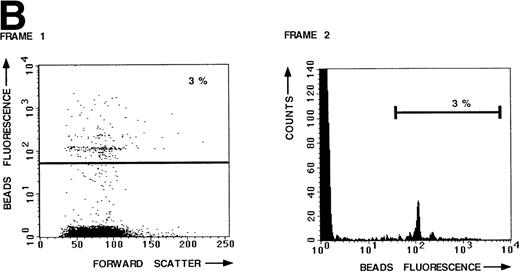
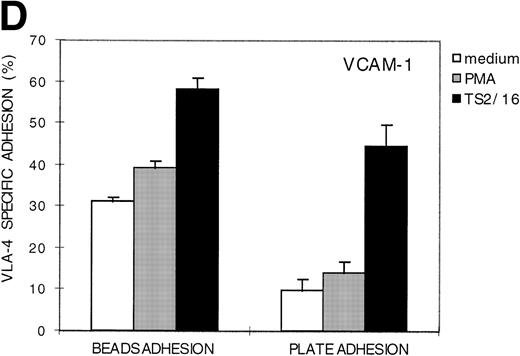
To measure the LFA-1– and VLA-4–mediated adhesion of the leukemic cell population of a large number of B-lineage ALL patients, we developed a new adhesion assay using fluorescent beads indirectly coated with integrin ligands. This new assay was compared with the standard plate adhesion assay by measuring the LFA-1– and VLA-4–mediated adhesion of resting peripheral blood lymphocytes (PBL) in both assays (Fig 1). LFA-1–mediated adhesion, as measured with the novel fluorescent beads adhesion assay, is shown in Fig 1A. Thirty-one percent of the PBL have bound ICAM-1 beads after stimulation of LFA-1 with PMA (frame 1). The defined peaks in frame 2 represent cells that have bound 1 bead, 2 beads, and more beads, respectively. Binding was LFA-1 specific, because it could be blocked by the blocking anti–LFA-1 MoAb NKI-L15 (Fig 1B, frames 1 and 2). Similar results were obtained with the plate adhesion assay (Fig1C). VLA-4–mediated adhesion, as measured with both assays, is shown in Fig 1D. Comparison of both assays demonstrates that the results obtained with the fluorescent beads adhesion assay are similar to those obtained with the standard plate adhesion assay, except that the adhesion measured with the fluorescent beads adhesion assay is substantially higher and more sensitive. Because the fluorescent beads adhesion assay is less time-consuming and directly measurable on the flow cytometer, it is very well suited for screening large numbers of samples. Furthermore, only the fluorescent beads adhesion assay allows us to screen the adhesive properties of different subpopulations of cells within one sample by performing double fluorescent labeling techniques with distinct FITC-labeled markers.
LFA-1– and VLA-4–mediated adhesion of PBL from a healthy donor both measured with the fluorescent beads and the plate adhesion assay. Binding of ICAM-1–coated fluorescent beads by PBL after stimulation with PMA (A) and in the presence of anti–LFA-1 blocking antibody NKI-L15 (B). (C and D) Comparison of the adhesion as measured with both assays. Adhesion was measured without any stimulus and after stimulation with PMA or an activating anti-β2 (KIM185) or anti-β1 (TS2/16) MoAb. The specificity was determined by measuring adhesion in the presence of blocking anti–LFA-1 (NKI-L15) or anti–VLA-4 (HP2/1) MoAb.
LFA-1– and VLA-4–mediated adhesion of PBL from a healthy donor both measured with the fluorescent beads and the plate adhesion assay. Binding of ICAM-1–coated fluorescent beads by PBL after stimulation with PMA (A) and in the presence of anti–LFA-1 blocking antibody NKI-L15 (B). (C and D) Comparison of the adhesion as measured with both assays. Adhesion was measured without any stimulus and after stimulation with PMA or an activating anti-β2 (KIM185) or anti-β1 (TS2/16) MoAb. The specificity was determined by measuring adhesion in the presence of blocking anti–LFA-1 (NKI-L15) or anti–VLA-4 (HP2/1) MoAb.
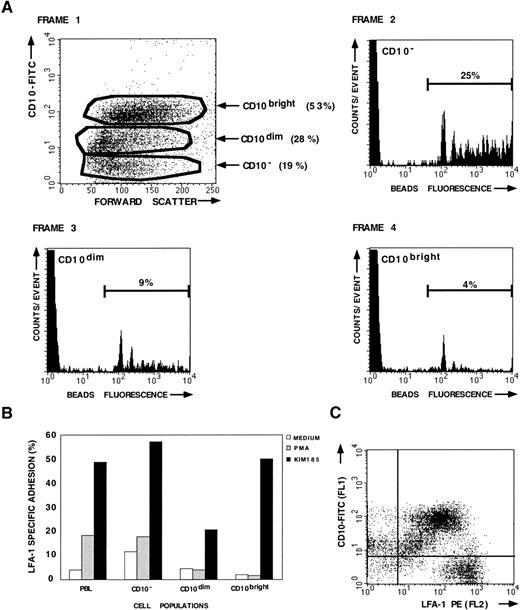
LFA-1–mediated adhesion of CD10+ ALL cells.
BM or peripheral blood samples were collected from patients that either suffer from cALL or pre-B-ALL. LFA-1–mediated adhesion of both the CD10− and leukemic (CD10+) cells in these B-lineage ALL samples was determined using the fluorescent beads adhesion assay. A representative set of data (patient no. 11) is shown in Fig 2. BM aspirate from patient no. 11 contains 3 cell populations with different CD10 antigen expression levels, ie, CD10− (19%), CD10dim (28%), and CD10bright (53%; Fig 2A, frame 1). Both the CD10dim and CD10bright cell population represent the leukemic cell population. As shown, 25% of the CD10− cells have bound the ICAM-1–coated beads after PMA activation (frame 2), whereas only 9% of the CD10dim(frame 3) and 4% of the CD10bright cells (frame 4) have bound ICAM-1–coated beads despite a high expression of LFA-1 (Fig 2C). Thus, LFA-1 expressed by the leukemic populations is not responsive to PMA, whereas LFA-1 expressed by the CD10− cell population is activated by PMA. However, adhesion of the CD10+ and CD10− cell populations could be stimulated with the activating anti-β2 antibody KIM185 (Fig 2B). These findings clearly demonstrate that functional adhesion defects can be observed in B-lineage ALL by measuring adhesion in defined cell populations (CD10 staining).
LFA-1–mediated adhesion of patient no. 11 and control PBL measured with the fluorescent beads adhesion assay. Double staining with CD10-FITC MoAb was used to distinguish between the leukemic (CD10+) cell population and the CD10− cell population within one sample. (A) Adhesion of the different CD10 cell populations after stimulation with PMA. (B) LFA-1–mediated adhesion of the leukemic (CD10dim and CD10bright) and the CD10− cell population, in comparison with the adhesion of PBL from a healthy donor. Standard deviations are less than 5%. (C) LFA-1 expression of the various CD10 cell populations.
LFA-1–mediated adhesion of patient no. 11 and control PBL measured with the fluorescent beads adhesion assay. Double staining with CD10-FITC MoAb was used to distinguish between the leukemic (CD10+) cell population and the CD10− cell population within one sample. (A) Adhesion of the different CD10 cell populations after stimulation with PMA. (B) LFA-1–mediated adhesion of the leukemic (CD10dim and CD10bright) and the CD10− cell population, in comparison with the adhesion of PBL from a healthy donor. Standard deviations are less than 5%. (C) LFA-1 expression of the various CD10 cell populations.
LFA-1–mediated adhesion of B-lineage CD10+ ALL cells.
Table 1 summarizes the data of the LFA-1 mediated adhesion and LFA-1 expression of the CD10+leukemic cell population from 20 B-lineage ALL patients (patients no. 1 through 20) and of the CD19+/CD34+/CD10+ cell population from 4 healthy donors (donors d1 through d4). The integrin-mediated adhesion of the CD19+/CD34+/CD10+cells from healthy donors was determined by isolating the CD34+ cells from BM with magnetic beads and subsequently by selecting for the CD10+ cell population in the fluorescent beads adhesion assay. These CD34+/CD10+ cells are appropriate controls for the B-lineage ALL cells, because the majority are also CD19+59-61 as was determined by triple CD19/CD10/CD34 fluorescence analysis (>96%; results not shown). The LFA-1 expression on the CD10+ populations (Table 1) was determined with a CD10/LFA-1 dual-color fluorescence analysis.
LFA-1–Mediated Adhesion and Expression of the Leukemic (CD10+) Cell Populations in B-Lineage ALL Patients
| Patient No. . | CD10+ (%) . | CD10* . | LFA-1 Adhesion† . | LFA-1* . | ||
|---|---|---|---|---|---|---|
| Medium . | PMA . | KIM185 . | ||||
| d1 | 12 | + | + | ++ | ++ | ++ |
| d2 | 17 | ++ | + | ++ | ++ | ++ |
| d3 | 29 | ++ | + | ++ | ++ | ++ |
| d4 | 20 | ++ | +/− | + | ++ | ++ |
| 1‡ | 19 | +++ | +/− | ++ | +++ | ++ |
| 2 | 94 | −1-153 | +/− | + | ++ | ++ |
| 3 | 66 | ++ | +/− | + | ++ | ++ |
| 16 | 33 | + | +/− | + | ++ | ++ |
| 4 | 66 | ++ | +/− | + | ++ | ++ |
| 61-155 | 84 | + | − | + | +++ | ND |
| 7 | 79 | +++ | − | +/− | +++ | +++ |
| 8‡ | 60 | +++ | − | +/− | +++ | ++ |
| 9 | 55 | ++ | − | +/− | +++ | ++ |
| 5 | 73 | +++ | − | +/− | +++ | + |
| 10 | 79 | ++ | − | +/− | ++ | + |
| 81-154 | 24 | + | − | +/− | + | + |
| 11‡ | 53 | +++ | − | − | ++ | ++ |
| 12 | 38 | +++ | − | − | ++ | + |
| 111-154 | 28 | + | − | − | + | +/− |
| 13 | 58 | + | − | − | + | + |
| 14 | 47 | +++ | − | − | + | + |
| 15 | 37 | ++ | − | − | + | + |
| 11-154 | 47 | + | − | − | +/− | − |
| 17 | 100 | ++ | − | − | − | +/− |
| 18 | 97 | −1-153 | − | − | − | − |
| 19 | 92 | ++ | − | − | − | − |
| 20 | 47 | ++ | − | − | − | − |
| Patient No. . | CD10+ (%) . | CD10* . | LFA-1 Adhesion† . | LFA-1* . | ||
|---|---|---|---|---|---|---|
| Medium . | PMA . | KIM185 . | ||||
| d1 | 12 | + | + | ++ | ++ | ++ |
| d2 | 17 | ++ | + | ++ | ++ | ++ |
| d3 | 29 | ++ | + | ++ | ++ | ++ |
| d4 | 20 | ++ | +/− | + | ++ | ++ |
| 1‡ | 19 | +++ | +/− | ++ | +++ | ++ |
| 2 | 94 | −1-153 | +/− | + | ++ | ++ |
| 3 | 66 | ++ | +/− | + | ++ | ++ |
| 16 | 33 | + | +/− | + | ++ | ++ |
| 4 | 66 | ++ | +/− | + | ++ | ++ |
| 61-155 | 84 | + | − | + | +++ | ND |
| 7 | 79 | +++ | − | +/− | +++ | +++ |
| 8‡ | 60 | +++ | − | +/− | +++ | ++ |
| 9 | 55 | ++ | − | +/− | +++ | ++ |
| 5 | 73 | +++ | − | +/− | +++ | + |
| 10 | 79 | ++ | − | +/− | ++ | + |
| 81-154 | 24 | + | − | +/− | + | + |
| 11‡ | 53 | +++ | − | − | ++ | ++ |
| 12 | 38 | +++ | − | − | ++ | + |
| 111-154 | 28 | + | − | − | + | +/− |
| 13 | 58 | + | − | − | + | + |
| 14 | 47 | +++ | − | − | + | + |
| 15 | 37 | ++ | − | − | + | + |
| 11-154 | 47 | + | − | − | +/− | − |
| 17 | 100 | ++ | − | − | − | +/− |
| 18 | 97 | −1-153 | − | − | − | − |
| 19 | 92 | ++ | − | − | − | − |
| 20 | 47 | ++ | − | − | − | − |
Abbreviation: ND, not determined.
Expression (mean fluorescence): −, 0 to 10; +/−, 11 to 30; +, 31 to 90; ++, 91 to 150; and +++, >150 (LFA-1 expression was measured on the CD10+ cells using dual fluorescence analysis).
Adhesion (%): −, 0 to 5; +/−, 6 to 10; +, 11 to 20; ++, 21 to 50; and +++, 51 to 100.
CD10bright.
Gated by forward/side scatter. The percentage of leukemic cells was based on morphological data and immunophenotyping.
CD34+.
CD10dim.
The leukemic cell populations of 6 patients show a normal LFA-1–mediated adhesion pattern similar to that of the CD19+/CD34+/CD10+ cell population from healthy donors. LFA-1 present on these cells is slightly active and can be further activated both with the phorbol ester PMA and with the activating antibody (KIM185). Ten patients show a KIM185-inducible activation of LFA-1 adhesion; however, the LFA-1 of these ALL patients fails to respond to intracellular signals (PMA), despite the fact that LFA-1 is expressed on the cell surface. Finally, 5 ALL patients do not show any LFA-1–mediated adhesion (neither after PMA nor KIM185 stimulation) due to the absence of LFA-1 on the surface of the leukemic cells (Table 1). Because 4 of these patients suffer from pre-B-ALL, we have analyzed the LFA-1 expression on pre-B cells from healthy donors by cytμ/LFA-1 dual fluorescence analysis on CD34+ cells (results not shown). This analysis showed that normal pre-B cells do express LFA-1.
It is of note that the CD10dim population of patient no. 1 does not express LFA-1, whereas the CD10bright cell population expresses functional LFA-1. In contrast, the CD10dim and CD10bright populations of both patients no. 8 and 11 have similar LFA-1–mediated adhesive properties.
We could exclude that unresponsiveness of LFA-1 towards PMA activation was due to low viability of the leukocytes, because the LFA-1–mediated adhesion of the CD10− cell population of these patients (no. 5, 9, 12, 14, and 15) was responsive to PMA similar to the results obtained in patient no. 11 (Fig 2; for other patients, data not shown).
VLA-4–mediated adhesion of B-lineage CD10+ ALL cells.
Similar to β2 integrins, we studied the capacity of ALL leukemic cells to bind VCAM-1–coated fluorescent beads to the β1 integrin VLA-4. The results are summarized in Table2. The VLA-4 expression on the CD10+ populations was determined using a CD10/VLA-4 dual-color fluorescence analysis.
The VLA-4–Mediated Adhesion and Expression of the Leukemic (CD10+) Cell Populations in B-ALL Patients
| Patient No. . | CD10* . | VLA-4 Adhesion† . | VLA-4* . | ||
|---|---|---|---|---|---|
| Medium . | PMA . | TS2/16 . | |||
| d1 | + | +++ | +++ | +++ | +++ |
| d2 | ++ | +++ | +++ | +++ | +++ |
| d3 | ++ | +++ | +++ | +++ | +++ |
| d4 | ++ | +++ | +++ | +++ | +++ |
| 7 | +++ | +++ | +++ | +++ | +++ |
| 1‡ | +++ | +++ | +++ | +++ | +++ |
| 9 | ++ | +++ | +++ | +++ | +++ |
| 4 | ++ | +++ | +++ | +++ | +++ |
| 62-153 | + | +++ | +++ | +++ | +++ |
| 14 | +++ | +++ | +++ | +++ | ++ |
| 5 | +++ | +++ | +++ | +++ | ++ |
| 15 | ++ | ++ | +++ | +++ | +++ |
| 12 | +++ | ++ | +++ | +++ | ++ |
| 8‡ | +++ | ++ | +++ | +++ | ++ |
| 20 | ++ | ++ | +++ | +++ | ++ |
| 17 | ++ | ++ | ++ | +++ | +++ |
| 82-155 | + | ++ | ++ | +++ | ++ |
| 16 | + | ++ | ++ | +++ | + |
| 12-155 | + | ++ | ++ | ++ | + |
| 2 | −2-154 | + | + | +++ | ++ |
| 11‡ | +++ | + | + | +++ | ++ |
| 10 | ++ | + | + | +++ | ++ |
| 3 | ++ | + | + | +++ | ++ |
| 13 | + | + | + | +++ | ++ |
| 112-155 | + | + | + | ++ | ++ |
| 19 | ++ | + | + | ++ | + |
| 18 | −2-154 | + | + | ++ | ++ |
| Patient No. . | CD10* . | VLA-4 Adhesion† . | VLA-4* . | ||
|---|---|---|---|---|---|
| Medium . | PMA . | TS2/16 . | |||
| d1 | + | +++ | +++ | +++ | +++ |
| d2 | ++ | +++ | +++ | +++ | +++ |
| d3 | ++ | +++ | +++ | +++ | +++ |
| d4 | ++ | +++ | +++ | +++ | +++ |
| 7 | +++ | +++ | +++ | +++ | +++ |
| 1‡ | +++ | +++ | +++ | +++ | +++ |
| 9 | ++ | +++ | +++ | +++ | +++ |
| 4 | ++ | +++ | +++ | +++ | +++ |
| 62-153 | + | +++ | +++ | +++ | +++ |
| 14 | +++ | +++ | +++ | +++ | ++ |
| 5 | +++ | +++ | +++ | +++ | ++ |
| 15 | ++ | ++ | +++ | +++ | +++ |
| 12 | +++ | ++ | +++ | +++ | ++ |
| 8‡ | +++ | ++ | +++ | +++ | ++ |
| 20 | ++ | ++ | +++ | +++ | ++ |
| 17 | ++ | ++ | ++ | +++ | +++ |
| 82-155 | + | ++ | ++ | +++ | ++ |
| 16 | + | ++ | ++ | +++ | + |
| 12-155 | + | ++ | ++ | ++ | + |
| 2 | −2-154 | + | + | +++ | ++ |
| 11‡ | +++ | + | + | +++ | ++ |
| 10 | ++ | + | + | +++ | ++ |
| 3 | ++ | + | + | +++ | ++ |
| 13 | + | + | + | +++ | ++ |
| 112-155 | + | + | + | ++ | ++ |
| 19 | ++ | + | + | ++ | + |
| 18 | −2-154 | + | + | ++ | ++ |
Expression (mean fluorescence): −, 0 to 10; +/−, 11 to 30; +, 31 to 90; ++, 91 to 150; and +++, >150 (VLA-4 expression was measured on the CD10+ cells using dual fluorescence analysis).
Adhesion (%): −, 0 to 5; +/−, 6 to 10; +, 11 to 20; ++, 21 to 50; and +++, 51 to 100.
CD10bright.
CD34+.
CD10dim.
Gated by forward/side scatter. The percentage of leukemic cells was based on morphological data and immunophenotyping.
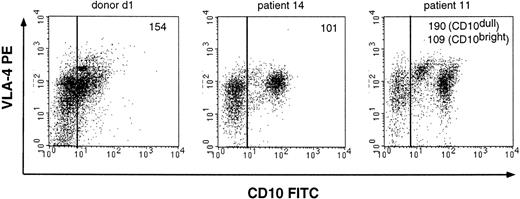
In 7 ALL patients, VLA-4 is constitutively active on CD10+cells. The adhesion is high without any stimulation and cannot be further activated by PMA or the activating anti-β1 MoAb TS2/16, indicating that VLA-4 is already maximally active. Similar results were obtained with CD19+/CD34+/CD10+cells from a healthy donor (donors d1 through d4), indicating that VLA-4 is constitutively active on normal CD19+/CD34+/CD10+ cells. In 7 other ALL patients, VLA-4 adhesion increases after stimulation with either PMA or TS2/16. In these patients, VLA-4 activity is very high but not maximal before stimulation. In 7 other patients, VLA-4 on the leukemic cells is minimally active without any activation. Furthermore, in these patients, VLA-4 cannot be activated by treating the cells with PMA, whereas VLA-4 is readily activated with the activating anti-β1 antibody TS2/16. Analysis of the expression pattern of VLA-4 in B-lineage ALL demonstrates that the defects are not due to lack of VLA-4 surface expression in these patients. As shown in Table 2, the leukemic cells of the ALL patients (no. 2, 3, 11, 10, 13, 18, and 19) express comparable levels of VLA-4 as other ALL patients, suggesting that VLA-4 is functionally defective in these 7 ALL. This is also shown in Fig 3, in which identical VLA-4 expression levels on the CD10+ cells from donor d1 and ALL patients no. 11 and 14 are shown, whereas the VLA-4–mediated adhesion of these patients is very different (Table 2).
VLA-4 expression of the CD10 cell populations from a healthy donor and 2 representative B-lineage ALL patients (no. 11 and 14). VLA-4 expression was measured with the anti–VLA-4 antibody HP2/1. The mean fluorescence of the VLA-4 expression of the CD10+ cell population is depicted in the top right corner of the figures.
VLA-4 expression of the CD10 cell populations from a healthy donor and 2 representative B-lineage ALL patients (no. 11 and 14). VLA-4 expression was measured with the anti–VLA-4 antibody HP2/1. The mean fluorescence of the VLA-4 expression of the CD10+ cell population is depicted in the top right corner of the figures.
The LFA-1– and VLA-4–mediated adhesion defects found in the leukemic cells from the different patients have been summarized in Table 3. The leukemic cells (CD10+) from 17 of 20 patients contain either LFA-1– and/or VLA-4–mediated adhesion defects, of which the LFA-1–mediated adhesion defects are most predominant. Furthermore, 5 of these patients contain leukemic blasts with both a LFA-1 and a VLA-4 adhesion defect.
LFA-1 and VLA-4 PMA Responsiveness of the Leukemic (CD10+) Cell Populations
| Patient No. . | Inside-Out Activation3-150 . | |
|---|---|---|
| LFA-1 . | VLA-4 . | |
| 2 | + | − |
| 3 | + | − |
| 13-151 | −3-152 | + |
| 5 | − | + |
| 7 | − | + |
| 83-151 | − | + |
| 83-153 | − | + |
| 9 | − | + |
| 12 | − | + |
| 14 | − | + |
| 15 | − | + |
| 17 | −3-152 | + |
| 20 | −3-152 | + |
| 10 | − | − |
| 113-153 | − | − |
| 113-151 | − | − |
| 13 | − | − |
| 18 | −3-152 | − |
| 19 | −3-152 | − |
| Patient No. . | Inside-Out Activation3-150 . | |
|---|---|---|
| LFA-1 . | VLA-4 . | |
| 2 | + | − |
| 3 | + | − |
| 13-151 | −3-152 | + |
| 5 | − | + |
| 7 | − | + |
| 83-151 | − | + |
| 83-153 | − | + |
| 9 | − | + |
| 12 | − | + |
| 14 | − | + |
| 15 | − | + |
| 17 | −3-152 | + |
| 20 | −3-152 | + |
| 10 | − | − |
| 113-153 | − | − |
| 113-151 | − | − |
| 13 | − | − |
| 18 | −3-152 | − |
| 19 | −3-152 | − |
+, PMA responsiveness; −, no PMA responsiveness.
CD10dim.
No LFA-1 expression.
CD10bright.
Comparison of the adhesion of CD10+ ALL cells present in BM and in peripheral blood.
The egress of specific leukemic cells from the BM into the periphery could be a result from different adhesive properties. Therefore, we compared the capacity of both BM- and peripheral blood-derived leukemic cells of 6 patients to mediate LFA-1– and VLA-4–mediated adhesion (Table 4). Patients no. 3, 5, 12, and 17 have a similar LFA-1– and VLA-4–mediated adhesion pattern independent of the source, ie, BM or peripheral blood. The leukemic cells of patients no. 2 and 18 have different adhesion characteristics depending on the source of cells. Furthermore, the CD10 expression of the circulating leukemic cells from both patients is higher than that of the leukemic cells derived from the BM.
LFA-1– and VLA-4–Mediated Adhesion of Leukemic Cells Derived From Both BM and Peripheral Blood
| Patient No. . | CD10+ (%) . | CD104-150 (mean) . | LFA-1 Adhesion4-151 . | VLA-4 Adhesion4-151 . | ||||
|---|---|---|---|---|---|---|---|---|
| Medium . | PMA . | KIM185 . | Medium . | PMA . | TS2/16 . | |||
| 2 | ||||||||
| BM | 94‡ | − | +/− | + | ++ | ++ | ++ | +++ |
| PB | 16 | + | − | − | − | − | − | +++ |
| 3 | ||||||||
| BM | 66 | ++ | +/− | + | ++ | ++ | ++ | +++ |
| PB | 61 | ++ | − | + | ++ | ++ | ++ | +++ |
| 5 | ||||||||
| BM | 73 | +++ | − | +/− | +++ | +++ | +++ | +++ |
| PB | 37 | +++ | − | − | ++ | ++ | ++ | +++ |
| 12 | ||||||||
| BM | 38 | +++ | − | − | ++ | ++ | +++ | +++ |
| PB | 69 | +++ | − | +/− | ++ | ++ | ++ | +++ |
| 17 | ||||||||
| BM | 100 | ++ | − | − | − | ++ | ++ | +++ |
| PB | 100 | ++ | − | − | − | ++ | ++ | +++ |
| 18 | ||||||||
| BM | 97‡ | − | − | − | − | + | ++ | ++ |
| PB | 10 | + | − | + | ++ | ++ | ++ | +++ |
| Patient No. . | CD10+ (%) . | CD104-150 (mean) . | LFA-1 Adhesion4-151 . | VLA-4 Adhesion4-151 . | ||||
|---|---|---|---|---|---|---|---|---|
| Medium . | PMA . | KIM185 . | Medium . | PMA . | TS2/16 . | |||
| 2 | ||||||||
| BM | 94‡ | − | +/− | + | ++ | ++ | ++ | +++ |
| PB | 16 | + | − | − | − | − | − | +++ |
| 3 | ||||||||
| BM | 66 | ++ | +/− | + | ++ | ++ | ++ | +++ |
| PB | 61 | ++ | − | + | ++ | ++ | ++ | +++ |
| 5 | ||||||||
| BM | 73 | +++ | − | +/− | +++ | +++ | +++ | +++ |
| PB | 37 | +++ | − | − | ++ | ++ | ++ | +++ |
| 12 | ||||||||
| BM | 38 | +++ | − | − | ++ | ++ | +++ | +++ |
| PB | 69 | +++ | − | +/− | ++ | ++ | ++ | +++ |
| 17 | ||||||||
| BM | 100 | ++ | − | − | − | ++ | ++ | +++ |
| PB | 100 | ++ | − | − | − | ++ | ++ | +++ |
| 18 | ||||||||
| BM | 97‡ | − | − | − | − | + | ++ | ++ |
| PB | 10 | + | − | + | ++ | ++ | ++ | +++ |
Expression (mean fluorescence): −, 0 to 10; +/−, 11 to 30; +, 31 to 90; ++, 91 to 150; and +++, >150.
Adhesion (%): −, 0 to 5; +/−, 6 to 10; +, 11 to 20; ++, 21 to 50; and +++, 51 to 100.
Leukemic cells according to morphological data and immunophenotyping not CD10 expression.
DISCUSSION
In this study, we have investigated the LFA-1– and VLA-4–mediated adhesion in B-lymphocyte lineage CD10+ ALL using a new fluorescent beads adhesion assay that enabled us to specifically measure adhesion of the leukemic cell population within a heterogeneous BM or peripheral blood sample. We show that the leukemic cells from 85% of the ALL patients investigated have a LFA-1– and/or VLA-4–mediated adhesion defect. The LFA-1–mediated adhesion defects observed in the ALL patients are most predominant. The LFA-1–mediated adhesion defects are either due to the lack of LFA-1 expression on the surface or due to the presence of nonfunctional LFA-1. The VLA-4–mediated adhesion defects are due to the cell surface expression of nonfunctional VLA-4. This study clearly demonstrates the importance of investigating integrin functionality in addition to integrin expression on specific leukemic cell populations.
In B-lineage ALL we have focused on both common ALL (cALL) and precursor B-ALL (pre-B-ALL) using CD10 antigen expression as a discriminating marker for the leukemic populations. Because samples from ALL patients contain CD10− and leukemic (CD10+) cell populations in variable ratios, it is necessary to distinguish between both populations when measuring adhesion. The standard plate adhesion method, involving integrin ligands coated onto plastic, is not suitable to measure the adhesion of different subpopulations within one BM sample simultaneously. Furthermore, due to the low amount of material, it is difficult to obtain enough CD10-sorted cells to perform plate adhesion assays. Therefore, we have developed a new adhesion assay involving fluorescent beads indirectly coated with integrin ligands, which can be measured in a flow cytometer, and by double staining the cells with an FITC-labeled marker it is possible to distinguish between different cell populations. We have shown that the LFA-1– and VLA-4–mediated adhesion of PBL from a healthy donor measured with the fluorescent beads adhesion assay is substantially higher than that measured with the standard plate adhesion assay (Fig 1). In a plate adhesion assay, the cells have to adhere to and subsequently spread on the ligand-coated plastic to resist being washed away. In the beads adhesion assay, the cells do not need to spread; therefore, the adhesion in the plate adhesion assay is lower. Furthermore, in a standard adhesion assay, the force of the multiple washing steps is very important in defining the amount of adhesion measured, and it is difficult to standardize these washing steps, whereas the single washing step in the beads adhesion assay does not influence the amount of beads bound and therefore is more reproducible between researchers (data not shown).
With this novel fluorescent beads adhesion assay, we could for the first time discriminate between adhesion of leukemic CD10+and CD10− cell populations within one BM or blood sample. Of the 20 ALL patients measured, 6 showed a normal LFA-1 function on the leukemic cells comparable with CD19+/CD34+/CD10+ cells from healthy donors (Table 1). The CD10+ cells from 5 ALL patients (Table 1; patients no. 1, 17, 18, 19, and 20) did not exhibit any LFA-1–mediated adhesion, due to the lack of LFA-1 expression. Interestingly, from the 5 pre-B-ALL patients investigated, 4 contained leukemic cells that did not express LFA-1 on the cell surface. LFA-1 is expressed on immature CD10+ B cells62 and, as we showed, on pre-B-cells from healthy donors, which indicates that the LFA-1 expression on pre-B-ALL cells from these patients is defective. A greater number of pre-B-ALL patients should be tested to determine whether the lack of LFA-1 expression is more often observed in pre-B-ALL patients than cALL. Surprisingly, the CD10+ cells from 10 B-lineage ALL patients expressed LFA-1 that could not be activated by PMA through inside-out signalling, whereas the CD10− cell population responded normally. The phorbol ester PMA activates protein kinase C and thereby triggers an intracellular signaling pathway resulting in the activation of both β1- and β2-integrins.29,30 Recently, we and others observed that certain leukemic T-cell lines (CEM and Jurkat) express LFA-1 that could not be activated by several stimuli known to induce ligand binding through intracellular signaling pathways.63,64 The unresponsiveness of LFA-1 to PMA in these cell lines could be caused by mutations in the intracellular part of either the αL or β2 chain of LFA-1 or by the absence of crucial cytoplasmic signaling elements that are involved in the inside-out activation of β2 integrins. Recently, Mobley et al65showed that a specific Jurkat mutant contains a mutation associated with an altered form of the mitogen-activated protein kinase ERK1 that explains the lack of PMA responsiveness of the integrins. The existence of essential elements necessary for intracellular activation of integrins is also shown by a defect in LFA-1–mediated adhesion after PMA triggering observed in the erythroid leukemic CML cell line K562 when transfected with wild-type LFA-1.66 This indicates that the leukemic cell line K562 lacks crucial signaling elements necessary to activate LFA-1 through the β2 cytoplasmic tail. It is tempting to speculate that CD10+ leukemic cells that express LFA-1 that cannot be activated by PMA might also contain mutations in protein kinases involved in integrin activation or in other intracellular signaling molecules such as cytohesin, which has recently been shown to be involved in integrin signaling.67The leukemic cells of these patients will be investigated in more detail to elucidate the signaling defect.
Examining VLA-4–mediated adhesion in B-lineage ALL shows that, in 7 patients, VLA-4 is constitutively active on the leukemic cells, similar to the CD19+/CD34+/CD10+ cells from healthy donors (Table 2). In the CD10+ cells from 7 other ALL patients, unstimulated VLA-4–mediated adhesion is high but can be further enhanced by PMA or activating antibodies. The leukemic cells from the remaining 7 patients express VLA-4 that is not functional. Because the defects are not due to the cell surface expression levels of VLA-4 (Fig 3 and Table 2), the aberrant adhesion could be caused by a signaling defect that maintains VLA-4 in a low-affinity state. The leukemic cells from 3 of these patients also express a nonfunctional form of LFA-1. This indicates that these leukemic cells have defects both in the β1- and β2-integrin activation pathways and that regulatory proteins involved in the adhesion of both β1- and β2-integrins could be defective, such as protein kinase C or certain cytoskeletal regulatory components.
The differences found in the activation of VLA-4 could be extremely important to survival, retention, and proliferation of the ALL blast cells in the BM. VLA-4 and VLA-5 have been shown to be important in the binding of ALL blasts to the BM stroma.49,51,52,68,69Manabe et al51 showed that leukemic cell contact with stromal cells can prevent apoptosis in ALL blasts. It has been suggested that VLA-4/VCAM-1 interactions could affect ALL blast survival and proliferation by transmitting signals (outside-in signaling) to the cells. Blocking studies demonstrated that adhesion of B-cell precursors to BM stroma is also mediated by VLA-4–VCAM-1 and could be regulated by cytokines that specifically increase or decrease cell-surface VCAM-1 expression.70 Furthermore, Arroyo et al71 demonstrated the importance of VLA-4 function in retaining progenitor cells in the BM for proper maturation. Hence, ALL blast cell survival may have major effects on leukemic cell growth and survival. The recent observation that binding to fibronectin may inhibit progenitor cell proliferation further supports this notion.72 Thus, differences in VLA-4 activities between different patients with ALL could reflect different proliferating and migratory properties, as has been demonstrated for chronic myeloid leukemia (CML). CML is a leukemia characterized by an abnormal, premature release of primitive progenitors and precursors in the blood and by the continuous proliferation of the malignant progenitor population. In vitro, CML progenitors fail to adhere to or be regulated by marrow stroma, and Verfaillie et al73 showed that this is due to aberrant VLA-4– and VLA-5–mediated adhesion. Treatment of the CML progenitors with interferon-α (IFN-α) restored the adhesive properties of these progenitors.74 Because IFN-α is known to induce remission in CML patients, it is feasible that the rescued integrin-mediated adhesion restores adhesion-mediated proliferation of the CML progenitors.
Comparison of the clinical characteristics observed in the B-lineage ALL patients, such as egress of the leukemic cells into the blood, homing, and cell turn-over, with the aberrant adhesion of the leukemic cells indicates a trend for a correlation between a high blood cell count and a defective VLA-4 function (P = .05). From the 20 patients investigated, 6 had high peripheral blood cell counts (>50 × 109/L; data not shown); interestingly, the leukemic cells from 4 of these patients show a VLA-4 defect. Furthermore, we demonstrated that, within 1 patient, leukemic cells derived from either the BM or the periphery have similar adhesive properties (Table 4; patients no. 3, 5, 12, and 17). In 2 patients (Table 4; patients no. 2 and 18), the CD10 antigen expression of the leukemic cells derived from the BM was different from those derived from the periphery. The integrin-mediated adhesion of the leukemic cells from both sources was also different, indicating that, within 1 patient, there may be different leukemic cell populations with different adhesive properties. Because the adhesive properties of the leukemic cells present in the periphery of both patients are different, it is likely that more factors regulate the egress of malignant lymphoblasts into the periphery.
In summary, we have demonstrated that not only the expression of integrins in relation to hematopoietic malignancies should be measured, but more importantly also the function of the integrin receptors. Using a newly developed fluorescent beads adhesion assay, we were able to analyze both the LFA-1– and VLA-4–mediated adhesion of the leukemic cells within B-lineage ALL patients, even though the samples were heterogeneous. We found that most ALL patients contained leukemic cell populations exhibiting adhesion defects either in LFA-1– and/or VLA-4–mediated adhesion, even though the leukemic cells had normal expression levels of these integrins. Studies to analyze the molecular basis of these defects in the different patients are in progress.
ACKNOWLEDGMENT
The authors thank Dr R. Lobb, Dr D. Simmons, Dr E. Martz, and Dr M. Robinson for kindly providing recombinant VCAM-1-Fc DNA construct, ICAM-1-Fc DNA construct, TS2/16 antibody, and KIM185 antibody, respectively. We are also grateful to M. Leenders from the Department of Hematology for reimmunophenotyping some of the ALL patients and R. Torensma for his help with the triple fluorescence analyses.
Supported by the Dutch Cancer Society (NKB; Grant No. 96-1358) and the Netherlands Organization for Scientific Research (NWO; Grant No. 901-09-244).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yvette van Kooyk, PhD, Tumor Immunology Laboratory, University Hospital Nijmegen, Philips van Leydenlaan 25, 6525 EX Nijmegen, The Netherlands; Y. vanKooyk@dent.kun.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal