Abstract
p27 cyclin-dependent kinase inhibitor downregulation is essential for transition to the S phase of the cell cycle. Thus, proliferating cells in reactive lymphoid tissue show no detectable p27 expression. Nevertheless, anomalous high p27 expression has been shown to be present in a group of aggressive B-cell lymphomas with high proliferation index and adverse clinical outcome. This suggests that abnormally accumulated p27 protein has been rendered functionally inactive. We analyzed the causes of this anomalous presence of p27 in a group of aggressive B-cell lymphomas, including 54 cases of diffuse large B-cell lymphomas and 20 Burkitt’s lymphomas. We simultaneously studied them for p27, cyclin D3, cyclin D2, cyclin D1, and cyclin E expression, because it has been stated that high levels of expression of cyclin D1 or E lead to increased p27 levels in some cell types. A statistically significant association between p27 and cyclin D3 expression was found for the group as a whole. Additionally, when dividing the cases according to the level of expression of cyclin D3 by reactive germinal centers, it was observed that cases with stronger cyclin D3 expression also show higher p27 expression. The relationship between both proteins was also shown at a subcellular level by laser confocal studies, showing that in cases with high expression of both proteins there was a marked colocalization. Additional evidence in favor of p27 sequestration by cyclin D3 was provided by coimmunoprecipitation studies in a Burkitt’s cell line (Raji) showing the existence of cyclin D3/p27 complexes and the absence of CDK2/p27 complexes. These results could support the hypothesis that there are cyclin D3/p27 complexes in a subset of aggressive B-cell lymphomas in which p27 lacks the inhibitory activity found when it is bound to cyclin E/CDK2 complexes. This interaction between both proteins could lead to an abnormal nuclear accumulation, detectable by immunohistochemical techniques.
CELL-CYCLE PROGRESSION is regulated by a group of cyclins and cyclin-dependent kinases (CDK) acting at different phases of the cell cycle. The G1 cyclins (D and E) complex with their CDK partners regulating G1/S transition. Thus the cyclin D family (D1, D2, and D3) form complexes with CDK4 or 6, whereas cyclin E assembles with CDK2. The kinase activity of these complexes is negatively regulated by two different families of CDK inhibitors (CDKI). The INK family proteins (p16, p15, p18, and p19) inhibit the complex cyclin D/CDK4-6, whereas the KIP family proteins (p21, p27 and p57) are able to inhibit a broader range of CDK.1-3
Although p27 can be found associated with several cyclin/CDK complexes, cyclin E/CDK2 seems to be the major target of its inhibitory activity.4,5 In vitro, p27 has been found bound to the complex cyclin D/CDK4-6, although this complex maintains its kinase activity,5,6,7 thus signaling the functional inactivation of p27 when bound to cyclin D/CDK4-6. On induction of an antimitogenic effect mediated through Transforming Growth Factor-β, p27 is released from cyclin D/CDK4-6 and redistributed to cyclin E/CDK2 complexes, which are inactivated after p27 binding. Cyclin E/CDK2 in turn triggers p27 phosphorilation, which is subsequently degraded by the ubiquitin-proteosome pathway.3 5
CDKI are potent negative regulators of the cell cycle, and it has been shown that some of them may play a role as tumor suppressor genes. In fact, some members of the INK family such as p15 and p16 have been found to suffer inactivation by different genetic or epigenetic mechanisms in a high percentage of human neoplasms of different cell lineage.8-10 Although members of the KIP family only very rarely suffer genetic alterations in human tumors,11-14their protein expression has been described as being regulated by p53 mutation15 (in the case of p21) or by increased ubiquitin degradation for p27.16
This absence of genetic alteration in the p27 locus suggests that p27 inactivation could take place by means of a different mechanism, because it is known to play a key role in the control of the G1/S transition, its degradation being one of the molecular events that mark the irreversible decision to enter the S phase.3
p27 expression has been studied previously in normal and tumor lymphoid tissue.17,18 Reactive lymphoid tissue and most of the lymphoid neoplasms analyzed to date show an inverse relation between p27 expression and growth fraction, thus confirming the role of p27 as a negative regulator of cell-cycle progression. This role is also supported by most of the information accumulated about p27 expression in different human tumors16,19,20 and indicates that loss of p27 expression is an unfavorable prognostic marker associated with tumor progression. The pattern of expression of p27 in aggressive B-cell lymphomas is not fully consistent with these observations. Thus although most cases of Diffuse Large B-cell lymphoma (DLBCL) and Burkitt’s lymphoma (BL) show weak or null p27 expression, anomalous p27 overexpression has been described in a group of high-grade lymphomas associated with a loss of the ability of p53 to transactivate p21 and MDM2.17 Moreover, when considering the DLBCL group, p27 overexpression has been found to be an adverse prognostic factor, independent of other clinical parameters.21 Deregulated p27 overexpression has also recently been pointed out in breast and colorectal cancer and chronic B-cell lymphocytic leukemia (B-CLL),22-25 and an association between poorer overall prognosis and high p27 expression in B-CLL has also been described.25
The aim of this study is to analyze the causes of this anomalous p27 nuclear accumulation found in a subset of aggressive B-cell lymphomas. To this end we simultaneously determined the expression of p27 together with cyclin D and cyclin E proteins in a group of aggressive B-cell lymphomas, including DLBCL and BL, because the existence of an homeostatic feedback mechanism has been described by which high levels of expression of cyclin D1 or cyclin E in some cell types leads to increased p27 expression.22,26 We also included cyclin D3 and D2 in this study because the regulatory role that cyclin D1 plays in the G1/S transition in most cell types has been preferentially attributed to cyclin D3, but also to cyclin D2 in lymphoid cells, which normally do not express cyclin D1.27
Because the results obtained show simultaneous overexpression of cyclin D3 and high p27 in a subset of cases, we studied some of the tumor specimens with confocal microscopy to see whether colocalization of both proteins could also be observed at a cellular level. Reactive lymphoid tissue specimens were used as a reference of normal patterns of expression for these proteins. Finally, we have included some coimmunoprecipitation studies for analyzing the interaction between p27, cyclin D3, and CDK2.
MATERIALS AND METHODS
Tissue samples.
Twenty samples of reactive lymphoid tissue and tumor specimens from aggressive B-cell lymphoma, including 54 cases of DLBCL and 20 BL, were obtained from the routine files of the Virgen de la Salud Hospital, (Toledo, Spain) and diagnosed using the criteria of the Revised European-American Lymphoma (REAL) classification.28
Immunostaining techniques.
All immunostaining techniques were performed in paraffin-embedded tissue sections, using a previous step of heat-induced antigen retrieval technique for all antibodies (Ab). Thus, before incubation with the Ab, the slides were heated in a pressure cooker during 3 minutes in a solution of 0.01 mol/L sodium citrate.
After incubation with the primary Ab, immunodetection was performed with biotinylated antimouse immunoglobulins, followed by peroxidase-labeled streptavidine (LSAB-DAKO, Denmark) and diaminobencidine chromogen as substrate. All immunostaining was performed using the Techmate 500 (DAKO) automatic immunostaining device. Incubation omitting the specific Ab, as well as with unrelated Ab, was used as a control of the technique. For double-labeling experiments, incubation with Ab for CD20 (L26, Dakopatts) was performed after p27 immunodetection, followed by incubation with the LSAB system (DAKO), alkaline phosphatase, and Fast Red chromogen as substrate.
Ab for immunohistochemistry.
p27 protein was detected with a monoclonal antibody (MoAb) from Transduction Laboratories (Lexington, KY), K25020 (1:1000 dilution), generated against the full-length mouse KIP1 protein. Expression in small lymphocytes was used as internal control of immunostaining.
Cyclin D3 was detected with MoAb DCS-22 from Novocastra (Newcastle on Tyne, UK) (1:10 dilution). The specificity of the antibody was confirmed using Western blot, which produced a 34 kD band corresponding to the expected molecular weight of cyclin D3.29 The internal control was provided by the reactivity of endothelial cells. In some specimens with epithelial tissues, an internal positive control was found in the reactivity of the epithelium. Those cases negative for cyclin D3 without a signal in endothelial cells (internal control) were excluded from the series.
Cyclin D1 was detected with DCS-6 MoAb from Novocastra. Internal control was provided by the reactivity of macrophages and endothelial cells.
Cyclin D2 was immunostained using the MoAb DCS3.2 from Novocastra. The staining of scattered lymphoid and epithelial cells in reactive tonsillectomy specimens provided an external control.
Cyclin E was detected with MoAb 13A3 (Novocastra). The simultaneous staining of cytotrophoblast cells in placenta, a known positive control, provided an external control.
Quantitative studies.
Quantitative immunohistochemical investigation with the Quantitative Nuclear Antigen application of the Computerized Analyzer System (CAS 200; Cell Analysis System Inc, Elmhurst, IL) was used to score individual nuclei for the presence of cyclin D3 nuclear protein. This program measured the percentage of total optical density of positive cell nuclei in tissue sections, ie, the intensity of nuclear staining in comparison with the total nuclear area, thus making it possible to estimate the amount of intracellular protein. Measurement was performed focusing mainly on the most positively stained tumor areas, although the great majority of the cyclin D3 signal was coming from the tumor cells. For cyclin D3 the signal coming from endothelial cells was considered negligible.
Representative fields down to a minimum of 30,000 μm2 were selected (approximately 5 to 7 fields with a 45× objective and 10× ocular lenses).
High magnification fields were chosen for the evaluation of p27, focusing on tumor areas and counting up to 200 cells, excluding small T cells or other nontumor cells. All immunoreactive large lymphoid cells were measured. The percentage of p27-positive tumor cells was expressed as a ratio of positive cells to 100 cells. A manual procedure of counting p27-positive cells was used to exclude all positive nontumor subpopulations, using the morphology of the cells as the main criterion.
Statistical studies.
The significance of the association between cyclin D3 and p27 expression was assessed using the Spearman Correlation Coefficient and the nonparametric Kruskal-Wallis test. Association was considered significant if P < .05.
Double immunolabelling and laser scanning confocal microscopy (LSCM).
Tissue sections from 5 reactive lymphoid tissue (tonsils) and 15 neoplastic specimens with different levels of p27 expression were stained using double labeling with mouse MoAb anti-cyclin D3 1/25 (Novocastra), and rabbit polyclonal anti-p27 (C19) 1/25 (Santa Cruz, Santa Cruz, CA). After simultaneous overnight incubation at 4°C with the primary Ab, sections were incubated with biotin-conjugated donkey antirabbit immunoglobulin [IgG] (1/50, Jackson Immunoresearch Labs, West Grove, PA) and goat antimouse IgG (1/50, Jackson Immunoresearch Labs). After washing in tris-buffered saline (TBS), sections were incubated with streptavidine Cy2 (1/50, Amersham Pharmaciabiotech, UK) and Cy3 conjugated donkey antigoat IgG (1/50, Jackson Immunoresearch Labs). Sections were mounted with glycerol and examined with a MRC 1024 Bio-Rad confocal system (Bio-Rad Laboratories SA, Richmond, CA) mounted on a Zeiss Axiovert 135 microscope (Zeiss, Oberkochen, Germany). A 25 mw multiline argon laser producing two major lines at 488 and 514 nm was used. Images were stored on magnetoptical disks. Series of images were processed with the Lasersharp software package (Bio-Rad).
Immunoprecipitation assay.
Raji, a Burkitt’s cell line, was kept at 37°C in a 5% CO2 humidified incubator in cell culture flasks, at 2 × 106 cells/mL of RPMI-1640 medium, supplemented with 15% Fetal Bovine Serum, and 2 mmol/L L-glutamine. Cells were collected 24 hours after medium change, washed in cold PBS, and then dissolved in lysis buffer (50 mmol/L Tris-HCl pH 8.0; 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L dithiothreitol (DTT), beta-glicerophosphate 10mmol/L, NaF 1 mmol/L, Na3VO4 1 mmol/L, EDTA 1mmol/L, EGTA 5mmol/L, phenylmethylsulfonyl fluoride (PMSF) 1mmol/L, 5 μg/mL leupeptine, 2 μg/mL aprotinine). Frozen lymphoma tissue of two non-Hodgkin’s lymphoma samples was cut in a cryostat and dissolved in 250 μL of lysis buffer. The cell line and lymphoma tissues were incubated 45 minutes on ice, and supernatants were cleared by centrifugation at 12,000g at 4°C for 15 minutes. Approximately 250 μg of total protein was precleared and incubated with 5μg of the indicated antibodies and 10μL of Protein G Plus/Protein A Agarose (Calbiochem; Oncogene Research Products, La Jolla, CA). After gentle rotation for 2 to 3 hours at 4°C, the beads were pelleted and washed three times in lysis buffer and resuspended in 10 μL of 2× loading buffer. The samples were denatured for 5 minutes at 98°C and centrifuged for 5 minutes at 12000g. 10 μL of the supernatant were separated in 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and detected with the appropriate antibodies, as previously described.30
Ab for immunoprecipitation and Western blot.
The following antibodies were used: for p27: C-19 (Santa Cruz) and K25020 (Transduction Laboratories); for cyclin D3: C-16 and D-7 (Santa Cruz); and for CDK2: M-2 (Santa Cruz).
RESULTS
Immunohistochemical Study
p27.
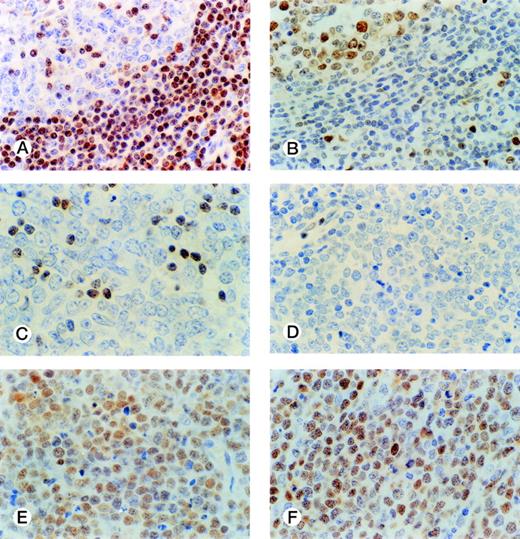
The distribution of p27-positive cells in reactive nontumor lymphoid tissue was as previously described.17 In brief, small quiescent lymphocytes showed strong nuclear p27 immunostaining, whereas proliferating cells in either the germinal center or interfollicular location were p27 negative (Fig 1A). Double immunostaining for p27 and CD20 showed p27 immunostaining in a subset of B cells in the germinal center. When investigating a group of aggressive B-cell lymphomas, including DLBCL and BL, the mean percentage ± Standard Deviation (SD) value of the series was 20.7 ± 27.8 (Fig 2A). Absence of reactivity in a comparable grade to that observed in benign proliferating B cells (ie, null or very low p27 staining in large proliferating cells) was observed in 29 of 74 cases. (Fig 1C, 2A). Twelve cases (4 of 54 DLBCL and 8 of 20 BL) were strongly positive for p27, and almost all tumor-proliferating cells were p27 positive in these (Fig 1E, 2A). p27 expression was observed more frequently in BL than in DLBCL (Table1) although this was not statistically significant.
p27 and cyclin D3 expression in reactive and tumor lymphoid tissue. Germinal center cells show low levels of p27 expression (A) with opposed high cyclin D3 staining (B). DLBCL case with a low level of p27 expression restricted to small lymphocytes (C); and cyclin D3–negative (D). DLBCL case with high simultaneous expression of p27 (E) and cyclin D3 (F) in tumor cells.
p27 and cyclin D3 expression in reactive and tumor lymphoid tissue. Germinal center cells show low levels of p27 expression (A) with opposed high cyclin D3 staining (B). DLBCL case with a low level of p27 expression restricted to small lymphocytes (C); and cyclin D3–negative (D). DLBCL case with high simultaneous expression of p27 (E) and cyclin D3 (F) in tumor cells.
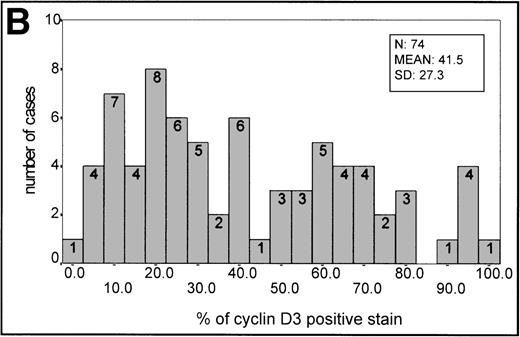
(A) Histogram of p27 expression. (B) Histogram of cyclin D3 expression.
The Expression of p27, Cyclin D3, and Correlation Between Both Proteins in Aggressive B-Cell Lymphomas
| . | P27 . | cyclin D3 . | p27 v cyclin D3 . |
|---|---|---|---|
| DLBCL | 13.8 ± 18.6 | 34.04 ± 22.4 | R .40, P .002 |
| BL | 39.3 ± 38.7 | 61.8 ± 29.6 | R .44, P.065 |
| DLBCL + BL | 20.7 ± 27.8 | 41.5 ± 27.3 | R .45, P .000 |
| . | P27 . | cyclin D3 . | p27 v cyclin D3 . |
|---|---|---|---|
| DLBCL | 13.8 ± 18.6 | 34.04 ± 22.4 | R .40, P .002 |
| BL | 39.3 ± 38.7 | 61.8 ± 29.6 | R .44, P.065 |
| DLBCL + BL | 20.7 ± 27.8 | 41.5 ± 27.3 | R .45, P .000 |
Values of p27 and cyclin D3 represent mean percentages of labeled cells and standard deviation.
r, Spearman correlation coefficient.
Cyclin D3 expression in benign lymph nodes and reactive tonsils was mainly observed in large lymphocytes, either in the germinal center or in the interfollicular area (Fig 1B).
The mean percentage ± SD value in reactive germinal centers was 42 ± 17.64 (20 samples). This staining intensity of the germinal center was used as an informative threshold of normal cyclin D3 expression in proliferating B cells.
In aggressive B-cell lymphomas, cyclin D3 nuclear expression was found in all cases, ranging from 2.4% to 100% of positive staining (Fig 1C,1F, 2B). The mean ± SD of the series was 41.5 ± 27.3. A stronger staining was observed in the large B cells, whereas cells of intermediate size had weaker staining. However, occasional small nuclei with a clear-cut expression could be observed. Cyclin D3 staining was also observed in some endothelial and epithelial cells when the sample analyzed included epithelium. A higher expression of cyclin D3 protein was observed more frequently in BL (Table 1).
Cyclin D2 expression was studied in 38 of 74 cases, showing nuclear staining of scattered positive cells in reactive tonsils, lymph nodes, and lymphoma specimens.
Cyclin D1 expression was also investigated in 70 of 74 cases of the series.
Most cases were mainly negative for this protein, although a weak cyclin D1 reactivity was found in scattered cells with tumor morphology in 7 of 70 cases. There is no difference in cyclin D3 expression between these cases and those completely negative for cyclin D1. Cyclin D1 expression by endothelial cells and macrophages, the internal positive control, was detected in all of the tissue sections analyzed.
Cyclin E.
Low levels of cyclin E could be detected in normal and tumor lymphoid tissue. In reactive tonsils some positive proliferating cells could be found in germinal centers, and less frequently in the mantle and interfollicular areas. Sporadic cells in the cryptal epithelium were also cyclin E–positive. Similarly, most tumor cases were negative for cyclin E expression, although a significant cyclin E expression by tumor cells was found in 7 of 68 cases analyzed. These cyclin E–positive cases did not differ in the expression of cyclin D1, D3, or p27 from those cases that lacked cyclin E expression. Only one BL had relatively high levels of cyclin E and concomitant high p27 expression (93%). The cyclin D3 value of this isolated case was within the range of the other cases with high cyclin D3 overexpression.
The Relationship Between the Expression of Cyclin D3 and p27 Proteins
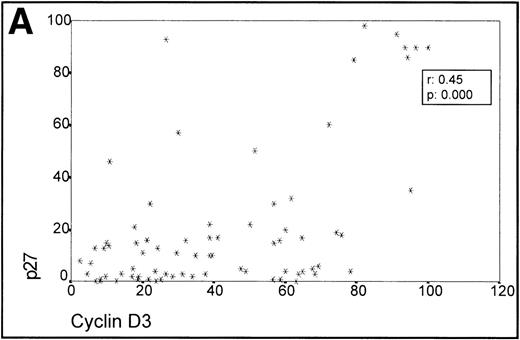
When analyzing the overall relationship between cyclin D3 and p27 expression using the Spearman Correlation Coefficient, it was found to be significant (r 0.45, P = .000). (Fig3A, Table 1).
(A) Scatterplot of the simultaneous expression of cyclin D3 and p27 proteins. A significant correlation exists between both parameters. (B) Kruskall-Wallis test: Cases of lymphoma with cyclin D3 expression over the level observed in benign reactive germinal centers (42%) have significantly higher p27 expression.
(A) Scatterplot of the simultaneous expression of cyclin D3 and p27 proteins. A significant correlation exists between both parameters. (B) Kruskall-Wallis test: Cases of lymphoma with cyclin D3 expression over the level observed in benign reactive germinal centers (42%) have significantly higher p27 expression.
Taking into account the expression of cyclin D3 in the proliferating cells of the germinal centers of reactive tonsils (mean 42%), two groups of lymphomas, low (<42%) and high expressers (>42%) of cyclin D3, were made (Fig 3B). Those cases with a high expression of cyclin D3 expressed p27 levels higher than those cases characterized by a low expression of cyclin D3, this difference being statistically significant (Kruskal-Wallis test, P = .0045).
There was no difference in the p27-cyclin D3 relationship between cases of DLBCL and BL (Table 1).
Colocalization of Cyclin D3 and p27
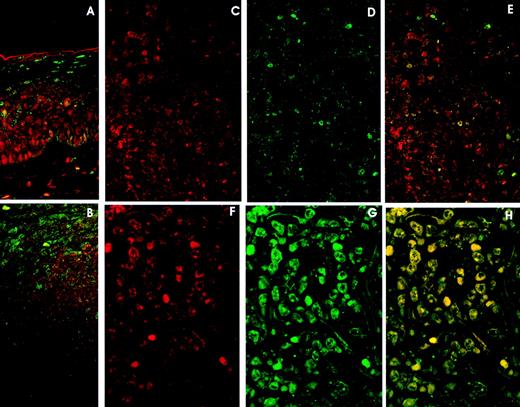
Double immunolabeling LSCM studies in reactive lymphoid tissue confirmed previous observations on p27: generalized expression by all the small lymphocytes in the mantle zone and interfollicular area, and focal staining by scattered germinal center cells. On the other hand, cyclin D3 was mainly expressed by germinal center cells, whereas very few mantle cells were cyclin D3–positive (Fig4A).
Double immunolabelling and LSCM studies. A and B. Reactive Lymphoid Tissue. (A) Tonsil surface epithelium: cyclin D3 expression (red) in the lower rds and p27 expression (green) in the upper third. Cyclin D3 and p27 colocalization (yellow) is very unusual. (B) Tonsil reactive follicle showing cyclin D3 expression (red) mainly restricted to germinal center cells and p27 expression in mantle cells and very few germinal center cells (green). Cyclin D3 and p27 colocalization is very infrequent (yellow). C, D, and E. DLBCL with high (60%) cyclin D3 expression (red) (C) and moderate (20%) p27 expression (green) (D). Cyclin D3 and p27 colocalization (E) (yellow) is observed in isolated cells. F, G, and H. DLBCL with (F) high (79%) cyclin D3 expression and (G) p27 overexpression (85%). Cyclin D3 and p27 colocalization (H) occurs in almost every cell.
Double immunolabelling and LSCM studies. A and B. Reactive Lymphoid Tissue. (A) Tonsil surface epithelium: cyclin D3 expression (red) in the lower rds and p27 expression (green) in the upper third. Cyclin D3 and p27 colocalization (yellow) is very unusual. (B) Tonsil reactive follicle showing cyclin D3 expression (red) mainly restricted to germinal center cells and p27 expression in mantle cells and very few germinal center cells (green). Cyclin D3 and p27 colocalization is very infrequent (yellow). C, D, and E. DLBCL with high (60%) cyclin D3 expression (red) (C) and moderate (20%) p27 expression (green) (D). Cyclin D3 and p27 colocalization (E) (yellow) is observed in isolated cells. F, G, and H. DLBCL with (F) high (79%) cyclin D3 expression and (G) p27 overexpression (85%). Cyclin D3 and p27 colocalization (H) occurs in almost every cell.
Tonsil surface epithelium displayed cyclin D3 expression in the lower two thirds, whereas p27 was observed in the upper third (Fig 4B).
Colocalization of p27 and cyclin D3 was very unusual, being observed only in isolated cells in the interfollicular area, germinal center, and epithelium (Fig 4A and B).
p27-Cyclin D3 colocalization was very rarely observed in lymphomas with low or moderate expression of p27 and cyclin D3 (Fig 4C, D, E).
However, when studying cases of lymphoma with high levels of p27 and cyclin D3 expression, the colocalization of both proteins by lymphoma cells was observed in all cases in the most positive cells. (Fig 4F, G, H).
Immunoprecipitation Assays
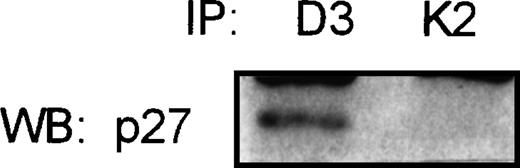
In the attempt to show the existence of the association of p27 with the complex cyclin D3/CDK4, the Raji cell line (a Burkitt’s cell line) was used for coimmunoprecipitation studies. This cell line expresses high levels of p27, and CDK2 and cyclin D3 proteins, as detected by Western blot. Total protein was immunoprecipitated with cyclin D3 and CDK2 antibodies. p27 was found associated with cyclin D3 but no detectable levels of p27-CDK2 complexes could be observed (Fig5).
Coimmunoprecipitation assay in Raji Burkitt’s cell line. Total protein was immunoprecipitated with cyclin D3 (D3) and CDK2 (K2) specific antibodies, followed by immunoblot with the p27 antibody. Not detectable association of p27 with CDK2 could be observed.
Coimmunoprecipitation assay in Raji Burkitt’s cell line. Total protein was immunoprecipitated with cyclin D3 (D3) and CDK2 (K2) specific antibodies, followed by immunoblot with the p27 antibody. Not detectable association of p27 with CDK2 could be observed.
Immunoprecipitation studies were also performed in two NHL samples, with intermediate levels of p27 and cyclin D3 expression, showing the simultaneous existence of both cyclin D3/p27 and CDK2/p27 complexes.
DISCUSSION
The data of the present study essentially confirms those previously published on p27 expression in DLBCL and BL.17 18 The vast majority of cases are either negative or show very weak p27 protein expression, and a subset of cases display an anomalous p27 high expression, which in opposition to that observed in benign lymphocytes, is associated with a high-growth fraction. This suggests that p27 protein in these cases could be rendered nonfunctional as an inhibitor of cell cycle progression through an unknown mechanism. The increase in the percentage of cases overexpressing p27 in comparison with our previous study seems to be due to the inclusion of a higher proportion of BL, which tends to express higher level of p27 protein.
These, and previous studies,31 32 have shown that normal and tumor lymphoid cells express cyclin D3 protein, the level of which is correlated with the cell growth fraction.
An association between high p27 expression and overexpression of cyclin D3 is the main result of this study. There is an overall and statistically significant tendency for both proteins to increase in parallel, thus suggesting that cyclin D3 could play a role in the anomalous stabilization of p27.
The strong immunostaining of both proteins, cyclin D3 and p27, in a group of cases is consistent with physical association between them. Moreover, identical nuclear localization patterns of p27 and cyclin D3 observed in most tumoral cells by confocal analysis microscopy, strongly argues in this direction. Reactive lymphoid tissue and tumors without high p27 expression display alternative cyclin D3 and p27 signals by different cells. Indeed, the colocalization of p27 and cyclin D3 is extremely unusual. However, this pattern dramatically changes in lymphoma cases with high p27 levels in which most cells show p27 and cyclin D3 colocalization. Therefore, those cases with an anomalous high expression of p27 could have an inactive p27 protein sequestered by cyclin D3, as shown by the presence of the complex p27-cyclin D3 and the absence of the complex p27-CDK2, because it has been found in the coimmunoprecipitation study in the Burkitt’s Raji cell line. A partial sequestration of p27 by cyclin D3 has also been observed in two lymphoma samples with intermediate levels of p27 and cyclin D3 expression. This inactivation of p27 is also supported by the recent finding of an association between high expression of p27 protein and adverse clinical outcome of DLBCL patients,21 which contrasts with the favorable clinical course usually associated with higher p27 expression.16,19 20
The association described here is also consistent with previous reports showing the existence of complexes p27-cyclin D3/CDK6 or p27-cyclin D/CDK4 with Rb-kinase activity in cell lines of different types,5-7 33 suggesting that p27 is not active when joined to cyclin D/CDK4-6 complexes. A redistribution of p27 from cyclin E/CDK2 to cyclin D/CDK4 complexes, which precludes its negative regulator action on the cell cycle, has also been described in growing cells in which p27 is associated with cyclin D/CDK4.
The interaction between cyclin D3 and p27 seems to differ in some respects from that observed between cyclin D1 and p27 in mantle cell lymphomas (MCL). Thus, in both types of lymphomas there are data showing p27 sequestration by cyclin D (cyclin D1 in MCL and cyclin D3 in DLBCL and BL), although the p27 protein detectable by IHC seems to be lower in MCL than in DLBCL or BL, for unknown reasons, because Western blot studies in MCL display an increased level of p27 protein.18 34
The results here obtained on cyclin E, D1, and D2 protein expression do not explain the anomalous p27 stabilization, because cyclin D1 staining is virtually negative in this type of lymphomas, the expression of cyclin D2 is very low, and only one of seven cases with a relatively high expression of cyclin E protein also have increased p27 levels.
To conclude, the data shown here seem to support the hypothesis that high cyclin D3 levels could lead to an anomalous p27 stabilization, probably associated with its functional inactivation. The search for a cause for the expression of both cyclin D3 and p27 in aggressive B-cell lymphomas above the levels observed in reactive lymphoid tissue should consider not only genetic alterations of cyclin D3 and p27 genes (rarely described), but also the interaction of both proteins with their partners (other CDKIs, kinases, …) in regulation of the cell cycle.
ACKNOWLEDGMENT
We thank M.A. Ollacarizqueta, PhD, for her technical assistance in LSCM studies, and D. Gómez-Donaire for her excellent help in double immunofluorescent staining. Prof Oriol Bachs and Dr Monserrat Jaumot have kindly collaborated in the coimmunoprecipitation study.
Supported by grants 96/1382 and 98/993 from the Fondo de Investigaciones Sanitarias, and 08.1/0027/1997 from Comunidad de Madrid, Spain.
MS-B and FIC contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal