Abstract
Shock is frequently accompanied by thrombocytopenia. To investigate the pathogenic role of platelets in shock, we examined the in vivo effects of monoclonal antibodies (MoAbs) against mouse platelet membrane proteins. Injection of the platelet-specific MoAb MWReg30 to the fibrinogen receptor (gpIIb/IIIa) rendered mice severely hypothermic within minutes. Isotype-matched control antibodies, even if they also recognized platelet surface antigens, did not induce comparable signs. MWReg30 induced early signs of acute lung injury with increased cellularity in the lung interstitium and rapid engorgement of alveolar septal vessels. Despite this in vivo activity, MWReg30 inhibited rather than stimulated platelet aggregation in vitro. MWReg30-binding to platelets led to phosphorylation of gpIIIa, but did not induce morphological signs of platelet activation. The MWReg30-induced reaction was abolished after treatment with MoAbs 2.4G2 to FcγRII/III and was absent in FcγRIII-deficient mice, clearly demonstrating the requirement for FcγRIII on involved leukocytes. Simultaneous administration of tumor necrosis factor exacerbated, whereas a tolerizing regimen of tumor necrosis factor or bacterial lipopolysaccharide completely prevented the reaction. These data suggest that platelet surface-deposited MWReg30-immune complexes lead to an acute Fc-mediated reaction with pulmonary congestion and life-threatening potential that could serve as an in vivo model of acute lung injury.
ACUTE LUNG INJURY occurring in association with septic shock appears to result from an inflammatory reaction involving particularly the lung microvasculature. At the same time, many other regulatory systems in the body become activated by inflammatory stimuli. In extreme situations such as septic shock, the molecular mechanisms leading to microvascular injury, tissue damage, and organ failure, therefore, become very complex. Most forms of shock, particularly after a variety of diseases, such as sepsis, major trauma, and malignancy, can be accompanied by thrombocytopenia and disseminated intravascular coagulation. Thrombocytopenia is most commonly regarded as a secondary complication, resulting from endothelial injury. Alternatively, platelets could also play an active role, because depletion of platelets resulted in reduced morbidity or mortality in different experimental settings related to shock.1-4 The interactions of platelet surface molecules with leukocyte or endothelial cell adhesion molecules can lead to platelet activation and to the release of biologically important mediators. The facts that platelets are so numerous and that degranulation is a rather rapid event may cause a very effective yet locally restricted reaction.5 Such a role for platelets can be predicted in some types of hypersensitivity reactions and immune complex-mediated diseases.
Hyperactive polymorphonuclear neutrophils play a central role in the pathogenesis of acute lung injury. Neutrophils from patients with severe bacterial infection display enhanced responses when stimulated in vitro.6 In vitro investigations and studies using the extracorporeal rat lung perfusion model of acute lung injury demonstrated that capillary leakage results from two independent insults, one leading to priming events with the result of sequestration of polymorphonuclear neutrophils and a second activating signal.7 Observations from the isolated lung perfusion model in the absence of blood constituents suggest the possibility that the priming effect can act on resident cells in the lung, eg, macrophages or endothelial cells.8 Such a priming for enhanced platelet activating factor (PAF) production by tumor necrosis factor (TNF) on macrophages, endothelial cells, and neutrophils has been demonstrated in vitro.9 In the transfusion-related acute lung injury (TRALI), the second signal can be provided by Igs directed against recipient granulocytes10 or by biologically active compounds generated during storage of cellular blood components involving the action of lipid mediators and triggering of the PAF receptor.7
The role of TNF mediating pathogenic effects in bacterial lipopolysaccharide (LPS)-induced shock has clearly been established.11-13 A trend towards protection by neutralizing TNF in patients with severe sepsis rather than septic shock has been reported.14 However, especially when administered late in full-blown shock, this treatment did not result in any beneficial effect, clearly indicating that TNF action alone does not account for the shock syndrome.15 Another important pathogenic element is the activation of Fcγ receptor III (FcγRIII, CD16) on granulocytes and macrophages, as described for the anaphylactic shock.16,17 Among numerous other pathways not mentioned here, an excessive activation of the complement system can also lead to severe inflammation and tissue destruction.18
We present here evidence that specific binding of an anti-gpIIb/IIIa monoclonal antibody (MoAb) MWReg30, but not of other antibodies to the platelet surface, formed immune complexes in situ that led to an FcγRIII-mediated shock-like reaction with clear signs of acute lung injury, the extent of which was modulated by inflammatory signals.
MATERIALS AND METHODS
Animals.
Specific-pathogen–free mice (NMRI, C3H/HeN, C3H/HeJ, and BALB/c) 5 to 7 weeks of age were obtained from Charles River (Sulzfeld, Germany) and kept in the animal facilities of the University of Regensburg (Regensburg, Germany) for the experiments. Mice deficient in FcγRIII were generated in collaboration with the group of J.S. Verbeek (Utrecht, The Netherlands) as described.19 These mice were bred and maintained under dry barrier conditions in the animal facilities at the Hannover Medical School (Hannover, Germany) and were studied at 2 to 4 months of age.
Reagents.
Recombinant mouse TNF (rmTNF) was prepared and purified in our laboratory with a specific activity of 8 × 107 U/mg as determined in the TNF bioassay. LPS (LPS-W from Salmonella minnesota 9700) was obtained from Difco Laboratories (Detroit, MI); methylene blue, Mianserin, cyproheptadine, and acid glycoprotein (bovine α-1 AGP) were from Sigma (Deisenhofen, Germany). Cobra venom factor preparations (CVF) were kindly provided by J. Köhl (Hannover, Germany); soluble complement receptor I (sCRI) was kindly provided by M. Kirschfink (Heidelberg, Germany); the peptides GRGDS and YIGSR were synthesized and kindly provided by R. Frank (Heidelberg, Germany).
Antibodies.
MoAbs were generated and purified following standard procedures from the following hybridomas: rat-antimouse-LFA-1 MoAb (H35.89.9),20 rat-antimouse–intercellular adhesion molecule-1 (ICAM-1) MoAb (YN1/1),21anti-granulocyte MoAb (R14),22 and rat-antimouse-FcγRII/III MoAb (2.4G2).23 The MoAbs anti-CD18 and anti-CD13 were obtained from Pharmingen (Hamburg, Germany). For in vivo application, 100 μg of the MoAb were injected intraperitoneally (IP) in 200 μL phosphate-buffered saline (PBS) 24 hours before the challenge. Rat antimouse platelet MWReg30 (IgG1) and MWReg31 (IgG2a) MoAbs were generated in our laboratory and screened as described.24
Induction of thrombocytopenia.
Rabbit antimouse platelet serum was produced by immunization of rabbits with purified platelets as described.25 Injection of 100 μL of this serum IP reduced the number of circulating platelets to less than 0.05 × 106/μL (normal value, 1.0 to 1.2 × 106/μL) within 1 hour and kept it at this level for at least 48 hours. Thrombocytopenic mice were taken into the experiments 24 hours after injection of the antiplatelet serum.
Hypothermia measurement.
Body temperature was measured at the indicated times with a rectal probe.
Platelets preparation and counting.
Mice were bled under ether anesthesia from the retroorbital plexus. Blood from 10 mice was pooled into a tube containing 0.5 mL 0.1 mol/L sodium citrate, and platelet-rich plasma (PRP) was obtained by centrifugation at 300g for 10 minutes at room temperature. The platelets were washed 3 times with PBS by centrifugation at 1,300g for 10 minutes and were used immediately. Isolated platelets did not show any signs of activation as tested by staining for P-selectin or morphological signs of degranulation. For determination of platelet counts, blood (20 μL) was obtained from the retroorbital plexus of anesthetized mice using siliconized microcapillaries and immediately diluted 1:100 in Unopette Kits (Becton Dickinson, Heidelberg, Germany). The diluted blood sample was allowed to settle for 20 minutes in an improved Neubauer hemocytometer, and platelets were counted under phase contrast at 400× magnification.
Flow cytometry.
MWReg30 MoAb was fluoresceinated at a fluorescein:protein ratio of 3:1 by standard procedures with fluorescein isothiocyanate (FITC; Sigma) and separated from free FITC by gel filtration on a PD-10 column (Pharmacia, Uppsala, Sweden). Cells were stained at a final concentration of 5 μg/mL fluoresceinated antibody in PBS for 20 minutes on ice. For determination of membrane-bound MWReg30, mice were bleed 3 hours after MWReg30 injection. Platelets were washed with PBS twice and stained with FITC-labeled goat antirat Ig (Pharmingen) for 20 minutes at 4°C in the dark. All samples were analyzed on a FACscan (Becton Dickinson), and platelets were gated by forward/side scatter characteristics.
Aggregometry.
To determine platelet aggregation, transmission was measured using PRP (200 μL with ∼106 platelets/μL). Ten microliters of rat IgG (300 μg/mL), MWReg30 (300 μg/mL), or rat antiserum to mouse gpIIb/IIIa (10 μL) and 10 μL of Tyrode’s buffer (Sigma) were mixed with PRP for 1 minute at 37°C before 10 μL of ADP (110 μmol/L; Sigma), phorbol 12-myristate 13-acetate (PMA; 1 μg/mL; Sigma), or collagen A (1 mg/mL; Biochrom, Berlin, Germany) as aggregation-inducing agents were added. For negative controls 10 μL of apyrase (100 U/mL; grade III; Sigma) was added. Transmission was recorded in a Fibrintimer 2 channel aggregometer (APACT Laborgeräte + Analysensysteme, Hamburg, Germany) over 10 minutes and was expressed as arbitrary units with 100% transmission adjusted with plasma.
Histology.
For histology, lung, liver, and kidney samples were fixed by immersion in 4% PBS-buffered formaldehyde and embedded in paraffin. Sections (2 to 3 μm) were stained with hematoxlin and eosin. Additional lung samples were fixed in cacodylate-buffered Karnovsky fixative (2.5% glutaraldehyde and 2% paraformaldehyde), postfixed in 1% osmium tetroxide, and embedded in EMbed-812 epoxy resin, and semithin sections (0.8 μm) were stained with 1% toluidine blue.
For immunohistochemistry, acetone-fixed cryosections of mouse spleens were stained with MWReg30 at a final concentration of 5 μg/mL for 20 minutes on ice, washed with PBS, and incubated for 1 hour at room temperature with biotinylated goat polyclonal antibodies antirat IgG (Southern Biotechnology, Bioreba, Reinach, Switzerland) followed by the addition of horseradish peroxide (HRPO)-avidin. Color reaction was obtained by the addition of AEC substrate-chromogen (Dako, Glostrup, Denmark), and the slides were counterstained with toluidine blue. Bone marrow cells (106) or tumor cells were stained with FITC-labeled MWReg30 at a final concentration of 5 μg/mL for 20 minutes on ice, washed with PBS, and investigated under the fluorescence microscope.
Immunoprecipitation.
Immunoprecipitation was performed as described previously.26 Briefly, 108 washed platelets were surface-labeled with PBS containing 100 μg/mL 6-(+)-biotinylamidohexanoic acid N-hydroxysulfosuccimide ester sodium salt (NHSS-LC-biotin; Serva, Heidelberg, Germany) and subsequently solubilized in 1 mL lysis buffer (Tris-buffered saline containing 20 mmol/L Tris/HCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 0.5 μg/mL leupeptin, and 0.5% Nonidet P-40 (all from Boehringer Mannheim, Mannheim, Germany). Cell debris was removed by centrifugation (12,000g for 20 minutes). After preclearing (8 hours), 10 μg MWReg30 or control MoAb was added together with 25 μL of protein G-Sepharose (Pharmacia), and precipitation took place overnight at 4°C. Samples were run on a 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) along with a biotinylated molecular weight marker and transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with streptavidin-horseradish peroxidase (1 μg/mL) for 1 hour after blocking. After extensive washing, biotinylated proteins were visualized by ECL (Amersham, Arlington Heights, IL).
Sequencing.
The antigen of 1011 unbiotinylated platelets was immunoprecipitated with MWReg30. After electrophoresis on SDS-PAGE and transfer to PVDF membrane, specific bands were cut out and digested with trypsin by standard methods. High-performance liquid chromatography (HPLC)-purified tryptic fragments were subjected to an Applied Biosystems (Foster City, CA) protein sequencer (model 477A) with an online PTH-analyzer (model 120A).
Tyrosine phosphorylation of gpIIIa.
Platelets were stimulated with MWReg30 (30 μg/mL) for 3 minutes and taken up in Laemmli sample buffer containing sodium vanadate as described.27 For each reaction, 200 μL of PRP (∼109/mL) was treated and lysed immediately by the addition of 200 μL of 2× sample buffer (to give a final concentration of 37 mmol/L Tris, pH 6.8, 11.8% glycerol, 2.36% SDS, 2 mmol/L sodium vanadate, and 0.002% bromphenol blue). Immunoprecipitation with protein G-Sepharose was performed for 1 hour at 4°C with the addition of MWReg30 to platelets either 3 minutes before or immediately after (control) platelet lysis with lysis buffer (see above). Samples were boiled for 3 minutes, run on a 7.5% SDS-PAGE along with a molecular weight marker, and transferred to PVDF membrane. After blocking, the membrane was incubated with antiphosphotyrosine antibody PY-20 (Santa Cruz Biotechnology, Santa Cruz, CA) at a final concentration of 2 μg/mL for 1 hour. Bound PY-20 was detected by horseradish peroxidase-conjugated sheep antimouse IgG (Dianova, Hamburg, Germany) and visualized by ECL (Amersham).
RESULTS
Induction of hypothermia by a platelet-specific MoAb, MWReg30.
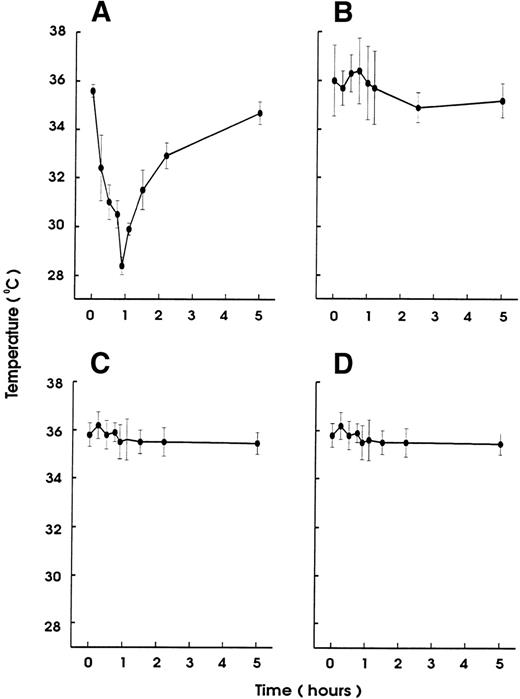
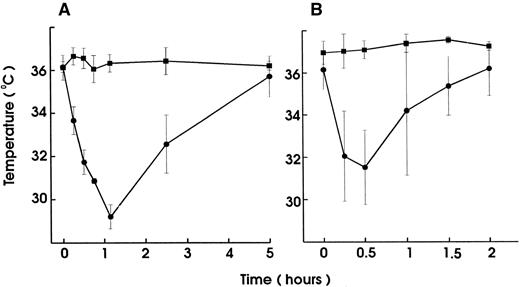
To investigate the role of platelets in hypersensitivity reactions and shock, antisera and MoAbs against mouse platelet membrane proteins were generated. Rats were immunized with purified platelets and the resultant antisera were tested for their ability to induce thrombocytopenia and shock signs in mice. All antisera markedly reduced the platelet counts. One antiserum induced, in addition, significant hypothermia in mice. Fusion of the spleen cells from this rat with mouse myeloma cells resulted in a number of hybridomas. Two different clones were identified to produce MoAb (MWReg30, IgG1 and MWReg31, IgG2a) directed against two distinct mouse platelet membrane antigens. Purified MoAb from these clones were tested for their ability to induce hypothermia in mice. Injection of 100 μg MWReg30 (Fig 1A) but not MWReg31 (Fig 1C) rapidly induced a strong hypothermia in normal mice. Mice rendered thrombocytopenic by injection of 100 μL of a rabbit antiplatelet serum 24 hours before MWReg30 challenge failed to develop hypothermia (Fig 1B). A rabbit antiserum to mouse platelets, ie, platelet depletion itself, did not show any effect on body temperature (Fig 1D). Isotype-matched (rat IgG1) control MoAb to aminopeptidase (anti-CD13) and to LFA-1 (anti-CD18), respectively, did not induce any hypothermia, even when 100 μg were injected intravenously (data not shown). The CD18 epitope recognized by the anti-CD18 MoAb is expressed on mouse platelets, as determined by flow cytometry, whereas aminopeptidase (CD13) is not. Injection of 100 μg rat IgG or rabbit IgG did not induce hypothermia.
Induction of hypothermia by MWReg30 but not other MoAbs—requirement of platelets. Normal (A) or thrombocytopenic (B) male NMRI mice received 100 μg of affinity-purified MWReg30 IP in 100 μL sterile PBS. As control for platelet-specific antibodies, normal mice received 100 μg affinity-purified MWReg31 (C) or 100 μL of a rabbit antimouse platelet serum (D). The mean values of the body temperatures of 3 mice per group at the indicated times after injection of the antibodies are given ± SD.
Induction of hypothermia by MWReg30 but not other MoAbs—requirement of platelets. Normal (A) or thrombocytopenic (B) male NMRI mice received 100 μg of affinity-purified MWReg30 IP in 100 μL sterile PBS. As control for platelet-specific antibodies, normal mice received 100 μg affinity-purified MWReg31 (C) or 100 μL of a rabbit antimouse platelet serum (D). The mean values of the body temperatures of 3 mice per group at the indicated times after injection of the antibodies are given ± SD.
Absence of linkage between hypothermia and thrombocytopenia.
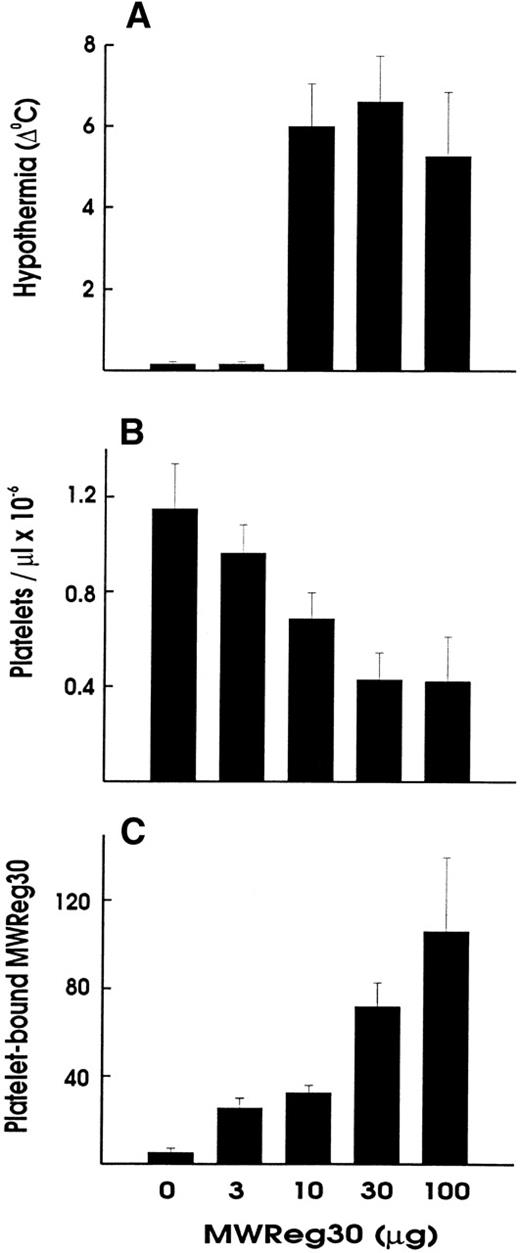
Upon intravenous (IV) injection of MWReg30, the central temperature decreased within 10 minutes, to reach a nadir at 60 minutes, with a return to normal within 2 to 3 hours. The hypothermic response after MWReg30 injection was not dose-dependent but rather occurred with a threshold between 3 and 10 μg (Fig 2A). The lowest dose required to exert hypothermia (defined as a temperature decrease of 5°C or more) in naive, healthy mice was 10 μg per mouse. In contrast, there was a clear dose-dependency in the MWReg30-induced thrombocytopenia (Fig 2B) and in the number of in vivo MWReg30-labeled platelets in mice determined after 3 hours (Fig 2C). The maximal thrombocytopenia, presenting as a 70% decrease in platelet counts, was reached with 30 μg. MWReg31 had a comparable thrombocytopenia-inducing activity to MWReg30, but failed to cause hypothermia, as shown in Fig 1C. Injection of a rabbit antimouse platelet antiserum led to an even more pronounced thrombocytopenia, with more than a 95% decrease in platelet counts, but not to hypothermia (Fig 1D).
Hypothermia is independent of thrombocytopenia induction. Dose-dependency of hypothermia (A), thrombocytopenia (B), and platelet-bound MWReg30 levels upon IV injection of the indicated amount of MWReg30 in 200 μL PBS. The hypothermia is expressed as the decrease in degrees Celcius measured 60 minutes after injection, whereas platelet counts and platelet antirat Ig staining were performed 3 hours after injection. Results are expressed as the means from 3 mice per group ± SD.
Hypothermia is independent of thrombocytopenia induction. Dose-dependency of hypothermia (A), thrombocytopenia (B), and platelet-bound MWReg30 levels upon IV injection of the indicated amount of MWReg30 in 200 μL PBS. The hypothermia is expressed as the decrease in degrees Celcius measured 60 minutes after injection, whereas platelet counts and platelet antirat Ig staining were performed 3 hours after injection. Results are expressed as the means from 3 mice per group ± SD.
Characterization of MWReg30.
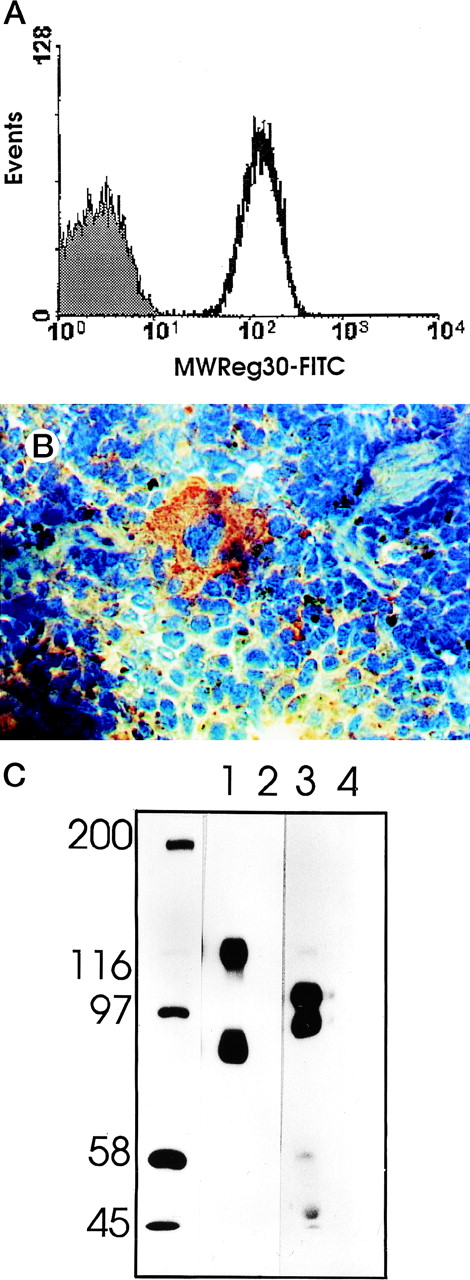
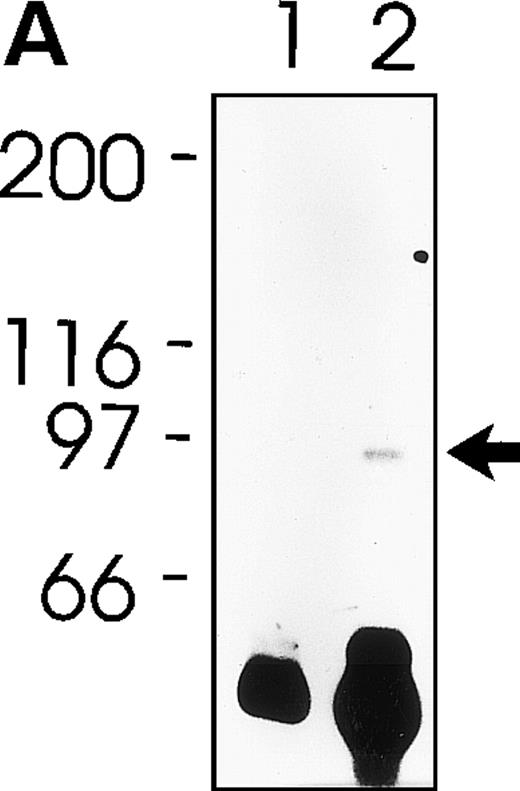
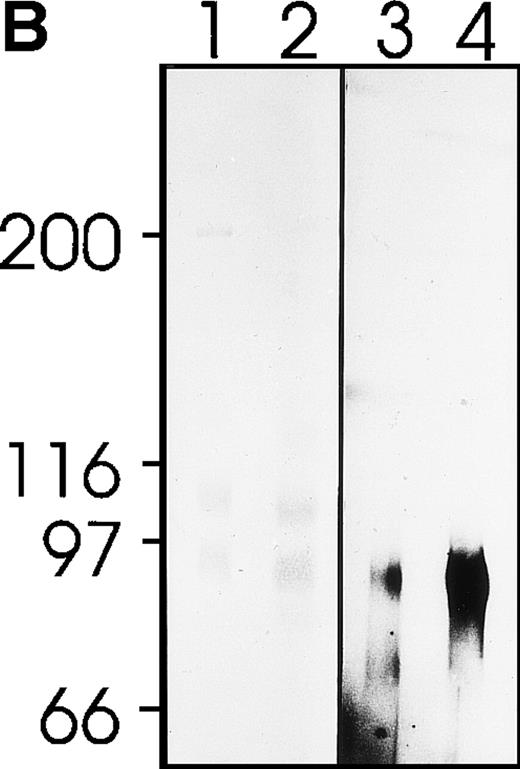
MWReg30 strongly and exclusively bound to platelets (Fig 3A) and megakaryocytes (Fig 3B). The antibody did not recognize αvβ3 integrin-positive tumor cells (bEnd3), and immunohistochemical staining of different organ sections and tumor cells (endothelioma, fibrosarcoma, thymoma, monocytic cell lines) demonstrated the platelet/megakaryocyte specificity of the MoAb. Immunoprecipitates obtained from surface-biotinylated platelets were analyzed by SDS-PAGE under nonreducing (Fig 3C, lanes 1 and 2) and reducing conditions (Fig 3C, lanes 3 and 4). MWReg30 precipitated a complex of two membrane proteins of 135 and 80 kD. Under reducing conditions, the 135-kD protein resolved into two bands of 110 and 25 kD (the latter being determined in gels of higher density; not shown), whereas the apparent molecular mass of the 80-kD chain slightly increased. Amino acid sequences of tryptic fragments identified the 80-kD protein (5′-DASHLLVFT-3′) as gpIIIa (integrin β3/CD61)28 and the 135-kD protein (5′-LRGEQMASYF-3′, 5′-PQALSTPTL-3′, 5′-DGYNDIAV-3′) as gpIIb (integrin aIIb/CD41),29 identifying the immunoprecipitated protein as the fibrinogen receptor (gpIIb/IIIa).
Characterization of the MWReg30 MoAb. Binding characteristics of the MWReg30 MoAb were investigated (A) by flow cytometric analysis, which shows that all circulating mouse platelets were intensively stained with FITC-labeled MWReg30. The shaded area indicates platelets stained with an FITC-labeled control rat MoAb. (B) Mouse spleen sections were stained with MWReg30. (C) Immunoprecipitates with MWReg30 (lanes 1 and 3) or control rat IgG (lanes 2 and 4) obtained from lysates of surface-biotinylated platelets were analyzed by SDS-PAGE under nonreducing (lanes 1 and 2) and reducing conditions (lanes 3 and 4) by staining of the blotted proteins.
Characterization of the MWReg30 MoAb. Binding characteristics of the MWReg30 MoAb were investigated (A) by flow cytometric analysis, which shows that all circulating mouse platelets were intensively stained with FITC-labeled MWReg30. The shaded area indicates platelets stained with an FITC-labeled control rat MoAb. (B) Mouse spleen sections were stained with MWReg30. (C) Immunoprecipitates with MWReg30 (lanes 1 and 3) or control rat IgG (lanes 2 and 4) obtained from lysates of surface-biotinylated platelets were analyzed by SDS-PAGE under nonreducing (lanes 1 and 2) and reducing conditions (lanes 3 and 4) by staining of the blotted proteins.
Histopathology of the MWReg30-induced reaction.
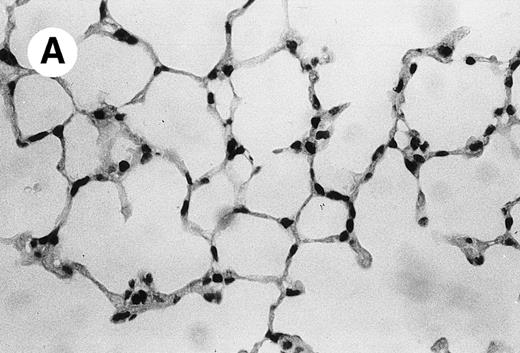
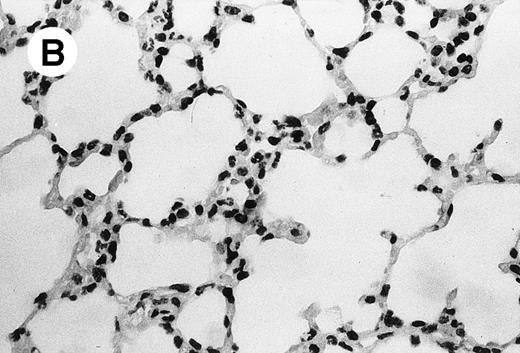
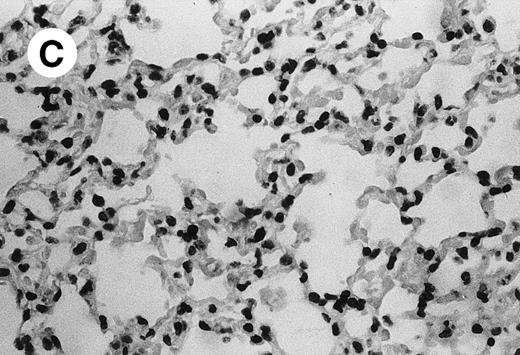
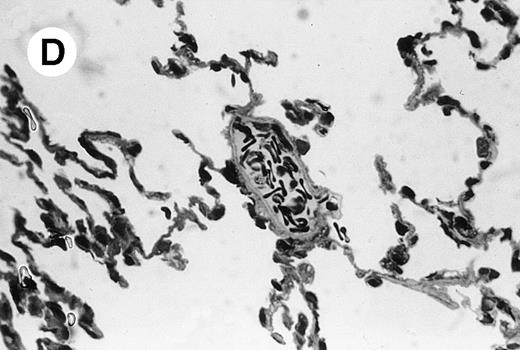
IV injection of MWReg30 caused within 15 minutes a peripheral vasodilatation, as evidenced by red extremities (tail and feet) and uncoordinated movements. These signs persisted for 60 to 90 minutes. Proportional to the injected dose of MWReg30, mice showed intestinal hemorrhages, ruffled fur, and reduced flow rate during blood sampling. Lung, kidney, and liver were sampled at different times after MWReg30 injection for histological examination. Histopathology of the whole lung performed 60 minutes after injection showed enhanced thickness of alveolar septa, due to edema and to increased cellularity, consisting of polymorphonuclear and mononuclear cell accumulation in capillaries, as compared with lungs from mice injected with PBS (Fig 4) or control IgG (not shown). This change was found to be uniform, not focal, in the two lungs (n = 6). After 24 hours, lungs showed a further enhancement of septa thickness, due to mononuclear, but not polymorphonuclear, cell accumulation in capillaries and interstitium, as well as plasma leakage into the alveolar space (Fig 4C). No changes were observed in peribronchiolar areas. As early as 20 minutes after IV injection, semithin sections showed that the vascular engorgement was essentially due to red blood cells, with very few leukocytes or platelets (Fig 4D). Twenty-four hours after injection, some mice showed centrolobular necrosis in the liver as well as glomerular shrinkage and enlargement of Bowman spaces in the kidney, compatible with hemodynamic changes of shock, but without evidence of acute tubular necrosis. Thrombi or microthrombi were not seen in any of the examined organs. Platelets recovered from the blood of mice 30 minutes after injection of MWReg30 did not express P-selectin as determined by FACS analysis. In sera from mice obtained 90 minutes after injection of MWReg30, no TNF was detectable.
Lung histopathology. Aspect of alveolar walls 60 minutes after IV injection of PBS (A) or 10 μg of MWReg30 (B); (C) 24 hours after IV injection of 10 μg of MWReg30; (D) vascular engorgement of septal capillaries with erythrocytes, 20 minutes after IV injection of 100 μg of MWReg30. (A through C) Hematoxylin/eosin staining of paraffin-embedded section (original magnification × 400). (D) Toluidine blue staining of Epoxy resin-embedded sections (original magnification × 1,000).
Lung histopathology. Aspect of alveolar walls 60 minutes after IV injection of PBS (A) or 10 μg of MWReg30 (B); (C) 24 hours after IV injection of 10 μg of MWReg30; (D) vascular engorgement of septal capillaries with erythrocytes, 20 minutes after IV injection of 100 μg of MWReg30. (A through C) Hematoxylin/eosin staining of paraffin-embedded section (original magnification × 400). (D) Toluidine blue staining of Epoxy resin-embedded sections (original magnification × 1,000).
In vitro effects of MWReg30 on platelet aggregation and activation.
MWReg30 did not have any aggregating effect by itself in vitro when tested up to a concentration of 30 μg/mL but interfered with platelet aggregation, as determined by aggregometry. MWReg30 and the antiserum to gpIIb/IIIa completely prevented PMA-induced platelet aggregation after 1 minute of preincubation of the platelets in PRP. Collagen-induced aggregation was only inhibited by 14% with MWReg30 as compared with the 50% inhibition with the anti-gpIIb/IIIa antiserum. ADP-induced aggregation, on the other hand, was stabilized by MWReg30, whereas the antiserum to gpIIb/IIIa completely prevented aggregation. Exposure of mouse platelets in suspension to MWReg30 did not induce any sign of activation. Neither degranulation nor pseudopodia formation was observed 10 minutes after binding of MWReg30, as established by electron microscopic examination. No P-selectin expression or surface-bound fibrinogen was detectable by cytofluorometric analysis.
Phosphorylation of a 80-kD protein occurred upon exposure of platelets to MWReg30 (Fig 5A). The electrophoretic mobility of this tyrosine-phosphorylated protein from platelet lysates was indistinguishable from gpIIIa (CD61). Immunoprecipitation with MWReg30 enriched for the phosphorylated 80-kD band that was much stronger when the platelets had been pre-exposed to MWReg30 before lysis (Fig 5B), indicating identity with the gpIIIa chain of the fibrinogen receptor trimer.
Protein phosphorylation upon platelet activation by MWReg30. Tyrosine-phosphorylated proteins from mouse platelets were separated by gel electrophoresis. (A) Plain platelet lysates without (lane 1) or after 5 minutes of pre-exposure of the platelets to MWReg30 (30 μg/mL) (lane 2) stained for phosphotyrosin after blotting. (B) Immunoprecipitates of gpIIb/IIIa from platelet lysates with MWReg30 without (lane 1 and 3) and after 5 minutes of pre-exposure of the platelets to MWReg30 (30 μL/mL) stained for protein detection with Coomassie (lanes 1 and 2) or for phosphotyrosin after blotting (lanes 3 and 4).
Protein phosphorylation upon platelet activation by MWReg30. Tyrosine-phosphorylated proteins from mouse platelets were separated by gel electrophoresis. (A) Plain platelet lysates without (lane 1) or after 5 minutes of pre-exposure of the platelets to MWReg30 (30 μg/mL) (lane 2) stained for phosphotyrosin after blotting. (B) Immunoprecipitates of gpIIb/IIIa from platelet lysates with MWReg30 without (lane 1 and 3) and after 5 minutes of pre-exposure of the platelets to MWReg30 (30 μL/mL) stained for protein detection with Coomassie (lanes 1 and 2) or for phosphotyrosin after blotting (lanes 3 and 4).
Mechanisms of the MWReg30-induced reaction: importance of FcγRIII triggering.
In vivo blocking of FcγRII/III by pretreatment with the 2.4G2 MoAb completely prevented the MWReg30-induced hypothermia (Fig 6A). Mouse platelets were not stained by the anti-FcγRII/III 2.4G2 MoAb, as determined by FACS analysis. Unlike human platelets, mouse platelets do not express FcγRII. Granulocyte-depletion with MoAbs to mouse granulocytes before MWReg30 injection partly inhibited the hypothermic response. The body temperature decrease in granulocyte-depleted mice was shorter and not as marked as in control animals (maximal decrease of 2.5°C ± 0.8°C after 30 minutes in granulocyte-depleted v 4.7°C ± 0.4°C after 45 minutes in normal mice). More importantly, MWReg30 did not induce any hypothermia in FcγRIII-deficient mice, clearly identifying this as the FcγR responsible for the hypothermic response (Fig 6B). Even though no hypothermia developed in these mice, MWReg30 injection reduced their platelet numbers significantly. The FcγRIII-deficient mice a priori have an elevated platelet count (1.6 ± 0.1 × 106/μL, n = 6) that was reduced by 25% (1.2 ± 0.1 × 106/μL, n = 6) within 2 hours after MWReg30 injection to platelet counts of normal mice.
Involvement of FcR triggering in the MWReg30-induced reaction. Hypothermia induced by IV injection of MWReg30 (100 μg) was determined (A) in mice after blocking their FcγRII/III with 2.4G2 MoAb (100 μg; IP) (▪) or in untreated controls (•) and (B) in FcγRIII-deficient mice (▪) compared with untreated control animals (•). The mean values of the body temperatures of 3 mice per group at the indicated times after injection of the antibodies from 1 of 2 experiments are given ± SD.
Involvement of FcR triggering in the MWReg30-induced reaction. Hypothermia induced by IV injection of MWReg30 (100 μg) was determined (A) in mice after blocking their FcγRII/III with 2.4G2 MoAb (100 μg; IP) (▪) or in untreated controls (•) and (B) in FcγRIII-deficient mice (▪) compared with untreated control animals (•). The mean values of the body temperatures of 3 mice per group at the indicated times after injection of the antibodies from 1 of 2 experiments are given ± SD.
Sensitization/desensitization by TNF and LPS.
To test whether the hypothermia induced by MWReg30 was modulated by inflammatory stimuli, mice received 5 μg rmTNF 90 minutes before MWReg30 injection. These mice (n = 6) became hypothermic even faster (decrease of >4°C within 15 minutes) than nonsensitized controls, and all animals died between 30 and 40 minutes after the MWReg30 injection. Control mice not pretreated with rmTNF recovered from MWReg30-induced hypothermia within 90 minutes and survived (n = 6). Also, rmTNF-pretreated mice (n = 4) did not develop any hypothermic reaction upon injection of either 100 μg anti-CD18 or 100 μg anti-CD13 MoAb as MWReg30-isotype control antibodies (data not shown). In addition, the hypothermic effect of MWReg30 injection was indistinguishable in LPS-responder (C3H/HeN) and LPS-low responder (C3H/HeJ) mice, indicating that possibly contaminating LPS did not contribute to the MWReg30 effect (data not shown).
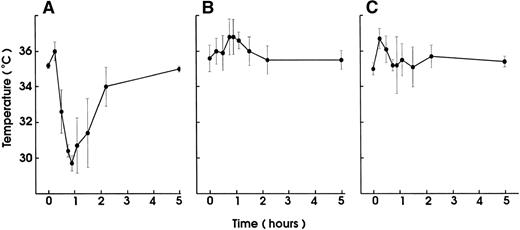
Injection of small amounts of rmTNF (600 ng) or LPS (10 μg) per mouse 24 hours before MWReg30 challenge, which by itself had no hypothermic effect, resulted in complete prevention of MWReg30-induced hypothermia (Fig 7). This protection was neither due to thrombocytopenia induced by the LPS or TNF pretreatment because platelet counts at the time of challenge were unchanged nor due to altered binding characteristics of MWReg30 to platelets, as determined by flow cytometry.
Desensitization for the MWReg30-induced hypothermia by LPS or TNF pretreatment. Hypothermia induced by IP injection of MWReg30 (100 μg) was determined in mice after pretreatment with (A) PBS (200 μL), (B) LPS (10 μg), or (C) rmTNF (600 ng) IP 24 hours before challenge. The mean values of the body temperatures of 3 mice per group at the indicated times after injection of the antibodies from 1 of 2 experiments are given ± SD.
Desensitization for the MWReg30-induced hypothermia by LPS or TNF pretreatment. Hypothermia induced by IP injection of MWReg30 (100 μg) was determined in mice after pretreatment with (A) PBS (200 μL), (B) LPS (10 μg), or (C) rmTNF (600 ng) IP 24 hours before challenge. The mean values of the body temperatures of 3 mice per group at the indicated times after injection of the antibodies from 1 of 2 experiments are given ± SD.
DISCUSSION
In this study, we show that the in vivo administration of the MoAb MWReg30 that is directed to the platelet-specific integrin αIIbβ3-(gpIIb/IIIa, fibrinogen receptor) induces acute hypothermia and pathological changes, especially in the lung. To exert these effects, an engagement of the MoAb with its antigen on platelets was required, speaking against a nonspecific activity of this MoAb. Hypothermia is one of the signs that characterize endotoxic shock in mice.30 31
The lung congestion, as well as the increased cellularity of the lung septa, due to mononuclear and polymorphonuclear cells, are akin to LPS-induced lung pathology. The vascular engorgement, essentially due to erythrocytes, was more pronounced than in mice injected with 1,000 μg LPS (not shown). Histological examination showed that these changes were neither focal nor associated with atelectasis: the increased cellularity was uniform and was observed in both lungs. In contrast, control lungs from mice injected either with PBS or with normal IgG were consistently free of accumulated cells in the septa. In MWReg30-injected animals, the increased cellularity appeared to be due to cell accumulation in both vascular and interstitial compartments, although electron microscopic studies are required to clarify this point. Preliminary studies show enrichment of pinocytotic vesicles (Männel et al, manuscript in preparation). Altogether, the picture is compatible with the very early steps of acute lung injury.
The syndrome described here might be the result of an in situ-formed immune complex reaction, with the antigen being the platelet surface gpIIb/IIIa. The reason why the MWReg30-induced effect took place so rapidly in vivo could be the immediate access of the MoAb to its target antigen in the vascular system. A mere opsonization of the platelets may not explain the effect, because different platelet-specific control antibodies, although inducing comparable thrombocytopenia, did not lead to the same reaction. Even the isotype-matched anti-CD18, which also reacts with a platelet surface protein and other newly developed MoAb to different platelet antigens (manuscript in preparation), did not induce hypothermia. One reason could obviously be that these antibodies find fewer epitopes on the platelet surface than the MWReg30, because the gpIIb/IIIa constitutes the most abundant platelet membrane protein. Apart from the antigen specificity of the antibody involved, the in situ immune complexes formed by identical isotypes may also differ in their FcγR binding capacity.32 At this point, it is still not clear whether platelets, besides serving as carriers for the immune complexes in situ, contribute actively to the pathology.
In our in vitro studies, the observed tyrosine phosphorylation of the integrin β subunit gpIIIa upon MWReg30 binding is the only indication for a signal mediated directly via gpIIb/IIIa. Such tyrosine phosphorylation of the integrin β subunit of the fibrinogen receptor has been suggested to be an important event in initiating outside-in signaling cascades into the platelet.27 However, electron microscopic analysis of MWReg30-stimulated platelets did not show any morphological evidence for platelet activation (intact α and dense granules, absence of pseudopod formation).
Unlike in the human system, in which antibodies to gpIIb/IIIa led to secondary responses such as secretion, second wave of platelet aggregation, and release of procoagulant membrane vesicles,33 MWReg30 directed to gpIIb/IIIa did not induce any measurable platelet activation or aggregation per se. Rather, stimulus-induced platelet aggregation was either inhibited or stabilized as in the case of ADP-stimulation. This might be explained by a steric interference once MWReg30 is bound to gpIIb/IIIa. Additionally, neither P-selectin expression nor surface-bound fibrinogen was detectable by flow cytometric analysis, nor was ATP-release measurable in a whole blood test system after the addition of MWReg30. Autocrine or juxtacrine activation with antibodies to membrane proteins of human platelets is a well-recognized phenomenon either by direct receptor activation or by cross-linking of the receptors, by FcγRII-mediated interplatelet or intraplatelet activation,34 or by complement activation.35 It has been suggested that the complement system plays an important role in this platelet activation.36 However, FcγRII/III have not been demonstrated on mouse platelets. In contrast to human platelets, which carry the FcγRII (A),37 this isoform is not produced in mice. Our staining attempts with the antibody for the FcγRII/III (2.4G2) on mouse platelets were also negative. Taken together, these findings do not support a platelet activation function of MWReg30.
Based on these data and especially on the results with the FcγRIII-deficient mice, FcγRIII on cell types other than platelets must be involved. The alleviation of the MWReg30-induced syndrome in mice depleted of granulocytes supports this concept. Besides granulocytes, FcγRIII-bearing monocytes, macrophages, natural killer cells, and mast cells are also likely candidates for the pathogenesis of this syndrome. In the FcγRIII-deficient mice, the syndrome did not develop, even though the platelet numbers were reduced. The capacity of MWReg30 to lower the platelet count is somewhat in contrast to the reported resistance of such mice to develop experimental immune thrombocytopenia induced by an autoimmune antimouse platelet MoAb of the same isotype, IgG1.38 Whether the MWReg30-induced hypothermia and reduction of platelet numbers involves different mechanisms than a regular FcγRIII-dependent thrombocytopenia, as described by these investigators, requires further studies.
Neither inhibition of hypotension with methylene blue, an inhibitor of the NO-induced activation of the cytosolic guanylate cyclase, nor the acute-phase protein α1-acid-glycoprotein (AGP),39 nor inhibition of integrin binding to RGD or YSIGR sequences containing sites, nor MoAb to LFA-1 or ICAM-1 conferred protection. Drugs used to antagonize serotonin or histamine effects were equally ineffective. The finding that neither decomplementation of the mice by CVF nor pretreatment with soluble CRI prevented the syndrome does not support the view of a major complement involvement in our system. On the other hand, the injection of CVF itself led to a similar hypothermic reaction as MWReg30 injection. Clearly, more investigations are needed to ascertain the precise role of complement in the development of the observed pathology.
The concept that several pathways have to act in concert for the development of the MWReg30-induced syndrome and that one pathway involves FcγRIII-positive cells is supported by the synergistic effect of TNF and MWReg30. The observation that sublethal amounts of TNF injected shortly before MWReg30 led to the death of the animals within 40 minutes demonstrates a strong sensitizing effect of inflammatory stimuli for the MWReg30-induced reaction. This priming effect of TNF for the MWReg30-induced reaction parallels the findings in experimental models of LPS-induced shock as well as of acute lung injury.8 It has been shown that concomitant injections of LPS and TNF,40 or IL-1 and TNF,41 lead to exacerbated pathological consequences upon a subsequent challenge with LPS. Also, the in vivo effect of MWReg30 injection alone is not the result of a synergistic action of MWReg30 with traces of LPS contamination, because the reactions of LPS responder and nonresponder mice are indistinguishable. For acute lung injury, it has been postulated that two sequential insults are required: one provided by an underlying predisposing clinical condition, eg, recent surgery or active infection leading to neutrophil priming and activation of the pulmonary endothelium and a second triggering stimulus. In transfusion-related acute lung injury, Igs that either bind to the recipient granulocytes or recognize HLA determinants on recipient or donor leukocytes10 or biologically active lipids produced during blood storage7 trigger the organ failure. In our model, this second signal could be provided by the MWReg30 injection.
Development of a refractory state by desensitization with LPS or TNF for an LPS challenge, on the other hand, requires time between the injections: mice are desensitized when low doses of LPS or TNF are administered at least 24 hours before the challenge.42 A similar desensitizing effect of TNF and LPS was also seen for the challenge with MWReg30. This could indicate that similar regulatory mechanisms govern our antibody-induced syndrome, the widely used LPS-induced experimental model for shock, and acute lung injury. The regulatory mechanisms accounting for these LPS or TNF effects remain to be analyzed. Transient upregulation of FcγRIII expression on monocytes or other cells43 could be considered as a cause for sensitization and, afterwards, active downregulation as cause for desensitization.44 It remains to be investigated whether a counteracting mechanism of the body to cope with the degree of insult, eg, NF-κB upregulation rendering possible target cells as for example endothelial cells refractory for damaging effects of platelets,45-47 is the reason for desensitization.
No direct effects of LPS or TNF on platelets have been described so far; however, the participation of platelets in shock, either directly or via mediators, such as PAF, PF4, thromboxane A2, or β-thromboglobulin, has been shown.5,7,8,48,49 Although we could not demonstrate any MWReg30-induced platelet activation, our data do not rule out any platelet release reactions occurring after interaction with FcγRIII-carrying cells. So far, clinical intervention studies based on findings in available experimental models on shock have been largely disappointing.50 The MWReg30-induced syndrome combining the findings from LPS-shock models and the results from investigations on acute lung injury might provide a new in vivo model that reproduces features of very early steps of shock development, thus setting the stage for the detrimental action of inflammatory mediators. Investigations of the regulatory mechanisms governing the extent of the pathological consequences can easily be performed in this model. Also, results from studies aiming at alleviating the MWReg30 action might be useful for future strategies designed to interfere with septic lung injury in humans.
ACKNOWLEDGMENT
The authors thank J. Köhl for CVF, M. Kirschfink for sCRI, R. Deutzmann for sequencing, E. Heinmöller for help in aggregometry, and R. Straub, C. Galanos, R. Urbaschek, and G. Bein for helpful discussions.
Supported in part by DFG (SFB265 Project No. B01) to R.E.S. and by BMBF (01KI9473 Project No. A3) to D.N.M.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Daniela N. Männel, PhD, Department of Pathology, University of Regensburg, D-93042 Regensburg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal