Abstract
Igs contain unique portions, collectively termed idiotypes (Id), that can be recognized by the immune system. Id expressed by tumor cells in B-cell malignancies can be regarded as tumor-specific antigens and a target for vaccine immunotherapy. We have started a vaccination trial in multiple myeloma (MM) using Id-specific proteins conjugated to keyhole limpet hemocyanin (KLH) as immunogens and low doses of subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-2 (IL-2) as immunoadjuvants. Twelve patients who had previously been treated with high-dose chemotherapy followed by peripheral blood progenitor cell (PBPC) transplantation entered this study from August 1995 to January 1998. All patients were in first remission at the time of vaccination. They received subcutaneous injections of Id vaccines and immunoadjuvants in an outpatient setting. The generation of Id-specific T-cell proliferative responses was documented in 2 patients, whereas a positive Id-specific delayed-type hypersensitivity (DTH) reaction was observed in 8 of the 10 patients studied. DTH specificity was confirmed in 1 patient by investigating the reactivity to synthetic peptides derived from the VDJ sequence of the tumor-specific Ig heavy chain. None of the patients generated soluble immune responses to Id, whereas the generation of soluble and cellular immune responses to KLH was observed in 100% and 80%, respectively. Eleven patients completed the treatment, whereas 1 patient failed to finish owing to progression of disease. Freedom from disease progression (FFDP), measured from the date of first Id/KLH injection to the date of first treatment after vaccination or last follow-up, ranged from 9 to 36 months. These data indicate that the immune competence status of MM patients is still susceptible to specific immunization after high-dose chemotherapy and PBPC transplantation. It remains to be determined whether generation of Id-specific immune responses can reduce the relapse rate of patients with minimal residual disease.
MULTIPLE MYELOMA (MM) is still a fatal B-cell neoplastic disease, with a median survival of less than 4 years.1,2 High-dose chemotherapy followed by autologous bone marrow or peripheral blood progenitor cell (PBPC) transplantation have recently increased the complete remission rate and remission duration.3-5 However, overall survival has only been slightly prolonged, and no evidence for a cure has been obtained.6 All patients ultimately relapse even under maintenance therapy with interferon-α (IFN-α) alone7 or in combination with steroids.8,9 A possible strategy to improve the clinical outcome is to prolong the duration of the remission phase by inducing an active specific immune response. MM is characterized by the clonal expansion of lymphoid cells with rearranged Ig genes. Igs contain unique portions, collectively termed idiotype (Id), that can be recognized by the immune system. Id expressed by tumor cells in MM can be regarded as a tumor-specific antigen and a target for active specific immunotherapy. MM has other immunologic features that can be advantageously exploited in the setting up of active specific immunotherapy. First, there is a T-cell population open to exploitation as a source of specific antitumor effector cells.10-14 Second, the T-cell effector mechanisms are not exhausted by chronic tumor cell stimulation.15,16 However, despite the evidence of activation and immune recognition, it is clear that T cells do not perform adequately in vivo and are unable to hold the disease in check. Normal T cells can recognize and eliminate tumor cells, but this ability is impaired in MM patients due to several mechanisms, including inadequate tumor antigen presentation leading to T-cell apoptosis.17 Immunotherapeutic strategies aimed at conferring immunogenicity on autologous Id may achieve two goals: the first is to rescue T cells from apoptosis; the second is to induce an active specific immune response against tumor cells. Lynch and Eisen were the first to prove in mice that Id can be rendered immunogenic.18,19 In the light of these and other experimental data, Id-specific proteins have come into medical use in patients with follicular lymphoma.20,21 These pioneering studies have provided the rationale for exploring the use of autologous Id as a therapeutic vaccine in MM. Clinical results in the allogeneic transplantation setting, including donor lymphocyte infusions, have provided proof in principle that an adequate antitumor immune response, unlike chemotherapy, may eradicate the disease.22-25 We have therefore started a vaccination trial using Id-specific protein coupled to keyhole limpet hemocyanin (KLH) and low doses of subcutaneous interleukin-2 (IL-2) or granulocyte-macrophage colony-stimulating factor (GM-CSF). Treatment of an initial series of 8 MM patients in relapse or with resistant disease has shown that Id vaccines are safe and can be administered in an outpatient setting. Two patients had stable disease for 20 months with no further chemotherapy. We now report the results of a subsequent study in which Id vaccines were administered in first remission as a maintenance treatment after high-dose chemotherapy and PBPC transplantation.
MATERIALS AND METHODS
Patients.
Twelve MM patients (Table 1) entered this study from from August 1995 to January 1998. Approval was obtained from the Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. MM was diagnosed as previously reported.26 According to the Durie and Salmon staging system,27 9 patients were classified as stage III and 3 were stage II; 1 was substage B. Seven were IgG, 4 were IgA, and 1 was Bence Jones myeloma. All patients had received previous high-dose chemotherapy followed by PBPC transplantation according to the Italian Myeloma Study Group regimens3 28 and were in first remission after induction chemotherapy. Peripheral blood samples were collected from age-matched normal donors (kindly provided by the local Blood Bank) to set the reference values for some of the immunologic analyses.
Patients’ Characteristics
| UPN . | Age/Gender . | Isotype . | Previous Treatments . | Time Off Therapy to Vaccination (mo) . | Disease Status at Vaccination . |
|---|---|---|---|---|---|
| 14 | 60/M | IgAλ | HDS*, DEX + IFN-α | 3 | RC |
| 240 | 61/F | IgGλ | HDS*, DEX + IFN-α | 3 | RC |
| 453 | 63/F | IgGλ | CM*, DEX + IFN-α | 4 | 1R50 |
| 485 | 62/F | IgGλ | CM* | 8 | RC |
| 510 | 47/F | IgAκ | HDS*, IFN-α | 2 | RC |
| 522 | 53/M | IgGλ | CM* | 3 | 1R75 |
| 535 | 57/M | IgAκ | HDS*, IFN-α | 17 | RC |
| 637 | 54/M | IgAλ | HDS* | 4 | RC |
| 734 | 60/F | IgGκ | Mev*, DEX | 2 | 1R75 |
| 743 | 65/M | κ† | Mev*, DEX | 2 | RC |
| 749 | 64/F | IgGκ | Mev*, DEX | 2 | RC |
| 847 | 60/F | IgGλ | Mev*, DEX | 2 | RC |
| UPN . | Age/Gender . | Isotype . | Previous Treatments . | Time Off Therapy to Vaccination (mo) . | Disease Status at Vaccination . |
|---|---|---|---|---|---|
| 14 | 60/M | IgAλ | HDS*, DEX + IFN-α | 3 | RC |
| 240 | 61/F | IgGλ | HDS*, DEX + IFN-α | 3 | RC |
| 453 | 63/F | IgGλ | CM*, DEX + IFN-α | 4 | 1R50 |
| 485 | 62/F | IgGλ | CM* | 8 | RC |
| 510 | 47/F | IgAκ | HDS*, IFN-α | 2 | RC |
| 522 | 53/M | IgGλ | CM* | 3 | 1R75 |
| 535 | 57/M | IgAκ | HDS*, IFN-α | 17 | RC |
| 637 | 54/M | IgAλ | HDS* | 4 | RC |
| 734 | 60/F | IgGκ | Mev*, DEX | 2 | 1R75 |
| 743 | 65/M | κ† | Mev*, DEX | 2 | RC |
| 749 | 64/F | IgGκ | Mev*, DEX | 2 | RC |
| 847 | 60/F | IgGλ | Mev*, DEX | 2 | RC |
Abbreviations: CM, high-dose cyclophosphamide ×3, high-dose melphalan ×3; HDS, high-dose sequential chemotherapy; Mev, high-dose cyclophosphamide ×1, high-dose melphalan ×2/3; DEX, dexamethasone; IFN-α, interferon-α; RC, first complete remission; 1R75, first remission (75% reduction serum M protein level); 1R50, first partial remission (50% reduction).
These regimens are followed by PBPC infusion.
Light chain disease.
Vaccine preparation.
Id purification from serum and urine was accomplished by means of precipitation and chromatography techniques that exploit Id-specific molecular weight and isoelectric point. To reduce microbiological contamination, chromatography separations were generally performed in disposable conical tubes rather than in columns. Different strategies were used to purify IgG, IgA, and light chains, as previously reported.29 Purified IgG were obtained by ion exchange chromatography followed by affinity chromatography. IgA were separated by ammonium sulfate precipitation followed by gel filtration. κ or λ light chains were isolated from urine by means of ammonium sulfate precipitation. The precipitate was dissolved in physiologic saline and dialyzed versus three changes of physiologic saline overnight. Further purification was not required, because glomerular filtration itself separates molecules on the basis of their size. When purified Id were reanalyzed by high-resolution agarose gel electrophoresis, no extra bands were observed above the nephelometry threshold. The median recovery of IgG, IgA, and free light chain from 30 samples was 50%, 15%, and 16%, respectively. KLH (Biosyn, Arzneimittel GmbH, Fellbach, Germany), purified from the hemolymph of the keyhole limpet (Megathura crenulata), was conjugated to Ids as previously reported.20,21 29 Briefly, equal amounts of Id and 1 mg/mL KLH in physiologic saline were mixed with 0.1% sterile glutaraldehyde (Sigma, Milano, Italy) for 4 hours at room temperature. Final aliquots of Id/KLH conjugates contained 0.5 mg of Id and KLH each per milliliter of physiologic saline. Id/KLH conjugates were tested for Mycoplasma, fungi, bacteria, and endotoxin contamination before vialing and storing at −20°C.
On average, endotoxin contamination was about 3,000 USP-EU/mL, which is approximately 10-fold higher than the European threshold forparentalia. To decrease it, a final affinity chromatography was performed by mixing 2 mL of conjugate with 2 mL of polymyxin (Bio-Rad Laboratories, Irvine, CA) in conical tubes. After incubation at 4°C for 12 hours, tubes were centrifuged for 5 minutes at 2,000 rpm and the supernatant was carefully collected. This procedure was repeated twice and yielded a mean concentration of 37 USP-EU/mL (range, l to 90 USP-EU/mL). Id/KLH conjugates were then sterilized by passage through a 0.2-μm filter and stored at −20°C until use. Id/KLH conjugates were successfully manufactured in all 16 cases attempted. In 2 patients, vaccine treatment was not started because early relapse occurred. Two patients refused to be enrolled in the study, after initial informed consent and preparation of clinical grade Id vaccines ready-for-use. Thus, the number of vaccines actually used versus the number of preparations manufactured was 12 to 16.
Commercially available human polyclonal Igs (IgVena; Sclavo, Pisa, Italy) were used as a control in delayed-type hypersensitivity (DTH) skin tests. They were dialyzed versus physiologic saline, incubated twice with polymyxin, passed through a 0.2-μm filter, and stored at −20°C until use.
Treatment schedule.
Patients received subcutaneous injections of 0.5 mg of Id-KLH conjugates at time 0 and at 2, 6, 10, 14, 24, and 28 weeks. IL-2 (Proleukin; EuroCetus, Milano, Italy) at 1.5 IU/m2/d (2 patients) or GM-CSF (Leucomax; Sandoz, Milano, Italy) at 150 μg/m2/d (10 patients) was administered subcutaneously close to the vaccine site for the next 5 days. The preferential use of GM-CSF was based on both our initial series of 8 MM patients in relapse or with resistant disease and the first 4 patients of the present series, in whom GM-CSF proved to be a better immunoadjuvant than IL-2 in terms of anti-KLH antibody responses and DTH skin tests.
Clinical evaluation.
The serum level of the tumor-related heavy chain, the serum κ/λ light chain ratio, and Bence Jones proteinuria were determined by nephelometry (Sanofi Diagnostics Pasteur, Paris, France). Plasma cell infiltration was determined by microscopic evaluation of bone marrow aspirates after May-Grünwald-Giemsa staining. In patients with normal high-resolution agarose gel electrophoresis and normal κ/λ ratio, the disease was detected by immunofixation or by polymerase chain reaction (PCR) using oligonucleotide primers and probes derived from the tumor-specific Ig heavy-chain gene sequences.6,30 31
Freedom from disease progression (FFDP) was measured from the date of first immunization to the date of progression or last follow-up. Survival was measured from the date of first immunization to the date of death or last follow-up.
Amplification and sequencing of the tumor-specific variable heavy chain.
Bone marrow mononuclear cells were separated on a Ficoll-Hypaque density gradient. RNA was isolated using the RNAzol B method (Biotech Laboratories, Houston, TX), and total RNA (5 μg) was reverse-transcribed into Ig cDNA with an isotype-specific primer as previously described.6 Amplification and sequencing of the tumor-specific VDJ were performed as previously described.31 Briefly, 1 μL of Ig cDNA was amplified using a VH3 consensus primer derived from the IgH framework region (FR1) and an antisense primer derived from the 3′ end of the JH region (JH3). The reaction was performed for 33 cycles (denaturation at 94°C for 30 seconds, annealing at 65°C for 30 seconds, and extension at 72°C for 30 seconds), with a final extension at 72°C for 7 minutes. PCR products were run on a 2% preparative agarose gel. The expected size was excised and phenol-extracted. Direct sequencing of the amplified products was performed using the Promega fmol system (Promega, Madison, WI) according to the manufacturer’s instructions. Reactions were performed in a thermocycler at 68°C annealing temperature for 15 cycles. Because the direct sequencing did not allow a complete reading of the complementarity determining regions (CDRs), DNA was reamplified with primers containing EcoRI and HindIII restriction sites and cloned in a Bluescript SK vector (Stratagene, San Diego, CA). Restriction enzyme analysis was performed on plasmid DNAs prepared by the alkaline lysis method, and miniprep plasmid DNAs were then sequenced. Sequence analysis was performed with the PC-GENE software (Intelligenetics, Inc, Mountain View, CA).
Monitoring of minimal residual disease.
Bone marrow and peripheral blood were evaluated for the presence of residual myeloma cells by PCR, using oligonucleotide primers and probes derived from the tumor-specific Ig heavy-chain gene sequences, as described above. Postswitch B cells were detected as previously described.30 Briefly, 2 μL of 50 μL of total cDNA was amplified with a 5′ primer derived from the CDR2 and a 3′ primer from the Cα or Cγ first exon sequence. A nested-PCR strategy was used to detect preswitch B cells. The first amplification was performed with a consensus primer for the variable region (VH.L or VH.D) and a primer from the Cμ first exon (Cμ-5). Of this amplification, 5 μL was reamplified using the internal primers CDR2 and Cμ-7. Twenty percent of the PCR product was analyzed by agarose gel electrophoresis, blotted overnight, and hybridized to CDR3 probes end-labeled with [γ-32P] (Amersham, Milano, Italy) adenosine triphosphate (ATP). To avoid false-negatives, all of the cDNA samples not producing PCR products were reamplified and the cDNA quality was tested by amplifying the sequence of p53 exon 5 or n-ras exon 2.
Peptide synthesis.
The VDJ sequence of the tumor-specific Ig heavy chain of patient 510 was translated into the amino acid sequence with the PC-GENE software (Intelligenetics, Inc). The deduced amino acid sequence was analyzed with a program predicting immunogenicity based on the potential beta turn formation within the sequence. The sequences around the glycine residues in position 17, 38 (part of CDR3), and 47 were identified as the most potentially immunogenic regions. A sequence around glycine in position 38 and including the CDR3 sequence (position 32-42) was selected as the most tumor-specific sequence, whereas a sequence corresponding to the FR3 region (position 22-31) was selected as an internal control. The sequences in standard single-letter code with the N-acetylated amino terminus on the left are Ac-ESRHAVYYCA-OH and Ac-EAVGYGARFD-OH for the CDR3- and FR3-derived peptides, respectively.
Peptides were synthesized on polyethylene pins that had been radiation grafted with hydroxyethylmethacrylic acid (HEMA).32 These crowns were functionalized with Fmoc-protected amino acid esters of 4-hydroxymethylphenoxyacetic (HMP) that yield a free acid c-terminus on cleavage. Amino acid coupling was performed in distilled N1N-dimethylformamide (DMF) at 120 mmol/L by means of HBTU/HoBT/NMM activation (120:120:180 mmol/L). After the completion of the synthesis cycle, peptides were N-terminal–acetylated with acetic anhydride. They were then simultaneously side chain deprotected and cleaved using a mixture of 95% trifluoracetyl (TFA)/2.5% anisole/2.5% ethanedithiol (EDT). The peptide TFA solutions then were reduced under vacuum and the cleaved peptide was precipitated with diethyl ether/petroleum ether at 40°C to 60°C (1:2 vol/vol). The precipitate was washed twice with the ether/petrol mixture. The peptides were then air-dried, dissolved in a solution of 10% AcOH/MeCN, and sampled for analysis by mass spectroscopy and high-performance liquid chromatography (HPLC). The remaining solution was frozen and lyophilized.
Peptides were purified by reverse-phase chromatography with a Vydac RP C18 250 × 10 mm column (Metachem, Torrence, CA) installed in a Waters system (Cornerstone Water System, Phoenix, AZ). The chromatogram was developed at a flow rate of 4 mL/min using 0.1% TFA in water and 0.1% TFA in MeCN as the limiting solvent. Analytical HPLC was performed using a Merck LiChrosphere (Merck, Whitehouse Station, NY) 100 RP C18 (250 × 4 mm; flow rate, 1.5 mL/min) installed in a Waters system. Solvents used were similar to those used to purify the peptide. Ion spray mass spectral analysis was performed on a Perkin Elmer Sciex API 111 biomolecular mass analyzer (Perkin-Elmer, Foster City, CA). Purity was 96.5% and 98.4% for the CDR3- and the FR3-derived peptide, respectively.
Dry peptides were solubilized in 10 mmol/L phosphate buffer and 100 mmol/L sodium chloride at pH 6.0 to give a final concentration of 1 mg/mL. A final affinity chromatography step was performed by mixing 2 mL of peptides with 2 mL of polymyxin (Affi-Prep; Bio-Rad Laboratories) in conical tubes. After incubation at 4°C for 12 hours, tubes were centrifuged for 5 minutes at 2,000 rpm and the supernatant was carefully collected. This procedure was repeated twice and yielded an endotoxin concentration of 0.2 USP-EU/mL (CDR3-derived peptide) and 0 USP-EU/mL (FR3-derived peptide). Final aliquots of unconjugated Id as well as CDR3- and FR3-derived peptides were tested for Mycoplasma, fungi, and bacteria before vialing and storing at −20°C.
Humoral responses.
Microtiter plates (Costar, Milano, Italy) were coated with 10 μg/mL KLH, incubated for 12 hours at room temperature, and washed 3 times with 0.05% Triton X-100 (Sigma). After incubation for 1 hour at room temperature with phosphate-buffered saline (PBS) + 2% bovine serum albumin (BSA), they were washed, blocked with 0.2% Tween-20 in PBS for 30 minutes, and washed. Preimmune and postimmune sera were diluted in PBS + 5% fetal calf serum (FCS), dispensed into microwells, and incubated for 1 hour at room temperature. After washing, horseradish peroxidase (HRP)-conjugated-goat antihuman IgG or IgM (Cappel; Organon Teknica Corp, West Chester, PA) was added and incubated for 1 hour at room temperature. The enzyme substrate solution was added after extensive washing and incubated for 10 minutes at 37°C. Absorption was evaluated at an optical density of 405 nm with an enzyme-linked immunosorbent assay (ELISA) microtiter plate reader (Titertek Multiskan Plus; Flow Laboratories, Milano, Italy).
Anti-Id antibody responses were evaluated as follows. Microtiter plates were coated with monoclonal antibodies (MoAbs) specific for the human κ or λ chain present in the patient’s Id. Plates were incubated for 2 hours at room temperature and then overnight at 4°C. After blocking with 0.2% Tween-20 in PBS for 30 minutes, 10 μg/mL purified Id in PBS +2% FCS was added and incubated for 1 hour at room temperature. Preimmune and postimmune sera were diluted in PBS + 2% FCS, dispensed into microwells, and incubated for 1 hour at room temperature. After washing, HRP-conjugated goat antihuman κ or λ chain (opposite to the light chain of the coating Id) was added and incubated for 1 hour at room temperature. The enzyme substrate solution was added after extensive washing and incubated for 10 minutes at 37°C. Absorption was evaluated at an optical density of 405 nm with an ELISA microtiter plate reader.
Cellular proliferation assays.
Cellular proliferations to autologous Id, control Id (unrelated isotype-matched Id), and KLH were evaluated at the end of the vaccination procedure. Briefly, 5 × 106 PBMC/mL were incubated in RPMI + 20% FCS at 37°C in a humidified atmosphere of 5% CO2 in air. After 2 hours of incubation, nonadherent cells were recovered and T cells were isolated by rosetting with sheep red blood cells at 29°C for 1 hour to exclude the majority of CD3−, CD2+ rosette-forming cells.15 Adherent cells (AC) were gently recovered using a cell scraper. AC were incubated overnight at 1 × 106/mL in RPMI + 10% pooled human AB serum supplemented with 50 ng/mL GM-CSF alone (control culture) or with GM-CSF and 100 μg/mL autologous Id, 100 μg/mL control Id, or 100 μg/mL KLH (experimental cultures). Tumor necrosis factor-α (TNF-α; Genzyme, Cambridge, UK) at 10 ng/mL was added for the last 3 hours of incubation. AC were then washed, irradiated, and cultured in flat-bottomed microtiter plates with autologous purified T cells (1:5 ratio) for 5 days in RPMI + 10% FCS and 5 U/mL IL-2. Cell proliferation was evaluated by pulsing 200 μL of cells with 5 μCi3[H]TdR (47 MBq/mmol; Amersham, Milano, Italy) and harvesting 4 hours later with a semiautomated sample harvester. The filters were counted in a liquid scintillation counter. Controls were 14 normal blood donors (kindly provided by the local Blood Bank) and were matched for sex and age. AC derived from normal donors were incubated with GM-CSF alone or with GM-CSF and control Id or KLH. Experiments were set up in such a way that T cells from at least one normal donor were always studied simultaneously with T cells from the patients. Results are expressed as the following stimulation index: (3[H]TdR incorporation in the experimental culture)/(3[H]TdR incorporation in the control culture) = Stimulation index.
The stimulation index to KLH was scored positive in MM when it was above the mean plus 3 standard deviations (AVG + 3SD) observed in the controls. The stimulation index to autologous Id was scored positive if it was at least threefold higher than that observed to the control Id in the same experiment.
DTH skin tests.
DTH skin tests were performed in 5 patients before vaccination and in 10 patients 1 month after the last injection of Id/KLH conjugates. Unconjugated autologous Id was prepared as described above. At the KLH conjugation step, one aliquot was left unconjugated and used for DTH skin testing, whereas the remainder was used to prepare the vaccine. Unconjugated autologous Id alone (0.5 mg) was injected intradermally into the forearm. In parallel, an equivalent amount of human polyclonal Ig (IgVena; Sclavo) was injected into the opposite forearm as a control. DTH skin tests with the CDR3- and FR3-derived peptides were performed by injecting intradermally 0.1 mg of each peptide side-by-side on the same forearm. Final aliquots of unconjugated Id as well as CDR3- and FR3-derived peptides were treated with polymyxin and tested for Mycoplasma, fungi, bacteria, and endotoxin content before vialing and storing at −20°C.
Skin biopsies were taken with a punch from selected patients at sites of Id and/or control injections. Formalin-fixed, paraffin-embedded skin biopsy sections were stained with the following antibodies: polyclonal anti-CD3 (Dako, Milano, Italy; 1:100 final dilution), C8/144B MoAb (CD8, IgG1-κ; Dako), OPD4 MoAb (CD4/CD45R0, IgG1-κ; Dako; 1:50 final dilution), L-26 MoAb (CD20, IgG2a-κ; Dako), and Leu7 MoAb (CD57, HNK1, IgM-κ; Becton Dickinson, Milano, Italy; 1:20 final dilution). Staining was performed after antigen retrieval with a microwave. Reactions were shown by the avidin-biotin-peroxidase complex technique.
Analysis of TCRBV repertoire by molecular evaluation of CDR3 size distribution.
RNA was extracted from 5 to 10 × 106 unfractionated peripheral blood or bone marrow mononuclear cells using the TRIzol Reagent (Life Technologies, S. Giuliano Milanese, Italy). RNA from skin biopsies was obtained by homogenization in the presence of guanidine isothiocyanate solution, followed by ultracentrifugation on a cesium chloride discontinuous density gradient.33 Reverse transcription of RNA into cDNA was performed at 42°C by AMV reverse transcriptase (Promega kit). The CDR3 size distribution of the BV chain was determined with a two-step PCR reaction.34 Briefly, the first step consists of 24 reactions, each containing one specific human BV subfamily primer coupled with a consensus antisense BC primer. Sequences of BV and BC primers were derived from Genevée et al,35 whereas numbering of BV subfamilies was obtained from Wei et al.36 The PCR was performed in 96 polycarbonate microwell plates (MJ Research, Watertown, MA) using a PTC-100 thermal cycler (MJ Research). PCR conditions were as follows: denaturation at 94°C for 2 minutes; 40 cycles of amplification each consisting of 20 seconds of denaturation at 94°C, annealing at 60°C for 20 seconds, and extension at 72°C for 20 seconds; and a final extension at 72 °C for 5 minutes. The second step, named run-off, was performed to obtain a readable fluorescent product. Briefly, 2 μL of the first PCR was further amplified for 5 cycles in the presence of a nested consensus fluorescent BC primer or 13 BJ-specific fluorescent primers. Sequences of BJ primers were derived from Pusieux et al.37 The run-off reaction products were visualized on a 4.25% polyacrylamide sequencing gel in a 377 ABI DNA Sequencer (Perkin-Elmer), and the size of the fragments obtained was compared with a set of fluorescent size markers ranging from 35 to 350 nucleotides in length (Genescan 350 Tamra; Perkin-Elmer). Automatic size analyses and CDR3 size distributions were determined with the Immunoscope software package.38
RESULTS
Toxicity.
All courses were delivered on an outpatient basis without any acute World Health Organization (WHO) grade III/IV toxicity. Local reactions included erythema, induration, and local discomfort without any skin breakdown at sites of injection. Mild axillary lymph node enlargement occurred in 2 patients. Systemic toxicity was mostly associated with the injection of cytokines and consisted in bone pain, myalgia, and arthralgia in patients receiving GM-CSF (WHO grade I/II) and fever in patients receiving IL-2 (WHO grade I/II). These symptoms were easily controlled with oral acetaminophen.
Patients used a self-report diary to record side-effects and provide an overall evaluation about toxicity, feasibility, and tolerability. Most patients stated that their quality of life remained good or very good during the vaccine treatment.
Clinical observations.
Eleven patients completed the treatment, whereas 1 patient failed to finish owing to progression of disease. The clinical impact was determined by evaluating the tumor mass, FFDP, and survival (Table 2). The serum tumor-related heavy chain level, the serum κ/λ ratio and Bence Jones proteinuria (unique patient no. [UPN] 743), and bone marrow plasma cell infiltration were used to determine the tumor mass before vaccination and 1 month after the last immunization. Immunofixation was used to detect the disease in 9 patients with normal serum M protein level and normal serum κ/λ ratio (UPNs 14, 240, 453, 485, 510, 522, 535, 743, and 749). A PCR analysis was also used in 2 patients to detect Cα- and Cμ-associated tumor-specific VDJ sequences in the peripheral blood and bone marrow (UPNs 14 and 510; data not shown). Use of the C region defines the cell differentiation stage and allows discrimination between clonally related preswitch B cells (which can be regarded as potential myeloma cell precursors) and postswitch tumor B cells.30 The vaccine treatment did not reduce the tumor mass, irrespective of its magnitude.
Effect of Id Vaccines on the Tumor Mass and Clinical Outcome
| UPN . | Immuno Adjuvant . | Serum M Protein (mg/dL) . | Serum κ/λ Ratio . | % BM Plasma Cells . | Immuno Fixation . | FFDP (mo) . | OS (mo) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | Pre . | Post . | ||||
| 14 | GM-CSF | 196 | 299 | 1.2 | 1.6 | 1 | 1 | Pos | Pos | 17 | 32+ |
| 240 | GM-CSF | 1,359 | 1,528 | 1.8 | 1.8 | 1 | 1 | Pos | Pos | 31+ | 31+ |
| 453 | IL-2 | 1,734 | 1,755 | 0.8 | 0.6 | 1 | 3 | Pos | Pos | 36 | 39+ |
| 485 | IL-2 | 1,207 | 1,189 | 2.4 | 2 | ND | ND | Neg | Neg | 14 | 33 |
| 510 | GM-CSF | 167 | 311 | 1.4 | 1.6 | 1 | 1 | Pos | Pos | 32+ | 32+ |
| 522 | GM-CSF | 1,285 | 1,388 | 0.6 | 0.4 | 2 | 5 | Pos | Pos | 19 | 36+ |
| 535 | GM-CSF | 63 | 122 | 1.6 | 1.4 | 1 | 1 | Pos | Pos | 15 | 19+ |
| 637* | GM-CSF | 148 | 1,258 | 1.8 | 0.7 | 1 | 2 | Pos | Pos | 4* | 20 |
| 734 | GM-CSF | 1,563 | 1,486 | 14.9 | 10.3 | 5 | 5 | ND | ND | 17+ | 17+ |
| 743 | GM-CSF | 0† | 0.5† | 1.2 | 1.4 | 1 | 10 | Neg | Neg | 9 | 20+ |
| 749 | GM-CSF | 627 | 738 | 2.1 | 2 | 1 | 1 | Pos | Pos | 20+ | 20+ |
| 847 | GM-CSF | 1,632 | 1,412 | 4.4 | 3.3 | 2 | 2 | Pos | Pos | 11+ | 11+ |
| UPN . | Immuno Adjuvant . | Serum M Protein (mg/dL) . | Serum κ/λ Ratio . | % BM Plasma Cells . | Immuno Fixation . | FFDP (mo) . | OS (mo) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | Pre . | Post . | ||||
| 14 | GM-CSF | 196 | 299 | 1.2 | 1.6 | 1 | 1 | Pos | Pos | 17 | 32+ |
| 240 | GM-CSF | 1,359 | 1,528 | 1.8 | 1.8 | 1 | 1 | Pos | Pos | 31+ | 31+ |
| 453 | IL-2 | 1,734 | 1,755 | 0.8 | 0.6 | 1 | 3 | Pos | Pos | 36 | 39+ |
| 485 | IL-2 | 1,207 | 1,189 | 2.4 | 2 | ND | ND | Neg | Neg | 14 | 33 |
| 510 | GM-CSF | 167 | 311 | 1.4 | 1.6 | 1 | 1 | Pos | Pos | 32+ | 32+ |
| 522 | GM-CSF | 1,285 | 1,388 | 0.6 | 0.4 | 2 | 5 | Pos | Pos | 19 | 36+ |
| 535 | GM-CSF | 63 | 122 | 1.6 | 1.4 | 1 | 1 | Pos | Pos | 15 | 19+ |
| 637* | GM-CSF | 148 | 1,258 | 1.8 | 0.7 | 1 | 2 | Pos | Pos | 4* | 20 |
| 734 | GM-CSF | 1,563 | 1,486 | 14.9 | 10.3 | 5 | 5 | ND | ND | 17+ | 17+ |
| 743 | GM-CSF | 0† | 0.5† | 1.2 | 1.4 | 1 | 10 | Neg | Neg | 9 | 20+ |
| 749 | GM-CSF | 627 | 738 | 2.1 | 2 | 1 | 1 | Pos | Pos | 20+ | 20+ |
| 847 | GM-CSF | 1,632 | 1,412 | 4.4 | 3.3 | 2 | 2 | Pos | Pos | 11+ | 11+ |
Abbreviations: Pre, prevaccine value; Post, postvaccine value; ND, not done; FFDP, freedom from disease progression (calculated from the first immunization to the date of first treatment after vaccination or last follow-up); OS, overall survival (calculated from the first immunization to the date of death or last follow-up).
Treatment was not completed by this patient owing to disease progression.
Light chain disease (values are expressed as grams per 24 hours).
Five of the 11 patients who completed the treatment have maintained the remission at 9 to 30 months after the first immunization, whereas 6 patients relapsed at 9 to 36 months (Table 2). Ten patients are alive. At a median follow-up of 26 months, the median survival was not reached and ranged from 11 to 39 months (Table 2).
Humoral responses.
Antibody responses to KLH were evaluated by comparing prevaccine with postvaccine serum samples collected 1 month after the last injection of Id/KLH conjugates (Table 3). Anti-KLH responses were observed in all patients. On average, there was an eightfold increase in anti-KLH antibody titer (8.12 ± 4.22; range, 3.2 to 15.7). The kinetics of anti-KLH antibody response was analyzed in 2 patients. IgM antibodies were detected after the first immunization and boosted with the second and third, after which they started to decline. The appearance of IgG antibodies was slightly delayed; they were boosted by subsequent immunizations and remained high up to the end of the vaccination procedure (data not shown).
Soluble and Cellular Immune Responses in MM Patients Receiving Id Vaccines
| UPN . | Anti-KLH . | Anti-Id . | |||
|---|---|---|---|---|---|
| Ab . | Cell . | Ab . | Cell . | DTH . | |
| 14 | Pos | Pos | Neg | Neg | Pos |
| 240 | Pos | Pos | Neg | Neg | Pos |
| 453 | Pos | Neg | Neg | Neg | Pos |
| 485 | Pos | Pos | Neg | Pos | Pos |
| 510 | Pos | Pos | Neg | Neg | Pos |
| 522 | Pos | Pos | Neg | Neg | Pos |
| 535 | Pos | Pos | Neg | Neg | Neg |
| 637 | Pos | ND | Neg | ND | Pos |
| 734 | Pos | Pos | Neg | Pos | Pos |
| 743 | Pos | Neg | Neg | Neg | ND |
| 749 | Pos | Pos | Neg | Neg | ND |
| 874 | Pos | Pos | Neg | Neg | Neg |
| Total immune responses | 12/12 | 9/11 | 0/12 | 2/11 | 8/10 |
| UPN . | Anti-KLH . | Anti-Id . | |||
|---|---|---|---|---|---|
| Ab . | Cell . | Ab . | Cell . | DTH . | |
| 14 | Pos | Pos | Neg | Neg | Pos |
| 240 | Pos | Pos | Neg | Neg | Pos |
| 453 | Pos | Neg | Neg | Neg | Pos |
| 485 | Pos | Pos | Neg | Pos | Pos |
| 510 | Pos | Pos | Neg | Neg | Pos |
| 522 | Pos | Pos | Neg | Neg | Pos |
| 535 | Pos | Pos | Neg | Neg | Neg |
| 637 | Pos | ND | Neg | ND | Pos |
| 734 | Pos | Pos | Neg | Pos | Pos |
| 743 | Pos | Neg | Neg | Neg | ND |
| 749 | Pos | Pos | Neg | Neg | ND |
| 874 | Pos | Pos | Neg | Neg | Neg |
| Total immune responses | 12/12 | 9/11 | 0/12 | 2/11 | 8/10 |
Abbreviations: Ab, soluble antibodies determined by ELISA; Cell, cellular proliferation determined by 3[H]TdR incorporation.
Antibody responses to Id were evaluated by comparing the reactivity of prevaccine and postvaccine serum samples with autologous and unrelated isotype-matched Id. No increase was observed in the titers of specific anti-Id antibodies (Table 3).
Cellular proliferation assays.
T-cell proliferative responses to KLH, control Id (unrelated isotype-matched Id), and autologous Id were evaluated 1 month after the last injection of Id/KLH conjugates (Table 3). Proliferations to KLH and control Id were also determined in 14 age-matched normal donors and were used as reference values. On average, the stimulation indexes to KLH and control Id in the normal donors were 1.00 ± 0.15 and 0.75 ± 0.4, respectively. Nine of 11 patients tested showed a positive response to KLH, whereas only 2 of 11 showed a positive response to autologous Id (Table 3).
DTH skin tests.
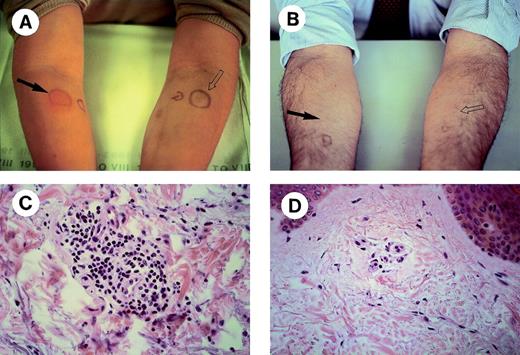
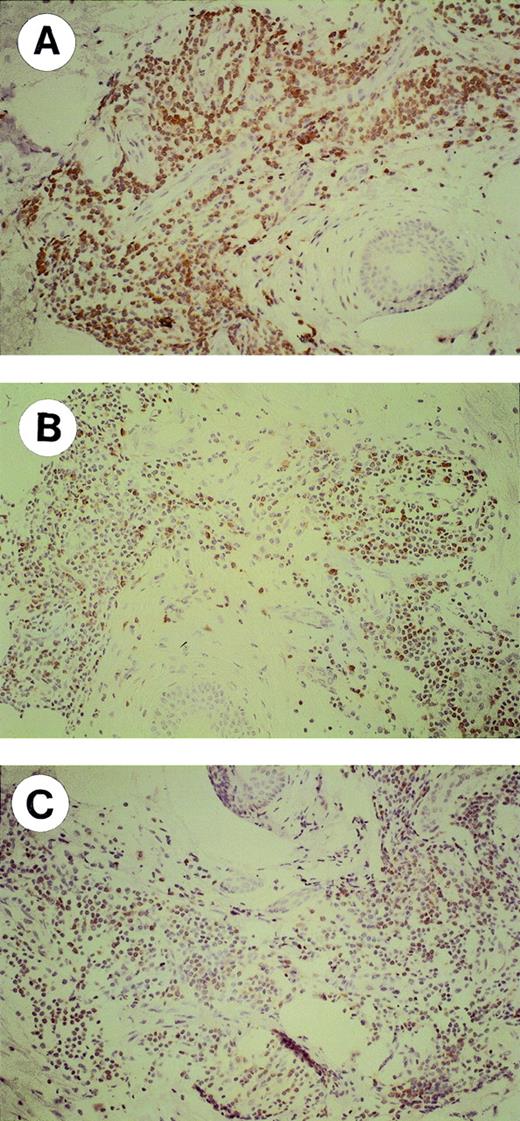
DTH skin tests were performed in 10 patients 1 month after the last injection of Id/KLH conjugates. A local reaction characterized by erythema and induration was observed in 8 patients after 24 hours at sites of Id injection only (Table 3). Two representative MM with a positive and a negative reaction are shown in Fig 1. Skin biopsies were performed in 6 patients: in 3, biopsies were taken from the Id sites only, whereas in the other 3 they were taken from both Id and control sites. A perivascular lymphocyte infiltration was observed at sites of Id injection only (Fig 1C and D). Immunohistochemical characterization of the lymphocytic infiltrates showed that most lymphocytes were CD3+ in the Id challenge site with a slight prevalence of CD8+ versus CD4+ cells (Fig 2). The proportions of CD56+ and CD20+ cells were negligible (data not shown). The prevaccine DTH skin tests were negative (data not shown).
DTH skin tests in MM receiving Id vaccines. Two representative patients with a positive (A) and a negative (B) reaction are shown. Unconjugated autologous Id alone (solid arrow) or an equivalent amount of polyclonal human Ig as a control (open arrow) were injected intradermally into the opposite forearms. Histologic assessments (original magnification × 40) of skin biopsies taken at sites of autologous Id challenge are shown in (C) and (D).
DTH skin tests in MM receiving Id vaccines. Two representative patients with a positive (A) and a negative (B) reaction are shown. Unconjugated autologous Id alone (solid arrow) or an equivalent amount of polyclonal human Ig as a control (open arrow) were injected intradermally into the opposite forearms. Histologic assessments (original magnification × 40) of skin biopsies taken at sites of autologous Id challenge are shown in (C) and (D).
Immunohistochemical characterization (original magnification × 20) of lymphocytes infiltrating the skin at sites of autologous Id challenge. A representative MM with a positive DTH reaction is shown. Formalin-fixed, paraffin-embedded skin biopsy sections were stained with the following antibodies: (A) polyclonal anti-CD3; (B) OPD4 MoAb (CD4/CD45R0); and (C) C8/144B MoAb (CD8).
Immunohistochemical characterization (original magnification × 20) of lymphocytes infiltrating the skin at sites of autologous Id challenge. A representative MM with a positive DTH reaction is shown. Formalin-fixed, paraffin-embedded skin biopsy sections were stained with the following antibodies: (A) polyclonal anti-CD3; (B) OPD4 MoAb (CD4/CD45R0); and (C) C8/144B MoAb (CD8).
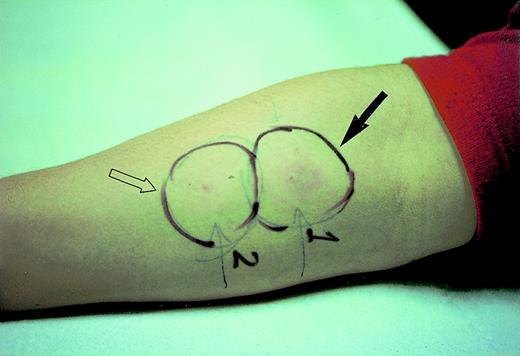
In 3 patients, the DTH skin test remained positive up to 1 year after the last immunization. In 1 of these patients (UPN 510), the VDJ sequence of the tumor-specific Ig heavy chain was available. Thus, synthetic peptides were derived from the CDR3 and FR3 sequences and used for DTH skin testing. A positive reaction was observed at site of challenge with the CDR3-derived peptide only (Fig 3). The DTH skin test to KLH was performed at the end of the vaccine treatment in 2 patients only. Both showed a very strong local reaction and the test was not repeated (data not shown).
DTH skin tests with synthetic peptides derived from the VDJ sequence of the tumor-specific Ig heavy chain: CDR3-derived peptide (solid arrow) or an equivalent amount of FR3-derived peptide (open arrow) were injected intradermally side-by-side into the forearm.
DTH skin tests with synthetic peptides derived from the VDJ sequence of the tumor-specific Ig heavy chain: CDR3-derived peptide (solid arrow) or an equivalent amount of FR3-derived peptide (open arrow) were injected intradermally side-by-side into the forearm.
TCRBV repertoire analysis.
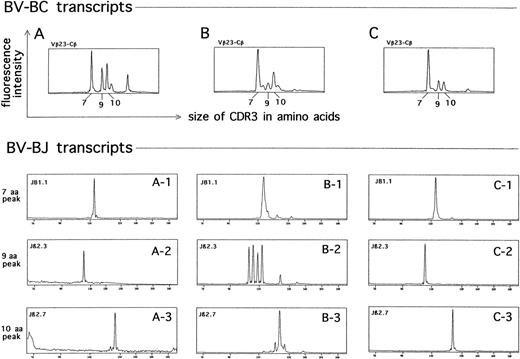
The TCRBV repertoire expressed by T lymphocytes at sites of DTH skin tests was analyzed in 2 patients, and a very high frequency of abnormal CDR3 size distribution patterns was observed (Fig 4). We distinguished 4 CDR3 size distribution patterns: (1) normal, characterized by a Gaussian distribution of BV-BC CDR3 fragments; (2) deteriorated, characterized by one or more peaks above the normal Gaussian background; (3) disrupted, in which the Gaussian distribution was replaced by 2 or more predominant peaks; and (4) single-peak, characterized by a prominent single-peak. The disrupted and single-peak patterns were indicative of the presence of multiple clonal T-cell expansions, as shown by the restricted usage of individual BJ gene segments in their predominant peaks (data not shown and Fig 5). A side-by-side comparison was performed with the TCRBV repertoires expressed by lymphocytes derived from the peripheral blood and the bone marrow. These preparations also showed a large frequency of disrupted and single-peak patterns compared with age-matched normal donors (manuscript in preparation). A restricted number of abnormal prominent peaks of the same size within the same BV subfamily were identified (Fig 5). Further analysis at the level of BV-BJ transcripts showed that these peaks were almost identical in the usage of individual BJ segments (Fig 5).
Analysis of the TCR repertoire expressed by T cells at sites of DTH skin tests with autologous Id. CDR3 size distribution patterns at the level of BV-BC transcripts from a representative MM (UPN 14) are shown. (X-axis) Sizes in amino acids of the CDR3 regions. (Y-axis) Fluorescence intensities, reflecting the number of clones using each CDR3 size combination. The high frequency of abnormal patterns (ie, CDR3 profiles without a Gaussian-like distribution) is indicative of the presence of multiple clonal T-cell populations.
Analysis of the TCR repertoire expressed by T cells at sites of DTH skin tests with autologous Id. CDR3 size distribution patterns at the level of BV-BC transcripts from a representative MM (UPN 14) are shown. (X-axis) Sizes in amino acids of the CDR3 regions. (Y-axis) Fluorescence intensities, reflecting the number of clones using each CDR3 size combination. The high frequency of abnormal patterns (ie, CDR3 profiles without a Gaussian-like distribution) is indicative of the presence of multiple clonal T-cell populations.
Identification of clonally related T-cell subsets in the skin (A), peripheral blood (B), and bone marrow (C) from a representative MM patient (UPN 14). (Upper panel) CDR3 size distribution at the level of BV23-BC transcripts. (X-axis) Sizes in amino acids of the CDR3 regions. (Y-axis) Fluorescence intensities, reflecting the number of clones using each CDR3 size combination. Three recurrent predominant peaks (7 aa, 9 aa, and 10 aa) are identified in each sample. (Lower panel) CDR3 size distribution at the level of BV23-BJ transcripts. The usage of individual BJ segments in the 7 aa, 9 aa, and 10 aa peaks from the skin (A1-A3), peripheral blood (B1-B3), and bone marrow (C1-C3) samples are shown.
Identification of clonally related T-cell subsets in the skin (A), peripheral blood (B), and bone marrow (C) from a representative MM patient (UPN 14). (Upper panel) CDR3 size distribution at the level of BV23-BC transcripts. (X-axis) Sizes in amino acids of the CDR3 regions. (Y-axis) Fluorescence intensities, reflecting the number of clones using each CDR3 size combination. Three recurrent predominant peaks (7 aa, 9 aa, and 10 aa) are identified in each sample. (Lower panel) CDR3 size distribution at the level of BV23-BJ transcripts. The usage of individual BJ segments in the 7 aa, 9 aa, and 10 aa peaks from the skin (A1-A3), peripheral blood (B1-B3), and bone marrow (C1-C3) samples are shown.
DISCUSSION
Id vaccination is a safe treatment in MM and can be delivered in an outpatient setting. Local and systemic toxicities were mild (WHO grade I/II) and more related to cytokines than to the Id/KLH conjugates themselves. All patients rated the quality of life during the period of vaccine treatment as good or very good. Failure to complete the treatment owing to disease progression occurred in 1 patient only.
The tumor mass was not reduced by the vaccines, irrespective of its pretreatment magnitude. A PCR analysis was used in 2 patients with minimal residual disease (UPNs 14 and 510) to detect Cα- and Cμ-associated tumor-specific VDJ sequences and evaluate the effect of Id vaccines at the level of pre-B and B cells other than plasma cells. These populations are clonally related to plasma cells30and are optimal targets for vaccine treatment, because they carry membrane-bound Id. The analysis was performed in the peripheral blood and the bone marrow without detecting any difference before and after vaccination.
However, the lack of effects on the tumor mass was not associated with a detrimental effect on the FFDP and survival. The date of the first immunization was the reference time point used to calculate the FFDP. Of the 11 patients who completed the treatment, 5 have maintained the remission at 9 to 30 months after the first immunization, whereas 6 patients relapsed at 9 to 36 months. Current options for MM in first remission are no treatment, IFN-α alone, or IFN-α and steroids. Clinical results with IFN-α alone are controversial, with some studies showing improved FFDP7,39,40 and others no effect whatsoever.8,41,42 The combination of IFN-α and steroids is more effective,9 but still unsatisfactory with a median duration of 19 months. A major difference between Id vaccination and other maintenance treatments is that the former was delivered as a time-limited treatment (28 weeks), whereas the latter are delivered indefinitely until relapse or toxicity. Patients maintained the remission at 3 to 30 months after the last immunization without any further maintenance treatment. It may be worth investigating whether long-term delivery until relapse is more effective than a time-limited schedule. Of the 12 patients treated, 10 are alive at 11 to 39 months after the first immunization (4 to 32 months after the last immunization).
So far, there are only 2 studies limited to 5 patients, each about the clinical use of Id vaccines in MM.43,44 Six patients had early stage disease and were untreated, whereas the remainder had received conventional chemotherapy from 2 to 5 years before vaccination. Vaccines consisted of autologous unconjugated Id precipitated in aluminium phosphate43 or administered in the presence of GM-CSF.44 One patient experienced a 65% decrease in the serum M protein level that lasted about 9 months, but no other clinical data were reported.
Unlike chemotherapy, which requires tumor cytoreduction to be effective, immunotherapy exploits more subtle mechanisms to achieve tumor control in the absence of tumor mass reduction. In this regard, immunologic monitoring is crucial to determine to what extent immunotherapy has acted on the immune system and generated tumor-specific responses, if any. We initially evaluated humoral and cellular responses to KLH. KLH is commonly used as a protein carrier to make Id immunogenic, but it can also be exploited as an internal control to evaluate the efficacy of the immunization schedule. A significant production of antibodies to KLH was observed in all patients. Cellular responses to KLH were also generated and detected using a cell proliferation assay or the DTH skin test. Thus, the immune competence status of MM patients is not permanently impaired by chemotherapy, even when multiple courses of high-dose chemotherapy followed by autologous PBPC transplantation are delivered.
The detection of humoral and cellular responses to autologous Id was more difficult. Id is a much weaker antigen than KLH: it belongs to self and is under the protection of self-tolerance mechanisms. We could not detect the presence of antibodies to Id with an ELISA assay. By using an enzyme-linked immunospot assay (ELISPOT), Bergenbrant et al43 have detected B cells producing anti-Id antibodies in MM patients receiving autologous unconjugated Id. The number of these cells increased after immunization and then diminished by the end of the vaccine treatment. Conflicting results have also been reported in follicular lymphomas. Antibodies to Id were documented in 17 of 41 patients receiving Id vaccines consisting of Id coupled to KLH and emulsified in an immunologic adjuvant,21 but in none of 4 patients receiving a more effective vaccine formulation consisting of dendritic cells pulsed with tumor-specific Id protein.45Beside experimental variations, it is possible that we could not detect antibodies to Id because they are bound by residual tumor cells or by circulating Id that are not eradicated, even after multiple PBPC transplantations.6
Cellular responses to Id were equally difficult to detect. A cell proliferation assay detected Id-specific responses in 2 of 11 patients. Similar data have recently been reported by Osterborg et al,44 who detected Id-specific T-cell responses in 1 of 5 patients. Cell proliferation may not be the most appropriate readout to investigate cell-mediated immunity. First, the generation of cytotoxic T lymphocytes (which is the ultimate goal of vaccine treatment) may occur in the absence of significant proliferation, but rather in the presence of the appropriate cytokines. Second, the frequency of Id-reactive T cells can be so low as to be undetectable in a short-term cell proliferation assay.44 By using an ELISPOT assay detecting cytokine production at the single-cell level, Osterborg et al44 showed that the frequency of Id-reactive cells increased by about twofold to threefold in MM receiving unconjugated Id vaccines and GM-CSF.
In our hands, the DTH skin test was the most reliable assay to demonstrate the in vivo generation of Id-specific immune responses. This is based on the local recognition by CD4 and CD8 cells of antigens against which the host has been immunized by prior exposure and is gaining increasing attention as a convenient method to detect antigen-specific immunity.46,47 Skin biopsies were performed at sites of DTH skin tests, and the perivenular infiltration of CD4+ and CD8+ lymphocytes, documented at sites of Id challenge, was correlated with the degree of local induration. This phenotype, in the absence of a granulocytic component, is consistent with previous descriptions of antigen-specific DTH reactions.48 The finding that both subsets were present indicates that the immunization schedule was effective enough to involve both arms of the immune system. The DTH skin tests were negative in the study of Osterborg et al,44 in which MM patients received autologous Id in the absence of KLH. This discrepancy can be explained by a number of differences in the immunization procedures, such as KLH conjugation, dose of GM-CSF, and number of immunizations.
The following findings strongly argue that DTH responses were Id-specific and generated by the vaccine treatment. (1) The DTH skin tests performed before vaccination were negative. The same aliquot of unconjugated Id was used for DTH skin testing before and after vaccination and to prepare the vaccine. Thus, the responses were not triggered by the intradermal injection per se, and the prolonged Id exposure did not generate any detectable reactivity a priori during the course of the disease. (2) No reactivity was observed against an equivalent amount of polyclonal human Ig. Our ethical committee did not allow the use of allogeneic isotype-related Id as a control. To minimize any bias in the preparation procedure, polyclonal Ig were treated like unconjugated Id, ie, dialyzed versus physiologic saline, incubated twice with polymyxin, passed through a 0.2-μm filter, and stored at −20°C until use. Even if these preparations were not the optimal control for IgA MM, they surely represented a very reliable control to rule out any isotype-specific reactivity for IgG MM patients. (3) We performed DTH skin tests using synthetic peptides of clinical grade corresponding to the CDR3 and FR3 sequences of the tumor-specific Ig heavy chain. The former only induced a detectable DTH reaction. The CDR3 is the most variable and is therefore the region most likely to be unique to the Ig produced by tumor cells. It has recently been reported by Wen et al49,50 that synthetic peptides derived from the CDR3 sequence of the tumor-specific Ig heavy chain can induce T-cell proliferative and cytotoxic responses in either B-cell lymphoma or MM. These results have been obtained in vitro by repeated rounds of appropriate stimulation using T cells isolated from unvaccinated patients. We have detected in vivo a similar response using the DTH skin test in a vaccinated patient 1 year after the last immunization. So far, this is the most compelling in vivo demonstration of the specificity of the immune response generated by Id vaccines in MM. Thus, tumor-specific CDR3-reactive T cells have not been deleted from the immune repertoire of MM patients and can be recruited by an appropriate immunization procedure. Wen and Lim51 have very recently reported a positive DTH reaction in a B-cell lymphoma patient immunized with a naked CDR3-derived synthetic peptide in the presence of KLH and GM-CSF.
We have performed a molecular characterization of the T cells infiltrating the skin at sites of Id challenge by analyzing the expression of their TCRBV repertoire. The high frequency of disrupted and deteriorated patterns indicated the presence of multiple clonal T-cell expansions.34,37,38 Such a multiple clonotypic response strongly suggests that both CD4+ and CD8+ cells are recruited by an antigen-driven process rather than by a nonspecific inflammatory reaction.52-54The high proportion of abnormal BV subfamilies involved in the anti-Id response was expected, because it is well known that T-cell responses to nominal antigens are oligoclonal and involve multiple TCRBV subfamilies.53,54 Multiple clonal T-cell expansions have also been detected in the peripheral blood and bone marrow of MM patients compared with age-matched normal donors (S. Mariani, manuscript in preparation). Most of the CDR3 distribution patterns were different in the skin versus the bone marrow and the peripheral blood (which, in turn, were similar), further evidence against nonselective T-cell extravasation at sites of Id challenge. A few abnormal BV subfamilies containing predominant peaks of the same size were identified. Further analysis at the level of BV-BJ transcripts showed that these peaks were almost identical in their usage of individual BJ segments. Even though sequencing was not performed, it is very likely that these are clonally related T cells sharing the same antigen specificity.55 These data confirm that the analysis of the TCRBV repertoire expressed by T cells elicited at sites of DTH skin tests can be used as a tool with which to “fish” for tumor-specific T-cell clones.55
In conclusion, we have been able to generate specific anti-Id immune responses in MM patients in first remission after high-dose chemotherapy and PBPC transplantation. In our hands, DTH skin tests were a convenient read-out to pick up the in vivo generation of anti-Id immune responses. However, it is currently unknown whether CD4+ and CD8+ cells specifically elicited in the skin by Id challenge are indeed the same cells that can hold myeloma cells in check in the bone marrow. Identification and monitoring of clinically relevant tumor-specific immune responses remains a major challenge in the setting of Id vaccination. Finally, it remains to be determined whether the generation of such responses can provide a better outcome in the setting of minimal residual disease compared with other maintenance treatments.
ACKNOWLEDGMENT
The authors thank Prof John Iliffe for editorial assistance. They also thank Drs Henri Gilbert and Gordon Tribbick (Chiron Technologies, Clayton Victoria, Australia) for peptide synthesis.
Supported by AIRC (Milano, Italy), MURST 60% (Roma, Italy), and Compagnia San Paolo di Torino (Torino, Italy). Fellowship recipients are S.P. (Comitato Gigi Ghirotti, Torino, Italy), S.M. (AIL, Torino, Italy), and B.B. (Associazione Italiana Amici José Carreras, Torino, Italy). The support of FIRC (Milano, Italy) to M.M. is also acknowledged.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
Subsequent to the submission of this work, Reichardt et al56 have reported a series of 12 MM treated with Id-pulsed autologous dendritic cells after high-dose chemotherapy and PBPC transplantation. Eleven of 12 patients made strong anti-KLH cellular responses and 2 of 12 developed cellular Id-specific proliferative responses.
Author notes
Address reprint requests to Massimo Massaia, MD, Divisione Universitaria di Ematologia, Via Genova 3, 10126 Torino, Italy; e-mail:maxmass@iol.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal