Cytogenetic investigations in two cases of anaplastic large cell lymphoma (ALCL) showed novel variants of the classical (2;5)(p23;q35) translocation, namely a t(1;2)(q21;p23) and a t(2;3)(p23;q21). The tumor cells in both cases gave positive immunohistochemical labeling for ALK protein (with both monoclonal and polyclonal antibodies), demonstrating that these translocations induce aberrant expression of this kinase and suggesting that genes other than NPM can activate the ALK gene in ALCL. These two cases were shown by an in vitro kinase assay to express ALK kinases (104 kD and 97 kD, respectively), which differed in size from the classical NPM-ALK fusion product (80 kD). Moreover, ALK expression was confined to the cytoplasm of the tumor cells in each case, supporting the hypothesis that the observed nuclear localization of NPM-ALK in classical ALCL is not the site of oncogenic activity of the ALK kinase.
ANAPLASTIC LARGE cell lymphoma (ALCL), first described by Stein et al,1 constitutes a subgroup of non-Hodgkin’s lymphoma characterized by unusual morphologic features and expression of the CD30 (Ki-1) antigen. Thirty percent to 40% of nodal ALCL carry the reciprocal t(2;5)(p23;q35) chromosomal translocation.2-4 This genetic abnormality juxtaposes theNPM gene at 5q35 to the ALK gene at 2p23,5leading to the generation of a novel chimeric NPM-ALK 80-kD kinase. ALK protein is not expressed in normal lymphocytes, and monoclonal and polyclonal anti-ALK antibodies (ALK1 and p80NPM/ALK) have therefore been widely used to provide immunohistochemical evidence for the presence of the NPM-ALK fusion protein in lymphoma cells.6 7
Two cases of ALCL have been reported recently in which a positive immunohistochemical reaction for ALK protein was found in the absence of the t(2;5)(p23;q35) (as detected by classical cytogenetics).8,9 These cases were shown to carry variants of the classical t(2;5), namely a t(1;2)(q25;p23) and an inv(2)(p23q35). One further variant translocation, a t(2;13)(p23;q34), has been described by Sainati et al,10 although no immunohistochemical data are available on ALK expression.
We here report on two novel variant translocations in ALCL and additional immunohistochemical and in vitro kinase assay studies.
MATERIALS AND METHODS
Patients.
Patient 1 was the 13-year-old boy D.F. who showed painful enlargement of cervical, axillary, and inguinal lymph nodes in June 1997. After diagnosis of a CD30+ ALCL of T-cell phenotype, he underwent chemotherapy according to the German NHL-BFM’95 protocol (group III/K3).11 After the final cycle the patient has remained in complete remission without symptoms.
A lymph node biopsy specimen obtained from patient 2 (10-year-old girl S.N.) in July 1994 showed a CD30+ ALCL of null cell phenotype. After initial chemotherapy (German NHL-BFM’90 protocol11) resulting in complete remission, a relapse occured in November 1995. Autologous bone marrow transplantation was performed in December 1995, and since then she is in complete remission.
Immunohistochemical and cytogenetic studies.
Immunohistochemical investigations on lymph node biopsy specimens included B- and T-cell markers, CD30 (BerH2), EMA, TIA-1, and the p80NPM/ALK 6 and ALK17 antibodies.
Cytogenetic analysis was performed on unstimulated short-term cultures of tumor tissue. Metaphases were stained by a conventional trypsin-Giemsa technique and evaluated according to the ISCN guidelines.12
In vitro kinase assay.
Frozen tissue sections were subjected to an in vitro kinase assay technique that is detailed elsewhere.13 A frozen tumor of an ALCL-derived t(2;5)+ cell line SU-DHL-1 grown in severe combined immunodeficient (SCID) or nude mice and normal tonsils was used as positive and negative control.
RESULTS AND DISCUSSION
Histological evaluation of the lymph node biopsy specimen from patient D.F. identified an ALCL of T-type with the tumor cells being positive for CD5, CD30, and TIA-1 and exhibiting strong cytoplasmic staining for ALK protein (with both the polyclonal antiserum p80NPM/ALKand the monoclonal antibody ALK1; Fig 1a).
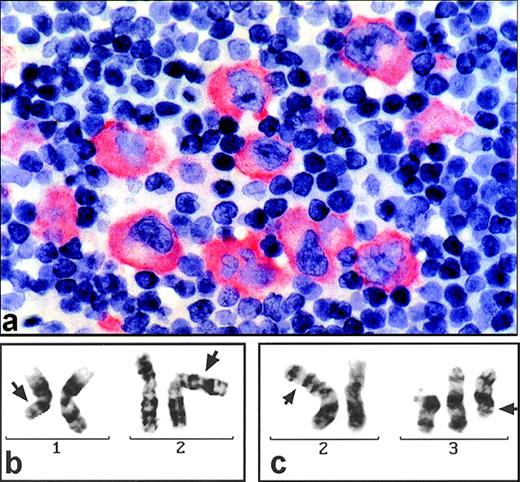
(a) Section of a paraffin-embedded lymph node from patient D.F. stained for ALK1 expression (alkaline phophatase-technique). Large pleomorphic tumor cells show marked cytoplasmic but no nuclear immunostaining. (b) G-banded partial karyotype of patient D.F. exhibiting the translocation t(1;2)(q21;p23) (arrows). (c) G-banded partial karyotype of patient S.N. showing the t(2;3)(p23;q21) (arrows) and, additionally, a +del(3)(p21).
(a) Section of a paraffin-embedded lymph node from patient D.F. stained for ALK1 expression (alkaline phophatase-technique). Large pleomorphic tumor cells show marked cytoplasmic but no nuclear immunostaining. (b) G-banded partial karyotype of patient D.F. exhibiting the translocation t(1;2)(q21;p23) (arrows). (c) G-banded partial karyotype of patient S.N. showing the t(2;3)(p23;q21) (arrows) and, additionally, a +del(3)(p21).
Cytogenetics showed a karyotype of 46,XY,t(1;2)(q21;p23)[12]/92,XXYY, t(1;2)(q21;p23)x2[4]/46,XY[1] (Fig 1b).
The lymph node biopsy specimen of S.N. led to the diagnosis of an ALCL of null cell phenotype. Again, strong cytoplasmic staining was noted for ALK protein (not shown). The karyotype, obtained from conventional cytogenetics, was: 47,XX,t(2;3)(p23;q21),+del(3)(p21)[5]/46,XX[3] (Fig 1c).
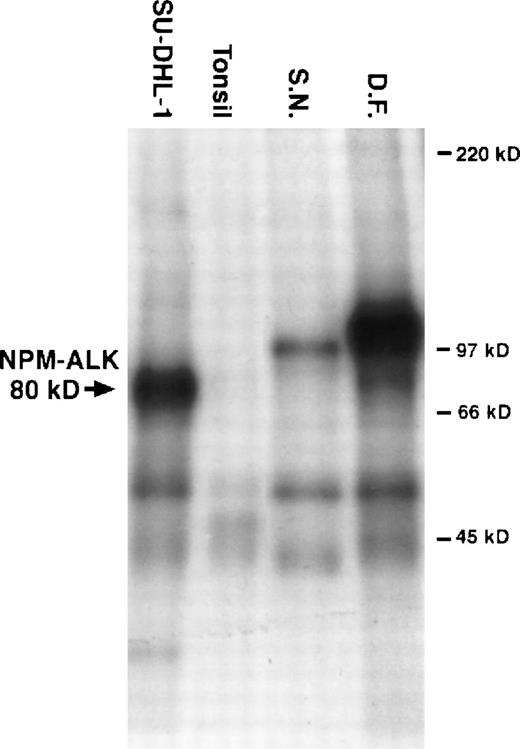
In the in vitro kinase assay, the most prominent phosphorylated proteins were a 104-kD band in the tumor from D.F. and a 97-kD band in cells from S.N. (for details see Fig 2).
Results from an in vitro kinase assay. Proteins were extracted from cryostat tissue sections, immunoprecipitated with anti-ALK, and incubated with radioactive kinase substrate. The samples were then resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by autoradiography. Phosphorylated proteins of 97 kD and 104 kD were detected in tumor tissue from patients S.N. and D.F., respectively. 80-kD phosphorylated NPM-ALK was present in the t(2;5)+ cell line SU-DHL-1. No ALK proteins were present in the tonsil lysates used as negative controls.
Results from an in vitro kinase assay. Proteins were extracted from cryostat tissue sections, immunoprecipitated with anti-ALK, and incubated with radioactive kinase substrate. The samples were then resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by autoradiography. Phosphorylated proteins of 97 kD and 104 kD were detected in tumor tissue from patients S.N. and D.F., respectively. 80-kD phosphorylated NPM-ALK was present in the t(2;5)+ cell line SU-DHL-1. No ALK proteins were present in the tonsil lysates used as negative controls.
The t(2;5)(p23;q35) is regarded as a critical step in the genesis of nodal ALCL leading to the generation of a chimeric 80-kD NPM-ALK protein that is thought to cooperate in malignant transformation by aberrant activation of its ALK kinase domain.14-16 There is now clear evidence that the NPM-ALK fusion protein is directed from the cytoplasm to the nuclei of the tumor cells via heterodimerization with normal NPM.15,16 This results in the characteristic staining pattern for NPM-ALK in cells exhibiting the t(2;5)(p23;q35), in which both the nuclei and cytoplasm are labeled.7,16 17
However, in a minority of ALCL cases, ALK protein expression is restricted to the cytoplasm of tumor cells.17,18 A possible interpretation of this staining pattern is the presence of chromosomal abnormalities affecting the ALK gene but not involvingNPM, and there is direct evidence for this in the report of a cytoplasmic distribution of ALK protein in an ALCL bearing a t(1;2)(q25;p23).7,16 Moreover, an ALK fusion protein in which NPM is replaced by a TPR sequence19 is capable of transforming cells in vitro and in vivo but is expressed only in the cytoplasm of transfected COS cells.15 16
These results indicate that mechanisms other than the classical t(2;5) can lead to the activation of the ALK gene and that such cases should be recognizable because of their ‘cytoplasm-only’ pattern of ALK expression. Our two cases of ALCL carrying novel variant translocations support this suggestion. Furthermore, it was possible to show using an in vitro kinase assay that these variant translocations gave rise to ALK protein which differed in size from classical NPM-ALK (80 kD).5-7 The tumor carrying the t(1;2)(q21;p23) contained a 104-kD ALK protein and the t(2;3)(p23;q21) was associated with a 97-kD ALK protein.
Reverse transcriptase-polymerase chain reaction and fluorescence in situ hybridization techniques have also been applied to detect the classical t(2;5).9,20 21 Such procedures would not, however, have provided any information on variant translocations described in the present study. In view of this, the detection of ALK proteins using immunolabeling techniques combined with the in vitro kinase assay would seem to constitute the easiest way to provide evidence for novel ALK gene rearrangements in ALCL when cytogenetic data are not available.
It has recently been proposed that ALK+ lymphomas (‘ALKomas’) constitute a distinct clinicopathological entity.17 Variant translocations involving the ALKgene may permit the identification of new genes that could be involved in this entity. It will be of interest to see, with increasing experience, whether cases of ALCL with variant translocations behave biologically and/or clinically in a different way from cases of ‘ALKoma’ carrying the classical t(2;5).
ACKNOWLEDGMENT
The authors thank Heike Brückner, Andrea Trumpfheller, Maria Reichert, Sabine Roth, and Petra Stempfle for expert technical assistance.
Supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 172, Teilprojekt C8 to G.O. and H.K.M.-H., and by the Leukaemia Research Fund, Grant No. 9646.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to German Ott, MD, Pathologisches Institut der Universität, Josef-Schneider-Str. 2, D-97080 Würzburg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal