The thrombopoietin receptor, Mpl, is a member of the cytokine receptor superfamily. The extracellular domain of Mpl contains two copies of the cytokine receptor homology module (CRM). Mpl is encoded by c-mpl, the cellular homologue of the oncogene v-mpl.The oncogenic potential of v-mpl may arise from deletion of all but the 43 most membrane-proximal amino acids of the extracellular domain of the wild-type receptor. To test the hypothesis that the extracellular domain of Mpl plays a role in controlling receptor activity, we created mutants of murine Mpl in which the membrane-distal CRM was either deleted or replaced by the membrane-proximal CRM. Introduction of these mutant receptors into factor-dependent BaF3 cells led to constitutive cell growth in the absence of growth factor. Both mutant receptors failed to bind 125I-Tpo. These results suggest that the membrane-distal CRM of Mpl acts as a brake on cell proliferation and that this region is required for ligand binding.
MPL, THE THROMBOPOIETIN (Tpo) receptor,1,2 is a member of the cytokine receptor superfamily.3 One characteristic of these receptors is the presence of one or more 200 amino acid extracellular cytokine receptor homology modules (CRMs).4 Like the common β chain of the human interleukin-3 (IL-3), IL-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor (βc), Mpl contains two CRMs. One potential role for the membrane-distal CRM of Mpl is suggested by the structure of the oncogene v-mpl.5v-mpl differs from c-mpl primarily due to deletion of all but the 43 most membrane-proximal extracellular amino acids.3 The exact mechanism by which v-mpl is activating is not known. To test the hypothesis that the extracellular domain of Mpl plays a role in controlling receptor activity, we created mutants of murine Mpl in which the membrane-distal CRM (CRM-1) was either deleted or replaced by the membrane-proximal CRM (CRM-2), which has 33.3% sequence similarity to CRM-1. These mutants were introduced into BaF3 cells and their effects on cell growth and ligand binding were evaluated.
MATERIALS AND METHODS
Cell culture and growth factors.
IL-3–dependent BaF3 cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO/BRL, Grand Island, NY) with 0.9% Antibiotic/Antimycotic (GIBCO/BRL), 10% fetal calf serum (FCS; Summit Biotechnology, Fort Collins, CO), and 400 U/mL IL-3.6Proliferation assays were performed by culturing 3 × 104cells/mL for 5 days with either no added growth factor or 30 ng/mL murine Tpo.6 Viable cell number was determined using a hemocytometer.
Construction and expression of murine Mpl mutants.
Full-length murine c-mpl cDNA was used for mutagenesis.6 Nucleotide numbering refers to the sequence published by Vigon et al.3 Silent point mutations were introduced at bp 60 and bp 813 to create an AatII site and aBglII site, respectively. To introduce the FLAG epitope tag, a 137-bp oligonucleotide (5′-GCTAGAATTCGATCCCCACCATGTTCCATGTTTCTTTTAGATATATCTTTGGAATTCCTCCACTGATCCTTGTTCT-GCTGCCTGTCACATCATCTGACTACAAAGACGATGACGACAAGGCTGCTCAAGACGTCTTC-3′), encoding the IL-7 secretory leader sequence,7 the FLAG epitope tag, two alanine residues and the first three nucleotides of the second exon of c-mpl was synthesized (Universal DNA, Tigard, OR) and inserted as an EcoRI/AatII fragment into c-mpl. To create ΔCRM-1, an AatII/BamHI fragment encoding bp 823 to 1127 was substituted for bp 64 to 1127. To create CRM-2/CRM-2, an AatII/BglII insert encoding bp 823 to 1449 was substituted for bp 64 to 813. Primers used for PCR synthesis of the inserts were ΔCRM-1: 5′-GCTTGCGACGTCGGAGATGCAGTGACAATTGG-3′ and 5′-CGGGATCCAAAAAGGGGAGCCCAGG-3′, and CRM-2/CRM-2: 5′-GCGACGTCGGAGATGCAGTGACA-3′ and 5′-GGCAGATCTACCCAAGCAGTCTCGGAGCC-3′. All inserts were sequenced using the ABI Prism Dye Terminator Kit (Perkin Elmer, Foster City, CA). Full-length cDNAs were subcloned into the expression vector pHZ-1, and stably transfected by electroporation into BaF3 cells.6Selection was made on the basis of neomycin resistance by growth in G418 at a concentration of 1 mg/mL.
Flow cytometry.
Flow cytometry studies were performed using the M2 antibody (Sigma, St Louis, MO), an isotype control and fluorescein isothiocyanate (FITC)-linked goat anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL).8
Immunoprecipitation and Western blot analysis.
Mpl was immunoprecipitated from cell lysates prepared from 30 to 50 × 106 cells using polyclonal antibody to Mpl.6Samples were analyzed by Western blot analysis using enhanced chemiluminescence (ECL) per the manufacturer’s instructions (Amersham Pharmacia, Piscataway, NJ) with the M2 antibody.
Binding studies.
Binding studies were performed using purified recombinant 70-kD murine Tpo, provided by Drs Dan Eaton and Fred de Sauvage (Genentech Inc, South San Francisco, CA). 1 × 106 cells were incubated with 125I-Tpo (170 pmol/L) in the presence or absence of 14 nmol/L unlabeled Tpo for 1 hour at 37°C.8
RESULTS AND DISCUSSION
Construction and expression of FLAG-tagged mutant c-mpl cDNAs.
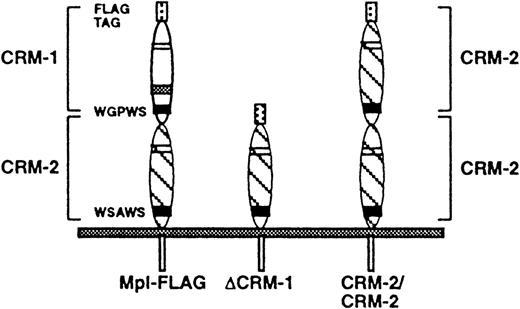
An amino-terminal FLAG epitope tag was introduced by substituting the IL-7 secretory leader followed by the FLAG epitope for the first exon of c-mpl, which encodes only the native Mpl secretory leader.9 A construct in which the FLAG epitope immediately followed the native c-mpl secretory leader did not produce a surface-expressed protein. Two mutant cDNAs were constructed: ΔCRM-1, in which CRM-1 of Mpl was deleted, and CRM-2/CRM-2, in which the membrane-proximal CRM-2 of Mpl was substituted for CRM-1 (Fig1). BaF3 cells stably transfected with either FLAG-tagged Mpl (Mpl-FLAG), ΔCRM-1, or CRM-2/CRM-2 were obtained by G418 selection. Surface expression of receptors was confirmed by flow cytometry. Western blot analysis of Mpl-FLAG, ΔCRM-1, and CRM/CRM2 cells revealed the presence of 95-kD, 47-kD, and 86-kD proteins, respectively. The molecular weights obtained for ΔCRM-1 and CRM2/CRM2 correspond to the sizes predicted for the mutant proteins.
Schematic diagram of Mpl-FLAG, ▵CRM-1, and CRM-2/CRM-2 mutants. Mpl-FLAG represents FLAG-tagged wild-type Mpl, ▵CRM-1, a mutant in which the membrane-distal CRM-1 has been deleted, and CRM-2/CRM-2, a mutant in which the membrane-proximal CRM-2 of Mpl has been substituted for CRM-1.
Schematic diagram of Mpl-FLAG, ▵CRM-1, and CRM-2/CRM-2 mutants. Mpl-FLAG represents FLAG-tagged wild-type Mpl, ▵CRM-1, a mutant in which the membrane-distal CRM-1 has been deleted, and CRM-2/CRM-2, a mutant in which the membrane-proximal CRM-2 of Mpl has been substituted for CRM-1.
Effect of mutations on cell proliferation and ligand binding.
In the absence of growth factor, parental BaF3 and Mpl-FLAG cells failed to proliferate while ΔCRM-1 and CRM-2/CRM-2 cells were able to grow (Fig 2A). Similar results were obtained in either one (CRM2/CRM2) or two (ΔCRM-1) additional independent transfection experiments. In all experiments, ΔCRM-1 or CRM-2/CRM-2 cells became factor-independent within 1 to 2 weeks of G418 selection. The rapid appearance of factor-independent cells in separate experiments suggests that these results were not caused by the presence of a single clone containing a spontaneous mutation or DNA rearrangement. These studies show that deletion of CRM-1 from Mpl is an activating mutation as defined by Gonda and D’Andrea,10and suggest that one role of CRM-1 is to act as a brake on cell proliferation. This inhibitory effect appears to be specific to CRM-1, as substitution of CRM-2 did not restore factor dependence. The mechanism of activation may involve changes in receptor conformation that mimic those induced by ligand binding, such as formation of active receptor dimers. We were unable to show dimerization of either the mutant receptors or of wild-type receptor stimulated by Tpo by chemical cross-linking (unpublished data, January 1999). Similarly, dimerization of Mpl by Tpo could not be shown in normal human platelets.8
Proliferative response and ligand binding by ΔCRM-1 and CRM-2/CRM-2 cells. (A) BaF3, Mpl-FLAG, ΔCRM-1, and CRM-2/CRM-2 cells were grown either in the absence of growth factor (□) or presence (▪) of recombinant murine Tpo 30 ng/mL for 5 days. All values were determined in triplicate. Error bars represent SEM. (B) Binding of125I-Tpo by BaF3, Mpl-FLAG, ▵CRM-1, and CRM-2/CRM-2 cells. Cells were incubated with 125I-Tpo in the absence (▪) or the presence (□) of excess unlabeled 70-kD Tpo. The values represent mean of triplicate measurements. Error bars indicate standard error of the mean. Similar results were obtained in two additional experiments.
Proliferative response and ligand binding by ΔCRM-1 and CRM-2/CRM-2 cells. (A) BaF3, Mpl-FLAG, ΔCRM-1, and CRM-2/CRM-2 cells were grown either in the absence of growth factor (□) or presence (▪) of recombinant murine Tpo 30 ng/mL for 5 days. All values were determined in triplicate. Error bars represent SEM. (B) Binding of125I-Tpo by BaF3, Mpl-FLAG, ▵CRM-1, and CRM-2/CRM-2 cells. Cells were incubated with 125I-Tpo in the absence (▪) or the presence (□) of excess unlabeled 70-kD Tpo. The values represent mean of triplicate measurements. Error bars indicate standard error of the mean. Similar results were obtained in two additional experiments.
βc also contains two CRMs. Although deletion of the membrane-distal CRM of βc alone was not activating, deletion of both this region and a portion of the membrane-proximal CRM was.11 These results suggest that, like Mpl, βc contains regions that inhibit receptor activation in the absence of ligand although the specific regions involved may differ.
Because growth in Tpo had no effect on the proliferation of ΔCRM-1 and CRM-2/CRM-2 cells (Fig 2A), binding studies were performed. Neither ΔCRM-1 or CRM-2/CRM-2 cells were able to bind 70-kD125I-Tpo (Fig 2B). These results suggest that the membrane-distal domain of Mpl is important for ligand binding. Failure to bind was not due to absence of surface expression, as both mutants were detected on the cell surface in flow cytometric studies. The membrane-distal CRM of the murine IL-3–specific β chain, AIC2A, has also been shown to be necessary for ligand binding.12However, in contrast to our results, binding was restored when the membrane-distal domain of the alternative murine β chain, AIC2B, was substituted for the membrane-distal domain of AIC2A.
We found that the membrane-distal CRM of Mpl plays important roles both in controlling Mpl-induced cell growth and in ligand binding. Our results are consistent with a model in which the oncogenic potential of v-mpl may arise from deletion of CRM-1 of Mpl. Future studies will be needed to further delineate the mechanisms by which these processes are mediated.
Supported by National Institutes of Health Grants No. DK02448 and DK49855. D.F.S. is a recipient of an American Society of Clinical Oncology Young Investigator Award.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Diana F. Sabath, MD, University of Washington, Division of Hematology, Box 357710, Seattle, WA 98195; e-mail: dfsabath@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal