Human embryonic ζ and ɛ globin chains are synthesized in yolk sac–derived primitive erythroid cells, and decrease rapidly during definitive erythropoiesis. Examination of ζ and ɛ globin expression at the cellular level using dual-color immunofluorescence staining with specific monoclonal antibodies showed that embryonic globin proteins are present in definitive erythroid cells. More than half of fetal erythrocytes were positive for ζ and ∼5% for ɛ globin. Approximately one third of newborn red blood cells were ζ-positive and less than 1% ɛ-positive. Adult erythrocytes did not have embryonic globins. Erythroblasts that developed in liquid cultures also contained embryonic globin in amounts which declined with ontogenic age, and the proportion of positive cells in vitro was less than in the comparable erythrocytes that developed in vivo. Thus, embryonic globin chains are synthesized in definitive erythroid cells and decrease with ontogeny. Modulation of embryonic globin gene expression is not solely due to a switch from primitive to definitive erythropoiesis.
YOLK SAC–DERIVED primitive erythroid cells remain nucleated and definitive erythroid cells terminally enucleate. Hemoglobin (Hb) expression evolves simultaneously from embryonic Hbs Gower 1 (ζ2ε2), Gower 2 (α2ε2), and Portland 1 (ζ2γ2) to fetal Hb F (α2γ2) and then to adult Hb A (α2β2).1-4 Small amounts of embryonic globins have been detected in hemolysates from definitive fetal and newborn erythrocytes; ζ usually exceeded ε.5-10 Fetal, cord, and adult erythroblasts and reticulocytes had small amounts of embryonic globin mRNA, with more ζ than ε.11-14 The only adults who expressed ζ globin had α-thalassemia trait because of the −−SEA/ deletion of 20.5 kb including ψζ, α1, and α2, which leaves the ζ gene intact.15-17 Single cell immunocytology has confirmed the absence of embryonic-positive erythrocytes in normal adults, as well as in children with juvenile chronic myelogenous leukemia (JCML) in which Hb F is increased.16,18 Lysates from cultured fetal erythroblasts have also been shown to contain small amounts of embryonic globin, with ζ usually exceeding ε.10,19,20 A few cells from JCML cultures were embryonic positive, with more ζ+ than ε+ cells.18
None of those studies systematically examined embryonic globin expression in normal definitive erythrocytes and erythroblasts at all ontogenic stages. To address this, we used multicolor single-cell immunofluorescence with monoclonal antibodies (MoAbs) specific for ζ and ε globin chains7,17,21 to examine erythrocytes derived in vivo and erythroblasts developed in vitro in cytokine-induced liquid cultures from fetal, newborn, and adult blood.22 We show that the proportion of embryonic globin positive cells decreases during ontogeny, and ζ globin expression exceeds ε.
Heparinized blood was obtained from normal adults, term deliveries, and fetuses at termination of pregnancy for nonhematologic indications. All procedures were approved by the Institutional Review Board of the University of Texas Medical Branch. Mononuclear cells (MNC) were cultured in a serum-free modification of a two phase liquid system22 by using BIT (StemCell Technologies, Inc, Vancouver, British Columbia, Canada). Phase 1 (days 0 to 7) contained 106 MNC/mL and stem cell factor (SCF, 4 ng/mL; Boehringer Mannheim, Corp, Indianapolis, IN), and Phase 2 (days 7-14) had 2 × 105 cells/mL, 4 ng/mL SCF, 2 U/mL erythropoietin (Ep; Amgen, Inc, Thousand Oaks, CA), 20 u/mL interleukin-3 (IL-3), and 100 u/mL IL-6 (both from Boehringer Mannheim). Anti-ε and anti-ζ MoAbs were developed previously.7 17 Blood smears or culture cytospin slides were dried overnight and fixed with acetone:methanol (8:2) for 10 minutes (erythrocytes) or acetone:methanol:ethanol (3:1:1) for 20 minutes (cultured erythroblasts). Cells were stained for 30 minutes at 37°C with MoAbs labeled with fluorescein isothiocyanate (FITC; anti-ζ) or Texas Red (TR; anti-ε) (Molecular Probes, Inc, Eugene, OR). The stained slides were thrice washed with phosphate buffered saline and mounted with Vectoshield (Vector, Burlingame, CA). Cells were examined with a Nikon fluorescence microscope (Melville, NY) with a dual color bandpass filter. Fluorescence images were captured with a Vysis SmartCapture FISH Imaging System (Vysis, Downers Grove, IL).
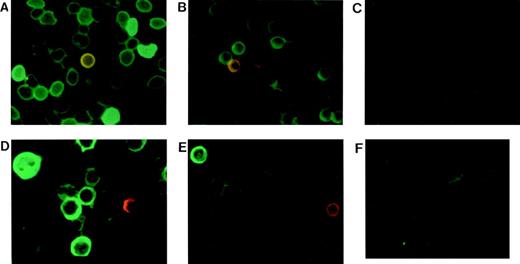
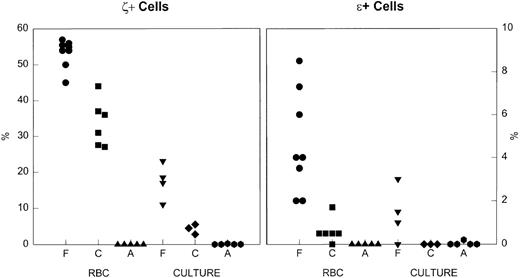
Fetal definitive erythrocytes contain variable amounts of embryonic ζ globin. The proportion with strong green fluorescence (ζ+) was 53 ± 4% in eight samples at 15 to 22 weeks’ gestation, while only 5% ± 2% of the fetal erythrocytes were red or yellow, ie, ε+ or ζ+/ε+ (Fig 1A). Cultured fetal erythroblasts from four experiments were 18% ± 4% ζ+ and only 1% ε+ or ζ+/ε+ (Fig 1D). The proportion of embryonic positive cells was lower in cultured erythroblasts than in erythrocytes, perhaps because the progenitors that differentiated in culture were ontogenically older than the progenitors which had led to erythrocytes in vivo. Both embryonic globin chains are clearly present in definitive cells after the first trimester, although there is a difference in their regulation.
Embryonic globin protein detection with dual color immunocytofluorescence staining. Samples were from 22-week-old fetus, term cord, and adult blood. Blood smears or cytospins were stained with MoAbs specific for ζ and ɛ globins conjugated with FITC and TR, respectively. (A) Fetal, (B) cord, and (C) adult erythrocytes. (D) Fetal, (E) cord, and (F) adult cultured erythroblasts.
Embryonic globin protein detection with dual color immunocytofluorescence staining. Samples were from 22-week-old fetus, term cord, and adult blood. Blood smears or cytospins were stained with MoAbs specific for ζ and ɛ globins conjugated with FITC and TR, respectively. (A) Fetal, (B) cord, and (C) adult erythrocytes. (D) Fetal, (E) cord, and (F) adult cultured erythroblasts.
In six cord blood samples there were 34 ± 6% ζ+ erythrocytes, significantly fewer than in fetal samples. Five of six cord samples had 0.6 ± 0.5% ε+ or ζ+/ε+ erythrocytes (Fig 1B). In cultures from three cord blood samples 4% ± 1% of the erythroblasts were ζ+ and rare cells were ε+ (Fig 1E). Thus, embryonic globins are clearly present in erythroid cells at term birth. There were no embryonic-positive adult erythrocytes (Fig 1C), and cultured adult erythroblasts positive for ζ or ε comprised 0.2% of the cells in only 1 of 7 experiments (Fig 1F).
The ontogenic decline in the proportion of embryonic-positive cells is documented in Fig 2. The differences between the percent ζ+ cells in fetal, cord, and adult erythrocytes and erythroblasts are significant at P < .001. The data for the expression of ε globin are also compelling. The amount of embryonic globin per cell appears qualitatively to decrease during ontogeny.
The percent of cells that were positive for embryonic globins in erythrocytes and cultured erythroblasts. Left, ζ+ cells. Right, ɛ+ cells. RBC, red blood cells; Culture, day 14 of culture; F, fetal; C, cord; A, adult. Note the difference in the y-axis scales for the two embryonic globins. There is an ontogenic decline in the percent positive cells, both in vivo and in culture.
The percent of cells that were positive for embryonic globins in erythrocytes and cultured erythroblasts. Left, ζ+ cells. Right, ɛ+ cells. RBC, red blood cells; Culture, day 14 of culture; F, fetal; C, cord; A, adult. Note the difference in the y-axis scales for the two embryonic globins. There is an ontogenic decline in the percent positive cells, both in vivo and in culture.
The α and non-α globin gene clusters may be regulated differently. The non-α cluster consists of ε, Gγ, Aγ, ψβ, δ, and β genes and has embryonic, fetal, and adult stages. The locus control region (LCR) 5 to 20 kb upstream from ε has open chromatin only in erythroid cells, and ε gene regulation is autonomous. In transgenic mice, ε is expressed only in primitive erythroid cells and undergoes developmental silencing in definitive cells. With a yeast artificial chromosome (YAC) construct of ε, γ, and β genes, all primitive cells contained γ RNA, although many also had ε RNA, which is consistent with sequential or simultaneous transcription of the globin genes. In definitive cells, developmental stage-specifictransacting factors may affect the interaction of the LCR with γ or β, but not with the ε gene.23-26 Our single cell data suggest that ε silencing may be leaky, because a few definitive cells in fetal and newborn blood were strongly positive for ε globin protein.
The α cluster consists of a DNAse I hypersensitive region (HS-40), which is 40 kb upstream, followed by ζ2, ψζ1, ψα2, ψα1, α2, α1, and θ genes. Unlike the β LCR, the α HS has open chromatin in both erythroid and nonerythroid cells. Developmental silencing of ζ is regulated by synergy between the 5′ ζ promoter and 3′ flanking sequences, with an additional autonomous component. There are both transcriptional and posttranscriptional regulators of normal ζ and α-globin gene expression.23 27-30
We have documented embryonic globin chains in definitive cells, a developmental decline in the proportion of positive cells, and an apparent decrease in the amount per cell. ε expression decreases more rapidly than ζ, perhaps related to ε transcription autonomy, and ζ is modulated at both transcriptional and posttranscriptional levels. One reason for these differences may be that there is a clear fetal stage for non-α genes (ie, γ), but there is not one for α genes. The persistence of ζ-gene expression in fetal definitive cells may be comparable to the appearance of γ globin chains.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to B.P. Alter, MD, Division of Pediatric Hematology/Oncology, Children’s Hospital C3.270, 301 University Blvd, University of Texas Medical Branch, Galveston, TX 77555-0361; e-mail:balter@utmb.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal