We have previously shown that selection for resistance to the anthracenes, doxorubicin or mitoxantrone, results in coselection for resistance to CD95-mediated apoptosis (Landowski et al: Blood89:1854, 1997). In the present study, we were interested in determining if the converse is also true; that is, does selection for CD95 resistance coselect for resistance to chemotherapeutic drugs. To address this question, we used two isogenic models of CD95-resistant versus CD95-sensitive cell lines: 8226/S myeloma cells selected for resistance to CD95-mediated apoptosis; and K562 cells expressing ectopic CD95. Repeated exposure of the CD95-sensitive human myeloma cell line, 8226/S, to agonistic anti-CD95 antibody resulted in a cell line devoid of CD95 receptor surface expression and completely resistant to CD95-mediated apoptosis. Multiple clonal populations derived from the CD95-resistant cell line showed no difference in sensitivity to doxorubicin, mitoxantrone, Ara-C, or etoposide, demonstrating that cross-resistance between Fas-mediated apoptosis and drug-induced apoptosis occurs only when cytotoxic drugs are used as the selecting agent. Using the inverse approach, we transfected the CD95-negative cell line, K562, with a CD95 expression vector. Clones expressing variable levels of cell-surface CD95 were isolated by limiting dilution, and analyzed for sensitivity to CD95-mediated apoptosis and response to chemotherapeutic drugs. We show that CD95 surface expression confers sensitivity to CD95-mediated apoptosis; however, it does not alter response to chemotherapeutic drugs. Similarly, doxorubicin-induced activation of caspases 3 and 8 was identical in the CD95-sensitive and CD95-resistant cell lines in both isogenic cell systems. In addition, prior treatment with the CD95 receptor-blocking antibody, ZB4, inhibited CD95-activated apoptosis in 8226/S cells, but had no effect on doxorubicin cytotoxicity. These results show that CD95 and chemotherapeutic drugs use common apoptotic effectors, but the point of convergence in these two pathways is downstream of CD95 receptor/ligand interaction.
IN RECENT YEARS, evidence has been accumulating to support the hypothesis that chemotherapeutic drugs induce an apoptotic response in target cells.1-3 This cytotoxic response reportedly uses signal transduction pathways common to physiological mechanisms of programmed cell death. For example, treatment of the human leukemia cell line U937 with either etoposide or agonistic anti-CD95 monoclonal antibody (MoAb) results in the activation of cysteine proteases and DNA fragmentation.4The cytotoxic effects of both these agents can be inhibited by the caspase 3 (CPP32/apopain) specific tetrapeptide inhibitor DEVD, demonstrating the participation of caspase 3 in both signal transduction cascades. In a second study, treatment of human glioma cell lines with the broad specificity caspase inhibitor YVAD was shown to reduce cell sensitivity to both cisplatin- and CD95-mediated cell death.5 Although these studies, and others, indicate that chemotherapeutic drugs and immune effectors share common mediators, specific enzyme activation events and the point of convergence in the apoptotic signal transduction pathway remain to be defined.
Conflicting data has been reported regarding the role of CD95/CD95 ligand interactions in drug-induced cell death. Studies using a variety of cell lines in which the CD95 receptor and CD95 ligand were shown to be induced by drug treatment have been interpreted as an indication that drug-induced cell death is mediated by the CD95/CD95 ligand system.6-9 This activity was reported to be inhibited by anti-APO-1 F(ab′)2 fragments. Subsequent studies by the same group demonstrated deficient activation of the caspases as a potential mechanism of cross-resistance between drug- and CD95-mediated cell death.10,11 In contrast, a study by Villunger et al12 showed that treatment with the inhibitory anti-CD95 MoAb ZB4 or the anti-CD95 ligand MoAb Nok2 had no effect on the cytotoxicity of cisplatin, doxorubicin, or fludarabine. However, both were capable of inhibiting apoptosis mediated by the agonistic CH-11 antibody or recombinant human CD95 ligand.12 Villunger et al provided further support for the hypothesis that cytotoxic drugs and CD95 cross-linking initiate independent signals to programmed cell death using CEM T-cell leukemia cells rendered resistant to CD95 by expression of the cowpox virus protein crmA. This protein has been shown to inhibit the activity of caspase 8, which is requisite to CD95-mediated apoptosis.13 However, its expression had no effect on the cell sensitivity to doxorubicin, cisplatin, or high-dose fludarabine in the expressing cells. Similarly, Eischen et al14 found no inhibitory effects of the nonagonist anti-CD95 antibody ZB4 in the cytotoxic effects of etoposide on Jurkat T cells; however, this antibody did block apoptosis induced by ligation of the T-cell receptor.
Early studies on CD95-mediated apoptosis showed that forced overexpression of the cytoplasmic death domain of the CD95 receptor was sufficient to induce programmed cell death, suggesting apoptotic signal transduction involved aggregation of this region.15 This observation has been further supported by a recent study showing that UV-irradiated keratinocytes initiated the caspase signal transduction pathway via CD95 receptor aggregation without ligation by CD95 ligand.16 UV activation of caspase 3 and subsequent apoptosis of the cells could be inhibited by a dominant negative mutant of FADD, but was not affected by antibodies directed at external epitopes of CD95 or CD95 ligand. Taken together, all of these studies show the complexity of the signal transduction pathway leading to apoptosis, and suggest multiple mechanisms of caspase activation in response to various stressful stimuli.
We recently showed that in vitro selection for resistance to the anthracenes, doxorubicin, or mitoxantrone, results in the coselection of cell lines resistant to CD95-mediated apoptosis.17 Two mechanisms of CD95 resistance were identified in drug-resistant cells. One mechanism is associated with a dose-dependent reduction in the surface expression of the CD95 receptor in cells chronically exposed to anthracenes. This reduction of CD95 receptor expression in drug-resistant cells occurred at the level of mRNA transcription. The second mechanism of resistance to CD95-mediated apoptosis was likely related to alterations in the apoptotic signal transduction pathway that may be common to CD95- and drug-induced apoptosis. In the present study, we were interested in determining whether cells selected for resistance to CD95-mediated apoptosis were also resistant to chemotherapeutic drugs. To investigate this possibility, the human myeloma cell line, RPMI 8226, was selected for CD95 resistance by repeatedly exposing cells to the apoptosis-inducing MoAb, CH-11, and examining these cells for sensitivity to various chemotherapeutic drugs. If chemotherapeutic agents induce programmed cell death via interactions of the CD95 receptor and CD95 ligand, as some reports have suggested, then we would anticipate cross-resistance to cytotoxic drugs in the CD95-resistant cell lines. Clonal populations derived from the CD95-resistant 8226 cell line showed no differences in sensitivity to drug when compared with the CD95-sensitive 8226 parental cell line.
To further define the role of CD95 in sensitivity to chemotherapeutic drugs, we used an erythroleukemia cell line, K562, which does not express CD95 and is inherently resistant to CD95-mediated apoptosis. This cell line also displays a relatively high level of resistance to most cytotoxic drugs, probably due to the expression of the Bcr-abl oncogene.18 If CD95 plays a direct role in apoptosis induced by chemotherapeutic drugs, we would anticipate enhanced sensitivity to cytotoxic drugs in cells with enforced expression of CD95. K562 cells were transfected with CD95 under the control of a cytomegalovirus (CMV) promoter, and clonal populations analyzed for sensitivity to CD95-mediated apoptosis and chemotherapeutic drugs. Although CD95 expression conferred sensitivity to the agonistic antibody CH-11, clones expressing high levels of CD95 were no more sensitive to doxorubicin, melphalan, or etoposide than were the untransfected or empty vector control cells. Results of our experiments indicate that (1) treatment with agonistic anti-CD95 MoAb selects for a cell line that fails to express the CD95 receptor, and is resistant to CD95-mediated apoptosis; and (2) the presence or absence of CD95 receptor expression has no effect on sensitivity to chemotherapeutic agents.
MATERIALS AND METHODS
Cell culture.
The human multiple myeloma cell line 8226 was originally obtained from American Type Culture Collection (ATCC; Rockville MD), and maintained in RPMI (GIBCO, Grand Island, NY) supplemented with 5% fetal calf serum (FCS), 100 mmol/L L-glutamine, and 100 U/mL penicillin/streptomycin (Gemini, Calabasas, CA). Selection for the CD95-resistant variant, 8226/F4, was done by repeated exposure to the apoptosis inducing anti-CD95 MoAb CH-11 (MBL, Watertown, MA). Antibody was added to the tissue culture medium at 200 ng/mL, and the cells incubated for 72 hours at 37°C. This treatment initially induced 55% to 70% apoptosis in the parental 8226/S cell line. Surviving cells were isolated on a Ficoll gradient (Pharmacia, Piscataway, NJ), and expanded in complete media without MoAb for 2 weeks. The process was repeated a total of four times, and the 8226/F4 cell line was maintained in culture without further selection until analysis. Clonal populations of the 8226/F4 cell line were obtained by limiting dilution.
Antibodies and measurement of apoptosis and cytotoxicity.
Surface expression of the CD95 receptor was determined by flow cytometry using the nonapoptosis-inducing MoAb UB-2 (MBL). Mouse anti-human IgG1 (Dako, Carpinteria, CA) serum served as the isotype control. CD95-mediated cell death was assayed by staining with Annexin V-FITC (Clontech, La Jolla, CA) and flow cytometry analysis.19 Cells were plated at 5 × 105/mL and incubated with CH-11 MoAb at the indicated concentrations and times. Samples were washed in phosphate-buffered saline (PBS) and stained with Annexin V-FITC and propidium iodide (PI) according to the manufacturer’s protocol (Clontech). Apoptosis was measured on a FACScan flow cytometer and analyzed with CellQuest software (Becton Dickinson, Mountain View, CA). Apoptosis was confirmed by fluorescent microscopic examination of CH-11 and doxorubicin-treated cells using Annexin V-FITC and Dapi counterstain (Vector Laboratories, Burlingame, CA).
Cytotoxicity of doxorubicin, etoposide, Ara-C (all from Sigma, St Louis, MO) and mitoxantrone (Wyeth-Ayerst, Pearl River, NY) were determined by MTT assay as previously described.20 Cells were plated at 8 × 104/mL for the 8226-derived cell lines, or 5 × 104 for K562 cell lines, in 96-well plates and incubated with serial dilutions of cytotoxic drug. After 96 hours of drug exposure, 50 μL of MTT dye (2 mg/mL in PBS; Sigma) was added to each well, and allowed to incubate 4 hours. Plates were centrifuged and the media replaced with 100 μL dimethyl sulfoxide (DMSO; Fisher Scientific, Pittsburgh, PA) to solubilize the insoluble formazan complex. Optical density at 540 nm was determined with a Dynex II Elisa plate reader (Dynatech, Chantilly, VA). IC50 was calculated by linear regression analysis of the log-linear plot for percent survival versus drug concentration. Student’s t-test was used to analyze differences in drug response from three independent experiments (.05 significance level). For competition with the inhibitory anti-CD95 MoAb, cells were preincubated for 1 hour at 37°C with 0.5 μg/mL ZB4 (MBL) before addition of the drug or CH-11 agonist antibody. Drug-induced apoptosis was confirmed by labeling fragmented DNA with terminal deoxynucleotidyl transferase (TdT) using the ApopTag kit (Oncor, Gaithersburg, MD) and flow cytometric analysis.
RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA was extracted from 107 cells in log growth phase by lysis in guanidine isothiocyanate followed by cesium chloride density centrifugation and ethanol precipitation. Total RNA was digested with RNase-free DNase (Boehringer Mannheim, Indianapolis, IN) for 15 minutes at 37°C and repurified by the RNeasy kit according to the manufacturer’s protocol (Qiagen, La Jolla, CA). CD95 antigen analysis was performed by 30 cycles of RT-PCR as previously described.17 For detection of CD95 ligand, DNase-treated RNA was transcribed to cDNA by extension of dT primers with 200 U of Superscript II RT (GIBCO) followed by 30 cycles of PCR with primers 5′-TAAAACCGTTTGCTGGGGC-3′ and 5′-CTCAGCTCCTTTTTTTCAGGGG-3′.21 The identity of all products were confirmed by direct sequencing, and a 215-bp fragment of Histone 3.3 was amplified as a control for mRNA integrity and quantitation.22 The full-length CD95 cDNA was inserted into pcDNA3.1 expression vector according to the manufacturer’s protocol. K562 cells were transfected with 2 μg of plasmid DNA with Superfect (Qiagen) and selected with 800 μg/mL G418. CD95-expressing cells were enriched by FACS sorting, followed by limiting dilution cloning.
RNase protection assay.
RNase protection was performed using the Pharmingen RiboQuant hAPO-3 kit according to the manufacturer’s protocol (Pharmingen, San Diego, CA). The multi-probe template was prepared by32P incorporation in an in vitro transcription reaction, and free nucleotide removed on a G50 column (5 Prime → 3 Prime, Inc., Boulder, CO). Purified probe (1 to 1.5 × 106 cpm specific activity) was combined with 20 μg of total RNA, isolated from 5 × 106 cells in 1 mL of Trizol reagent (GIBCO). Hybridization was allowed to proceed through a temperature range of 90°C to 56°C over 16 hours before RNase digestion. After RNase digestion for 45 minutes at 37°C, samples were separated on a 5% denaturing gel, and protected fragments quantitated by phosphor imaging using Image Quant software (Molecular Dynamics, Sunnyvale, CA).
Analysis of caspase activity.
For analysis of caspase 3 and caspase 8 activation, doxorubicin or anti-CD95 treated cells (2 to 4 × 106) were washed with PBS and resuspended in lysis buffer (30 mmol/L HEPES, 10 mmol/L NaCl, 5 mmol/L MgCl2, 25 mmol/L NaF, 1 mmol/L EGTA, 1 mmol/L EGTA, 1% Triton X-100, 10% glycerol, 2 mmol/L Na-orthovanadate, 25 μg/mL leupeptin, 10 μg/mL aprotinin, 2 mmol/L phenylmethyl sulfonyl fluoride [PMSF], and 10 μg/mL soybean trypsin inhibitor) on ice for 30 minutes. Lysates were centrifuged at 14,000 rpm for 15 minutes at 4°C. Total protein determination was done using Bio-Rad Bradford Reagent (Bio-Rad, Hercules, CA), and 100 μg of protein separated on 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membrane. Immune detection of caspase 3 was done using a rabbit polyclonal antibody which recognizes both p32 procaspase and the p20/p17 activated subunits (generously provided by Dr H-D. Wang, H. Lee Moffitt Cancer Center). Caspase 8 was detected using a goat polyconal antibody (Santa Cruz Biotech, Santa Cruz, CA). Secondary antibodies were horseradish peroxidase (HRP)-conjugated (Dako), and blots were developed with the ECL detection system (NEN, Boston, MA).
RESULTS
Selection of a CD95-resistant cell line.
Chemotherapeutic agents commonly used in the treatment of malignant disease have been shown to activate common apoptotic pathways in target cells.1-3 Although the intracellular activity of many of these compounds has been extensively studied, it is still unclear how the cellular damage incurred is translated into a signal for programmed cell death. We have previously reported that in vitro selection for resistance to the anthracenes, doxorubicin, or mitoxantrone results in a coselection for cells that are resistant to CD95-mediated apoptosis.17 To determine if selection for resistance to CD95-mediated apoptosis also selected for resistance to cytotoxic drugs, we subjected the human myeloma cell line 8226/S to repeated exposure of the agonistic anti-CD95 MoAb CH-11. Treatment of 8226/S with 200 ng/mL was found to evoke the maximal response in the 8226/S cell line, resulting in 55% to 70% apoptosis of the unselected population.17,23,24 Surviving cells were rescued on a Ficoll-Hypaque gradient and expanded in culture for four consecutive selections. After repeated exposure to CH-11, the anti-CD95–selected cell line was maintained in culture without further selection pressure for 4 months before analysis. This CD95-resistant cell line, designated 8226/F4, did not respond to cross-linking with agonistic anti-CD95 MoAb (Fig 1) or with soluble recombinant CD95 ligand (data not shown). To ensure the resistance to CD95-mediated apoptosis was not simply caused by clonal variation, we examined seven subclones of the 8226/F4 cell line derived by limiting dilution. Clonal populations of the 8226/F4 cell line were uniformly resistant to CD95-mediated apoptosis in response to CH-11 or soluble CD95 ligand. This is in contrast to 62% cell death in the parental 8226/S cell line, and 12% death in the multidrug-resistant cell line 8226/Dox40, which is maintained under continuous selection with 4 × 10−7 mol/L doxorubicin.25 Extended exposure of the cells for 48 hours with 200 or 1,000 ng/mL CH-11 MoAb did not increase the CD95-mediated cell death in any of the cell lines examined. Both 8226/F4 and 8226/Dox40 were highly resistant to cross-linking by the agonistic CD95 antibody at all time points and concentrations examined (Fig 1).
CD95-mediated apoptosis in 8226 myeloma cell lines. Cells were treated with 200 ng/mL or 1,000 ng/mL anti-CD95 antibody CH-11 and incubated for the indicated times. Apoptosis was determined by staining with Annexin V FITC and flow cytometry analysis.
CD95-mediated apoptosis in 8226 myeloma cell lines. Cells were treated with 200 ng/mL or 1,000 ng/mL anti-CD95 antibody CH-11 and incubated for the indicated times. Apoptosis was determined by staining with Annexin V FITC and flow cytometry analysis.
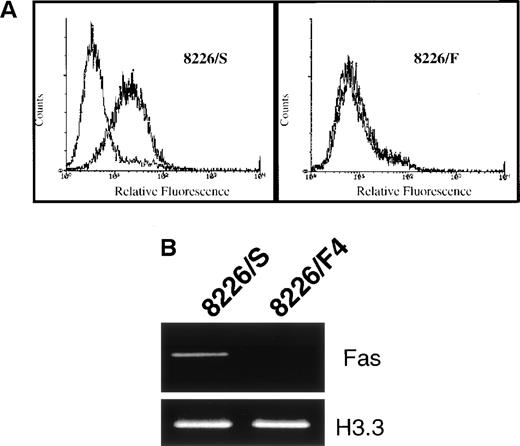
To identify the mechanism of CD95 resistance, we first examined the 8226/F4 cell line for surface expression of the CD95 receptor. These cells were stained with the nonapoptosis-inducing anti-CD95 MoAb UB2 and analyzed by flow cytometry. In contrast to the parental 8226/S cell line, which displays relatively high expression of the CD95 receptor, cell-surface expression of the CD95 receptor was entirely negative in the 8226/F4 cell line (Fig 2A). Using primers derived from the cytoplasmic region of the CD95 receptor, mRNA expression was examined by RT-PCR. Only very minimal levels of CD95 mRNA could be detected with 30 cycles of amplification (Fig 2B). Examination of seven clones derived from the 8226/F4 cell line showed similar results, with negligible CD95 expression and function in all cases (data not shown).
(A) Surface expression of the CD95 antigen in cells selected for resistance to CD95-mediated apoptosis. Cells were stained with the non–apoptosis-inducing antibody UB2 and analyzed by flow cytometry. In the 8226/F4 cell line, the UB2-specific staining completely overlaps the isotype control. (B) RT-PCR of CD95 antigen mRNA expression. Total RNA was extracted from 8226/S and 8226/F4 cells, and the CD95 antigen expression determined by RT-PCR as previously described. Histone 3.3 was amplified as a control for RNA integrity and equal gel loading.
(A) Surface expression of the CD95 antigen in cells selected for resistance to CD95-mediated apoptosis. Cells were stained with the non–apoptosis-inducing antibody UB2 and analyzed by flow cytometry. In the 8226/F4 cell line, the UB2-specific staining completely overlaps the isotype control. (B) RT-PCR of CD95 antigen mRNA expression. Total RNA was extracted from 8226/S and 8226/F4 cells, and the CD95 antigen expression determined by RT-PCR as previously described. Histone 3.3 was amplified as a control for RNA integrity and equal gel loading.
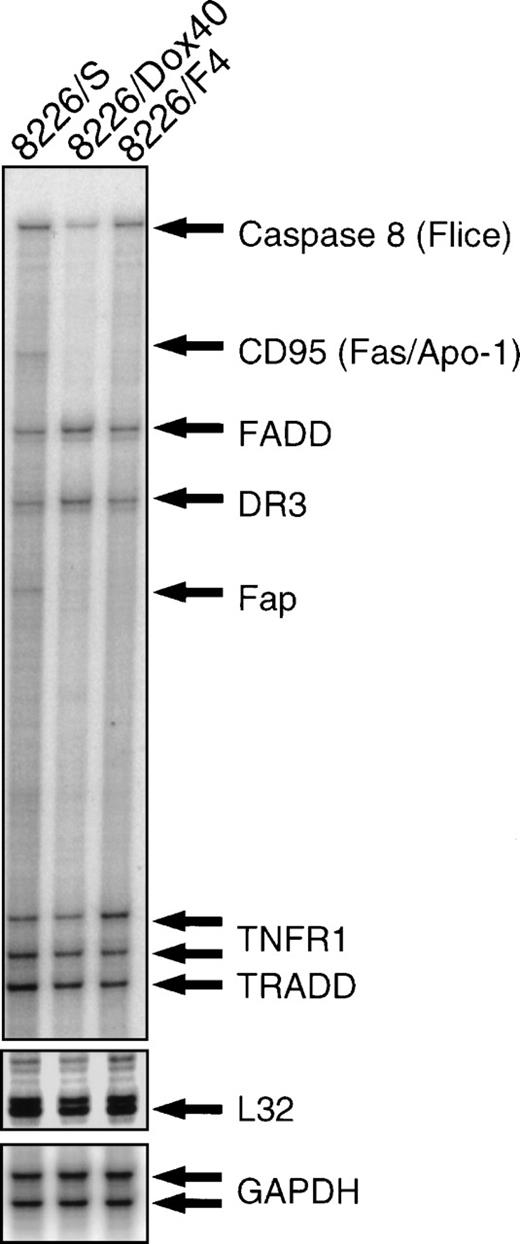
To examine the possibility that selection for CD95 resistance altered other components of the CD95 apoptotic pathway, we used an RNase protection assay with a multiprobe template for the expression of known effectors (Fig 3). This template includes the constitutive genes GAPDH and L32 for relative quantitation of protected RNA hybrids. Analysis of CD95 receptor mRNA by RNase protection correlated with RT-PCR and flow cytometry (see Fig 2), showing high expression in the parental 8226/S cell line, very low expression in the doxorubicin-resistant 8226/Dox40, and no CD95 receptor mRNA detected in the CD95-resistant 8226/F4. Comparison of the downstream mediators of CD95-mediated apoptosis demonstrates 30% and 37% reduction in the level of caspase 8 (FLICE/MACH/Mch5), in 8226/F4, and 8226/Dox40, respectively, compared with the parental 8226/S. Additionally, expression of the CD95-associated phosphatase FAP-1 is reduced in the cell lines with reduced CD95 expression. FAP-1 is reported to be constitutively associated with the cytoplasmic region of the CD95 receptor.26 The CD95-deficient cell lines 8226/F4 and 8226/Dox40 fail to express FAP-1, implying a potential coregulation of FAP with the CD95 receptor. However, this possibility remains to be investigated. No significant differences were found in the expression levels of the adapter proteins FADD and TRADD. Other death receptors, including TNFRI and DR3, are equally expressed in all three cell lines. Although the multiprobe template used in this study contains a CD95 ligand fragment, we were not able to detect CD95 ligand mRNA by RNase protection assay. Therefore, we examined CD95 ligand expression by RT-PCR using previously published conditions.21 All cell lines expressed low constitutive levels of CD95 ligand, which were likely below the limits of detection of the RNase protection assay. No significant differences in CD95 ligand expression were seen in any of the cell lines (data not shown).
RNase protection assay. Total RNA was isolated from 8226/S, 8226/F4, and 8226/Dox40 cell lines and hybridized with a multitemplate probe before digestion by RNase. Protected fragments were separated on a denaturing acrylamide gel and analyzed by PhosphorImage analysis. Fragment assignment was determined by migration distance relative to unprotected standards.
RNase protection assay. Total RNA was isolated from 8226/S, 8226/F4, and 8226/Dox40 cell lines and hybridized with a multitemplate probe before digestion by RNase. Protected fragments were separated on a denaturing acrylamide gel and analyzed by PhosphorImage analysis. Fragment assignment was determined by migration distance relative to unprotected standards.
Cytotoxicity analysis.
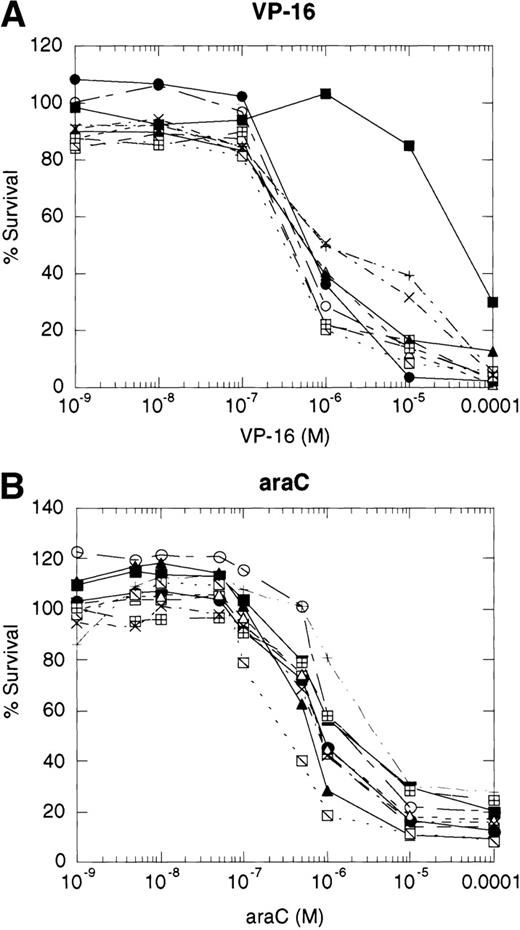
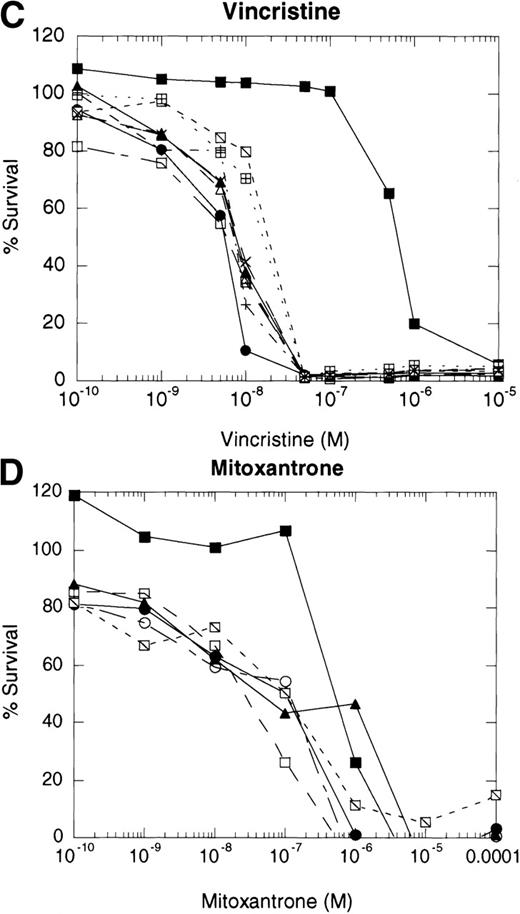
If cytotoxic drugs initiate cell death via CD95 receptor/ligand interactions, then we would predict that the CD95 receptor negative cell line, 8226/F4, would display resistance to cytotoxic agents as compared with the parental cell line, 8226/S. Using the MTT dye reduction assay, we examined the cytotoxicity of doxorubicin, mitoxantrone, Ara-C, vincristine, and VP-16 in 8226/F4 versus 8226/S. The P-glycoprotein–expressing cell line 8226/Dox40 was included as a control for multidrug resistance.25 Drug sensitivity profiles for the 8226/F4 cell line were virtually identical to the parental cell line 8226/S for all agents tested (Fig 4). Additionally, individual clones of the 8226/F4 cell line were equally sensitive, despite the absence of CD95 receptor expression in all cell lines examined. This response was highly reproducible over a wide range of drug concentrations. In contrast, 8226/Dox40, an MDR1-positive cell line, is cross-resistant to all agents except Ara-C, as previously described.17 25 Because the MTT dye reduction assay does not directly demonstrate apoptotic cell death, 8226/S and 8226/F4 cells were treated with 10−5 mol/L VP-16 for 24 hours, and flow cytometry used to analyze for TdT labeling. Again, there was no difference in the degree of DNA fragmentation in the CD95-positive cell ine, 8226/S, compared with the CD95-negative cell line, 8226/F4 (data not shown).
Dose-response profiles of 8226 myeloma cells selected for resistance to CD95-mediated apoptosis. Cells were plated in 96-well plates at 5 × 104/mL with the indicated concentrations of chemotherapeutic drugs. Percent survival was determined by MTT dye reduction after 96 hours of drug exposure, and the IC50calculated by linear regression analysis. Each drug was examined by three independent assays with eight replicates per assay. (A) VP-16; (B) ara-C; (C) vincristine; (D) mitoxantrone; (E) doxorubicin; (F) doxorubicin ± ZB4. (•), 8226/S; (▪), 8226/Dox40; (▴), 8226/F4. For plots A, B, C, D, and E, open symbols represent individual clones of 8226/F4. In plot F, open symbols show the effects of 1 hour of preincubation with 500 μg/mL ZB4 on doxorubicin cytotoxicity or CH-11–mediated apoptosis (inset).
Dose-response profiles of 8226 myeloma cells selected for resistance to CD95-mediated apoptosis. Cells were plated in 96-well plates at 5 × 104/mL with the indicated concentrations of chemotherapeutic drugs. Percent survival was determined by MTT dye reduction after 96 hours of drug exposure, and the IC50calculated by linear regression analysis. Each drug was examined by three independent assays with eight replicates per assay. (A) VP-16; (B) ara-C; (C) vincristine; (D) mitoxantrone; (E) doxorubicin; (F) doxorubicin ± ZB4. (•), 8226/S; (▪), 8226/Dox40; (▴), 8226/F4. For plots A, B, C, D, and E, open symbols represent individual clones of 8226/F4. In plot F, open symbols show the effects of 1 hour of preincubation with 500 μg/mL ZB4 on doxorubicin cytotoxicity or CH-11–mediated apoptosis (inset).
Previous reports have shown that antibodies which block CD95 receptor/ligand interactions inhibit drug cytotoxicity.8 9Therefore, we preincubated the parental 8226/S and four subclones of the 8226/F4 cell lines with the CD95 receptor-blocking antibody ZB4 for 1 hour before the addition of doxorubicin or the CD95-agonist MoAb, CH-11, in the MTT assay. Inactivation of the CD95 receptor by 0.5 μg/mL ZB4 did not inhibit the cytotoxicity of doxorubicin in either the 8226/S or the 8226/F4 cell lines. In contrast, apoptosis mediated by the CD95-agonist MoAb CH-11 is almost completely inhibited by this concentration of ZB4 (Fig 4). The inability of ZB4 to inhibit drug-induced cell death further supports the conclusions that CD95/CD95 ligand interactions do not participate in the apoptotic pathway initiated by cytotoxic drugs.
CD95 expression does not enhance chemosensitivity in K562 cells.
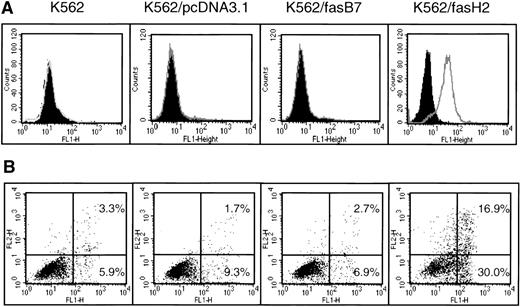
Because the K562 cell line expresses no CD95, and displays a relatively high level of intrinsic resistance to chemotherapeutic drugs, we were interested in determining if CD95 expression would enhance the efficacy of cytotoxic drugs. Using RT-PCR, we isolated full-length CD95 mRNA from normal peripheral blood lymphocytes (PBLs), inserted it into the pcDNA3.1 expression vector, and transfected K562 cells. After selection with G418, transfectants were cloned by limiting dilution and analyzed for CD95 expression and function (Fig 5). Data are shown for K562/fasH2, which expresses high levels of CD95; and K562/fasB7, which expresses minimally detectable levels of CD95 on the cell surface. Two additional clones with high CD95 expression and two clones with low or negative CD95 expression were analyzed, and found to correlate as well. Exposure to 500 ng/mL CD95 agonistic antibody, CH-11, induced no significant cell death in the parental K562 cell line, or cells transfected with the empty vector, pcDNA3.1. In contrast, clone K562/fasH2, which expresses high levels of CD95, showed 47% apoptosis after 24 hours of exposure to CH-11. Clone K562/fasB7, which expresses minimal levels of CD95, demonstrated apotosis equivalent to background cell death. In all clones examined, the degree of CH-11–induced cell death correlated directly with the level of CD95 surface expression.
Expression and function of CD95 in K562-transfected cell lines. K562 cells were transfected with pcDNAfas, selected with G418, and cloned by limiting dilution. (A) Surface expression of CD95 detection by staining with UB2-FITC or IgG1-FITC isotype control, and flow cytometry analysis. Filled peaks represent isotype control. (B) Annexin V-FITC analysis of CH-11–mediated apoptosis. Cells were treated with 500 ng/mL CH-11 anti-CD95 antibody for 24 hours followed by Annexin-V staining and flow cytometric analysis of programmed cell death. Horizontal axis is Annexin V-FITC, y-axis is propidium iodide staining. Background levels of apoptosis with mouse IgM was determined in an identical manner, and found to range from 2% to 12% in all cell lines.
Expression and function of CD95 in K562-transfected cell lines. K562 cells were transfected with pcDNAfas, selected with G418, and cloned by limiting dilution. (A) Surface expression of CD95 detection by staining with UB2-FITC or IgG1-FITC isotype control, and flow cytometry analysis. Filled peaks represent isotype control. (B) Annexin V-FITC analysis of CH-11–mediated apoptosis. Cells were treated with 500 ng/mL CH-11 anti-CD95 antibody for 24 hours followed by Annexin-V staining and flow cytometric analysis of programmed cell death. Horizontal axis is Annexin V-FITC, y-axis is propidium iodide staining. Background levels of apoptosis with mouse IgM was determined in an identical manner, and found to range from 2% to 12% in all cell lines.
Using both MTT and Annexin V analyses, the CD95-expressing K562 clones were further examined for differential sensitivity to doxorubicin, melphalan, or etoposide. Linear regression analysis of 96-hour MTT dose-response curves showed no significant differences in the IC50 values for all drugs tested comparing the CD95-expressing clones to untransfected or empty vector control cell lines (Table 1). Cell death after exposure to VP-16 was significantly delayed in all of the K562-derived cell lines compared with the 8226 myeloma-derived cell line. However, again, no significant differences were seen in the level of VP-16–induced apoptosis as measured by Annexin V staining in CD95 transfected cell lines as compared with the K562 parental cell line, or K562/pcDNA3.1 (data not shown).
Mean IC50 Concentrations of Cytotoxic Drugs in K562- and CD95-Transfected Cell Lines
| . | Doxorubicin . | VP-16 . | Melphalan . | |||
|---|---|---|---|---|---|---|
| IC50 . | Range . | IC50 . | Range . | IC50 . | Range . | |
| K562 (untransfected) | 1.02 × 10−8 | 3.23 × 10−9-1.72 × 10−8 | 7.86 × 10−7 | 1.80 × 10−7-1.39 × 10−6 | 5.37 × 10−6 | 3.72 × 10−6-7.02 × 10−6 |
| K562/fasH2 (high CD95) | 9.27 × 10−9 | 2.96 × 10−9-1.56 × 10−8 | 7.91 × 10−7 | 6.9 × 10−7-8.93 × 10−7 | 8.73 × 10−6 | 6.08 × 10−6-1.14 × 10−5 |
| K562/fasB7 (low CD95) | 2.37 × 10−8 | 1.61 × 10−8-3.13 × 10−8 | 6.27 × 10−7 | 1.74 × 10−7-1.08 × 10−6 | 1.22 × 10−6 | 8.36 × 10−6-1.60 × 10−5 |
| . | Doxorubicin . | VP-16 . | Melphalan . | |||
|---|---|---|---|---|---|---|
| IC50 . | Range . | IC50 . | Range . | IC50 . | Range . | |
| K562 (untransfected) | 1.02 × 10−8 | 3.23 × 10−9-1.72 × 10−8 | 7.86 × 10−7 | 1.80 × 10−7-1.39 × 10−6 | 5.37 × 10−6 | 3.72 × 10−6-7.02 × 10−6 |
| K562/fasH2 (high CD95) | 9.27 × 10−9 | 2.96 × 10−9-1.56 × 10−8 | 7.91 × 10−7 | 6.9 × 10−7-8.93 × 10−7 | 8.73 × 10−6 | 6.08 × 10−6-1.14 × 10−5 |
| K562/fasB7 (low CD95) | 2.37 × 10−8 | 1.61 × 10−8-3.13 × 10−8 | 6.27 × 10−7 | 1.74 × 10−7-1.08 × 10−6 | 1.22 × 10−6 | 8.36 × 10−6-1.60 × 10−5 |
Range = ±1 SD. Analysis using the paired Student’st-test showed no difference between groups in sensitivity to any drug (P < .05).
Analysis of caspase activity.
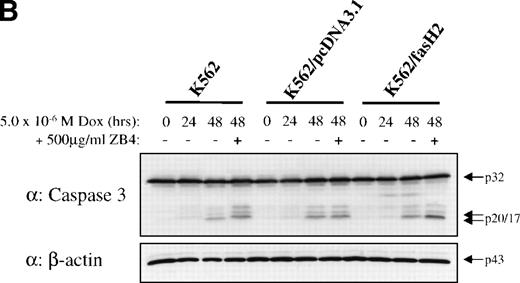
To establish that cytotoxic drugs used the caspase signal transduction cascade in these cells, 8226/S and 8226/F4 cells were exposed to 5 × 10−6 mol/L doxorubicin and assayed for activation of caspase 3 and caspase 8. Western blot analysis showed equivalent activation in the 8226/S and 8226/F4 cell lines with the appearance of catalytic fragments after 4 hours of doxorubicin exposure (Fig 6). As previously reported, caspase activation in the K562-derived cell lines was significantly delayed compared with 8226 myeloma cells.27 However, no differences were identified in the CD95-expressing cells as compared with the parental cell line or empty vector transfectants. These data are compatible with studies in many other cell lines showing that cytotoxic drugs use apoptotic pathways common to physiological stimuli. However, the lack of cross-resistance to chemotherapeutic drugs in cells that do not express CD95 indicates distinct pathways of caspase activation by CD95 cross-linking compared with activation by cytotoxic drugs.
Western blot analysis of caspase 3 and caspase 8 activation after doxorubicin treatment in 8226 and K562 cell lines. Cells were exposed to 5 × 10−6 mol/L doxorubicin for the indicated times, and cell lysates analyzed for caspase 3 and caspase 8 active subunits. (A) 8226/S and 8226/F4; (B) K562 untransfected, K562/pcDNA3.1 empty vector, and the CD95 high-expressing K562/fasH2. Although procaspase 8 can be detected in K562 cells, the active subunits are below the limits of detection at the time points examined. Data shown are representative of three independent experiments.
Western blot analysis of caspase 3 and caspase 8 activation after doxorubicin treatment in 8226 and K562 cell lines. Cells were exposed to 5 × 10−6 mol/L doxorubicin for the indicated times, and cell lysates analyzed for caspase 3 and caspase 8 active subunits. (A) 8226/S and 8226/F4; (B) K562 untransfected, K562/pcDNA3.1 empty vector, and the CD95 high-expressing K562/fasH2. Although procaspase 8 can be detected in K562 cells, the active subunits are below the limits of detection at the time points examined. Data shown are representative of three independent experiments.
DISCUSSION
Signal transduction pathways for cytotoxic drugs and physiological mediators of apoptosis have been shown to converge into a common final pathway.28 Thus, alterations in shared effectors could potentially result in cross-resistance between chemotherapeutic drugs and CD95-mediated apoptosis. We have previously shown that selection for resistance to chemotherapeutic drugs results in a coselection for resistance to CD95-induced apoptosis.17 In this report, we show that selection for resistance to CD95-mediated apoptosis does not coselect for resistance to chemotherapeutic drugs. Using the human myeloma cell line 8226, which has long served as a useful model for multidrug resistance, we selected a cell line resistant to CD95-mediated apoptosis, 8226/F4. Analysis of this cell line, and multiple subclonal populations derived from this cell line, showed no resistance to several cytotoxic agents as compared with the parental, CD95-sensitive cell line. Furthermore, no differences in the activation of distal effectors of apoptosis were observed between the CD95-sensitive and CD95-resistant cell lines when exposed to chemotherapeutic drugs. Thus, while selection for resistance to chemotherapeutic agents coselects for resistance to CD95-mediated apoptosis, we find that in the human myeloma cell line 8226, selection for resistance to CD95-mediated apoptosis does not select for resistance to chemotherapeutic drugs. In addition, constitutive expression of CD95 in the K562 cell line resulted in CD95-induced apoptosis, but no changes in the sensitivity to chemotherapeutic drugs. These data have important implications for current understanding of mechanisms contributing to cellular response to chemotherapeutic agents, and the drug-resistant phenotype.
Our data agree with that published by Eischen et al,14 who selected for CD95 resistance in Jurkat T-cell leukemia cells after pretreatment with mutagenic substances. Using immunoblotting and an affinity labeling technique to identify specific caspase activity, these investigators showed that the cytotoxic activity of etoposide, doxorubicin, topotecan, and cisplatin in CD95-resistant Jurkat cells is indistinguishable from that of the parental cell line. In their study, one CD95-resistant clone was reported to be negative for surface expression of the CD95 receptor, while four additional clones selected for CD95 resistance were reported to express normal levels of the CD95 receptor. Thus, it is likely that their protocol selected for alterations in signal transduction effector molecules that participate in CD95-mediated, but not drug-mediated, cell death.
Our selection protocol, which included no mutagenic pressure, resulted in a cell line that did not express the CD95 receptor. In addition, multiple subclones of the CD95-selected cell line were also negative for CD95 receptor. These results indicate that the mechanism of resistance to CD95-mediated apoptosis in the selected cells is directly related to the absence of receptor expression. To gain insight into mediators of apoptosis that may be differentially affected by selection for CD95 resistance versus drug resistance, we examined the expression of several known mediators of the apoptotic signal transduction cascade using an RNase protection assay. This assay showeded a slight reduction in caspase 8 mRNA expression in both CD95- and drug-resistant cell lines. Expression of the death-inducing signaling complex (DISC) components FADD and caspase 8 are not significantly changed in the 8226/F4 cell line, and the absence of CD95 receptor does not affect the activation of the proteolytic cascade in response to cytotoxic drugs. These data emphasize the existence of at least two distinct routes for caspase activation, and demonstrate that the point of convergence in the signaling pathways of drug- and CD95-mediated apoptosis is before the activation of caspase 3, but downstream of CD95 receptor/ligand interaction.
Several investigators have shown induced expression of the CD95 receptor and ligand by chemotherapeutic drugs.6,7,12,21This observation has led to the suggestion that the cytotoxicity of these agents is at least partially due to engagement of the CD95 receptor and initiation of the apoptotic cascade. However, attempts to provide direct evidence of receptor/ligand interactions in these studies have yielded conflicting reports. Kasibhatla et al21 showed a reduction of etoposide cytotoxicity in Jurkat T cells with the use of a recombinant CD95-Fc chimeric protein, which inhibits CD95 receptor/ligand interactions. Likewise, Fulda et al8 and Friesen et al6 reported that preincubation with Fab fragments of the anti-CD95 antibody APO-1 inhibited the cytotoxicity of doxorubicin, etoposide, and cisplatin. In contrast, Eischen et al14 and Villunger et al12showed no inhibitory effects of the non–cross-linking antibody ZB4, as we also found in this study. This discrepancy may be due to the particular reagents used for competition. The ZB4 and APO-1 antibodies are likely directed to different epitopes of the CD95 receptor, and one potential explanation for the disparate results reported in the literature may be related to the different MoAbs used in these studies.
A second possibility for the differential effects of CD95 inhibitory reagents in reducing drug cytotoxicity may be related to the particular cell lines analyzed. For example, Scaffidi et al29 recently proposed two classifications of tissues based on DISC formation and kinetics of caspase activation. In their study, the T-cell leukemias CEM and Jurkat were both classified as type II, based on their delayed induction of caspases 8 and 3 in response to cross-linking by anti-CD95 MoAb. In these cell lines, caspase activation and subsequent apoptosis were inhibited by forced overexpression of the anti-apoptotic proteins Bcl-2 or Bcl-XL, indicating that caspase 8 and caspase 3 activation occurs downstream of mitochondrial perturbation. In contrast, a variety of other cell types, including the B-cell line SKW6 and the T-lymphoma cell line H9 were classified as type I. Type I cells showed rapid DISC formation and direct activation of caspases 8 and 3 by CD95 agonist antibody. This activity could not be blocked by Bcl family members, indicating caspase 8 activity and commitment to apoptosis occurred independent of mitochondrial perturbation. These observations suggest two independent routes to caspase activation, one which is initiated by caspase 8 at the level of DISC formation before mitochondrial perturbation, and one which occurs downstream of the mitochondrial permeability transition and cytochrome c release. Our data suggest that cytotoxic drugs may use a DISC-independent route to caspase activation, similar to that identified in the type II cells. Further definition of these alternative pathways and identification of factors involved may be relevant to defining strategies to overcome the drug-resistant phenotype.
In our previous study, we showed that selection for resistance to the anthracenes doxorubicin or mitoxantrone coselects for resistance to CD95-mediated apoptosis. Although the primary mechanism of cross-resistance was found to be a reduction in the cell-surface expression of the CD95 receptor, some cell lines exhibited a reduced sensitivity to CD95 mediated without reduced receptor levels, indicating a potential alteration in downstream effectors. Similarly, a study published by Friesen et al11 demonstrated reduced expression of the CD95 receptor in CEM and SHEP neuroblastoma cells selected for resistance to doxorubicin. However, in contrast to their study, we did not find any cross-resistance to cytotoxic drugs in myeloma cell lines selected for resistance to CD95-mediated apoptosis, nor did we find chemosensitization in the cell lines engineered to express high levels of CD95. One potential explanation for the differences seen in these studies may be related to the particular cells used in each study and the type of stress response they display. For example, the K562 cell line expresses bcr-abl, which has been shown to confer resistance to apoptotic death in some cell types.18 In our hands, enforced expression of CD95 was sufficient to overcome the inhibitory effects of the Abl kinase and confer sensitivity to CD95-mediated apoptosis, but CD95 expression did not alter the response of the K562 cell lines to chemotherapeutic drugs. Therefore, using two isogenic cell lines with variable levels of CD95 expression, we have shown that the CD95 receptor is not required for drug cytotoxicity.
Because apoptosis is vital to the process of development and the maintenance of homeostasis, it is not surprising that multiple pathways exist to achieve a common end. For example, daunorubicin and CD95-mediated apoptosis have both been reported to induce apoptosis through a ceramide-activated ras signal transduction pathway.30,31 Likewise, Jun kinase (JNK), stress-activated kinase (SAPK), and extracellular signal-regulated kinases (ERK1 and 2) have also been implicated in signal transduction cascades leading to programmed cell death induced by drugs and physiological stimuli.32-35 Although many of these apoptotic signal transduction pathways appear to converge, the specific point of convergence and commonalities are not yet fully defined. Further definition of these mechanisms and their common effectors may contribute to the future development of strategies to enhance cytotoxicity of anticancer agents.
Submitted August 12, 1998; accepted March 1, 1999.
Supported in part by a grant from the National Cancer Institute, No. CA77859 (W.S.D.). T.H.L. is a Cure for Lymphoma Foundation Fellow.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to William S. Dalton, MD, PhD, Clinical Investigations Program, H. Lee Moffitt Cancer Center and Research Institute, University of South Florida, 12902 Magnolia Dr, Tampa FL 33612.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal