Abstract
To assess whether the progression of plasma cell tumors is accompanied by angiogenesis and secretion of matrix-degrading enzymes, bone marrow biopsy specimens from 20 patients with monoclonal gammopathy of undetermined significance (MGUS), 18 patients with nonactive multiple myeloma (MM), and 26 patients with active MM were evaluated for their angiogenic potential and matrix-metalloproteinase (MMP) production. A fivefold increase of the factor VIII+microvessel area was measured by a planimetric method of point counting in the bone marrow of patients with active MM as compared with nonactive MM and MGUS patients (P < .01). When serum-free conditioned media (CM) of plasma cells isolated from the bone marrow of each patient were tested in vivo for their angiogenic activity in the chick embryo chorioallantoic membrane (CAM) assay, the incidence of angiogenic samples was significantly higher (P< .01) in the active MM group (76%) compared with nonactive MM (33%) and MGUS (20%) groups. Moreover, a linear correlation (P < .01) was found between the extent of vascularization of the bone marrow of a given patient and the angiogenic activity exerted in the CAM assay by the plasma cells isolated from the same bone marrow. In vitro, a significantly higher fraction of the plasma cell CM samples from the active MM group stimulated human umbilical vein endothelial cell (HUVEC) proliferation (53%, P < .01), migration (42%, P < .05), and/or monocyte chemotaxis (38%,P < .05) when compared with nonactive MM and MGUS groups (ranging between 5% and 15% of the samples). Also, immunoassay of plasma cell extracts showed significantly higher (P < .01) levels of the angiogenic basic fibroblast growth factor (FGF)-2 in the active MM patients than in nonactive MM and MGUS patients (153 ± 59, 23 ± 17, and 31 ± 18 pg FGF-2/100 μg of protein, respectively). Accordingly, neutralizing anti–FGF-2 antibody caused a significant inhibition (ranging from 54% to 68%) of the biological activity exerted on cultured endothelial cells and in the CAM assay by plasma cell CM samples from active MM patients. Finally, in situ hybridization of bone marrow plasma cells and gelatin-zymography of their CM showed that active MM patients express significantly higher (P < .01) levels of MMP-2 mRNA and protein when compared with nonactive MM and MGUS patients, whereas MMP-9 expression was similar in all groups. Taken together, these findings indicate that the progression of plasma cell tumors is accompanied by an increase of bone marrow neovascularization. This is paralleled by an increased angiogenic and invasive potential of bone marrow plasma cells, which is dependent, at least in part, by FGF-2 and MMP-2 production. Induction of angiogenesis and secretion of MMPs by plasma cells in active disease may play a role in their medullary and extramedullary dissemination, raising the hypothesis that angiostatic/anti-MMP agents may be used for therapy of MM.
ANGIOGENESIS is a required step in the progression of tumor growth, invasion, and metastasis.1Progression also involves secretion of the extracellular matrix-degrading enzymes such as metalloproteinase-2 (MMP-2 or 72-kD type IV collagenase) and MMP-9 (92-kD type IV collagenase) by tumor cells.2 In human solid tumors such as colon, breast, lung carcinomas, and melanoma, angiogenesis and MMP-2/MMP-9 overexpression occur simultaneously during invasion and metastasis, but are downregulated or even absent in hyperplastic or normal tissue and in situ tumors.3-5 In contrast, little is known regarding angiogenesis in response to hematologic tumors. Angiogenesis correlates with plasma cell growth (S-phase fraction) in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM)6 and is associated with acute lymphoblastic leukemia7 and high-grade B-cell non-Hodgkin’s lymphomas.8 MMP-2 is activated by and MMP-9 is secreted by plasma cells in MM, potentially contributing to tumor invasion,9 whereas MMP-9 is secreted by some high-grade lymphomas, correlating with systemic spread and shorter survival.10
In this study, the extent of bone marrow angiogenesis was investigated in patients with MGUS, nonactive and active MM. The in vivo and in vitro angiogenic potential and the MMP-2/MMP-9 expression of bone marrow plasma cells isolated from the same patients were also assessed. The results showed increased bone marrow neovascularization during active MM paralleled by increased angiogenic potential and MMP-2 expression by the plasma cells isolated from active MM patients when compared with MGUS and nonactive MM patients.
MATERIALS AND METHODS
Patients and control subjects.
A total of 65 patients who fulfilled the South West Oncology Group (SWOG) diagnostic criteria for MM and MGUS11 were studied (Table 1). Myeloma patients were defined as active or nonactive, according to clinical features and M-component level.12 Active patients were those: (1) at diagnosis, with symptomatic disease and an increase in M-component level in the 3 months before analysis; (2) at relapse; (3) with unresponsive and rapidly progressive disease (leukemic progression), characterized by severe bone pain, hypercalcemia, and pancytopenia. Several patients at relapse or with leukemic progression displayed extramedullary localizations. Nonactive patients were those in: (1) posttreatment complete/objective response; (2) the off-treatment plateau phase. Control subjects were 12 patients with anemia due to iron or vitamin B12 deficiencies.
Patient Clinical and Immunologic Data
| Total no. | 64 |
| MM | 44 |
| Active | 26 |
| Average age (median, range) | 62 yr (64.5, 44-84) |
| Men/women | 16/10 |
| M-component IgG/IgA/IgD/κ or λ | 16/8/1/1 |
| Diagnosis | 10 |
| Stage I/II/III; A/B* | 1/1/8; 7/3 |
| Relapse† | 10 |
| Progression‡ | 6 |
| Nonactive | 18 |
| Average age (median, range) | 64 yr (68, 42-81) |
| Men/women | 12/6 |
| M-component IgG/IgA/κ or λ | 12/5/1 |
| Response | 11 |
| Plateau1-153 | 7 |
| MGUS | 20 |
| Average age (median, range) | 66 yr (63.5, 47-89) |
| Men/women | 12/8 |
| M-component IgG/IgA/IgM/κ or λ | 16/2/1/1 |
| Total no. | 64 |
| MM | 44 |
| Active | 26 |
| Average age (median, range) | 62 yr (64.5, 44-84) |
| Men/women | 16/10 |
| M-component IgG/IgA/IgD/κ or λ | 16/8/1/1 |
| Diagnosis | 10 |
| Stage I/II/III; A/B* | 1/1/8; 7/3 |
| Relapse† | 10 |
| Progression‡ | 6 |
| Nonactive | 18 |
| Average age (median, range) | 64 yr (68, 42-81) |
| Men/women | 12/6 |
| M-component IgG/IgA/κ or λ | 12/5/1 |
| Response | 11 |
| Plateau1-153 | 7 |
| MGUS | 20 |
| Average age (median, range) | 66 yr (63.5, 47-89) |
| Men/women | 12/8 |
| M-component IgG/IgA/IgM/κ or λ | 16/2/1/1 |
According to Durie and Salmon.11
Relapse defined as M-component increasing by >50% from the lowest value, or clinical and bone marrow relapse when the M-component did not reflect tumor load and disease activity. Three patients displayed extramedullary localizations: one, left kidney; one, retrobulbar tissue; one, blood.
Two patients displayed extramedullary localizations: one, skin; one, blood.
Plateau phase defined as posttreatment M-component decreasing by >50%, and lasting for at least 6 months without treatment.
The study was approved by the local ethics committee and all patients gave their informed consent.
Measurement of bone marrow angiogenesis.
Blood vessels were detected in 6-μm sections of 4% paraformaldehyde-fixed paraffin-embedded biopsy specimens by staining endothelial cells with the antifactor VIII murine monoclonal antibody (MoAb) M616 (IgG1; Dako, Glostrup, Denmark) and a three-layer biotin-avidin-peroxidase system described previously.13Megakaryocytes, very few in number, also stained by factor VIII, but were easily distinguishable by their morphology. Angiogenesis was measured as microvessel area without knowledge of the clinical diagnosis. Briefly, four to six 250× fields covering each of two sections per biopsy were examined with a superimposed 484-point square reticulum (12.5 × 10−2 mm2) for the presence of microvessels (capillaries and small venules). These were identified as endothelial cells, either single or clustered in nests or tubes, and clearly separated from one another, and either without or with a lumen (not exceeding 10 μm). A planimetric point count method14 with slight modifications for the computed image analysis (Leica Quantimet 500, Wetzlar, Germany) was applied to measure the microvessel area within the cellular area (reticulum area minus dense connective tissue, fat, bone lamellae, necrosis, and hemorrhage areas).13 Values are expressed as mean ± 1 standard deviation (SD) per patient, subgroups, and groups of patients.
Cell cultures and preparation of conditioned medium (CM).
Aspirates close to the biopsy site were subjected to Ficoll-Hypaque density gradient centrifugation and plasma cell enrichment. T cells were removed by twofold E-rosetting, monocytes and fibroblasts by adhesion determined by culturing in plastic flasks for 90 minutes in RPMI 1640 containing 10% fetal calf serum (FCS) at 37°C in 5% CO2 humidified atmosphere. Enriched plasma cells were then obtained by: (1) incubating residual cells with magnetic beads (Dynal, Oslo, Norway) coated with antibody to the plasma cell marker CD38 (Becton Dickinson, Mountain View, CA) for 30 minutes at +4°C; (2) magnetic subtraction; (3) bead detachment by culturing cells for 12 hours in RPMI-1640 supplemented with 10% FCS at 37°C in 5% CO2.13 Enriched plasma cells contained <2% of T cells and monocytes, as assessed by flow cytometry and with the anti-CD3 and anti-CD68 antibodies, respectively (FACScan; Becton Dickinson). They consisted of greater than 95% tumor plasma cells and their clonally related cells,15 or plasma cells in control subjects, as assessed by morphology in May-Grünwald-Giemsa and flow cytometry with the anti-CD38 antibody, or by immunocytochemical staining with anti-κ or anti-λ antibody (Dako) according to the light chain of the M-component. Cells of each patient and control subject were cultured (1 × 107 per 25-cm2flask) in RPMI-1640 medium (6 mL/flask) supplemented with 1% glutamine for 24 hours, and their viability assessed by trypan blue exclusion was greater than 90%. The CM was collected, sequentially centrifuged at 1,200 and 12,000 rpm for 10 minutes, respectively, filtered through sterilized 0.22-μm pore-size filters (Costar, Cambridge, MA), and stored at −80°C.
Human umbilical vein endothelial cells (HUVEC) prepared as described16 were grown in Petri dishes coated with 1% gelatin (Sigma Chemical Co, St Louis, MO) in M199 medium supplemented with 20% FCS, 0.02% bovine brain extract, and 0.01% porcine heparin (both from Sigma Chemical Co). A Kaposi’s sarcoma spindle cell line17 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS and 1% glutamine. Cells were harvested in trypsin/EDTA solution (0.05/0.02% in phosphate-buffered saline [PBS]), washed twice with PBS, and cultured (1 × 107 per 25-cm2 flask) in DMEM for 24 hours. Cell viability and collection of the CM were performed as described above.
HUVEC proliferation assay.
A total of 2.5 × 103 HUVEC (second passage) was plated in 96-well plates precoated with 1% gelatin. After 24 hours, the medium was removed and replaced on days 0, 2, and 4 in quadruplicate with complete fresh medium (positive control) or with starvation medium (containing only 2.5% FCS) supplemented 1:1 (vol:vol) with RPMI-1640 medium (negative control) or with the plasma cell CM. The cell number was estimated18 on day 6 by the crystal violet colorimetric method of Kueng et al19 with reading at 595 nm in the Microplate Reader Model 3550 (Bio-Rad Laboratories): the cell number was derived from a calibration curve set up with a known number of cells. Values were expressed as mean per sample.
HUVEC chemotaxis assay.
By using the Boyden chamber technique,20 200 μL of the plasma cell CM, of Kaposi CM as the positive control,17 or of 0.1% BSA in DMEM as the negative control (to evaluate random migration) were placed in triplicate in the lower compartment of the chamber (Costar), and 1.2 × 105 cells20in 400 μL of DMEM 0.1% BSA were seeded in the upper compartment. Compartments were separated by a 12-μm pore-size polycarbonate filter (polyvinylpyrrolidone-free; Costar) precoated with 0.1% gelatin. After 6 hours of incubation at 37°C, cells on the upper side of the filter were removed by scraping, whereas those that had migrated to the lower side were fixed in absolute ethanol, stained with toluidine blue (Merck, Darmstadt, Germany), and counted in 10 oil 400× immersion fields. Values were expressed as mean per sample.
Monocyte chemotaxis assay.
Monocytes were isolated from heparinized peripheral blood of healthy donors by centrifugation on Ficoll-Hypaque gradients. After NH4Cl lysis of residual erythrocytes, they were further purified by centrifugation on Percoll gradients (density = 1062) and resuspended in RPMI-1640 medium containing 0.1% BSA to a final concentration of 4 × 106 cells/mL. Chemotaxis was performed in a 48-microwell chamber consisting of two compartments (Costar) separated by a 5-μm pore-size polycarbonate filter (polyvinylpyrrolidone-free; Costar). The lower compartment was filled in sextuplicate with 27 μL of: (1) the plasma cell CM; (2) the RPMI-1640 medium/0.1% BSA as the negative control; (3) this medium supplemented with 10 nmol/L of the strong chemoattractant formylpeptide as the positive control.21 The upper compartment was filled with 2 × 105 cells in 50 μL. After 2 hours of incubation at 37°C in 5% CO2 humidified atmosphere, the cells on the lower surface of the filter were fixed, stained, and counted in the same way as in the HUVEC chemotaxis assay.
Chick embryo chorioallantoic membrane (CAM)-gelatin sponge assay.
CAM of fertilized White Leghorn chicken eggs was used.22 On day 3 of incubation, a square window was opened in the shell and 2 to 3 mL of albumin was removed to detach the developing CAM from the shell. On day 8, 3 μL of plasma cell CM was loaded onto 1 mm3gelatin sponges (Gelfoam; Upjohn Co, Kalamazoo, MI) that were then implanted on top of the CAM. Sponges loaded with RPMI-1640 medium alone or containing basic fibroblast growth factor (FGF-2) (200 ng/mL) were used as the negative and positive controls, respectively. The sponge trapped the sample, allowing a slow release of the products contained in the medium. CAM were examined daily until day 12, when the angiogenic response peaked,22 and photographed in ovo with a Zeiss SR stereomicroscope and the MC63 camera system (Zeiss, Oberkochen, Germany). To better highlight vessels, CAM were injected into the large allantoic vein with an India ink solution, fixed in Serra’s fluid, dehydrated in graded ethanols, and made transparent with methylbenzoate.23
Angiogenesis was measured in the sponges by a planimetric method22 similar to that applied for bone marrow biopsy specimens. Briefly, on day 12, the embryos and their membranes were fixed in ovo in Bouin’s fluid. Sponges and the underlying and immediately adjacent CAM portions were removed, embedded in paraffin, sectioned at 3 μm along a plane parallel to the CAM surface, and stained with 0.5% toluidine blue. Four to six 250× fields covering almost the whole of every third section within 30 serial sections of at least two sponges per patient were analyzed, and the microvessel area was calculated inside the reticulum test reference area as the mean ± 1 SD per patient, subgroups, and groups of patients.
Quantification of plasma cell FGF-2 and vascular endothelial growth factor (VEGF-A).
A total of 2 to 5 × 106 cells of each patient washed twice in PBS were sonicated in 1 mL of ice-cold PBS with three 15-second 60-W bursts (Labsonic 2000 U, B-Braun, Germany), and clarified by centrifugation at 12,000 rpm for 10 minutes at 4°C.24 For each sample, 100-μg proteins measured with the Bradford method (Bio-Rad Laboratories, Richmond, CA) were tested in duplicate for FGF-2 and VEGF-A concentrations by using the sandwich enzyme-linked immunosorbent assay (ELISA) (Quantikine Human FGF-2, Quantikine Human VEGF-A; R & D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
MMP-2 and MMP-9 sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) zymography.
Gelatin-zymography of plasma cell CM was performed to visualize the gelatinolytic activity of the secreted MMP-2 and MMP-9.25 A total of 10 μg of proteins from each CM were applied in duplicate to 7.5% SDS-PAGE gels copolymerized with type A gelatin from porcine skin (Sigma Chemical Co) at a final concentration of 0.6 mg/mL. After electrophoresis, gels were washed in 2.5% Triton 1× for 1 hour to remove SDS, incubated for 18 hours at 37°C, and stained in 0.1% Coomassie brilliant blue. The gelatinolytic regions were observed as white bands against a blue background. The levels of MMP activity were assessed by scoring the intensity of the bands by a computerized image analysis (APPLE, Cupertino, CA).
In situ hybridization of MMP-2 and MMP-9 mRNA.
This was performed as described previously.26Cytocentrifuged plasma cells (1 to 2 × 105 per slide) were fixed in 4% paraformaldehyde, washed in PBS, made permeable with 10 μg/mL of proteinase K (Sigma Chemical Co) in CaCl2 (2 mmol/L)-tris(hydroxymethyl)aminomethane (TRIS) (20 mmol/L) for 5 minutes at 37°C, acetylated with 0.25% acetate in 0.1 mol/L triethanolamine, washed in 2X standard saline citrate (SSC), dehydrated in graded ethanols and air-dried. Cells were hybridized overnight at 50°C with 5 μg/mL of two 5′-biotinylated oligonucleotides (Genenco Life Sciences, Florence, Italy), the first of 42 bases complementary to the ninth exon sequence 446-459 of the MMP-2 mRNA, the second of 48 bases complementary to the ninth exon sequence 445-460 of the MMP-9 mRNA.27 Fifty percent deionized formamide, 600 mmol/L NaCl, 80 mmol/L TRIS, 4 mmol/L EDTA, 10 mmol/L dithiothreitol, 1X Denhardt’s solution, and 100 μg/mL salmon sperm DNA were used as hybridization medium. After washing in 2X to 0.01X SSC and in PBS, cytospins were incubated overnight at 4°C with streptavidin-alkaline phosphatase conjugate (Promega Co, Madison, WI). After alkaline phosphatase activity was revealed by Western blue stabilized substrate (Promega Co), cytospins were mounted in buffered glycerin and evaluated by two observers with a double-headed Leitz Dialux 20 photomicroscope (Leitz, Wetzlar, Germany). Plasma cells were scored positive for the hybridization signal relative to the background signal of RNAse-treated control cytospins hybridized with the same oligonucleotides.
RESULTS
Bone marrow microvessel area.
Biopsy sections stained with antifactor VIII antibody were examined planimetrically to determine their microvessel area as normalized to the total cellular area. Table 2 shows that this area was much larger in patients with active MM than in those with nonactive MM, MGUS, or control subjects (virtually identical). Figure 1 shows that microvessels in active MM bone marrow were thin, winding, often without visible lumina, and that single, spread endothelial cells and small endothelial sprouts without lumen were evident, as opposed to straight vessels and no sprouts in nonactive MM, MGUS, and control subject bone marrow.
Bone Marrow Microvessel Area
| Patients/Status and Control Subjects (no.) . | Microvessel Area as mm2 × 10−2 Mean ± SD (range) . | Cellular Area as mm2 × 10−2 Mean ± SD (range) . |
|---|---|---|
| MM | ||
| Active (26) | 0.50 ± 0.23 (0.14-1.01)* | 11.2 ± 0.7 (9.3-12.2) |
| Diagnosis (10) | 0.34 ± 0.15 (0.14-0.62)* | 11.2 ± 0.6 (10.3-12.2) |
| Relapse (10) | 0.49 ± 0.17 (0.19-0.74)* | 11.0 ± 0.8 (9.3-12) |
| Progression (6) | 0.59 ± 0.32 (0.51-1.01)* | 11.4 ± 0.7 (10-12.1) |
| Nonactive (18) | 0.11 ± 0.02 (0.08-0.19) | 10.4 ± 0.7 (9.4-12) |
| Response (11) | 0.11 ± 0.03 (0.08-0.19) | 10.3 ± 0.7 (9.4-11.5) |
| Plateau (7) | 0.11 ± 0.01 (0.08-0.15) | 10.5 ± 0.8 (9.5-12) |
| MGUS (20) | 0.11 ± 0.02 (0.08-0.15) | 10.6 ± 0.7 (9.4-11.8) |
| Control subjects (12) | 0.08 ± 0.01 (0.07-0.09) | 10.3 ± 0.5 (9.1-10.5) |
| Patients/Status and Control Subjects (no.) . | Microvessel Area as mm2 × 10−2 Mean ± SD (range) . | Cellular Area as mm2 × 10−2 Mean ± SD (range) . |
|---|---|---|
| MM | ||
| Active (26) | 0.50 ± 0.23 (0.14-1.01)* | 11.2 ± 0.7 (9.3-12.2) |
| Diagnosis (10) | 0.34 ± 0.15 (0.14-0.62)* | 11.2 ± 0.6 (10.3-12.2) |
| Relapse (10) | 0.49 ± 0.17 (0.19-0.74)* | 11.0 ± 0.8 (9.3-12) |
| Progression (6) | 0.59 ± 0.32 (0.51-1.01)* | 11.4 ± 0.7 (10-12.1) |
| Nonactive (18) | 0.11 ± 0.02 (0.08-0.19) | 10.4 ± 0.7 (9.4-12) |
| Response (11) | 0.11 ± 0.03 (0.08-0.19) | 10.3 ± 0.7 (9.4-11.5) |
| Plateau (7) | 0.11 ± 0.01 (0.08-0.15) | 10.5 ± 0.8 (9.5-12) |
| MGUS (20) | 0.11 ± 0.02 (0.08-0.15) | 10.6 ± 0.7 (9.4-11.8) |
| Control subjects (12) | 0.08 ± 0.01 (0.07-0.09) | 10.3 ± 0.5 (9.1-10.5) |
P < .01 or lower versus nonactive myeloma as a group and subgroups (analysis of variance by Fisher and Kruskal-Wallis test followed by paired Duncan [t], Bonferroni [t], and Wilcoxon tests).
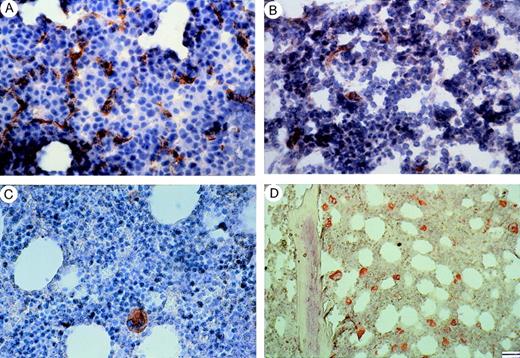
Staining with factor VIII of bone marrow from patients with (A) MM at relapse, (B) MM at plateau, (C) MGUS, and (D) a control subject (patient with pernicious anemia). Note in (A) numerous microvessels, whereas in (B), a microvessel and some rare endothelial cell clusters and in (C), (D) the lack of vessels in the presence of strongly stained megakaryocytes. Bar, (A) to (C) 40 μm; (D) 55 μm.
Staining with factor VIII of bone marrow from patients with (A) MM at relapse, (B) MM at plateau, (C) MGUS, and (D) a control subject (patient with pernicious anemia). Note in (A) numerous microvessels, whereas in (B), a microvessel and some rare endothelial cell clusters and in (C), (D) the lack of vessels in the presence of strongly stained megakaryocytes. Bar, (A) to (C) 40 μm; (D) 55 μm.
Angiogenic potential of bone marrow plasma cells.
The intense neovascularization in the bone marrow of active MM patients was followed by assessment of the angiogenic potential of their bone marrow plasma cells harvested in close proximity to the biopsy site. The CM of plasma cell cultures was evaluated in vivo to determine its ability to induce an angiogenic response in the chick embryo CAM-gelatin sponge assay.22 As shown in Table 3, plasma cell CM of 20/26 (76%) active MM patients induced a pronounced angiogenic response. This presented as a dense growth with numerous capillaries converging like spokes toward the sponge (Fig 2A and B). In contrast, only 33% and 20% of CM from nonactive MM and MGUS patients, respectively, induced the response and none from control subjects. Each CM was assayed on two to three eggs and gave similar results.
Angiogenic Response to the Plasma Cell CM Assessed by the CAM Gelatin Sponge Assay
| Sample . | No. of Samples3-150 . | Microvessel Area as mm2 × 10−2 Mean ± SD (range) . | Angiogenic Samples3-152 No. (%) . |
|---|---|---|---|
| Negative control medium3-151 | 4 | 0.53 ± 0.15 (0.25-0.75) | 0 |
| Positive control medium | 4 | 2.97 ± 1.24 (0.62-4.62) | 4 (100) |
| Plasma cell CM from active MM patients | |||
| Total | 26 | 2.48 ± 1.24 (0.5-4.07)3-153 | 20 (76)3-153 |
| Diagnosis | 10 | 2.74 ± 1.24 (0.5-4.07)3-153 | 8 (80)3-153 |
| Relapse | 10 | 2.11 ± 1.27 (0.5-3.87)3-153 | 7 (70)3-153 |
| Progression | 6 | 2.70 ± 1.08 (0.5-3.87)3-153 | 5 (83)3-153 |
| Plasma cell CM from nonactive MM patients | |||
| Total | 18 | 0.91 ± 0.61 (0.37-2.75) | 6 (33) |
| Response | 11 | 1.00 ± 0.69 (0.37-2.75) | 4 (36) |
| Plateau | 7 | 0.77 ± 0.44 (0.37-2.25) | 2 (28) |
| Plasma cell CM from MGUS patients | |||
| Total | 20 | 0.90 ± 0.81 (0.25-3.37) | 4 (20) |
| Sample . | No. of Samples3-150 . | Microvessel Area as mm2 × 10−2 Mean ± SD (range) . | Angiogenic Samples3-152 No. (%) . |
|---|---|---|---|
| Negative control medium3-151 | 4 | 0.53 ± 0.15 (0.25-0.75) | 0 |
| Positive control medium | 4 | 2.97 ± 1.24 (0.62-4.62) | 4 (100) |
| Plasma cell CM from active MM patients | |||
| Total | 26 | 2.48 ± 1.24 (0.5-4.07)3-153 | 20 (76)3-153 |
| Diagnosis | 10 | 2.74 ± 1.24 (0.5-4.07)3-153 | 8 (80)3-153 |
| Relapse | 10 | 2.11 ± 1.27 (0.5-3.87)3-153 | 7 (70)3-153 |
| Progression | 6 | 2.70 ± 1.08 (0.5-3.87)3-153 | 5 (83)3-153 |
| Plasma cell CM from nonactive MM patients | |||
| Total | 18 | 0.91 ± 0.61 (0.37-2.75) | 6 (33) |
| Response | 11 | 1.00 ± 0.69 (0.37-2.75) | 4 (36) |
| Plateau | 7 | 0.77 ± 0.44 (0.37-2.25) | 2 (28) |
| Plasma cell CM from MGUS patients | |||
| Total | 20 | 0.90 ± 0.81 (0.25-3.37) | 4 (20) |
Each tested on 2 to 3 eggs with similar results.
The negative and positive control media were RPMI-1640 medium alone or containing FGF-2 (200 μg/mL), respectively.
The angiogenic response was scored positive when the microvessel area was larger than the mean area plus 3 SD of the negative control medium.22
P < .01 or lower versus nonactive myeloma as a group and subgroups (analysis of variance by Fisher and Kruskal-Wallis test followed by Duncan [t], Bonferroni [t], and Wilcoxon paired tests).
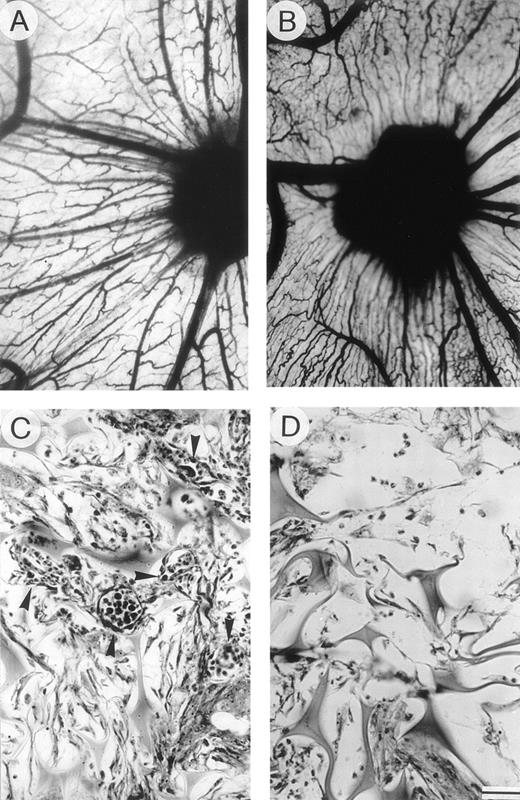
Angiogenic activity of plasma cell CM: chick embryo CAM-sponge assay. (A) The positive control (RPMI-1640+FGF-2) and (B) the CM of an active MM patient (progression) were loaded onto gelatin sponges implanted on top of the CAM on day 8. Macroscopic appearance of the CAM on day 12: note the presence of numerous blood vessels with a “spoked wheel” pattern around both sponges, highlighted by India ink injection. (C) Histologic section of the sponge sub (B), showing a collagenous matrix pierced by winding blood vessels (arrowheads) containing circulating cells and surrounded by a dense mononuclear cell infiltrate. (D) Histologic section of a sponge loaded with negative control medium (RPMI-1640) and devoid of vessels among the trabeculae. Bar, 3 mm in (A) and (B); 90 μm in (C) and (D).
Angiogenic activity of plasma cell CM: chick embryo CAM-sponge assay. (A) The positive control (RPMI-1640+FGF-2) and (B) the CM of an active MM patient (progression) were loaded onto gelatin sponges implanted on top of the CAM on day 8. Macroscopic appearance of the CAM on day 12: note the presence of numerous blood vessels with a “spoked wheel” pattern around both sponges, highlighted by India ink injection. (C) Histologic section of the sponge sub (B), showing a collagenous matrix pierced by winding blood vessels (arrowheads) containing circulating cells and surrounded by a dense mononuclear cell infiltrate. (D) Histologic section of a sponge loaded with negative control medium (RPMI-1640) and devoid of vessels among the trabeculae. Bar, 3 mm in (A) and (B); 90 μm in (C) and (D).
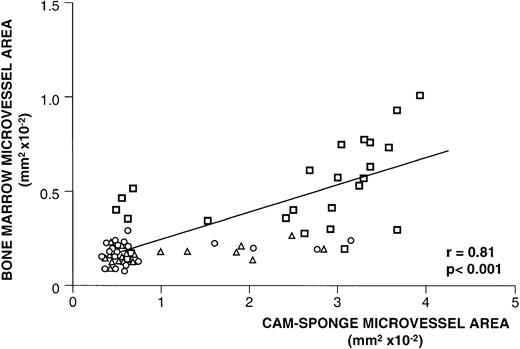
Planimetric quantification of the average microvessel area also showed the more intense neovascularization of the CAM loaded with CM of active MM patients (Table 3). As observed in their bone marrow, the angiogenic response elicited by their plasma cell CM consisted of thin, winding, and branching microvessels, either without or with a lumen containing circulating cells (Fig 2C). Interestingly, this angiogenic activity was always significantly correlated with the corresponding bone marrow microvessel area (Fig 3).
Relationship between bone marrow neovascularization and bone marrow plasma cell angiogenic activity. For each patient, bone marrow microvessel area was measured and plotted against the microvessel area measured in the CAM-sponge assay after loading of the CM of his/her plasma cells isolated close to the biopsy site. Each symbol (bold square, active MM; triangle, nonactive MM; circle, MGUS) corresponds to one patient. Significance of the regression analysis was calculated by the Pearson (r) test.
Relationship between bone marrow neovascularization and bone marrow plasma cell angiogenic activity. For each patient, bone marrow microvessel area was measured and plotted against the microvessel area measured in the CAM-sponge assay after loading of the CM of his/her plasma cells isolated close to the biopsy site. Each symbol (bold square, active MM; triangle, nonactive MM; circle, MGUS) corresponds to one patient. Significance of the regression analysis was calculated by the Pearson (r) test.
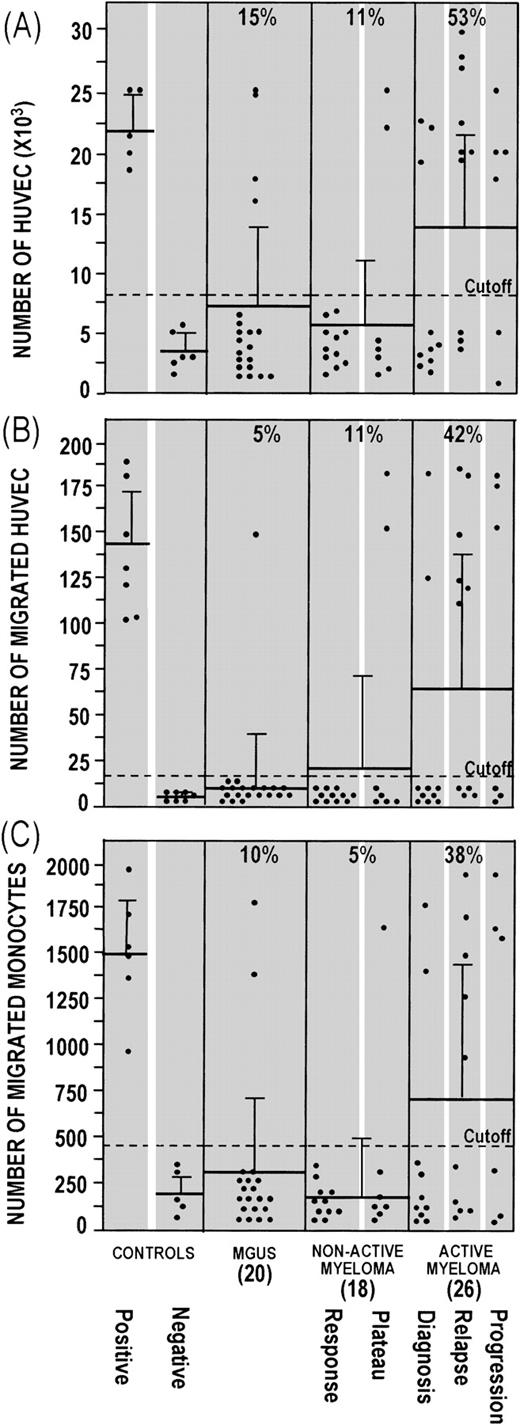
Furthermore, the plasma cell CM of active MM patients more strongly induced in vitro cell functions correlated with angiogenesis. CM of 14/26 (53%) active MM patients stimulated enhanced HUVEC proliferation (Fig 4A), as compared with CM of 2/18 (11%) nonactive MM and 3/20 (15%) MGUS patients (P< .01, χ2 Cochrane test), and a higher percentage (P < .05) of CM from active MM patients induced enhanced chemotaxis in both HUVEC (Fig 4B) and human monocytes (Fig4C). Plasma cell CM of control subjects overlapped the negative controls in all of the assays.
Effect of the CM of bone marrow plasma cells on (A) HUVEC proliferation, (B) HUVEC chemotaxis, and (C) human monocyte chemotaxis. In (A), low-density cultures of HUVEC (2.5 × 103 cells per well) were exposed on days 0, 2, and 4 with complete medium (positive control), starvation medium (negative control), and negative control medium supplemented 1:1 (vol/vol) with plasma cell CM. HUVEC were counted on day 6. Each dot represents the mean of four determinations for each CM or control. In (B), Kaposi cell CM (positive control), DMEM.1% BSA (negative control), and plasma cell CM were added to the lower compartment, and 1.2 × 105 HUVEC were placed in the upper compartment. Cells that migrated to the lower surface of a gelatin-coated filter separating the compartments were counted after 6 hours. Each dot is the mean of three determinations for each CM or control. In (C), a formylpeptide solution (positive control), RPMI-1640.1% BSA (negative control), and plasma cell CM were added to the lower compartment, and 2 × 105human monocytes were placed in the upper compartment. Cells that migrated to the lower surface of a polycarbonate filter were counted after 2 hours. Each dot is the mean of six determinations for each CM or control. In all assays, reproducibility was ≤±10% of the mean value of each CM. For all assays, the cutoff corresponds to the mean plus 3 SD of the negative control medium. The number of CM tested for each group of patients is given in brackets. The mean ± 1 SD is given for each group.
Effect of the CM of bone marrow plasma cells on (A) HUVEC proliferation, (B) HUVEC chemotaxis, and (C) human monocyte chemotaxis. In (A), low-density cultures of HUVEC (2.5 × 103 cells per well) were exposed on days 0, 2, and 4 with complete medium (positive control), starvation medium (negative control), and negative control medium supplemented 1:1 (vol/vol) with plasma cell CM. HUVEC were counted on day 6. Each dot represents the mean of four determinations for each CM or control. In (B), Kaposi cell CM (positive control), DMEM.1% BSA (negative control), and plasma cell CM were added to the lower compartment, and 1.2 × 105 HUVEC were placed in the upper compartment. Cells that migrated to the lower surface of a gelatin-coated filter separating the compartments were counted after 6 hours. Each dot is the mean of three determinations for each CM or control. In (C), a formylpeptide solution (positive control), RPMI-1640.1% BSA (negative control), and plasma cell CM were added to the lower compartment, and 2 × 105human monocytes were placed in the upper compartment. Cells that migrated to the lower surface of a polycarbonate filter were counted after 2 hours. Each dot is the mean of six determinations for each CM or control. In all assays, reproducibility was ≤±10% of the mean value of each CM. For all assays, the cutoff corresponds to the mean plus 3 SD of the negative control medium. The number of CM tested for each group of patients is given in brackets. The mean ± 1 SD is given for each group.
Seven samples from active MM patients induced all three responses compared with one from nonactive MM and none from MGUS patients. In addition, a small percentage of CM that induced neovascularization in the CAM assay failed to induce chemotaxis in HUVEC and/or human monocytes.
Plasma cell FGF-2 and FGF-2 antagonism.
FGF-2 is a potent angiogenic factor28 and stimulator of monocyte chemotaxis.29 Evaluation of its levels in plasma cell lysates by immunoassay showed that they were significantly higher (P < .01, Student’s t-test) in the plasma cells of active MM patients (153 ± 59 pg FGF-2/100 μg protein) compared with nonactive MM and MGUS patients (23 ± 17 and 31 ± 18 pg FGF-2/100 μg protein, respectively).
An assessment was therefore made of the effect of a neutralizing polyclonal anti–FGF-2 antibody (kindly provided by Dr D.B. Rifkin, New York University, New York, NY) on the CM samples from seven active MM patients that induced both endothelial cell and monocyte functions in vitro and angiogenesis in vivo in the CAM. The antibody (at 400 μg/mL) partly inhibited CM-induced HUVEC proliferation (from 22 ± 5 × 103 to 12 ± 7 × 103cells/dish, −46%), HUVEC chemotaxis (from 146 ± 33 to 65 ± 18 cells/filter, −56%), monocyte chemotaxis (from 1,473 ± 321 to 486 ± 175 cells/filter, −68%), and CAM neovascularization (from 2.72 ± 0.59 × 10−2to 1.27 ± 0.38 × 10−2 mm2 of microvessel area, −54%). Its inhibition of CM-induced CAM neovascularization is illustrated in Fig 5(see page 3067).
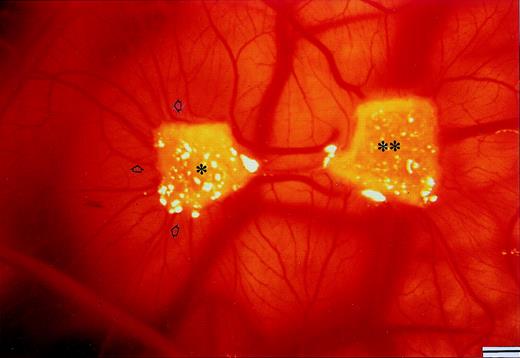
Macroscopic appearance of a CAM (day 12) implanted simultaneously at day 8 with a sponge loaded with the plasma cell CM of an active (progression) MM patient alone (*) and with a second sponge loaded with the same CM added with an anti–FGF-2 antibody (**). Note the angiogenesis toward the one-asterisk sponge (some neovessels are arrowheaded), and its inhibition by the anti–FGF-2 antibody. Bar, 2 mm.
Macroscopic appearance of a CAM (day 12) implanted simultaneously at day 8 with a sponge loaded with the plasma cell CM of an active (progression) MM patient alone (*) and with a second sponge loaded with the same CM added with an anti–FGF-2 antibody (**). Note the angiogenesis toward the one-asterisk sponge (some neovessels are arrowheaded), and its inhibition by the anti–FGF-2 antibody. Bar, 2 mm.
FGF-2 may thus play a role in plasma cell-mediated angiogenesis. When all patients were considered, however, there was no significant correlation between their individual plasma cell FGF-2 levels and bone marrow neovascularization, suggesting that other angiogenic factors may be involved. The angiogenic activity of VEGF-A can be excluded, as its levels were low in plasma cells from active MM patients (12 ± 9 pg VEGF-A/ 100 μg proteins), nonactive MM patients (19 ± 10 pg), and MGUS patients (21 ± 11 pg).
MMP-2 and MMP-9 production by bone marrow plasma cells.
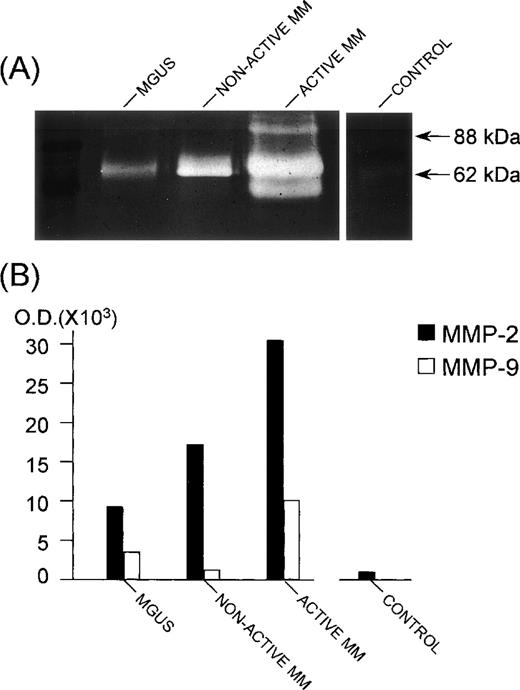
The invasive potential of bone marrow plasma cells during MM progression was assessed by SDS-PAGE gelatin-zymography of plasma cell CM. Bone marrow plasma cells from patients secrete activated (62-kD form) MMP-2 and lower levels of activated (88-kD form) MMP-9, whereas those from control subjects secrete only marginal levels of activated MMP-2 (Fig 6A). Accordingly, bone marrow plasma cells from patients expressed MMP-2 and MMP-9 mRNA, whereas in plasma cells from control subjects, the MMP-9 mRNA appeared to be absent and the MMP-2 mRNA to be either poorly expressed or absent, as evaluated by in situ hybridization (Fig 7). Quantification of secreted MMP-2 and MMP-9 was performed by computerized image analysis of the gelatinolytic bands, which showed that MMP-2 was present in plasma cell CM of 22/26 (84%) active MM patients at 22.5 ± 7.4 × 103 optical density (OD), compared with 5/18 (27%) nonactive MM patients at 12.4 ± 3.6 × 103 OD, 5/20 (25%) MGUS patients at 13.4 ± 5.4 × 103 OD, and 2/12 (16%) control subjects at 4.0 ± 1.7 × 103 OD (P < .01, χ2 Cochrane test). MMP-9 was cosecreted in 6 (23%) active MM, 3 (16%) nonactive MM, and 2 (10%) MGUS patients. Its levels, usually lower than MMP-2, overlapped between active MM (10.5 ± 2.7 × 103 OD), nonactive MM (10.0 ± 2.8 × 103 OD), and MGUS (9.0 ± 1.4 × 103 OD). The MMP-2 and MMP-9 quantification in representative patients and a control subject is shown in Fig 6B.
MMP-2 and MMP-9 secretion by bone marrow plasma cells. The patient with nonactive MM was in the plateau phase; the patient with active MM was at relapse; the control was a patient affected with anemia due to iron deficiency. (A) SDS-PAGE gelatin zymography of plasma cell CM samples. Note the white bands against a dark background with an apparent molecular weight of 62 kD and 88 kD, corresponding to the gelatinolytic regions of activated (cleaved) MMP-2 and MMP-9, respectively. Measurement of the intensity of the bands, as evaluated by computerized image analysis, is shown in (B). The assay was performed in duplicate for each CM. Reproducibility was ≤±20% of the mean intensity value. The lowest (third) band in the lane of the active MM patient represents the 54-kD cleaved form of MMP-2,2 which is sometimes present in the CM of both nonactive and active MM patients, but is not correlated with disease activity.
MMP-2 and MMP-9 secretion by bone marrow plasma cells. The patient with nonactive MM was in the plateau phase; the patient with active MM was at relapse; the control was a patient affected with anemia due to iron deficiency. (A) SDS-PAGE gelatin zymography of plasma cell CM samples. Note the white bands against a dark background with an apparent molecular weight of 62 kD and 88 kD, corresponding to the gelatinolytic regions of activated (cleaved) MMP-2 and MMP-9, respectively. Measurement of the intensity of the bands, as evaluated by computerized image analysis, is shown in (B). The assay was performed in duplicate for each CM. Reproducibility was ≤±20% of the mean intensity value. The lowest (third) band in the lane of the active MM patient represents the 54-kD cleaved form of MMP-2,2 which is sometimes present in the CM of both nonactive and active MM patients, but is not correlated with disease activity.
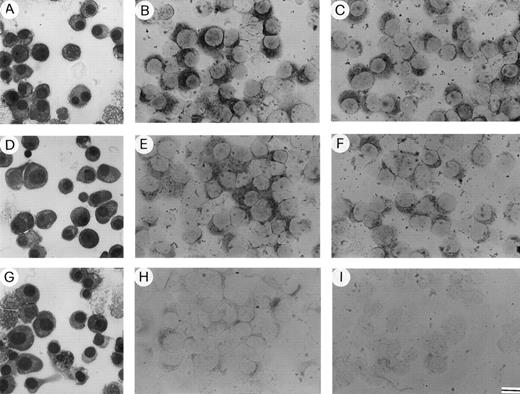
Enriched bone marrow plasma cells isolated from patients with (A) active (relapsing) MM, (D) nonactive (plateau) MM, and (G) MGUS stained with May-Grünwald-Giemsa. Expression of (B) MMP-2 and (C) MMP-9 mRNA by the plasma cells of the active MM patient; (E) MMP-2 and (F) MMP-9 mRNA by those of the nonactive MM patient; (H) MMP-2 mRNA by those of the MGUS patient; (I) MMP-2 mRNA by a control subject (a patient with pernicious anemia). Note the weaker MMP-2 expression in the nonactive MM patient, a weak expression in the MGUS patient, and no expression in the control. Bar, 22 μm in (A), (D), (G); 16 μm in (B), (C), (E), (F), (H), and (I).
Enriched bone marrow plasma cells isolated from patients with (A) active (relapsing) MM, (D) nonactive (plateau) MM, and (G) MGUS stained with May-Grünwald-Giemsa. Expression of (B) MMP-2 and (C) MMP-9 mRNA by the plasma cells of the active MM patient; (E) MMP-2 and (F) MMP-9 mRNA by those of the nonactive MM patient; (H) MMP-2 mRNA by those of the MGUS patient; (I) MMP-2 mRNA by a control subject (a patient with pernicious anemia). Note the weaker MMP-2 expression in the nonactive MM patient, a weak expression in the MGUS patient, and no expression in the control. Bar, 22 μm in (A), (D), (G); 16 μm in (B), (C), (E), (F), (H), and (I).
DISCUSSION
This study showed a significant increase of bone marrow angiogenesis (evaluated as microvessel area) in patients with active MM compared with nonactive MM and MGUS patients. Their bone marrow plasma cells also displayed a stronger angiogenic potential, as assessed by the ability of their CM to stimulate angiogenesis in the chick embryo CAM, cell proliferation of HUVEC, and HUVEC, and human monocyte chemotaxis. They also produced higher levels of angiogenic FGF-2 and of MMP-2. These properties of active MM plasma cells were associated with MM activity rather than with the tumor cell mass or burden, as very similar numbers of plasma cells were assayed for each patient of each group.
As the progression from in situ to invasive and metastatic solid tumors is accompanied and enhanced by the switch from the prevascular to the vascular phase,30 our findings suggest that active MM may represent the “vascular phase” of plasma cell tumors, and nonactive MM and MGUS the “prevascular phase.” Bone marrow angiogenesis may therefore be involved in progression from MGUS or nonactive MM to active MM.
The angiogenic activity exerted by the CM of plasma cells isolated from a given patient correlated significantly with the corresponding bone marrow microvessel area in all patients. Plasma cells may thus be a major source of angiogenic stimuli in the bone marrow microenvironment, although macrophages, T cells, and mast cells may also play a role. Plasma cell CM, in fact, stimulates endothelial cell proliferation and motility, functions required for vascular sprouting31 and monocyte chemotaxis, with the consequent recruitment of activated cells able to secrete a variety of angiogenic factors.32
It must be pointed out, however, that significant variability in the ability of plasma cell CM to exert a biological response on endothelium and/or monocytes was observed in the same experimental group. For instance, 76% of the plasma cell CM samples obtained from the 26 active MM patients induced an angiogenic response in the CAM assay, while 53%, 42%, and 38% induced HUVEC proliferation, HUVEC chemotaxis, or human monocyte chemotaxis. Thus, several CM samples were unable to exert a response in all of the assays, a characteristic shared by 7 of the 26 active MM samples tested. This may reflect differences in the sensitivity of our biological assays and/or the presence of different angiogenesis factors in the plasma cell CM of different patients.
Although none of the parameters tested may represent an independent prognostic factor, our data clearly indicate a significant increase of bone marrow plasma cell angiogenic potential in the cohort of active MM patients when compared with nonactive MM patients and MGUS patients. This is reflected by the increased neovascularization of the bone marrow in MM progression. Interestingly (data not shown), the plasma cell labeling index (LI), a progression marker12 studied in 45 patients, was found to be highly correlated with their bone marrow microvessel area (Pearson’s [r] = .83, P < .001). Indeed, an LI (%) of 3.43 ± 1.86 was associated with an area of 0.42 ± 0.21 mm2 × 10−2 in 19 patients with active MM, whereas LI (%)/area (mm2 × 10−2) of 0.57 ± 0.38/0.09 ± 0.03, and 0.36 ± 0.25/0.11 ± 0.02 matched in 12 nonactive MM and 14 MGUS patients, respectively. Conceivably, other progression markers, such as interleukin-6 (IL-6) and IL-1β,33 which also act as angiogenic factors,30-32 might be correlated with the bone marrow microvessel area.
Plasma cells isolated from the bone marrow of active MM patients produce higher levels of FGF-2, suggesting that this angiogenic factor plays a role in bone marrow neovascularization during MM progression. Interestingly, we have found that neutralizing anti–FGF-2 antibody causes a partial, but significant, decrease in the angiogenic activity exerted by the plasma cell CM in both the chick embryo CAM in vivo assay and in vitro assays. No significant correlation was observed, however, between FGF-2 levels in plasma cell extracts and bone marrow microvessel area, or between FGF-2 levels and the angiogenic potential exerted by plasma cell CM samples in vitro and/or in vivo. Taken together, these data suggest that other factors secreted by plasma cells able to induce angiogenesis directly and/or indirectly (for instance, via monocyte recruitment and activation), namely VEGF-A,34 tumor necrosis factor-α (TNF-α),35 macrophage-colony stimulating factor,36 IL-1β,37 and transforming growth factor-β (TGF-β),38 may act together with FGF-2. Further studies are required to characterize these factors. Preliminary observations have shown that production of VEGF-A by plasma cells does not increase significantly during MM progression (see above), although its ability to act in synergy with FGF-239,40 suggests that even if its levels remain fairly constant, increased FGF-2 production may result in a potent stimulation of bone marrow angiogenesis in active MM patients. The support of the human bone marrow microenvironment for the growth of both myeloma plasma cells and new vessels into the tumor mass has recently been shown.41
As observed for tumor cells from invasive and metastatic solid tumors,2 the bone marrow plasma cells of active MM patients express and secrete high levels of MMP-2. In some patients, sizable levels of MMP-9 were coexpressed and cosecreted. Both enzymes were present in the plasma cell CM in their cleaved, activated form,42 suggesting that they are rapidly cleaved after secretion, possibly by membrane-type (MT)-MMP,43 with which plasma cells are likely equipped. Yet, additional activators such as plasmin, kallicrein, trypsinlike serine proteinase, cathepsin G, and α-chymotrypsin,2 possibly carried by fetal calf serum, are unlikely to be involved because the CM contained no serum. Previous results showed a prominent production of activated MMP-9 in MM.9 Differences in the sensitivity of the detecting system may account for this discrepancy. Nevertheless, given the ability of both activated MMP-2 and MMP-9 to degrade types IV, V, VII, and X collagens, as well as fibronectin,2 the data suggest that plasma cells of active MM patients can invade interstitial stroma and the subendothelial basement membrane. Additional proof of the invasive capability will be the positive balance between one or both MMPs and their antagonists, or tissue inhibitors of MMPs. This is under ongoing study.
The increased bone marrow neovascularization, increased angiogenic and proteolytic potential of plasma cells, together with their poor adhesiveness to fibronectin (due to the lower expression of VLA-4 integrin),44 and their enhanced ability to adhere to the vascular endothelium and thereby extravasate (due to the high expression levels of LFA-1 and CD44)13 may explain the frequent occurrence of extramedullary localizations in active MM, eg, peripheral blood, skin, liver, lung, and kidney,45 as in five of our patients. Thus, combination therapy with angiostatic/protease inhibitor molecules plus cytolytic drugs previously shown to be effective in animal solid tumor models46 could perhaps be considered for clinical application in active MM.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro-A.I.R.C., Milan (Project Diagnosis and Prognosis in Clinical Oncology to F.D. and Special Project Angiogenesis to M.P.), and Ministry of Education-M.U.R.S.T., (Grants 40% 1997 to A.V. and M.P.), Rome, Italy. M.M. is the recipient of a fellowship from the Fondazione Italiana per la Ricerca sul Cancro (F.I.R.C.), Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Franco Dammacco, MD, Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine and Clinical Oncology, Policlinico, Piazza G. Cesare, 11, I-70124 Bari, Italy; e-mail: dimoclin@cimedoc.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal