Abstract
CD40 is present on B cells, monocytes, dendritic cells, and endothelial cells, as well as a variety of neoplastic cell types, including carcinomas. CD40 stimulation by an antibody has previously been demonstrated to induce activation-induced cell death in aggressive histology human B-cell lymphoma cell lines. Therefore, we wanted to assess the effects of a recombinant soluble human CD40 ligand (srhCD40L) on human breast carcinoma cell lines. Human breast carcinoma cell lines were examined for CD40 expression by flow cytometry. CD40 expression could be detected on several human breast cancer cell lines and this could be augmented with interferon-γ. The cell lines were then incubated with a srhCD40L to assess effects on in vitro growth. srhCD40L significantly inhibited the proliferation of the CD40+ human breast cancer cell lines. This inhibition could also be augmented with interferon-γ. Viability was also affected and this was shown to be due to increased apoptosis of the cell lines in response to the ligand. Treatment of tumor-bearing mice was then performed to assess the in vivo efficacy of the ligand. Treatment of tumor-bearing SCID mice with the ligand resulted in significant increases in survival. Thus, CD40 stimulation by its ligand directly inhibits human breast carcinoma cells in vitro and in vivo. These results suggest that srhCD40L may be of clinical use to inhibit human breast carcinoma growth.
CD40 IS A MEMBER of the tumor necrosis factor (TNF)/nerve growth factor (/NGF) receptor superfamily of molecules, which includes fas and CD30.1,2 CD40 has been demonstrated to be present on a variety of cell types ranging from B cells to dendritic and endothelial cells.3,4 CD40 has been shown to play a critical role in normal B-cell development and function.2,5 The ligand for CD40 (CD40L) is expressed predominantly on activated T cells.2,5 Interestingly, CD40 has also been demonstrated to be expressed at high levels on a variety of human carcinomas, including bladder, breast, and ovarian cancers, as well as melanomas.6 7 Little is known concerning the function of CD40 on these neoplastic cells.
We and others have previously shown that stimuli inducing activation of normal lymphocytes (eg, anti-CD3, IgM, etc) often can lead to death of transformed lymphocytes.8,9 This is postulated to occur by activation induced cell death, which may or may not involve apoptosis.8,9 We have shown that CD40 stimulation can promote normal B-cell growth and development yet inhibit neoplastic B-cell growth both in vitro and in vivo.10 11 Even though the role of CD40 on carcinoma cells remains largely speculative, its high-level expression on neoplastic epithelial cells may make it an attractive target for therapy. A recombinant soluble human CD40 ligand (srhCD40L) has been developed and we therefore wanted to evaluate its biologic effects on human breast carcinoma cells in vitro and, if effective, in vivo.
Breast cancer remains a significant cause of mortality among women. Current treatments include chemotherapy, hormone therapy, and bone marrow transplantation, but mortality rates remain high for women with advanced disease.12 The demonstration that CD40 is expressed at high levels on human carcinomas and not on corresponding normal epithelial tissue lends promise for the idea of using CD40 to target therapy to the tumor. Since CD40 is expressed on a variety of hematopoietically derived cells, the use of monoclonal antibodies (MoAbs) (either nonconjugated or conjugated with a toxin or radioisotope) directed towards CD40 could result in their depletion. If stimulation of CD40 by its ligand could be demonstrated to adversely affect neoplastic cell growth, the use of a recombinant soluble CD40 ligand would be an attractive alternative in that the normal CD40+ cells would be spared and in fact may be functionally stimulated. Additionally, use of soluble recombinant human ligand is least likely to induce potentially neutralizing antibodies that often occur when murine proteins are used clinically.
MATERIALS AND METHODS
Mice.
C.B-17 scid/scid mice with severe combined immune deficiency (SCID) were obtained from the Animal Production Area (National Cancer Institute-Frederick Cancer Research and Development Center [NCI-FCRDC], Frederick, MD) and were not used until 6 to 8 weeks of age. SCID mice were kept under specific-pathogen-free conditions at all times. The mice were housed in microisolator cages and all food, water, and bedding were autoclaved before use. SCID mice received trimethoprim/sulfamethoxazole (40 mg trimethoprim and 200 mg sulfamethoxazole per 320 mL drinking water) in suspension in their drinking water.
Antibodies, srhCD40L, and cytokines.
Anti-human CD40 (M3 hybridoma, a mouse IgG1 antibody) was purified and characterized as described.13 Normal mouse sera and mouse IgG1 myeloma proteins (msIgG1) used as control antibodies were purchased from Cappel (West Chester, PA). srhCD40L derived from Chinese hamster ovary (CHO) cells was manufactured by Immunex (Seattle, WA), and contains the extracellular domain of the CD40L fused to an amino-proximal modified leucine zipper motif.13Goat–anti-mouse IgG1 antibody (GamIgG1) used for cross-linking anti-CD40 was purchased from Southern Biotechnology Associates (Birmingham, AL). Anti-fas MoAb was obtained from Pharmingen (San Diego, CA). Recombinant human interferon-γ was obtained by the Repository at the NCI-FCRDC. For studies involving interferon-γ, 500 U/mL was used, either as a pretreatment in the surface expression studies or concurrently as in the proliferation/viability studies.
Breast cancer and normal mammary epithelial cell lines.
The human breast carcinoma line, MDA-231, was kindly provided by Dr Jack Pearson (NCI-FCRDC). MCF-7, BT-20, and T-47D were obtained from American Type Culture Collection (Rockville, MD). Normal mammary epithelial cells were obtained by Clonetics (BioWhittaker, Walkersville, MD) and were cultured in Mammary Epithelial Cell Growth Medium Bullet kit (MEGM-Bullet kit) also obtained by Clonetics. MDA-231 cells were isolated from a pleura effusion of a 51-year-old woman with poorly differentiated adenocarcinoma in 1973.14 BT-20 cells were isolated from original tumor of a 74-year-old woman with infiltrating ductal carcinoma in 1958.14 T-47D cells were isolated from a pleural effusion of a 54-year-old woman with infiltrating ductal carcinoma.14 The cell lines were maintained in monolayers cultures using RPMI-1640 medium (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), L-glutamine (BioWhittaker, Walkersville, MD), penicillin, and streptomycin (BioWhittaker, Walkersville, MD). The cells were incubated at 37°C in a humidified, 5% CO2 atmosphere and subcultured every 3 to 7 days. Only cultures in the log phase of growth were used.
Flow cytometric analysis and immunofluorescence studies.
Expression of CD40 on various human breast cancer cell lines was determined by flow cytometric analysis. The protocol for flow cytometric analysis has been described previously.11Briefly, the cells were washed and counted using Coulter counter (Coulter Electronic, Hialeah, FL). The cells were then adjusted to the appropriate cell concentration and were blocked with 2% human AB serum to prevent nonspecific binding of immunoglobulin. The cells were then incubated with the appropriate primary antibody of either anti-CD40 or an isotype-matched mouse IgG1 myeloma protein. The cells were then washed and incubated with a fluoresceinated (fluorescein isothiocyanate [FITC]) secondary antibody; a goat–anti-mouse IgG (kindly provided by Dr Kristin Komschilies, Science Applications International Corporation [SAIC] Frederick, Frederick, MD) or HLA-ABC from Olympus (Lake Success, NY) as control. After washing, the cells were fixed in 1% paraformaldehyde (Fluka Chemical, Ronkonkoma, NY) and analyzed on an EPICS flow cytometer (Coulter Electronic).
In vitro proliferation assay.
Proliferation was measured by using a microculture tetrazolium (MTT) assay as described by Carmichael et al15 or by3H-thymidine incorporation.10 Briefly, 3 × 103 to 1 × 104 cells were incubated in each well of 96-well flat-bottom microtiter plates (Corning, Corning, NY) in a volume of 200 μL containing various doses of reagents for up to 72 hours. After incubation, either 1 μCi of3H-thymidine (specific activity, 6.7 Ci/mmol; New England Nuclear Research Products, Boston, MA) or 100 μg of MTT (3-[4,5 dimethylthiozol-2-yl]-2,5-diphenyl tetrazolium bromide; Boehringer Mannheim, Mannheim, Germany) was added to each well at 37°C in a humidified, 5% CO2 atmosphere. After 4 hours of culture, 100 μL of 10% sodium dodecyl sulfate (SDS) in 0.01 mol/L HCl (Boehringer Mannheim) was added to solubilize the MTT formazan crystals. The spectrophotometric absorbance at 570 nm was determined using a scanning multiwell spectrophotometer (EI 900; Bio-Tec Instruments, Winooski, VT) or counts per minutes (cpm) was determined by liquid scintillation, and the surviving fraction of cells and the percentage growth inhibition were calculated. When no significant departure from linearity was detected, the regression of slopes for the control and treated samples were compared by t-test. Cell viability was assessed using the trypan blue exclusion method (Life Technology, Grand Island, NY). After culturing breast cancer cells for 24 to 72 hours in the presence of various amounts of anti-CD40, srhCD40L, or control MsIgG1, the cells were stained with the vital dye trypan blue and the number of dead cells, which do not exclude the trypan blue dye, was counted under a phase contrast microscope (CD2, Olympus, Tokyo, Japan). Each experiment was performed 3-4 times with a representative experiment being shown.
In vivo experiments.
All SCID mice received 20 μL of anti-asialo GM1 (anti-ASGM1; Wako Chemicals, Richmond, VA) in 0.2 mL phosphate-buffered saline (PBS) by intravenous (IV) injection 1 day before tumor transfer to remove host natural killer (NK) cells.11 One million MDA-231 breast cancer cells were then administrated by IV injection. SCID recipients then received either 10 μg of anti-CD40, 100 μg of srhCD40L, or isotype-matched control antibody in 0.2 mL Hanks’ balanced saline solution (HBSS) intraperitoneally (IP) every day for a total of five injections starting 2 days after tumor injection. Tumor-bearing mice were then monitored for tumor development and progression. Moribund mice were euthanized and all mice underwent necropsy for evidence of tumor. There were eight to 10 mice per group. Survival data were plotted by the Kaplan-Meier method and analyzed by the log-rank test. AP value ≤.05 was considered significant.
Immunohistology of primary tumors.
Sections cut from formalin-fixed and paraffin-embedded tissues were dewaxed by incubating in xylene for 10 minutes. Xylene was then removed with three changes of acetone and the tissue sections were rehydrated by incubating in 80%, 50%, and 20% acetone solutions for 1 minute each. Endogenous peroxidase activity was blocked by incubating the tissue sections in 3% hydrogen peroxide/methanol for 10 minutes. After rinsing under a running tap for 2 minutes, the tissues were immersed in 300 mL of citric acid buffer (pH 6.0) at room temperature and subjected to microwave irradiation at full power until boiling. The tissue sections were then subjected to a further 5 minutes of microwave irradiation. The citric acid buffer was then replenished and the tissue sections were subjected to another 5 minutes of microwave irradiation. The tissue sections were then cooled under a running tap, immersed in PBS, ringed with a Dako pen (Dako, Carpenteria, CA), and incubated with the rabbit polyclonal antibody N-16 (Santa Cruz Biotech, Santa Cruz, CA) at 1/160 PBS overnight in a humidified atmosphere. The tissue sections were then washed in PBS for 10 minutes at room temperature (RT); incubated with biotinylated goat–anti-rabbit (Dako) in 10% heat inactivated goat serum in PBS for 30 minutes at RT; washed in PBS for 10 minutes at RT incubated with biotinylated horse radish peroxidase (HRP)/streptavidin complex (Dako) 30 minutes at RT, and washed in PBS for 10 minutes at RT. Bound antibody was then visualized by incubation with a substrate solution containing 1 mg/mL diaminobenzidine (Sigma, St Louis, MO), 3% hydrogen peroxide/PBS for 10 minutes. Tissue sections were then lightly counterstained with hematoxylin and mounted with Immunomount (Shandon, Inc, Pittsburgh, PA).
Assays for assessment of apoptosis.
MDA-231 cells were grown in RPMI 1640 with 5% FBS as an adherent monolayer. The tumor cells were harvested in log-phase growth (705 to 80% confluent) by brief trypsinization and replated at 50,000 cells per well in 96-well flat-bottom tissue culture-treated microplate wells (Corning, Acton, MA) in the presence or absence of media alone or media containing srhCD40L at 3 μg/mL and cultured for an additional 18 hours. The cells were harvested by brief trypsinization and then were assessed for levels of apoptosis/necrosis by flow cytometry on a FACScan (Becton Dickinson, Sunnyvale, CA) using the Annexin V-FITC apoptosis kit according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Apoptosis was also assessed by examining nuclear matrix protein (NMP) levels in the culture supernatants of treated cells using an enzyme-linked immunosorbent assay (ELISA) kit (Oncogene Research Products, Cambridge, MA) according to the manufacturer’s instructions. The results are expressed as NMP units per milliliter of culture supernatant.
RESULTS
CD40 expression on human breast carcinoma cell lines and upregulation by interferon-γ.
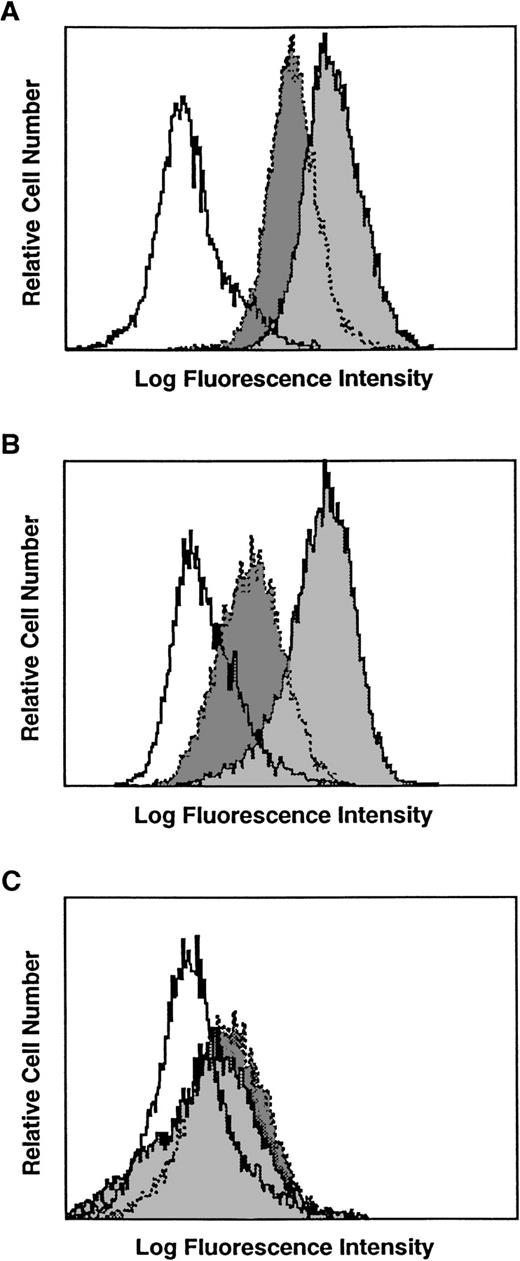
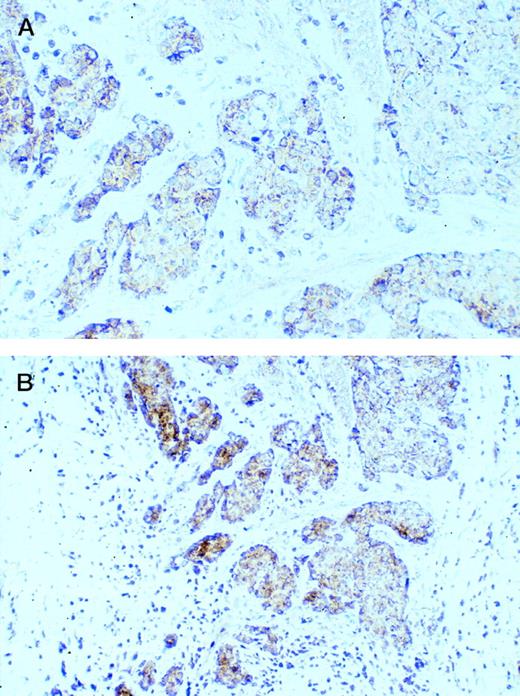
Several human breast carcinoma cell lines were examined for CD40 expression by flow cytometry. The MDA-231 and T-47D human breast carcinoma cell lines demonstrated surface expression of CD40 (Fig1A and B). Another common breast carcinoma line MCF-7 was negative for CD40 staining (data not shown). Normal mammary epithelial cells were only slightly positive for CD40 expression (Fig 1C). It has been reported that CD40 expression can be augmented with interferon-γ.6 16 We therefore cultured the breast cancer cell lines with interferon-γ for 12 hours before staining for CD40 expression. The results demonstrate that interferon-γ pretreatment of the cell lines resulted in an increase in CD40 expression (Fig 1A and B). Interestingly, normal mammary epithelial cells still expressed low levels of CD40 despite interferon-γ pretreatment (Fig 1C). These results indicate that human breast carcinoma cell lines can express CD40 and that CD40 expression can be increased by interferon-γ. We then examined primary human breast carcinomas for CD40 expression (Fig2). CD40 expression was detected in the majority of breast carcinoma cases examined (25 of 27 cases). CD40 staining of this ductal carcinoma was predominantly on the membrane of carcinoma cells with a weaker cytoplasmic component (Fig 2). Smaller CD40+ infiltrating lymphocytes, probably B cells, were also frequently observed. These results demonstrate that CD40 is expressed on breast carcinomas, both cell lines and primary tumors.
Expression of CD40 on various human breast carcinoma cell lines. White shade with solid line is the msIgG1 control staining. Dark gray with dotted lines is CD40 staining. Light gray shade with solid lines is CD40 staining after overnight incubation of the cells with 500 U/mL interferon-γ. (A) T-47D, (B) MDA-231, and (C) normal mammary epithelial cell lines.
Expression of CD40 on various human breast carcinoma cell lines. White shade with solid line is the msIgG1 control staining. Dark gray with dotted lines is CD40 staining. Light gray shade with solid lines is CD40 staining after overnight incubation of the cells with 500 U/mL interferon-γ. (A) T-47D, (B) MDA-231, and (C) normal mammary epithelial cell lines.
Expression of CD40 on vitually all tumor cells in a case of ductal carcinoma. Lower magnification (bottom) demonstrated that CD40 can also be observed in smaller infiltrating lymphocytes. Original magnification: top, ×400; bottom, ×200.
Expression of CD40 on vitually all tumor cells in a case of ductal carcinoma. Lower magnification (bottom) demonstrated that CD40 can also be observed in smaller infiltrating lymphocytes. Original magnification: top, ×400; bottom, ×200.
srhCD40L inhibits the proliferation of human breast carcinoma cell lines in vitro.
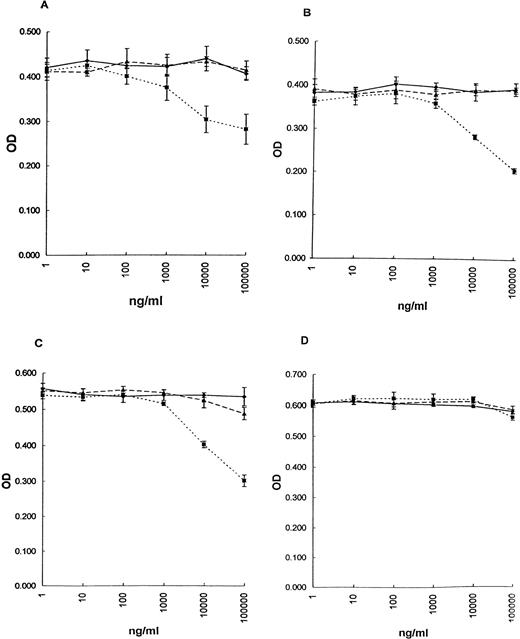
The effect of srhCD40L or an anti-CD40 MoAb on the growth of breast carcinoma cell lines was examined using an MTT assay or3H-thymidine incorporation. The results demonstrate that srhCD40L significantly inhibited the proliferation of all of the CD40+ cell lines tested (Fig 3A through C; MDA-231, BT-20, and T-47D respectively; and Table1), with an optimal inhibition occurring at human CD40L concentrations of 1 to 10 μg/mL. No effect of the ligand was observed with the CD40− line MCF-7 (Fig 3D and Table 1). Interestingly, in direct contrast to results previously reported with B-cell lymphomas, the anti-CD40 MoAb (M3 clone) did not appear capable of exerting significant inhibitory effects on the breast carcinoma cell lines (Fig 3).
Effect of srhCD40L on proliferation of breast carcinoma cell lines in vitro. After 72 hours, proliferation was assessed by MTT. (▩) srhCD40L, (▧) anti-CD40 MoAb, and (⧫) msIgG1 control. (A) MDA-231, (B) T-47D, (C) BT20, and (D) MCF-7 (CD40−) cell line. Incubation with either 1 or 10 μg/mL srhCD40L resulted in significant (P < .001) inhibition of proliferation of the 3 CD40+ lines (A, B, and C).
Effect of srhCD40L on proliferation of breast carcinoma cell lines in vitro. After 72 hours, proliferation was assessed by MTT. (▩) srhCD40L, (▧) anti-CD40 MoAb, and (⧫) msIgG1 control. (A) MDA-231, (B) T-47D, (C) BT20, and (D) MCF-7 (CD40−) cell line. Incubation with either 1 or 10 μg/mL srhCD40L resulted in significant (P < .001) inhibition of proliferation of the 3 CD40+ lines (A, B, and C).
Effect of srhCD40L on Proliferation and the Induction of Apoptosis of Human Breast Carcinoma Cell Lines
| Cell Line . | Treatment . | CPM (±SD) . | NMP (U/mL) . |
|---|---|---|---|
| MDA-231 (CD40+) | — | 14,567 (216) | <2 |
| srhCD40L (1 μg/mL) | 6,875 (158)† | 26* | |
| srhCD40L (1 μg/mL) | 9,778 (364)* | 18* | |
| Anti-fas (10 ng/mL) | 5,098 (206)† | 38* | |
| T47D (CD40+) | — | 37,658 (1,897) | 8 |
| srhCD40L (10 μg/mL) | 22,592 (2,025)* | 36* | |
| srhCD40L (1 μg/mL) | 27,798 (2,046)* | 27* | |
| Anti-fas (10 μg/mL) | 11,674 (1,923)† | 58* | |
| MCF-7 (CD40−) | — | 19,867 (1,216) | 0 |
| srhCD40L (10 μg/mL) | 18,976 (321) | 0 | |
| srhCD40L (1 μg/mL) | 20,981 (1,786) | 0 | |
| Anti-fas (10 μg/mL) | 7,930 (483)† | 37* |
| Cell Line . | Treatment . | CPM (±SD) . | NMP (U/mL) . |
|---|---|---|---|
| MDA-231 (CD40+) | — | 14,567 (216) | <2 |
| srhCD40L (1 μg/mL) | 6,875 (158)† | 26* | |
| srhCD40L (1 μg/mL) | 9,778 (364)* | 18* | |
| Anti-fas (10 ng/mL) | 5,098 (206)† | 38* | |
| T47D (CD40+) | — | 37,658 (1,897) | 8 |
| srhCD40L (10 μg/mL) | 22,592 (2,025)* | 36* | |
| srhCD40L (1 μg/mL) | 27,798 (2,046)* | 27* | |
| Anti-fas (10 μg/mL) | 11,674 (1,923)† | 58* | |
| MCF-7 (CD40−) | — | 19,867 (1,216) | 0 |
| srhCD40L (10 μg/mL) | 18,976 (321) | 0 | |
| srhCD40L (1 μg/mL) | 20,981 (1,786) | 0 | |
| Anti-fas (10 μg/mL) | 7,930 (483)† | 37* |
Cell lines were incubated for 72 hours with either srhCD40L or anti-fas as indicated in the Methods. Proliferation was assessed by 3H-thymidine incorporation and apoptosis was by NMP levels in the supernatants.
P > .05.
P > .01.
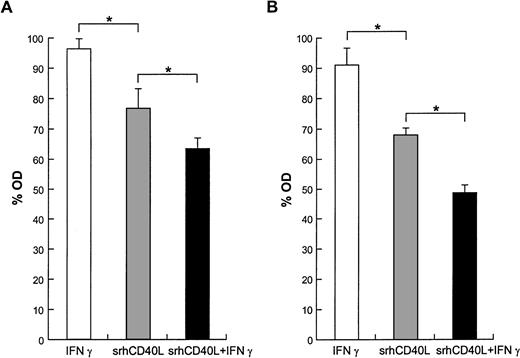
We have previously demonstrated that prior crosslinking of the antibody was necessary to produce optimal inhibition of lymphomas.10Crosslinking of the antibody had no effect on breast cancer cell line proliferation and, consistent with this, prior crosslinking of the ligand had no effect on the inhibition by the srhCD40L (data not shown). These results indicate that binding of CD40 by its ligand can result in direct antiproliferative effects on human breast carcinoma cell lines. Because interferon-γ can increase CD40 expression on the cell lines, we then examined whether interferon-γ incubation would result in greater growth inhibition of the cells. The results demonstrate that coincubation of the T-47D and MDA-231 cell lines with interferon-γ and srhCD40L resulted in greater inhibition of cell proliferation (Fig 4). Interferon-γ alone had no effect on viability of this dose (data not shown). Thus, interferon-γ can both increase CD40 expression and augment the growth inhibitory effects of srhCD40L with human breast carcinoma cell lines in vitro.
Effect of interferon-γ and srhCD40L on proliferation. In some wells, interferon-γ (500 U/mL) was added with srhCD40L (10 μg/mL) during the assay; 72 hours later, an MTT assay was performed. Values presented as percent control(msIgG1). (A) MDA-231 and (B) T-47D. Significant (P < .05) differences in proliferation were detected.
Effect of interferon-γ and srhCD40L on proliferation. In some wells, interferon-γ (500 U/mL) was added with srhCD40L (10 μg/mL) during the assay; 72 hours later, an MTT assay was performed. Values presented as percent control(msIgG1). (A) MDA-231 and (B) T-47D. Significant (P < .05) differences in proliferation were detected.
srhCD40L induces apoptosis in human breast carcinoma cell lines.
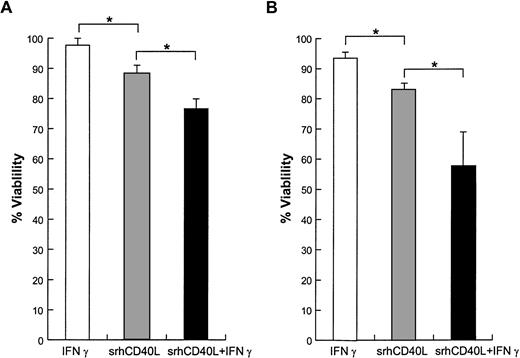
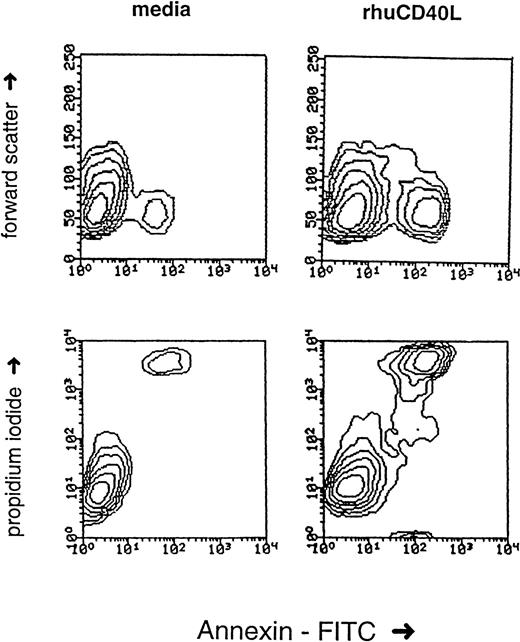
It has been shown that signals which cause the activation of normal cells can result in growth inhibition of transformed cells. This “activation-induced cell death” (AICD) has been extensively characterized on lymphocytes and has been shown to involve apoptosis, cell-cycle arrest, and necrosis.8 9 To ascertain the mechanism underlying the antiproliferative effects of srhCD40L, we examined the effects of the ligand on cell viability and apoptosis on human breast carcinoma cell lines. The results demonstrate that incubation of the T-47D and MDA-231 cell lines with srhCD40L resulted in a significant reduction of viable cells after 72 hours (Fig5). Coincubation with interferon-γ augmented this ligand-mediated decrease in cell viability. When the cells were assessed by flow cytometry for effects on apoptosis using Annexin-V dye binding, it was found that srhCD40L induced apoptosis and necrosis of the MDA-231 to a significant extent. The percentage of ligand-treated cells in the Annexin-V single-positive (indicative of apoptosis) quadrant increased to 5% and the amount in the Annexin-V/propidium iodide (PI) double-positive quadrant (indicative of apoptosis and necrosis) increased to 31% (Fig 6). NMP release, another indicator of apoptosis, was also assessed. It was found that srhCD40L induced NMP release comparable with an anti-fas MoAb (Table 1). Thus, the antiproliferative effects of the srhCD40L on human breast carcinoma cell lines are due, at least in part, to the induction of apoptosis and necrosis in the growth-inhibited cells.
Effect of srhCD40L on cell viability. (A) MDA-231 or (B) T-47D cells were incubated with 10 μg/mL srhCD40L and/or 500 U/mL interferon γ. After 72 hours, viability was assessed by trypan blue exclusion. Significant differences (P < .05) in viability were noted.
Effect of srhCD40L on cell viability. (A) MDA-231 or (B) T-47D cells were incubated with 10 μg/mL srhCD40L and/or 500 U/mL interferon γ. After 72 hours, viability was assessed by trypan blue exclusion. Significant differences (P < .05) in viability were noted.
Effect of srhCD40L on apoptosis. MDA-231 cells were cultured with 6 μg srhCD40L as described in Materials and Methods. Annexin /PI staining was then performed to assess apoptosis/necrosis 24 hours after treatment. No interferon-γ was present in this assay.
Effect of srhCD40L on apoptosis. MDA-231 cells were cultured with 6 μg srhCD40L as described in Materials and Methods. Annexin /PI staining was then performed to assess apoptosis/necrosis 24 hours after treatment. No interferon-γ was present in this assay.
srhCD40L exerts antitumor effects in SCID mice bearing human breast carcinoma cells.
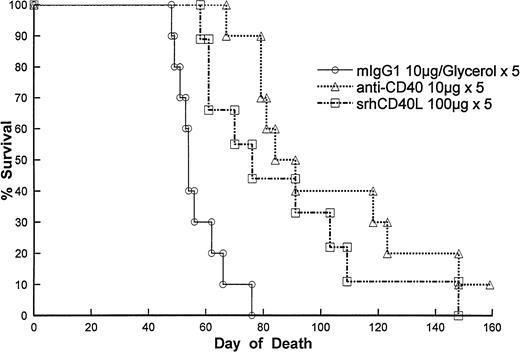
We then wanted to assess the efficacy of the ligand against human breast carcinoma cells in vivo. Immunodeficient SCID mice were injected with MDA-231 cells IV. After 2 days, the mice were treated with either srhCD40L (100 μg IP given every day for five injections) or an anti-CD40 MoAb (10 μg IP using the same regimen). The results demonstrate that treatment with either the anti-CD40 antibody or the srhCD40L resulted in significant increases in survival of the tumor-bearing recipients (Fig 7). The antitumor effects of the ligand were dose-dependent as lowering the amount of the ligand resulted in diminished antitumor effects (data not shown). No adverse effects of the ligand were detected at this dose and schedule. These results indicate that srhCD40L exerts antitumor effects against human breast carcinoma cells in vitro and in vivo.
Effect of srhCD40L on survival in MDA-231 tumor-bearing SCID mice. Mice were treated as described in Materials and Methods. Mice were treated 2 days after tumor cell injection. Treatment with srhCD40L or anti-CD40 resulted in significant (P < .01) increases in survival.
Effect of srhCD40L on survival in MDA-231 tumor-bearing SCID mice. Mice were treated as described in Materials and Methods. Mice were treated 2 days after tumor cell injection. Treatment with srhCD40L or anti-CD40 resulted in significant (P < .01) increases in survival.
DISCUSSION
Breast cancer remains a significant cause of mortality among women. Current treatments include surgery, chemotherapy, radiation therapy, and hormone therapy. There has been increasing interest in the use of immunotherapy for breast cancer, particularly in the treatment of the clinically undetectable residual disease that remains after conventional cytoreductive therapy. The results presented here suggest that stimulation of CD40 by its ligand may offer promise in the treatment of breast cancer. More work needs to be performed assessing the expression of CD40 on primary breast carcinomas and correlating it with disease progression. The results presented here also suggest that CD40 may be a target receptor for attacking the neoplastic cell itself. CD40 stimulation appears to induce apoptosis and inhibit growth in transformed epithelial cells. More work also needs to be performed to understand the mechanism underlying the growth-inhibitory effects of the CD40-CD40L interaction in carcinomas. It is possible that srhCD40L may be capable of predisposing the breast carcinoma cells for apoptosis by inducing fas expression.17 In that regard, it is also of interest that the inhibition and cell death can be enhanced with interferon-γ. Interferon-γ may simply induce more CD40 expression on the surface of the tumor making it more accessible for the ligand. Alternatively, interferon-γ can also induce fasexpression or may even be playing a more direct role in the increased inhibition seen. We are currently performing experiments to assess the effects of interferon-γ and srhCD40L in vivo. The observations that interferon-γ can augment CD40 expression and growth inhibition by the ligand in vitro suggests that combination immunotherapy involving srhCD40L and cytokines, such as interferon-γ, may offer additional benefits clinically.
It is of interest that while in vitro culture of the breast carcinoma cell lines with the anti-CD40 MoAb did not result in growth inhibition, there were significant antitumor effects noted in the tumor-bearing SCID recipients. The anti-CD40 MoAb is a murine IgG1 and therefore capable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC) in the antibody-deficient SCID recipients. Indeed, we have previously demonstrated that the antitumor effects of this anti-CD40 MoAb in SCID recipients bearing human lymphomas could be partially attributed by its ability to induce ADCC in vivo.18 Thus, the ability of the srhCD40L to inhibit breast carcinoma growth in vivo appears to correlate with its direct inhibitory effects as seen in vitro, whereas the anti-CD40 MoAb we assessed appears to exert antitumor effects in vivo not seen in vitro. The MoAb used in our studies is a partial antagonist and may therefore not bind regions capable of triggering apoptotic signals. However, other anti-CD40 MoAbs that recognize other epitopes may be found to be able to exert growth-inhibitory effects on carcinoma cells. It may also be of interest to combine the ligand with anti-CD40 MoAbs to assess potential synergistic effects.
The reason that CD40 is expressed at higher levels on certain carcinoma cells as compared with normal epithelium is unclear. CD40 has been reported on bladder, ovarian, and breast carcinomas, as well as on melanomas.6,7 In another study, CD40 was found to be expressed on 31% of breast, 40% of lung, and 24% of ovarian primary tumors.19 Thus, CD40 stimulation may also be of benefit in the treatment of other solid tumors as well. Preliminary data indicate that ovarian carcinomas and bladder carcinomas are also inhibited by the ligand for CD40 in vitro (data not shown). It will be important to determine the role of CD40 in the progression of the tumors and to assess whether tumors heterogeneous for CD40 expression can be selected for outgrowth of CD40− cells under the influence of exposure to CD40L.
The use of a recombinant human soluble ligand offers significant advantages over the current use of MoAbs to target the cancer cell. MoAbs (either conjugated with a toxin or radioisotope or nonconjugated) often have the potential to deplete normal cells positive for the marker towards which they are directed. Thus, antibodies to CD40 might deplete normal B cells,1-3 monocytes,20endothelial cells,4 dendritic cells,3 and other normal cell types and, thus, could be deleterious. Another advantage of using the recombinant human ligand would be the reduced risk of inducing potentially neutralizing human anti-mouse antibodies that often develop when murine MoAbs are used clinically. Other additional benefits of the ligand would be that not only are immune cell types not depleted, but certain functions of these cells may be augmented after ligand administration. A potential disadvantage of a ligand or agonist MoAb may be the triggering of nonspecific and polyclonal host B cells or induction of cytokine release by monocytes. As the human ligand does not bind murine CD40 with the same affinity of human CD40 (unpublished observations, January 1998) it is difficult to assess toxicity to murine cells in the xenograft model. Another potential problem with the xenograft model is the lack of binding of the srhCD40L to nontransformed cells (ie, B cells, etc), which would occur clinically and thus potentially allow for an overestimation of its efficacy. It would be useful therefore to assess the antitumor effects of a soluble murine CD40L in a mouse model. With regard to toxicity we have recently demonstrated that treatment of mice with a recombinant murine CD40L after syngeneic bone marrow transplantation resulted in accelerated immune and hematopoietic recovery with no overt toxicity.21 Thus, the srhCD40L may augment immune function, as well as provide direct antitumor effects clinically. Recent evidence also appears to suggest that CD40L is capable of augmenting antitumor immunity in tumor-bearing mice.22 Taken together, these results suggest that srhCD40L may offer potential clinical benefits in the treatment of CD40+ carcinomas.
ACKNOWLEDGMENT
We thank Dr Frank Ruscetti for critically reviewing the manuscript. We are also grateful for the excellent technical assistance by Steve Stull and the superb secretarial assistance by Laura Knott and Karen Hughes. Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 86-23. 1985)
Supported in part with Federal funds from the National Cancer Institute, National Institutes of Health under Contract No. N01-CO-56000.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to William J. Murphy, PhD, SAIC-Frederick, Bldg 567, Room 210, Frederick, MD; e-mail:murphyw@ncifcrf.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal