Abstract
Plasma fibrinogen levels have been identified as an important risk factor for cardiovascular diseases. Among the few compounds known to lower circulating fibrinogen levels in humans are certain fibrates. We have studied the regulation of fibrinogen gene expression by fibrates in rodents. Treatment of adult male rats with fenofibrate (0.5% [wt/wt] in the diet) for 7 days decreased hepatic A-, Bβ-, and γ-chain mRNA levels to 52% ± 7%, 46% ± 8%, and 81% ± 19% of control values, respectively. In parallel, plasma fibrinogen concentrations were decreased to 63% ± 7% of controls. The suppression of fibrinogen expression was dose-dependent and was already evident after 1 day at the highest dose of fenofibrate tested (0.5% [wt/wt]). Nuclear run-on experiments showed that the decrease in fibrinogen expression after fenofibrate occurred at the transcriptional level, as exemplified for the gene for the A-chain. Other fibrates tested showed similar effects on fibrinogen expression and transcription. The effect of fibrates is specific for peroxisome proliferator-activated receptor- (PPAR) because a high-affinity ligand for PPARγ, the thiazolidinedione BRL 49653, lowered triglyceride levels, but was unable to suppress fibrinogen expression. Direct evidence for the involvement of PPAR in the suppression of fibrinogen by fibrates was obtained using PPAR-null (−/−) mice. Compared with (+/+) mice, plasma fibrinogen levels in (−/−) mice were significantly higher (3.20 ± 0.48 v 2.67 ± 0.42 g/L). Also, hepatic fibrinogen A-chain mRNA levels were 25% ± 11% higher in the (−/−) mice. On treatment with 0.2% (wt/wt) fenofibrate, a significant decrease in plasma fibrinogen to 77% ± 10% of control levels and in hepatic fibrinogen A-chain mRNA levels to 65% ± 12% of control levels was seen in (+/+) mice, but not in (−/−) mice. These studies show that PPAR regulates basal levels of plasma fibrinogen and establish that fibrate-suppressed expression of fibrinogen in rodents is mediated through PPAR.
MANY CROSS-SECTIONAL and case-control studies and numerous prospective cohort studies have identified elevated plasma fibrinogen levels as an independent risk factor for coronary heart disease, stroke, and peripheral vascular disease. In addition, several cardiovascular and metabolic risk factors such as smoking, hypertension, hyperlipoproteinemia, and diabetes are also associated with high plasma fibrinogen concentrations (for a review see Handley and Hughes1 and references therein). Interpretations of the relationship between fibrinogen and coronary heart disease are interesting and unresolved, but most likely reflect low grade inflammatory processes associated with atherogenesis.2
The recognition that fibrinogen is an important factor in the promotion of various disease states has led to the search for specific therapies intended to reduce plasma fibrinogen levels. Although many different pharmacologic approaches and strategies for therapeutic modulation of fibrinogen have been tested, the efficacy of the different treatments to lower plasma fibrinogen in humans is limited and the mode of action unidentified.1,3 Among the few compounds that consistently lower circulating fibrinogen levels are some, but not all of the fibrates.1,4 Fibrates are widely used hypolipidemic drugs, very effective in lowering elevated plasma triglyceride and cholesterol levels.5 There is increasing evidence that at least part of the action of fibrates on lipid metabolism is exerted via the peroxisome proliferator-activated receptor-α (PPARα).6PPARα is a member of the nuclear receptor family of transcription factors, a diverse group of proteins that mediate ligand-dependent transcriptional activation and repression.7 Several studies have shown a direct involvement of PPARα in the fibrate-modulated gene expression of hepatic apo A-I and apo A-II, the major apolipoproteins in high-density lipoprotein (HDL), of lipoprotein lipase and apo C-III, both major determinants of plasma triglyceride levels, and of several enzymes implicated in fatty acid β-oxidation such as acyl-CoA oxidase (ACO).6 The importance of PPARα in these fibrate-induced changes in gene expression and in lowering plasma triglyceride and cholesterol levels was confirmed in experiments using PPARα-deficient mice.8-10 More recently, activation of PPARα by fibrates was also shown to inhibit the action of inflammatory cytokines by antagonizing the activities of the transcription factor, nuclear factor-κB (NF-κB).11
Given the reported suppressive effect of certain fibrates on plasma fibrinogen levels in humans, the hypothesis that PPARα is involved in regulating fibrinogen gene expression was tested. To that end, we first established the effect of fibrates on fibrinogen expression in rats. The fibrate-induced decrease of fibrinogen expression is regulated at the transcriptional level, as shown by nuclear run-on experiments, and is accompanied by a concomitant increase in ACO mRNA level and gene transcription, indicating PPARα activation. To establish the role of PPARα in fibrinogen gene expression, we studied the effect of fibrates in PPARα-null mice. Plasma fibrinogen concentrations were significantly higher in PPARα-null (−/−) mice than in wild-type (+/+) mice. On treatment with fibrate, a significant decrease in plasma fibrinogen levels and hepatic fibrinogen gene expression was observed in (+/+) mice, but not in (−/−) mice. Our data provide strong evidence for an important role of PPARα in the suppression of fibrinogen gene expression and may explain fibrate-induced reductions of fibrinogen in humans.
MATERIALS AND METHODS
Reagents.
Fenofibric acid, gemfibrozil, and ciprofibrate were kind gifts of Drs A. Edgar (Laboratoires Fournier, Daix, France), B. Bierman (Warner-Lambert, Hoofddorp, The Netherlands), and M. Riteco (Sanofi Winthrop, Maassluis, The Netherlands), respectively. BRL 49653 was generously provided by Dr J.-J. Berthelon (Lipha Merck, Lyon, France). Bezafibrate was obtained from Boehringer Mannheim (Almere, The Netherlands).
Animal studies.
Animal studies were performed in compliance with European Community specifications regarding the use of laboratory animals. Details of experimental conditions have been described previously.12Briefly, male Wistar rats (3 months old) were divided in groups of four animals each and treated for 7 days with fenofibrate mixed at the indicated concentrations (by mass) in standard rat chow. The food intake was recorded every 2 days throughout the treatment period. None of the treatments caused major changes in the amount of food consumed by the animals. Because each rat consumed approximately 20 g of chow per day, doses of 0.5%, 0.05%, and 0.005% (wt/wt) corresponded to 320, 32, and 3 mg of fibrate/kg of body weight/day. In a subsequent experiment, rats were treated with 0.5% (wt/wt) fenofibrate for different time periods up to 14 days, followed by a wash-out period varying from 1 to 14 days. In a second series of experiments, male Sprague-Dawley rats (3 months old) were divided in groups of four animals each and treated for 3 days with BRL 49653 (10 mg/kg of body weight/d), fenofibrate (200 mg/kg of body weight/d), or 10% (wt/vol) carboxymethylcellulose (vehicle for gavage) by gavage, twice a day. At the end of the treatment period, rats were fasted overnight, weighed, and killed by exsanguination under ether anesthesia between 8 and 10 am. Blood was collected by aortic puncture, and part of it was used for serum preparation. The other portion was incubated with 0.1 vol of trisodium citrate (3.8% [wt/vol]) to prevent coagulation, and platelet-free plasma was prepared for determination of fibrinogen. Livers were removed immediately, rinsed with 0.9% (wt/vol) NaCl, weighed, frozen in liquid nitrogen, and stored at -70°C until RNA preparation. In a third series of experiments, male Sv/129 homozygous wild-type (+/+) and PPARα-null (−/−) mice8(10 to 12 weeks of age) were fed for 17 days with either a standard mouse chow or one containing 0.2% (wt/wt) fenofibrate. At the end of the treatment period, the animals were fasted for 4 hours, weighed, and killed by exsanguination under ether anesthesia. For determination of plasma fibrinogen levels, blood was collected from a small tail-cut using potassium-EDTA Microvette CB 300 tubes (Sarstedt, Nümbrecht, Germany). Livers were removed immediately, weighed, rinsed with 0.9% (wt/vol) NaCl, frozen in liquid nitrogen, and stored at −70°C until RNA preparation.
Rat hepatocyte isolation and culture.
Rat hepatocytes were isolated and cultured as described previously.13 Briefly, hepatocytes were isolated by perfusion with 0.05% (wt/vol) collagenase and 0.005% (wt/vol) trypsin inhibitor. After a 4-hour attachment period in Williams E medium supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 135 nmol/L insulin, 50 nmol/L dexamethasone, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin, the nonadherent cells were washed from the plates and the remaining cells refed. After 16 hours, the medium was changed to incubation medium in which the amount of insulin was lowered to 10 nmol/L. Experiments were started 20 hours after isolation. Conditioned media were obtained by incubating cells at 37°C for 72 hours with incubation medium containing the appropriate test compound or stock solvent (dimethyl sulfoxide [DMSO]; final concentration 0.1% [vol/vol]). The media were changed every 24 hours. Conditioned media were centrifuged for 4 minutes at 5,000g to remove cells and cellular debris, and the samples were kept at −20°C until use. The cells were washed twice with ice-cold phosphate-buffered saline (PBS) and used for isolation of RNA.
Serum triglycerides and plasma fibrinogen measurements.
Serum triglycerides were determined using a commercially available kit to measure total serum triglycerides (Boehringer Mannheim). Fibrinogen concentrations in plasma and conditioned media were measured by an enzyme-linked immunosorbent assay (ELISA) procedure using polyclonal antibodies to rat fibrinogen both as catching and tagging antibodies.14
RNA analysis.
RNA was isolated from liver and cultured cells by the acid guanidinium thiocyanate/phenol/chloroform method.15 Northern and dot-blot analysis of total cellular RNA was performed as described.12 All probes were labeled with a Megaprime kit, yielding an average activity of 0.5 μCi/ng DNA. Filters were hybridized with 3 ng of [α-32P] deoxycytidine triphosphate (dCTP)-labeled probe per mL as described.16 Mouse fibrinogen cDNA probes used were provided by F. Razaee from our institute and were a 1.2-kb fragment of the mouse fibrinogen Aα-chain cDNA; a 1.0-kb fragment of the mouse fibrinogen Bβ-chain cDNA; a 0.6-kb fragment of the mouse fibrinogen γ-chain cDNA. Other cDNA probes used were a 2.0-kb Sac I fragment of the rat ACO cDNA, provided by Dr T. Osumi,17and a 3.8-megadalton (mDa) EcoRI fragment of the human 18S ribosomal DNA.18 The intensities of the signals were determined using a Fujix Bas 1000 phosphoimager (Fuji Photo Film Co, Tokyo, Japan) and expressed relative to the signal of the 18S ribosomal RNA band.
Isolation of nuclei and transcriptional rate assay.
Nuclei were prepared from livers of untreated rats and rats treated for 14 days with 0.5% (wt/wt) of different fibrates in rat chow, exactly as described by Gorski et al.19 Transcription run-on assays were performed as described by Nevins.20 Equivalent amounts of labeled nuclear RNA were hybridized for 36 hours at 42°C to 5 μg of purified cDNA probes immobilized on Hybond C Extra filters (Amersham, Arlington Heights, IL). The following cDNAs were spotted: a mouse fibrinogen Aα-chain cDNA probe, a rat ACO cDNA probe, and a chicken β-actin cDNA probe. As a control, 5 μg of vector DNA was applied to the filter. After hybridization, filters were washed at room temperature for 10 minutes in 0.5 × SSC (1 × SSC being 0.15 mol/L NaCl, 0.015 mol/L Na3 citrate) and 0.1% (wt/vol) sodium dodecyl sulfate (SDS), and twice for 30 minutes at 65°C, and subsequently exposed to x-ray (X-OMAT-AR, Eastman-Kodak, Rochester, NY) film. The intensities of the signals present were determined by scanning densitometry (Bio-Rad GS670 densitometer; Bio-Rad, Paris, France) and expressed relative to the signal of the β-actin mRNA band.
Statistical analysis.
The data are presented as means ± standard deviation (SD). The significance of treatment was assessed by an unpaired Student’st-test, with exception of the dose-response and time-course experiments in which analysis of variance was used to evaluate the results. For comparison of the wild-type and PPARα-deficient mice, an unpaired Student’s t-test was also used. Differences were considered significant at P < .05.
RESULTS
Fibrates decrease hepatic fibrinogen gene expression and plasma fibrinogen concentrations.
Adult male rats were treated for 7 days with different concentrations (0.005, 0.05, or 0.5% [wt/wt]) of fenofibrate mixed in standard rat chow and analyzed for hepatic fibrinogen gene expression and plasma fibrinogen levels (Fig 1). Fibrinogen is secreted as a fully assembled dimer, with each half composed of three nonidentical polypeptide chains, Aα, Bβ, and γ. Fenofibrate treatment decreased hepatic fibrinogen Aα-, Bβ-, and γ-chain mRNA as well as plasma fibrinogen levels in a dose-dependent fashion. At the highest dose (0.5%) of fenofibrate tested, fibrinogen mRNA levels were reduced to 52% ± 7%, 46% ± 8%, and 81% ± 19% of control values for the Aα-, Bβ-, and γ-chain, respectively (Fig1A and B). This weaker effect of fibrates on the γ-chain was consistently found in all experiments performed. In parallel, plasma fibrinogen concentrations were decreased to 63% ± 7% of control values (Fig 1C). At the lowest dose (0.005%) of fenofibrate tested, fibrinogen mRNAs in the liver and plasma fibrinogen levels were not significantly affected. Hepatic mRNA levels of ACO, the rate-limiting enzyme in peroxisomal β-oxidation, the induction of which by fibrates is strictly PPARα-mediated,8 showed a dose-dependent response to fenofibrate-treatment comparable to that of fibrinogen, reaching an approximately sixfold increase at a fenofibrate dose of 0.5%.
Dose-dependent effect of fenofibrate on hepatic fibrinogen mRNAs and plasma fibrinogen levels. Adult male rats were treated with 0.005%, 0.05%, or 0.5% of fenofibrate ([wt/wt] in rat chow) for 7 days and compared with animals on rat chow only. Total RNA was extracted from livers and analyzed by Northern blotting for fibrinogen A-, Bβ-, and γ-chain mRNA and ACO mRNA. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Plasma fibrinogen levels were measured as described in Materials and Methods. Data shown are from a representative experiment with four animals per experimental group. (A) Representative Northern blot analysis of fibrinogen (Fbg) A-, Bβ-, and γ-chain mRNA, ACO mRNA, and 18S rRNA. (B) Signals for the three fibrinogen chain mRNAs and ACO mRNA were quantified by densitometry and adjusted for the corresponding rRNA signals. Data are expressed relative to that found in untreated animals. Results are means ± SD of four animals. (C) Plasma fibrinogen data are means ± SD of four animals. Statistically significant differences (P < .05) are indicated by an asterisk; # P = .13.
Dose-dependent effect of fenofibrate on hepatic fibrinogen mRNAs and plasma fibrinogen levels. Adult male rats were treated with 0.005%, 0.05%, or 0.5% of fenofibrate ([wt/wt] in rat chow) for 7 days and compared with animals on rat chow only. Total RNA was extracted from livers and analyzed by Northern blotting for fibrinogen A-, Bβ-, and γ-chain mRNA and ACO mRNA. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Plasma fibrinogen levels were measured as described in Materials and Methods. Data shown are from a representative experiment with four animals per experimental group. (A) Representative Northern blot analysis of fibrinogen (Fbg) A-, Bβ-, and γ-chain mRNA, ACO mRNA, and 18S rRNA. (B) Signals for the three fibrinogen chain mRNAs and ACO mRNA were quantified by densitometry and adjusted for the corresponding rRNA signals. Data are expressed relative to that found in untreated animals. Results are means ± SD of four animals. (C) Plasma fibrinogen data are means ± SD of four animals. Statistically significant differences (P < .05) are indicated by an asterisk; # P = .13.
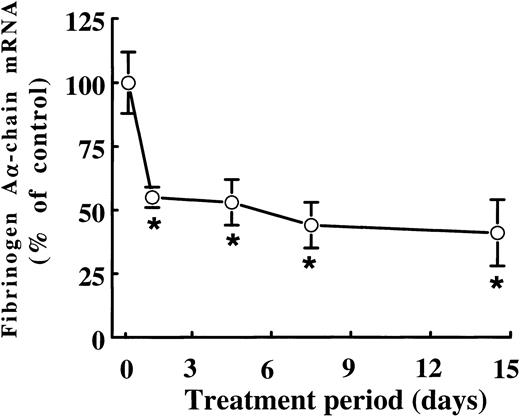
When rats were treated with 0.5% (wt/wt) fenofibrate for different periods of time, the mRNA levels of fibrinogen Aα-chain, the presumed rate-limiting chain in the assembly of the mature fibrinogen molecule in rats,21 were found to be decreased to 55% ± 3% of control levels after just 1 day of fenofibrate treatment (Fig 2). Fibrinogen Aα-chain mRNA concentrations decreased only slightly on further prolonged treatment, reaching 44% ± 9% and 41% ± 3% of control values after 7 and 14 days of treatment, respectively.
Time-dependent effect of fenofibrate on fibrinogen A-chain mRNA levels. Adult male rats were treated with 0.5% (wt/wt) fenofibrate for different time periods. Total RNA was extracted from livers and analyzed for fibrinogen A-chain mRNA levels by dot-blot analysis as described in Materials and Methods. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of three animals and presented as percentage of control values. Statistically significant differences (P < .05) are indicated by an asterisk.
Time-dependent effect of fenofibrate on fibrinogen A-chain mRNA levels. Adult male rats were treated with 0.5% (wt/wt) fenofibrate for different time periods. Total RNA was extracted from livers and analyzed for fibrinogen A-chain mRNA levels by dot-blot analysis as described in Materials and Methods. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of three animals and presented as percentage of control values. Statistically significant differences (P < .05) are indicated by an asterisk.
To examine whether the observed downregulation of plasma fibrinogen and hepatic fibrinogen gene expression is a general characteristic of fibrates rather than a specific effect of fenofibrate, we also tested the effect of other fibrates. In rats exposed for 14 days to 0.5% (wt/wt) of gemfibrozil or bezafibrate, or 0.05% (wt/wt) of ciprofibrate, fibrinogen Aα-chain mRNA levels were reduced to 74% ± 12%, 53% ± 3%, and 59% ± 2% of control values, respectively.
Downregulation of fibrinogen expression is due to a direct effect of fibrates on hepatocytes.
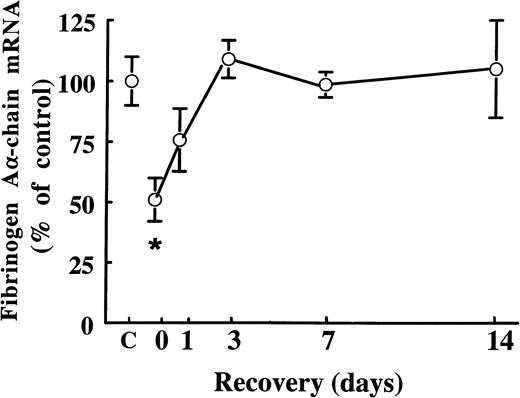
Fibrates are known to cause extensive peroxisome proliferation and hepatomegaly in rodents.6 In the present study, we found that treatment of rats with 0.05% (wt/wt) and 0.5% (wt/wt) of fenofibrate for 14 days increased liver weights 1.4-fold and 2.0-fold, respectively. To exclude the possibility that the suppressive effects of fenofibrate on fibrinogen expression are due to changes in liver structure and/or function, we performed wash-out experiments: male rats were treated for 14 days with 0.5% (wt/wt) of fenofibrate, after which the fibrate was withdrawn from the food. At the start of the wash-out period, hepatic fibrinogen Aα-chain mRNA levels were 51% ± 9% of control levels (Fig 3). Fibrinogen Aα-chain mRNA levels increased to 76% ± 13% of control values within 1 day after withdrawal of fenofibrate and reached control levels after 4 days, staying constant thereafter. These findings indicate that fibrates decrease fibrinogen expression reversibly and independent of changes in liver structure and/or function.
Effect of cessation of fenofibrate treatment on fibrinogen A-chain mRNA levels. Adult male rats were treated with 0.5% (wt/wt) of fenofibrate for 14 days, after which fenofibrate was withdrawn from the food. Total RNA was extracted from livers and analyzed for fibrinogen A-chain mRNA at different time points after cessation of fenofibrate treatment. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of three animals per group and presented as percentage of control values obtained from untreated animals (C). Statistically significant differences (P < .05) are indicated by an asterisk.
Effect of cessation of fenofibrate treatment on fibrinogen A-chain mRNA levels. Adult male rats were treated with 0.5% (wt/wt) of fenofibrate for 14 days, after which fenofibrate was withdrawn from the food. Total RNA was extracted from livers and analyzed for fibrinogen A-chain mRNA at different time points after cessation of fenofibrate treatment. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of three animals per group and presented as percentage of control values obtained from untreated animals (C). Statistically significant differences (P < .05) are indicated by an asterisk.
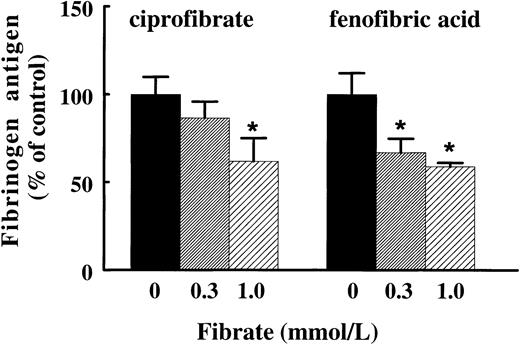
To find further evidence for a direct effect of fibrates on hepatic fibrinogen expression, we investigated whether these effects are also observed in primary cultures of rat hepatocytes. Treatment of rat hepatocytes for 72 hours with ciprofibrate or the active form of fenofibrate, fenofibric acid, reduced fibrinogen production dose-dependently to 62% ± 13% and 59% ± 2% of control values, respectively, at the highest concentration of the fibrate tested (1 mmol/L) (Fig 4). The reduction of fibrinogen antigen levels was reflected in a reduction of fibrinogen Aα-chain mRNA levels (data not shown), indicating that fibrates suppress fibrinogen gene expression in a direct manner.
Effect of ciprofibrate and fenofibric acid on fibrinogen production in primary cultures of rat hepatocytes. Isolated rat hepatocytes were incubated with 0.3 or 1 mmol/L ciprofibrate or fenofibric acid or vehicle for three consecutive periods of 24 hours. The conditioned media were analyzed for fibrinogen antigen as described in Materials and Methods. Results are means ± SD of three independent experiments performed in duplicate. The data are expressed as percentage values of controls. Statistically significant differences (P < .05) are indicated by an asterisk.
Effect of ciprofibrate and fenofibric acid on fibrinogen production in primary cultures of rat hepatocytes. Isolated rat hepatocytes were incubated with 0.3 or 1 mmol/L ciprofibrate or fenofibric acid or vehicle for three consecutive periods of 24 hours. The conditioned media were analyzed for fibrinogen antigen as described in Materials and Methods. Results are means ± SD of three independent experiments performed in duplicate. The data are expressed as percentage values of controls. Statistically significant differences (P < .05) are indicated by an asterisk.
Fibrates suppress fibrinogen gene transcription.
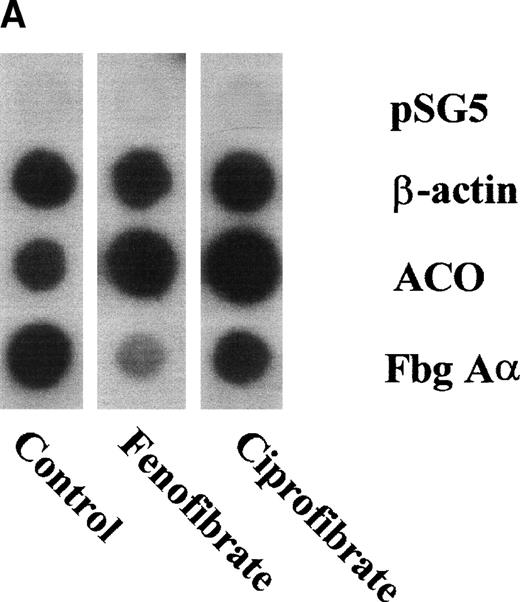
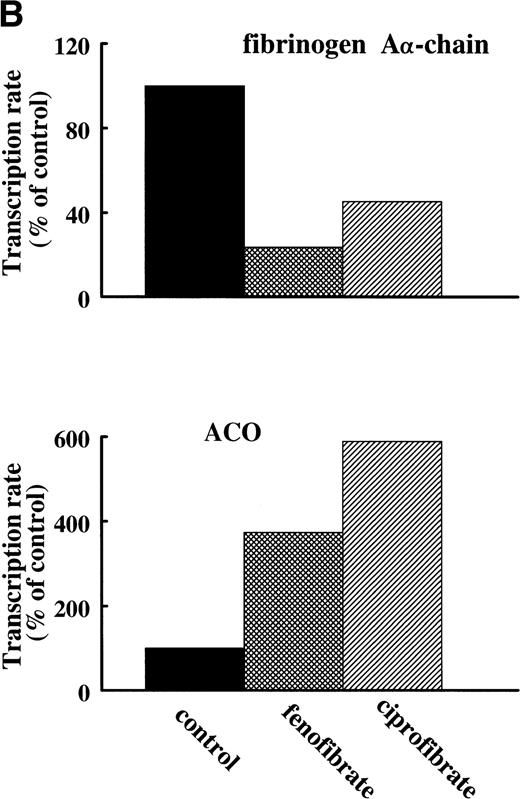
To assess the effects of various fibrates on fibrinogen gene transcription rate, nuclear run-on transcription assays were performed on nuclei prepared from livers of untreated (control) rats or rats treated for 14 days with 0.5% (wt/wt) of fenofibrate or ciprofibrate. Both fibrates decreased fibrinogen Aα-chain transcription rate (to 24% and 45% of control levels for fenofibrate and ciprofibrate, respectively) and increased ACO transcription rate (to 373% and 589% of control levels for fenofibrate and ciprofibrate, respectively) (Fig 5A and B), reflecting activation of PPARα.
Effect of fibrates on fibrinogen and ACO gene transcription. Nuclear run-on assays were performed on nuclei obtained from livers of control rats and rats treated with 0.5% (wt/wt) fenofibrate or 0.5% (wt/wt) ciprofibrate for 14 days as described in Materials and Methods. The data shown are of a representative experiment. (A) Autoradiogram showing vector (pSG5), β-actin, ACO, and fibrinogen A-chain (Fbg A) signals. (B) Signals were quantified by densitometric scanning and adjusted for the corresponding β-actin signal. Data are expressed as percentage values relative to that in control nuclei.
Effect of fibrates on fibrinogen and ACO gene transcription. Nuclear run-on assays were performed on nuclei obtained from livers of control rats and rats treated with 0.5% (wt/wt) fenofibrate or 0.5% (wt/wt) ciprofibrate for 14 days as described in Materials and Methods. The data shown are of a representative experiment. (A) Autoradiogram showing vector (pSG5), β-actin, ACO, and fibrinogen A-chain (Fbg A) signals. (B) Signals were quantified by densitometric scanning and adjusted for the corresponding β-actin signal. Data are expressed as percentage values relative to that in control nuclei.
Fibrinogen gene expression is suppressed by PPARα, but not by PPARγ activators.
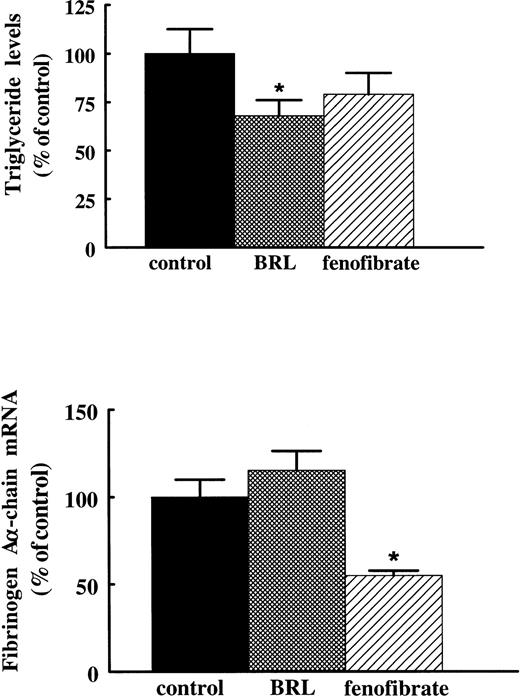
In addition to their PPARα activating capacity, fibrates are also known to activate, albeit much more weakly, PPARγ.22 To verify that the suppressive effect of fibrates on fibrinogen is mediated via activation of PPARα rather than PPARγ, we compared the effects of fenofibrate with the effects of the antidiabetic drug thiazolidinedione, BRL 49653, previously shown to be a high affinity ligand for PPARγ.23 Rats treated for 3 days with 400 mg/kg/d fenofibrate or 10 mg/kg/d BRL 49653 by gavage showed significantly reduced plasma triglyceride levels (to 69% ± 12% and 68% ± 8% of control levels for fenofibrate and BRL 49653, respectively). However, whereas treatment with fenofibrate reduced fibrinogen Aα-chain mRNA levels to 55% ± 3% of control levels, BRL 49653 did not affect fibrinogen expression (115% ± 11% of control levels) (Fig 6), indicating that the suppressive effect of fibrates on fibrinogen levels requires PPARα activation.
Effect of BRL 49653 and fenofibrate on fibrinogen A-chain mRNA levels in rats. Adult male rats were treated with 10 mg/kg of body weight/day BRL 49653 or 400 mg/kg of body weight/day fenofibrate by gavage twice a day for 3 days. Plasma triglyceride levels were determined as described in Materials and Methods. Total RNA was extracted from livers and analyzed for fibrinogen A-chain mRNA by Northern blotting. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of two independent experiments (with four animals per group) and presented as percentage of control values. Statistically significant differences (P < .05) are indicated by an asterisk.
Effect of BRL 49653 and fenofibrate on fibrinogen A-chain mRNA levels in rats. Adult male rats were treated with 10 mg/kg of body weight/day BRL 49653 or 400 mg/kg of body weight/day fenofibrate by gavage twice a day for 3 days. Plasma triglyceride levels were determined as described in Materials and Methods. Total RNA was extracted from livers and analyzed for fibrinogen A-chain mRNA by Northern blotting. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of two independent experiments (with four animals per group) and presented as percentage of control values. Statistically significant differences (P < .05) are indicated by an asterisk.
PPARα-null mice are refractory to the suppressive effects of fibrates on fibrinogen expression.
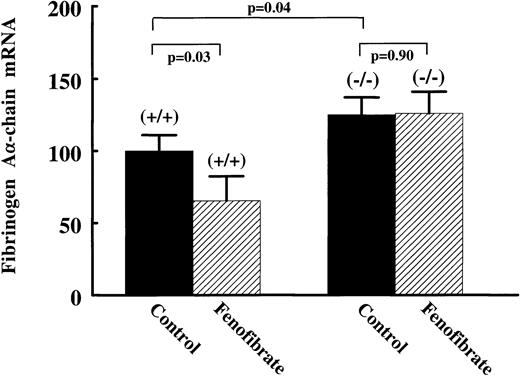
To establish a direct role of PPARα in the regulation of fibrinogen gene expression, we studied fibrinogen expression and its response to fenofibrate in PPARα-null (−/−) mice. Compared with wild-type (+/+) mice, plasma fibrinogen levels were significantly higher in (−/−) mice, being 2.67 ± 0.42 g/L and 3.20 ± 0.48 g/L, respectively (Table 1). Hepatic fibrinogen Aα-chain mRNA levels were 25% ± 11% higher in the (−/−) mice (Fig 7). On treatment with 0.2% (wt/wt) fenofibrate for 17 days, liver weights were increased to 277% of controls in (+/+) mice, while no change in liver weights of (−/−) mice was observed (data not shown). Fenofibrate significantly decreased plasma fibrinogen levels in (+/+) mice (−0.61 ± 0.31 g/L; P = .007), but not in (−/−) mice (−0.33 ± 0.36 g/L; P = .13) (Table 1). Consistent with the antigen data, fibrinogen Aα-chain mRNA levels were significantly decreased in (+/+) mice (−35% ± 12%; P = .04), but not in the fibrate-treated (−/−) mice (+1% ± 13%; P = .9) (Fig7). These results indicate that PPARα is involved in the suppression of basal levels of plasma fibrinogen, and that fibrate-suppressed expression of fibrinogen in wild-type mice is dependent on PPARα activation.
Effects of Fenofibrate on Plasma Fibrinogen Levels in PPAR-Null (−/−) and Wild-Type (+/+) Mice
| Treatment . | Fibrinogen (g/L) . | |
|---|---|---|
| Wild-Type (+/+) . | PPARα-Null (−/−) . | |
| Control (n = 7) | 2.67 ± 0.42 | 3.20 ± 0.48* |
| Fenofibrate (n = 7) | 2.06 ± 0.26 | 2.87 ± 0.23* |
| Difference | −0.61 ± 0.31 | −0.33 ± 0.36 |
| (P = .007) | (P = .13) | |
| Treatment . | Fibrinogen (g/L) . | |
|---|---|---|
| Wild-Type (+/+) . | PPARα-Null (−/−) . | |
| Control (n = 7) | 2.67 ± 0.42 | 3.20 ± 0.48* |
| Fenofibrate (n = 7) | 2.06 ± 0.26 | 2.87 ± 0.23* |
| Difference | −0.61 ± 0.31 | −0.33 ± 0.36 |
| (P = .007) | (P = .13) | |
Wild-type (+/+) and PPARα-null (−/−) mice (n = 7) were treated with 0.2% (wt/wt) fenofibrate mixed in chow for 17 days. Values are means ± SD.
Statistically significant (P < .05) difference between wild-type versus PPARα-null mice.
Effect of fenofibrate on fibrinogen A-chain mRNA levels in wild-type versus PPAR-null mice. Wild-type (+/+) and PPAR-null (−/−) mice were treated with 0.2% (wt/wt) fenofibrate mixed in chow for 17 days. Total RNA was extracted from livers and analyzed for fibrinogen A-chain by Northern blotting. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of seven animals per group and presented as percentage values of control, untreated wild-type mice.
Effect of fenofibrate on fibrinogen A-chain mRNA levels in wild-type versus PPAR-null mice. Wild-type (+/+) and PPAR-null (−/−) mice were treated with 0.2% (wt/wt) fenofibrate mixed in chow for 17 days. Total RNA was extracted from livers and analyzed for fibrinogen A-chain by Northern blotting. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Values are means ± SD of seven animals per group and presented as percentage values of control, untreated wild-type mice.
DISCUSSION
Fibrates reportedly lower plasma fibrinogen in humans, but the regulatory mechanism of this effect remains to be clarified. Here, we show that activation of the nuclear hormone receptor PPARα mediates the suppression of fibrinogen gene transcription by fibrates in rodents. A direct involvement of PPARα in fibrinogen gene expression was provided using PPARα-null (−/−) mice. Basal levels of plasma fibrinogen were significantly higher in the (−/−) mice than in (+/+) mice, and fibrates suppressed fibrinogen gene expression and plasma levels in (+/+) mice only. These observations clearly establish PPARα as a key regulatory factor in fibrinogen gene expression in rodents and may explain the suppressive effect of fibrates on plasma fibrinogen levels in humans.
The fibrinogen molecule is arranged as a dimer, with each monomer composed of three nonidentical polypeptide chains: Aα, Bβ, and γ.24 The three fibrinogen chains are encoded by three separate, closely linked genes situated on the same chromosome and located in the sequence γ, Aα, and Bβ, with the last one in opposite transcriptional orientation to the first one.25 It has been reported that in rat hepatocytes, the amount of Aα-chain limits the rate of assembly of the fibrinogen molecule,21whereas in human hepatoma cells, the amount of Bβ-chain appears to limit assembly.26,27 We found that, at least in rats, the inhibition of gene expression by fibrates was evidently not confined to the Aα-chain, but equally affected the Bβ-chain and, albeit to a lesser extent, the γ-chain. Recently, Binsack et al28reported that in the human hepatoma cell line, HepG2, bezafibrate suppressed Aα-, Bβ-, and γ-chain mRNA levels. These findings corroborate the concept of the coordinated expression of the three fibrinogen chains in both rats and humans.27
Our results show that PPARα has an important role in mediating the effects of fibrates on fibrinogen expression. Whereas several genes involved in lipid metabolism, apo A-I, lipoprotein lipase, and acyl-CoA synthetase, are positively regulated by PPARα,29-31 the genes encoding the three fibrinogen chains are negatively regulated by PPARα, like apo CIII.6 Although the coordinate suppression of gene transcription of the three fibrinogen chains by fibrates would suggest a shared regulatory mechanism, the exact molecular mechanism by which PPARα acts is not understood. Further experiments, including functional analysis of the regulatory regions of the genes encoding for the fibrinogen chains, will be necessary to elucidate the precise mechanism of transcriptional repression of fibrinogen gene expression by PPARα.
Fibrates are also implicated in suppressing elevated fibrinogen levels under inflammatory conditions. Many reports link the inflammatory mediator interleukin-6 (IL-6) to elevated fibrinogen expression,32,33 and indeed IL-6–responsive elements have been identified in the promoters of the different rat and human fibrinogen genes.34-36 Recent evidence indicates that activated PPARα can interfere negatively with cytokine-induced signaling pathways.11 37 It is thus conceivable that PPARα also plays an important role in downregulating cytokine-increased fibrinogen gene expression.
We found significantly higher basal plasma fibrinogen levels in PPARα-null (−/−) mice than in wild-type (+/+) mice, suggesting that PPARα is involved in modulating basal fibrinogen expression. Several endogenous ligands have been identified such as long chain fatty acids (palmitic acid, linoleic acid, arachidonic acid) and eicosanoids [leukotriene B4, 8(S)-hydroxyeicosatetraenoic acid],22,38 which could account for PPARα activation under basal conditions. Therefore, changes in endogenous fatty acid profiles as a result of changes in environmental and life-style factors may explain reported intraindividual variation in fibrinogen levels of about 10% to 15%.39-41 Similarly, the recent identification of structural and functional polymorphisms in human PPARα42 may be relevant for understanding regulation of plasma fibrinogen levels. It would be interesting to delineate the role of abnormal PPARα activity in patients with disturbed fibrinogen and lipid levels by genetic linkage studies.
It is important to recognize that fibrates downregulate fibrinogen expression via the same transcription factor as that identified for reducing circulating triglyceride and cholesterol levels, ie, activated PPARα. Other lipid lowering drugs such as 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors (statins) and PPARγ activators (thiazolidinediones) show no significant consistent effects on fibrinogen levels. For example, lovastatin therapy has resulted in minor fibrinogen reductions in hypercholesterolemic patients41 or actually increased fibrinogen,43while reductions were seen in a single reported study with pravastatin therapy in familial hypercholesterolemia patients.44 In the present study, we found no effect of the thiazolidinedione, BRL 49653, on plasma fibrinogen levels in rats, while triglyceride levels were lowered to a similar extent as with fenofibrate. These results further emphasize that the lowering effect of fibrates on fibrinogen are not the result of lowered triglyceride levels. Because both elevated plasma fibrinogen levels and elevated plasma lipids have been identified as key risk factors for cardiovascular diseases,1 the identification of a common, specific molecular target, PPARα, that is suitable for application of modern drug discovery provides a new lead for therapy. Such a novel compound specifically activating PPARα may prove superior to existing fibrates, in the action of which other, as yet unidentified molecular mechanisms are also involved.4 45
ACKNOWLEDGMENT
We gratefully acknowledge Sabine Post for technical help with the rat hepatocyte isolation and Karin Toet for technical assistance. We also thank Farhad Rezaee for providing the mouse fibrinogen Aα-, Bβ-, and γ-chain cDNA probes.
Supported by Grant No. NWO: 902-23-181 from the Netherlands Organization for Scientific Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Teake Kooistra, PhD, Gaubius Laboratory, TNO Prevention and Health, PO Box 2215, 2301 CE Leiden, The Netherlands.

![Fig. 1. Dose-dependent effect of fenofibrate on hepatic fibrinogen mRNAs and plasma fibrinogen levels. Adult male rats were treated with 0.005%, 0.05%, or 0.5% of fenofibrate ([wt/wt] in rat chow) for 7 days and compared with animals on rat chow only. Total RNA was extracted from livers and analyzed by Northern blotting for fibrinogen A-, Bβ-, and γ-chain mRNA and ACO mRNA. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Plasma fibrinogen levels were measured as described in Materials and Methods. Data shown are from a representative experiment with four animals per experimental group. (A) Representative Northern blot analysis of fibrinogen (Fbg) A-, Bβ-, and γ-chain mRNA, ACO mRNA, and 18S rRNA. (B) Signals for the three fibrinogen chain mRNAs and ACO mRNA were quantified by densitometry and adjusted for the corresponding rRNA signals. Data are expressed relative to that found in untreated animals. Results are means ± SD of four animals. (C) Plasma fibrinogen data are means ± SD of four animals. Statistically significant differences (P < .05) are indicated by an asterisk; # P = .13.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2991/4/m_blod40903001aw.jpeg?Expires=1769432799&Signature=LNTiUFXsdilwuv-UQL5VanxlLI25BflLXCufVQdOfcs92Zeg3qlrbYFc7IU8uEEY-iC2pew9DBzDYCZBE8-6aIJseDr4S0D6TEMC5E63-fVJEFvdCU0UB8i6DItaH0VvkgcVx7u90tV0E3rsRmj~P1bElIInLO-BJHC-2bMmGg5qrOQqqYDeUT4k2J365CIu1GRw9pLcsM4FLzOkqqotk8h1GPkyYlLzcbX18mJ048x5bJjjEJYc8POMPJWNFcIyyjKQSx9ARZFaI54EAm-q43bi6U9dJ9YcCAUXh0GQjsiHRayhnPn9lRlLYU0t1Zr8mTXze~VVAtG2XvaxARxxqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Dose-dependent effect of fenofibrate on hepatic fibrinogen mRNAs and plasma fibrinogen levels. Adult male rats were treated with 0.005%, 0.05%, or 0.5% of fenofibrate ([wt/wt] in rat chow) for 7 days and compared with animals on rat chow only. Total RNA was extracted from livers and analyzed by Northern blotting for fibrinogen A-, Bβ-, and γ-chain mRNA and ACO mRNA. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Plasma fibrinogen levels were measured as described in Materials and Methods. Data shown are from a representative experiment with four animals per experimental group. (A) Representative Northern blot analysis of fibrinogen (Fbg) A-, Bβ-, and γ-chain mRNA, ACO mRNA, and 18S rRNA. (B) Signals for the three fibrinogen chain mRNAs and ACO mRNA were quantified by densitometry and adjusted for the corresponding rRNA signals. Data are expressed relative to that found in untreated animals. Results are means ± SD of four animals. (C) Plasma fibrinogen data are means ± SD of four animals. Statistically significant differences (P < .05) are indicated by an asterisk; # P = .13.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2991/4/m_blod40903001cx.jpeg?Expires=1769432799&Signature=FsywivL5q0z-LHlfmsJ16q5tIC7ZY8kQ7KbvI3i2C~ssRDLHjmmniHgcOu12h7ihacqKbz2qocE3BsoHpyL-c1wdW3eTjxxiOp7174j3RuJOAQbZ8gbiC~O3r1wVpYq95joB22RZcRsx3TrPINiYu7WHje19qY~t4UZ3XrCPe-7xGEfJLboI7eT2Z-o702Jql642rzg0VejvnR3TirkbXYeU2nJdtvHtzPGJrppRR1pV0PqQW1oc2~9yIMJq8pdZ87a-4pGkotP2NyJLiKlLGEOHODvwu-HRPrCCPun1B6yiODc7av7UurRjUgof6amfKK7HnlYquPPzu-aIjNLltQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Dose-dependent effect of fenofibrate on hepatic fibrinogen mRNAs and plasma fibrinogen levels. Adult male rats were treated with 0.005%, 0.05%, or 0.5% of fenofibrate ([wt/wt] in rat chow) for 7 days and compared with animals on rat chow only. Total RNA was extracted from livers and analyzed by Northern blotting for fibrinogen A-, Bβ-, and γ-chain mRNA and ACO mRNA. Equal loading was checked by hybridizing with an 18S rRNA cDNA probe. Plasma fibrinogen levels were measured as described in Materials and Methods. Data shown are from a representative experiment with four animals per experimental group. (A) Representative Northern blot analysis of fibrinogen (Fbg) A-, Bβ-, and γ-chain mRNA, ACO mRNA, and 18S rRNA. (B) Signals for the three fibrinogen chain mRNAs and ACO mRNA were quantified by densitometry and adjusted for the corresponding rRNA signals. Data are expressed relative to that found in untreated animals. Results are means ± SD of four animals. (C) Plasma fibrinogen data are means ± SD of four animals. Statistically significant differences (P < .05) are indicated by an asterisk; # P = .13.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2991/4/m_blod40903001bx.jpeg?Expires=1769432799&Signature=fnYkUj0AUVA7fotDe9y2FoOeHjj5WpIGJqo2VxEQXQAX~ievJTaFcQzwLtSOrP0w81brsuM2wUZx8o~wovT9bRpKwij2Mge2I~LAPG9kheSndL0c1p6ESsxTt7UqEuFv608-tXBVkPRl6XB~pQAZWNYuNw0BT-oSNNESFHRMUVGhUThVQn9YaTB3R45Y6Kto8RuRVJR3VW4zPO5MlmjZNV3iB1EMHjMmoKoJtPCvmrz1aYerjWJqO39T~qYEdtq~~ouDBEkoUx9ujEUGD5NWaNW8lnRivSV~UF2M0OFhtS7azo6WzXtLLvXPDcjV5Y7v8-xz9c3s5zwWPmWB6-REPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal