Abstract
CREB-binding protein (CBP) and the closely related adenovirus E1A-associated 300-kD protein (p300) function as coactivators of transcription factors such as CREB, c-Fos, c-Jun, c-Myb, and several nuclear receptors. To study the roles of CBP in embryonic development, we generated CBP homozygous mutant mouse embryos that expressed a truncated form of CBP protein (1-1084 out of 2441 residues). The embryos died between embryonic days 9.5 (E9.5) and E10.5 and exhibited a defect in neural tube closure. They appeared pale and showed decreases in erythroid cells and colony-forming cells (CFCs) in the yolk sac, suggesting defects in primitive hematopoiesis. Immunohistochemistry with an anti-PECAM antibody showed a lack of vascular network formation. Organ culture of para-aortic splanchnopleural mesoderm (P-Sp) with stromal cells (OP9) showed an autonomous abnormality of putative endothelial precursors, which may induce the microenvironmental defect in hematopoiesis. In addition, these defects were partially rescued by the addition of VEGF to this culture. Our analyses demonstrate that CBP plays an essential role in hematopoiesis and vasculo-angiogenesis.
CREB-BINDING PROTEIN (CBP) and the closely related adenovirus E1A-associated 300-kD protein (p300) function as versatile coactivators of transcription factors such as CREB, c-Fos, c-Jun, c-Myb, and several nuclear receptors.1-7 Both CBP and p300 also play roles in chromatin remodeling, signaling pathways, and basic cellular functions such as DNA repair, cell growth, differentiation, embryonic development, and tumor suppression.8,9 The mutations in CBP gene were reported to be associated especially with the Rubinstein-Taybi syndrome in humans10 and mice.11 12 The role of CBP in embryonal development is not, however, well understood.
CBP heterozygous mutant mice are viable and fertile and show clinical features of Rubinstein-Taybi syndrome,11,12 but the CBP homozygous mutantation is embryonic lethal. Recently, Yao et al13 reported that p300-deficient mice show embryonic lethality due to poor cardiac development in the presence of normal levels of CBP. These data indicate that physiological and biochemical functions of CBP and p300 do not fully overlap, at least during embryonic development.
During mouse embryogenesis, hematopoiesis begins in the yolk sac at E7.5 and it shifts to the fetal liver and then to the spleen and bone marrow.14 Hematopoiesis before formation of the fetal liver is known as primitive hematopoiesis and is distinguished from the adult-type definitive hematopoiesis by specific expression of an embryonic-type of globin in nucleated erythrocytes. In the mouse embryo, a pre-liver intraembryonic site of definitive hematopoietic activity has been identified.15 This mesodermally derived region of the mouse embryo contains the para-aortic splanchnopleural mesoderm (P-Sp) region or dorsal aorta, genital ridge/gonads and pro/mesonephros (aorta-gonad-mesonephros, AGM) and has been shown to harbor adult-type multipotent hematopoietic stem cells.15-18 The development of hematopoiesis is closely linked to that of vasculo-angiogenesis. Endothelial and hematopoietic cells in blood islands are proposed to originate from a common precursor, termed the hemangioblast, based on their simultaneous emergence. To clarify the function of CBP in embryonal development, we performed a close examination of the CBP mutant embryos with special attention to defects in the hematopoietic and vascular system.
MATERIALS AND METHODS
Analysis of DNA and RNA.
For preparation of genomic DNA, embryonic stem (ES) cells were lysed with sodium dodecyl sulfate (SDS) proteinase K and with phenol/chloroform, 1:1 (vol/vol) twice. The genomic DNA was precipitated with ethanol and dissolved in 10 mmol/L Tris-HCl, pH 7.5/1 mmol/L EDTA. Six micrograms of genomic DNA was digested with appropriate restriction enzymes, electrophoresed on a 0.9% agarose gel, and blotted onto a nylon membrane (Boehringer Mannheim, Mannheim, Germany). Hybridization was performed using a DIG DNA Labeling and Detection Kit (Boehringer Mannheim).
For genotyping of the embryos, we used reverse transcriptase-polymerase chain reaction (RT-PCR) to detect fragments of the wild-type CBP and recombinant CBP/β-geo fusion transcript. Total tissue RNA from E9.5 embryos or yolk sacs was extracted with TRIZOL (GIBCO-BRL, Gaithersburg, MD). cDNA from total RNA was synthesized with Superscript II Preamplification System (GIBCO-BRL). In brief, 3 μg of total RNA was digested with RNase free-DNase, heated at 70°C for inactivation of the enzyme, and denatured and hybridized with oligo dT primers. First-strand cDNA was synthesized by RT, and the RNA was digested with RNase H. The first-strand cDNA was used as the template for PCR with forward (5′-gtctgagatgatggaagagg-3′) and reverse (5′-gacctccacagtcttgtctg-3′) primers in a reaction consisting of 28 cycles of denaturation at 94°C for 60 seconds, annealing at 58°C for 90 seconds, and extension at 72°C for 90 seconds. Amplification of the wild-type transcript with these primers generates a 1,060-bp CBP fragment. To detect the CBP/β-geo fusion transcript, the same CBP forward primer and a splicing acceptor (SA) reverse primer (5′-tgctctgtcaggtacctgttgg-3′) were used for PCR under conditions described above. Amplification with this set of primers generates a 328-bp CBP/β-geo fusion cDNA fragment.
Western blotting.
Homogenates from CBP+/+ andCBP+/Mut adult mouse brain andCBPMut/Mut embryos were prepared as previously described.11,19 Thirty micrograms of brain or 5 μg of whole embryo homogenate proteins were subjected to 0.1% SDS/7.5% polyacrylamide gel electrophoresis (PAGE) as described by Laemmli.20 Proteins were then transferred to a nitrocellulose filter (Millipore, Bedford, MA) and detected using anti-CBP (CBP-A22 or CBP-C20; Santa Cruz, Santa Cruz, CA) antibodies21 and the ECL Detection System (Amersham, Arlington Heights, IL).
In vitro culture of P-Sp.
P-Sp explants derived from E9.5 CBP heterozygous and homozygous mutant embryos were cultured at 37°C in humidified 5% CO2 air on a layer of OP9 stromal cells. OP9 cells were maintained in α-modified minimum essential media (α-MEM; GIBCO-BRL) supplemented with 20% fetal calf serum (FCS; JRH Biosciences, Renexa, KS). Explants of E9.5 P-Sp containing a part of the omphalomesenteric artery (OA) were cultured on OP9 stromal cells in 10% FCS and 10−5mol/L 2-mercaptoethanol (2ME; Sigma, St Louis, MO) with or without full-length VEGF (Pepro Tech EC Ltd, London, UK). After 14 days in culture, an anti–PECAM-1 antibody (MEC13.3, rat anti-mouse monoclonal; Pharmingen, San Diego, CA) was used to stain vascular cells.22
To examine definitive hematopoiesis, explants of P-Sp were cultured on OP9 cells in RPMI1640 (GIBCO-BRL) supplemented with interleukin-6 (IL-6) (20 ng/mL), IL-7 (20 ng/mL) (gifts from Dr T. Sudo, Toray Industries Inc, Kamakura, Japan), stem cell factor (SCF) (50 ng/mL) (a gift from Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan), and erythropoietin (Epo) (2 U/mL) (a gift from Snow-Brand Milk Product Co, Tochigi, Japan) at 37°C in a humidified 5% CO2 air. The in vitro colony assay was performed in methylcellulose-containing medium as described previously.22 23 Briefly, cells disaggregated from the culture of P-Sp explants were obtained after 10 days, then plated in 1 mL of culture medium containing α-MEM, 1.2% methylcellulose (Aldrich Chemical Co, Milwaukee, WI), 30% FCS, 1% deionized bovine serum albumin (BSA; Sigma), 50 mmol/L 2ME, 200 U/mL IL-3, 2 U/mL Epo, and 50 ng/mL SCF. On the seventh day of culturing, aggregates consisting of 40 or more cells were counted as a single colony.
Whole-mount immunohistochemistry.
For whole-mount immunohistochemistry embryos were fixed in 4% paraformaldehyde at 4°C overnight. The fixed embryos were then rinsed in phosphate-buffered saline (PBS), dehydrated in a methanol series, and stored in 100% methanol at −80°C. The dehydrated embryos were bleached in methnol plus 5% vol/vol hydrogen peroxide for 4 to 5 hours at 4°C. The bleached embryos were rehydrated and blocked in PBSMT (2% instant skim milk, 0.1% Triton X-100, PBS) for 1 hour twice. The embryos were then incubated with 1:500 diluted anti–PECAM-1 antibody (MEC13.3) in PBSMT at 4°C overnight. The next day, the embryos were washed with PBSMT at 4°C five times (1 hour each) and incubated overnight at 4°C with 1 μg/mL horseradish peroxidase–conjugated goat anti-rat IgG in PBSMT. The next day, the embryos were washed with PBSMT at 4°C five times (1 hour each) and in PBST (0.1% Triton X-100, PBS) for 6 minutes three times at room temperature. Peroxidase staining was performed by incubating embryos in 0.3 mg/mL DAB (Sigma), 0.8 mg/mL NiCl2 in PBST for 20 minutes, followed by addition of hydrogen peroxide to a final concentration of 0.05%. The best signal-to-background ratio was typically achieved by a 5- to 10-minute incubation. The staining reaction was stopped by rinsing in PBST followed by postfixing in 0.1% glutaraldehyde in PBS at 4°C overnight.
RESULTS
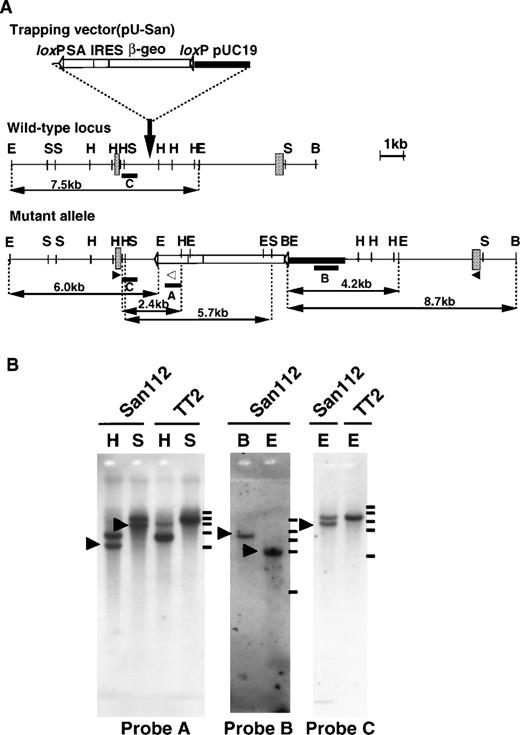
To identify functionally unique genes in embryogenesis, we took advantage of a random mutagenesis system using a gene-trapping strategy.24,25 First, we introduced the trapping vector, pU-San (Fig 1A), into TT2 ES cell through electroporation and established several ES clones containing insertional mutations. Using these clones, we generated chimeric mice and subsequent heterozygous mutants. Of the latter, the Ayu-San112 line, which showed apparent growth retardation after birth, was chosen for further analysis.11 Southern blot analysis with a vector fragment and an En-2 fragment as probes indicated that a single copy of the vector was integrated (Fig 1B). Analysis of the mutated genomic region and 5′RACE showed that the trapping vector was inserted into an intron of the CBP gene located between exons containing nucleotides 3064-3253 and 3254-3373 (Fig 1A). Restriction mapping and Southern blot analyses showed that gross deletion or rearrangement had not occurred at the inserted region (Fig 1B). The production of a truncated protein (residues 1-1084) was predicted from the integration pattern of the trapping vector into the CBP gene (Fig 1A). This truncated protein contains the CREB binding domain (residues 462-661), but not the histone acetyl transferase (HAT) domain (Fig 2C). We confirmed the expression of a fusion message of CBP and the reporter gene of pU-San in CBP+/mut and CBPmut/mut mouse embryos by RT-PCR analysis (Fig 2A). Furthermore, Western blot analysis using a specific antibody (CBP-A22) against the N-terminal region of CBP detected a 121-kD protein, which corresponds to the predicted size of the truncated protein in CBP+/mut brain and CBPmut/mut embryo, but not CBP+/+ embryos (Fig 2C). In CBPmut/mut embryos, wild-type CBP protein was not observed, indicating that only the truncated protein was produced (Fig 2C). In addition, with a C-terminal specific antibody (CBP-C20), the smaller fragment was not detected in three kinds of genotypes (data not shown). The heterozygotes showed various phenotypes of human Rubinstein-Taybi syndrome and were fertile,11 but no homozygotes were found among 98 newborn animals from heterozygous crosses, indicating that the homozygous mutation induces embryonic lethality. To determine the stage at which the CBP mutation was lethal, embryos from F3 or F4 intercrosses were examined. As shown in Fig 2D and E, E9.5 CBP homozygous mutants were smaller than their wild-type littermates and exhibited an open neural tube, both of which are seen in p300-deficient mice. Viable CBP homozygous mutants were not observed at E10.5.
Insertional mutation of the CBP gene. (A) The structures of the trapping vector (top), the wild-type allele (middle), and the mutant allele (bottom) are shown. The trapping vector, pU-San, contains two loxP sequences, a splice acceptor region (SA) of the mouse En-2 gene, an internal ribosomal entry site (IRES), a β-galactosidase/neomycin phosphotransferase fusion gene, the SV40 polyadenylation sequence (β-geo-pA), and the pUC19 vector as indicated. The gray boxes represent exons. Restriction enzyme sites (B, BamHI; E, EcoRI; H,HindIII; S, SphI), the location of probes (bars) used to confirm single integration of trapping vector, and the expected fragment sizes are indicated. Probe A is anSpeI-BgIII fragment in the SA of the trapping vector; probe B is an ScaI-XbaI fragment in pUC sequences of the trapping vector; probe C is theHindIII-XhoI fragment immediately upstream of the vector integrated region. The open and closed arrowheads indicate the location of primers used in RT-PCR for genotyping. (B) Southern blot analysis of an Ayu-San112 ES clone and a normal TT2 ES cell. (Left) A blot using HindIII (H) or SphI-digested (S) DNA hybridized with probe A. (Middle) A blot BamHI- (B) or EcoRI-digested (E) DNA with probe B. (Right) A blot of using EcoRI-restricted DNA hybridized with probe C. Molecular-weight makers are shown on the right. The bars indicate the positions of size marker: 23130, 9416, 6557, 4361, and 2322 bp from the top.
Insertional mutation of the CBP gene. (A) The structures of the trapping vector (top), the wild-type allele (middle), and the mutant allele (bottom) are shown. The trapping vector, pU-San, contains two loxP sequences, a splice acceptor region (SA) of the mouse En-2 gene, an internal ribosomal entry site (IRES), a β-galactosidase/neomycin phosphotransferase fusion gene, the SV40 polyadenylation sequence (β-geo-pA), and the pUC19 vector as indicated. The gray boxes represent exons. Restriction enzyme sites (B, BamHI; E, EcoRI; H,HindIII; S, SphI), the location of probes (bars) used to confirm single integration of trapping vector, and the expected fragment sizes are indicated. Probe A is anSpeI-BgIII fragment in the SA of the trapping vector; probe B is an ScaI-XbaI fragment in pUC sequences of the trapping vector; probe C is theHindIII-XhoI fragment immediately upstream of the vector integrated region. The open and closed arrowheads indicate the location of primers used in RT-PCR for genotyping. (B) Southern blot analysis of an Ayu-San112 ES clone and a normal TT2 ES cell. (Left) A blot using HindIII (H) or SphI-digested (S) DNA hybridized with probe A. (Middle) A blot BamHI- (B) or EcoRI-digested (E) DNA with probe B. (Right) A blot of using EcoRI-restricted DNA hybridized with probe C. Molecular-weight makers are shown on the right. The bars indicate the positions of size marker: 23130, 9416, 6557, 4361, and 2322 bp from the top.
Characterization of CBP homozygous mutant embryos. (A) RT-PCR analysis of RNAs isolated from yolk sacs of wild-type embryos, heterozygous embryos, and homozygous embryos. A CBP 5′-end forward primer and a 3′-end reverse primer, indicated in Fig 1A, are used to detect wild-type CBP transcripts. The same CBP 5′ forward primer and a reverse primer in the splice acceptor region are used to detect CBP/β-geo fusion transcripts. (B) Western blotting of extracts (30 μg protein) from the brains of adult mice (right) and extracts (5 μg protein) from E9.5 embryos (left) with N-terminal specific anti-CBP antibodies (CBP-A22). An arrowhead indicates the 265-kD wild-type CBP protein. An arrow indicates the truncated form of CBP at 121 kD. (C) The schematic structure of putative truncated and wild-type CBP and p300. Percentages refer to amino acid (aa) identify between proteins. (D) Phenotypes of wild-type and CBP homozygous mutant embryos at E9.5. A mutant is smaller and much paler than a wild-type littermate. The morphology of the cranial region (arrow) of mutants is distinct from that of wild-type littermates. (E) A CBP homozygous mutant displays an open neural tube (arrowhead) in the cranial region.
Characterization of CBP homozygous mutant embryos. (A) RT-PCR analysis of RNAs isolated from yolk sacs of wild-type embryos, heterozygous embryos, and homozygous embryos. A CBP 5′-end forward primer and a 3′-end reverse primer, indicated in Fig 1A, are used to detect wild-type CBP transcripts. The same CBP 5′ forward primer and a reverse primer in the splice acceptor region are used to detect CBP/β-geo fusion transcripts. (B) Western blotting of extracts (30 μg protein) from the brains of adult mice (right) and extracts (5 μg protein) from E9.5 embryos (left) with N-terminal specific anti-CBP antibodies (CBP-A22). An arrowhead indicates the 265-kD wild-type CBP protein. An arrow indicates the truncated form of CBP at 121 kD. (C) The schematic structure of putative truncated and wild-type CBP and p300. Percentages refer to amino acid (aa) identify between proteins. (D) Phenotypes of wild-type and CBP homozygous mutant embryos at E9.5. A mutant is smaller and much paler than a wild-type littermate. The morphology of the cranial region (arrow) of mutants is distinct from that of wild-type littermates. (E) A CBP homozygous mutant displays an open neural tube (arrowhead) in the cranial region.
The yolk sac of E9.5 CBP homozygous mutant embryos was obviously pale compared with that of wild-type littermates and the vessels appeared scarce (Fig 3A). The mutants themselves were pale and showed arrested growth at E8.75-9.0, allowing unequivocal identification of homozygous mutants (Fig 2D). Histological examination of the yolk sac showed that erythroid cells were remarkably reduced in mutants (Fig 3B). Although the total number of blood cells from the yolk sac was reduced to 20% of control in CBP homozygous mutants (Fig 3C), ε and β hemoglobin staining showed that the blood cells in the yolk sac contained a mature stage of primitive erythroid cells (Fig 3D). Despite normal maturation of red blood cells, CBP homozygous mutant embryos showed marked anemia and failed to survive beyond the stage of primitive hematopoiesis. To determine how development of primitive hematopoiesis is disturbed, we examined a colony-forming capacity of E9.5 homozygous yolk sac cells.26 They showed 22% and 69%, respectively, of erythroid and granulocyte-macrophage (GM) colony-forming capacity compared with both heterozygous and wild-type counterparts (Table1).
Phenotypes of CBP homozygous mutant embryos. (A) The E9.5 yolk sac from wild-type (left) and CBP (right) homozygous mutant embryos. (B) Section of the yolk sac from a wild-type (top) and a CBP homozygous mutant (bottom) at E9.5. Note the small number of erythrocytes in the yolk sac derived from CBP homozygous mutants. Scale bar indicates 100 μm. (C) Numbers of nucleated erythroid cells present in yolk sac cells from embryos at E9.5. (D) Staining of blood cells from a wild-type (left) and CBP homozygous mutant (right) yolk sac with antibodies to ɛ1-globin (top) and β-globin (bottom). Scale bar indicates 30 μm.
Phenotypes of CBP homozygous mutant embryos. (A) The E9.5 yolk sac from wild-type (left) and CBP (right) homozygous mutant embryos. (B) Section of the yolk sac from a wild-type (top) and a CBP homozygous mutant (bottom) at E9.5. Note the small number of erythrocytes in the yolk sac derived from CBP homozygous mutants. Scale bar indicates 100 μm. (C) Numbers of nucleated erythroid cells present in yolk sac cells from embryos at E9.5. (D) Staining of blood cells from a wild-type (left) and CBP homozygous mutant (right) yolk sac with antibodies to ɛ1-globin (top) and β-globin (bottom). Scale bar indicates 30 μm.
Yolk Sac Hematopoietic Progenitors in E9.5 Embryos
| Colonies per Yolk Sac . | |||||
|---|---|---|---|---|---|
| Wild type . | Heterozygote . | Homozygote . | |||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 232 ± 39 | 143 ± 35 | 221 ± 39 | 146 ± 34 | 50 ± 17 | 100 ± 52 |
| Colonies per Yolk Sac . | |||||
|---|---|---|---|---|---|
| Wild type . | Heterozygote . | Homozygote . | |||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 232 ± 39 | 143 ± 35 | 221 ± 39 | 146 ± 34 | 50 ± 17 | 100 ± 52 |
Hematopoietic colony assays from total yolk sac cells of CBP homozygous mutant embryos and wild-type littermates at E9.5. Numbers of hematopoietic colonies were scored after 7 days of culture of yolk sac cells. Total numbers of colonies (>40 cells) per one yolk sac were obtained from three independent experiments and expressed as the mean ± SEM (n = 3). Methylcellulose culture was performed in the presence of IL-3 (200 U/mL), SCF (50 ng/mL), and Epo (2 U/mL).
The pattern of the vascular system of CBP homozygous mutants was studied by staining E9.5 embryos in whole mount or tissue sections with the anti-PECAM monoclonal antibody, which detects differentiated endothelial cells.27 We found that all homozygous mutant embryos failed to form an organized vascular network at E9.5 (Fig 4A and B). Gross defects of vascular branching were observed especially in the head and trunk including the P-Sp region (Fig 4C through F). Because embryos have already finished “turning” at this stage, abnormalities in the vascular system cannot be caused by growth retardation of homozygous mutants. Immunohistochemical analysis showed scarce and disoriented vessels and a decrease in PECAM-1+ endothelial cells in the brain and P-Sp regions (Fig 5A through D [see page 2774]).
Vascular network formation in CBP homozygous mutant embryos. (A and B) E9.5 whole embryos stained with anti–PECAM-1 antibody to visualize all vessel endothelial cells. Note lack of vascular network formation in CBP homozygous mutant embryos. (C and D) Higher magnification of the head region of E9.5 embryo. Note lack of large vessels and smaller vascular branches in the homozygous embryo. (E and F) Higher magnification of the P-Sp region of E9.5 embryos. Note poor vascular network formation of P-Sp region in CBP homozygous mutant embryos.
Vascular network formation in CBP homozygous mutant embryos. (A and B) E9.5 whole embryos stained with anti–PECAM-1 antibody to visualize all vessel endothelial cells. Note lack of vascular network formation in CBP homozygous mutant embryos. (C and D) Higher magnification of the head region of E9.5 embryo. Note lack of large vessels and smaller vascular branches in the homozygous embryo. (E and F) Higher magnification of the P-Sp region of E9.5 embryos. Note poor vascular network formation of P-Sp region in CBP homozygous mutant embryos.
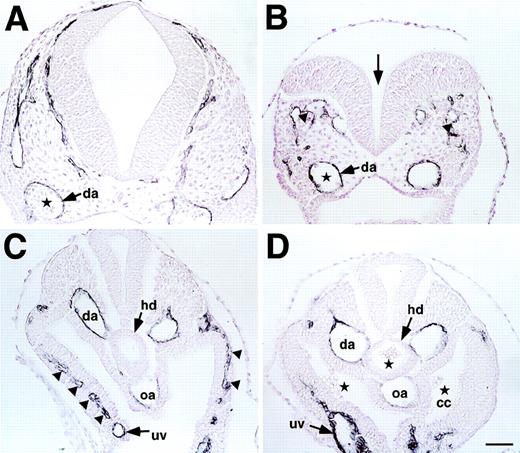
(A through D) Histological analysis of E9.5 wild-type (A and C) and CBP homozygous (B and D) mutant embryos stained with anti-PECAM antibody and counter-stained with hematoxylin. Open neural tube (arrow in B), disoriented posterior branch of primary head vein (arrowheads in B), and decreased numbers of blood cells in dorsal aorta (da; stars in A and B) were observed in the head region of CBP homozygous mutant embryos. In the trunk, lack of sprouting vessels from the dorsal aorta or umbilical vein (uv) in parietal mesoderm (arrowheads in C) and a decrease in PECAM-1+endothelial cells in the omphalomesenteric artery (oa) and dorsal aorta (arrows in D) were observed in CBP homozygous mutant embryos. Note ectopic blood cells in hindgut diverticulum (hd) and coelomic cavity (cc) in CBP homozygous mutant embryos (stars in D). Scale bar indicates 50 μm.
(A through D) Histological analysis of E9.5 wild-type (A and C) and CBP homozygous (B and D) mutant embryos stained with anti-PECAM antibody and counter-stained with hematoxylin. Open neural tube (arrow in B), disoriented posterior branch of primary head vein (arrowheads in B), and decreased numbers of blood cells in dorsal aorta (da; stars in A and B) were observed in the head region of CBP homozygous mutant embryos. In the trunk, lack of sprouting vessels from the dorsal aorta or umbilical vein (uv) in parietal mesoderm (arrowheads in C) and a decrease in PECAM-1+endothelial cells in the omphalomesenteric artery (oa) and dorsal aorta (arrows in D) were observed in CBP homozygous mutant embryos. Note ectopic blood cells in hindgut diverticulum (hd) and coelomic cavity (cc) in CBP homozygous mutant embryos (stars in D). Scale bar indicates 50 μm.
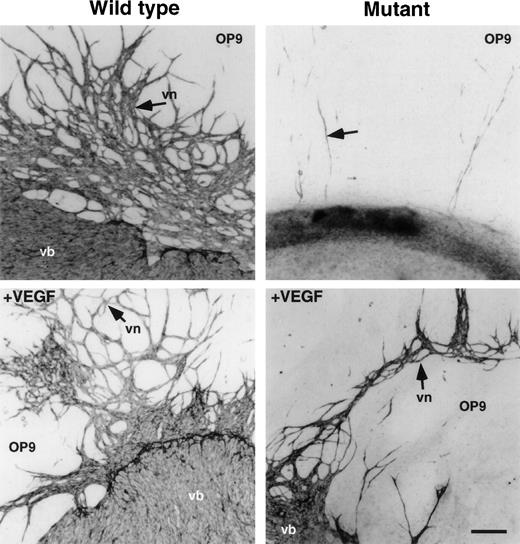
To determine how vascular networks are defective in CBP homozygous mutant embryos, organ culture of P-Sp from E9.5 embryos was undertaken on OP9 stromal cells.18,22 28 No vascular bed or network formation was detected in P-Sp from CBP homozygous mutant embryos, while cultures from wild-type embryos developed normally (Fig6). Because P-Sp from E8.5 wild-type embryos develops vasculature (data not shown), we concluded that vascular formation by CBP homozygous mutant embryos is impaired rather than delayed. Transcripts for VEGF165 and VEGF receptors, Flk-1 and Flt-1, remained unchanged in embryos homozygous for the CBP mutation (data not shown). Addition of 100 ng/mL VEGF to this culture slightly rescued vascular bed and network formation in mutants (Fig 6). However, this recovery was less than that seen in wild-type littermate explants cultured with or without VEGF.
Vasculo-angiogenesis in P-Sp culture. P-Sp explants derived from E9.5 CBP homozygous mutant embryos and CBP wild-type littermates were cultured on OP9 stromal cells. Note that vascular bed and vascular network formation were defective in mutant embryo explants compared with that of wild-type littermates. VEGF (100 ng/mL) was added to the culture system as noted above. Vascular bed (vb) and vascular network (vn) formation were partially rescued in CBP homozygous mutants by addition of VEGF. However, note that vascular bed and network formation of P-Sp explants from CBP homozygous mutant embryos was poorer than that of wild-type littermates. Scale bar indicates 50 μm.
Vasculo-angiogenesis in P-Sp culture. P-Sp explants derived from E9.5 CBP homozygous mutant embryos and CBP wild-type littermates were cultured on OP9 stromal cells. Note that vascular bed and vascular network formation were defective in mutant embryo explants compared with that of wild-type littermates. VEGF (100 ng/mL) was added to the culture system as noted above. Vascular bed (vb) and vascular network (vn) formation were partially rescued in CBP homozygous mutants by addition of VEGF. However, note that vascular bed and network formation of P-Sp explants from CBP homozygous mutant embryos was poorer than that of wild-type littermates. Scale bar indicates 50 μm.
To examine definitive hematopoiesis of the mutant embryos, we performed coculture of E9.5 P-Sp on OP9 cells. After 7 days of culture, the colony-forming cells (CFCs) of nonadherent cells was examined in a methylcellulose medium. As shown in Table2, the total number of CFCs in cultured P-Sp cells from homozygous mutants was markedly reduced compared with those from wild-type P-Sp. Addition of 100 ng/mL VEGF to this culture partially rescued erythroid colony formation in mutants (Table 2), as the vascular formation was recovered. However, the number of CFCs did not reach the level of wild type.
Hematopoietic Progenitors in Cultured P-Sp Explants
| Colonies per P-Sp Explant . | |||||||
|---|---|---|---|---|---|---|---|
| Wild Type . | Heterozygote . | Homozygote . | Homozygote (+VEGF) . | ||||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 72 ± 46 | 705 ± 127 | 70 ± 27 | 703 ± 33 | 0 ± 0 | 147 ± 81 | 18 ± 5 | 193 ± 20 |
| Colonies per P-Sp Explant . | |||||||
|---|---|---|---|---|---|---|---|
| Wild Type . | Heterozygote . | Homozygote . | Homozygote (+VEGF) . | ||||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 72 ± 46 | 705 ± 127 | 70 ± 27 | 703 ± 33 | 0 ± 0 | 147 ± 81 | 18 ± 5 | 193 ± 20 |
P-Sp of E9.5 CBP homozygous mutant embryos and wild-type littermates were cultured on OP9 cells for 10 days. Nonadherent cells were then assayed for hematopoietic colonies in a methylcellulose medium. The number of hematopoietic colonies was scored after 7 days of culture. In vitro hematopoietic activity of P-Sp culture of homozygous embryos was partially rescued by 100 ng/mL VEGF (+VEGF). Total numbers of colonies (>40 cells) per one explant were obtained from three independent experiments and expressed as the mean ± SEM (n = 3).
DISCUSSION
In this report, we show that the transcription cofactor, CBP, is essential for embryogenic hematopoiesis and vasculo-angiogenesis. Because the development of hematopoiesis is associated with that of vasculo-angiogenesis, the phenotypes of CBP mutants are noteworthy.
Hematopoiesis consists of primitive hematopoiesis in the yolk sac and definitive hematopoiesis which develops in the P-Sp region.15-18 Here, we show a reduction in hematopoietic progenitor cells in the yolk sac and P-Sp of CBP homozygous mutant mice. Therefore, anemia seen in mutant mice is due not to a loss of blood but to an intrinsic defect in proliferation of progenitor cells in both primitive and definitive hematopoietic system. In CBP mutant mice, the erythroid lineage was more susceptible than the GM lineage (Tables 1 and 2). It has been shown that the E1A binding region of CBP binds to the zinc finger region of GATA-1 and that CBP markedly stimulates GATA-1’s transcriptional activity.29 It is also likely that a defect in vascular endothelial cells may cause the defect in hematopoiesis, because these cells play a critical role in the hematopoietic microenvironment. In vitro definitive hematopoiesis of homozygous embryos was partially rescued by addition of VEGF (Table 2). To determine whether the hematopoietic defect is intrinsic or a consequence of defective angiogenesis, mouse chimera analysis will be required.
Gross defects in vascular branching is evident in CBP homozygous mutant mice at E9.5. The number of PECAM-1+ endothelial cells is significantly decreased. These abnormalities are confirmed by in vitro coculture of P-Sp on OP9 stromal layers. Embryonic vessel formation consists of two steps: vasculogenesis, the de novo organization of endothelial cells into vessels, and angiogenesis, the continued expansion of the vascular tree as a result of endothelial cell sprouting from existing vessels. In the P-Sp explant culture system, both vasculogenesis and angiogenesis can be observed in vitro.22 Both processes were defective in P-Sp cultures from CBP mutant mice. Addition of VEGF to mutant cultures partially rescued these defects. Expression of angiogenic factors such as VEGF and angiopoietins and their receptors, Flk-1, Flt-1, and TIE2, in mutant mice were not changed compared with wild type (data not shown). Therefore, we conclude that the defect in vascular network formation in CBP homozygous mutant embryos is due to the inability of putative angioblasts in P-Sp to proliferate and differentiate. Recently, it has been reported that CBP transactivates by interacting with Ets-1, which is expressed in migrating and sprouting endothelial cells.30 Moreover, Smad proteins, which are involved in mediating the transforming growth factor-β (TGF-β)-response can functionally interact with CBP in endothelial cells.31 It remains to be clarified whether homozygous mutants exhibit altered expression of Ets-1 and/or Smads during embryonic angiogenesis.
In our study, CBP homozygous mutants, which made a truncated CBP protein (residues 1-1084) showed embryonic lethality. Investigation of the phenotypes of CBP homozygous null mutants compared with our disrupted CBP mutants will show the function of the N-terminal or C-terminal domain of CBP in vivo. Although specific details of the phenotype of CBP null mutants have not yet been published, the mice die at E8-10 and display neural tube defects, similar to our CBP disrupted mutants.13
In summary, mice homozygous for a truncated form of CBP mutants die between E9.5 and E10.5 and exhibit impaired hematopoiesis and vasculo-angiogenesis. The P-Sp culture shows an autonomous abnormality of putative endothelial precursors, which may induce the microenvironmental defect in hematopoiesis. Our analyses show that CBP plays an essential role in hematopoiesis and vasculo-angiogenesis in mammalian cells.
ACKNOWLEDGMENT
We thank Y. Kiyonaga, M. Tokushima, and I. Kawasaki for technical assistance.
Y.O. and N.T. made an equal contribution to this paper.
Supported by grants from the Ministry of Education, Science and Culture of Japan, a grant from the Yamanouchi Foundation for Research on Metabolic Disorders, a grant from the Osaka Foundation for Promotion of Clinical Immunology, and a grant from the Science and Technology Agency.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Toshio Suda, MD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Honjo 2-2-1, Kumamoto 860-0811, Japan; e-mail: sudato@gpo.kumamoto-u.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal