Abstract
Hematopoietic and endothelial cell lineages share common progenitors. Accordingly, cytokines formerly thought to be specific for the hematopoietic system have been shown to affect several functions in endothelial cells, including angiogenesis. In this study, we investigated the angiogenic potential of erythropoietin (Epo), the main hormone regulating proliferation, differentiation, and survival of erythroid cells. Epo receptors (EpoRs) have been identified in the human EA.hy926 endothelial cell line by Western blot analysis. Also, recombinant human Epo (rHuEpo) stimulates Janus Kinase-2 (JAK-2) phosphorylation, cell proliferation, and matrix metalloproteinase-2 (MMP-2) production in EA.hy926 cells and significantly enhances their differentiation into vascular structures when seeded on Matrigel. In vivo, rHuEpo induces a potent angiogenic response in the chick embryo chorioallantoic membrane (CAM). Accordingly, endothelial cells of the CAM vasculature express EpoRs, as shown by immunostaining with an anti-EpoR antibody. The angiogenic response of CAM blood vessels to rHuEpo was comparable to that elicited by the prototypic angiogenic cytokine basic fibroblast growth factor (FGF2), it occurred in the absence of a significant mononuclear cell infiltrate, and it was not mimicked by endothelin-1 (ET-1) treatment. Taken together, these data demonstrate the ability of Epo to interact directly with endothelial cells and to elicit an angiogenic response in vitro and in vivo and thus act as a bona fide direct angiogenic factor.
RECENT STUDIES HAVE indicated that several cytokines and interleukins (ILs) formerly thought to be specific for the hematopoietic system, including granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, IL-4, IL-6, and IL-8, are also capable of affecting certain functions of endothelial cells.1-9 This responsiveness of both vascular and hematopoietic systems to some cytokines and ILs may reflect the common ontogenesis of endothelial and hematopoietic cells. Indeed, differentiation of vascular endothelium during embryonic development is closely linked to the appearance of primitive hematopoietic cells, suggesting that both cell lineages share a common progenitor, the hemangioblast.10,11 This is supported by the finding that cell surface antigens present on endothelium are also expressed by hematopoietic cells, including quail QH1 and MB1 antigens12,13 and human and murine CD31 and CD34 antigens.14,15 Accordingly, deletion of the endothelial-specific vascular growth factor receptor 2 (VEGFR2) by gene targeting has shown that both endothelial and hematopoietic cells are absent in homozygous null mice.16 VEGFR2 expression also defines a population of early hematopoietic precursors17 and VEGFR2+ cells isolated from the chick embryo mesoderm at the gastrulation stage give rise to either hematopoietic or endothelial cell colonies.18 Moreover, endothelial cell precursors have been isolated from the peripheral blood on the basis of cell surface antigen expression.19
Erythropoietin (Epo) was formerly believed to exert its hematopoietic effects by stimulating the proliferation of early erythroid precursors and the differentiation of later precursors of the erythroid lineage.20 However, the appearance of side effects arising from the vascular system, including hypertension and thrombosis21 during the therapeutic use of recombinant human Epo (rHuEpo), has prompted investigation of a possible interaction of Epo with endothelial cells. The Epo receptor (EpoR), in fact, has been demonstrated in endothelial cells in vitro and in vivo.22 rHuEpo also stimulates cell migration, proliferation, endothelin-1 (ET-1) release, and an increase in cytosolic free calcium concentration in endothelial cell cultures.23-26
Angiogenesis, the formation of new capillaries from pre-existing vessels, is a process involved in vascularization of organs of ectodermal or mesenchymal origin during embryonic development.27 In the adult, the proliferation rate of endothelial cells is very low compared with other cell types in the body. Physiological exceptions in which angiogenesis occurs under tight regulation are found in the female reproductive system and during wound healing. Uncontrolled endothelial cell proliferation has pathological implications and plays a pivotal role in tumor progression as well as in inflammatory and viral diseases.28 During neovascularization, endothelial cells change their genetic program and express an angiogenic phenotype that includes the production of proteases, cell migration, and proliferation followed by redifferentiation, thus resulting in the formation of new blood vessels.27
Several angiogenic growth factors have been characterized so far, including members of the VEGF and fibroblast growth factor (FGF) families.29,30 Interestingly, hematopoietic stimulators, including G-CSF and GM-CSF,1,2 have also been shown to induce an angiogenic response in endothelial cells, suggesting that endothelial cell growth and survival may contribute to the maintenance of bone marrow microenvironment and hematopoiesis. Similar to well-known angiogenic growth factors, Epo affects some properties of endothelial cells in culture, eg, cell proliferation and motility (see above), that are related to neovascularization in vivo, thus suggesting that it acts as an angiogenic factor. Nevertheless, to our knowledge there is no direct experimental evidence of the ability of Epo to induce neovascularization in vivo. This appears to be of particular importance when considering that IL-4 exerts an anti-angiogenic activity in vivo31 despite its ability to induce cell proliferation and protease production in cultured endothelial cells.5 32 We have therefore investigated the ability of rHuEpo to induce a pro-angiogenic phenotype in cultured endothelial cells, namely an increase in cell proliferation, protease production and morphogenesis, and the stimulation of new blood vessel formation in the chick embryo chorioallantoic membrane (CAM). The results unequivocally demonstrate that rHuEpo is a potent angiogenic factor both in vitro and in vivo.
MATERIALS AND METHODS
Cells.
Immortalized EA.hy926 endothelial cells, derived from the fusion of human umbilical vein endothelial cells (HUVECs) with A549 lung carcinoma cells,33 were maintained in Dulbecco’s modified Minimal Essential Medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% glutamine. HUVECs were grown in M199 medium supplemented with 10% FCS.
Immunoprecipitation with anti-Janus Kinase-2 (JAK-2) and Western blot analysis of EpoR and JAK-2 phosphorylation.
Confluent cell cultures were lysed in phosphate-buffered saline (PBS) containing 2% Triton-X100. Cellular extracts (25 μg) were resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and subjected to 8% SDS-PAGE. Proteins were transferred electrophoretically to a polyvinylidene difluoride (PVDF) membrane (NEN, Boston, MA) and probed with anti-EpoR rabbit antiserum (Santa Cruz Biotechnology, Santa Cruz, CA). The membrane was then incubated sequentially with horseradish peroxidase-conjugated secondary antibody (DAKO, Glostrup, Denmark) and with Renaissance chemiluminescence reagents (Du Pont de Nemours, Boston, MA) according to the manufacturer’s instructions and exposed to Reflection film (Du Pont de Nemours). An EpoR synthetic peptide (Santa Cruz Biotechnology) was used as positive control.
For evaluation of JAK-2 phosphorylation, EA.hy926 cells were cultured in serum-free medium for 16 to 18 hours and exposed to 0.5 to 30 U/mL rHuEpo (Eprex; Janssen-Cilag, Cologno Monzese, Milan, Italy) for different periods of time. They were then lysed in ice-cold lysis buffer (20 mmol/L HEPES, pH 7.2, 150 mmol/L NaCl, 1% Triton-X100, 10% glycerol, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 1 μg/mL leupeptin, 100 U/mL aprotinin, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 1 mmol/L NaVO4, 2 mmol/L NaPPi) for 20 minutes on ice. Lysates were centrifuged at 12,000 rpm for 15 minutes and incubated with rabbit preimmune serum (Santa Cruz Biotechnology) and 50 μL of 50% protein A slurry (Sigma Chemical Co, St Louis, MO). Supernatants were collected and incubated overnight at 4°C with anti–JAK-2 rabbit antiserum (Santa Cruz Biotechnology) and 50 μL of 50% protein A slurry. Immunoprecipitates were washed with 20 mmol/L HEPES, pH 7.2, 150 mmol/L NaCl, 10% glycerol, 1% Triton-X100; resuspended in SDS-PAGE sample buffer; subjected to 8% SDS-PAGE; and probed with anti-phosphotyrosine monoclonal antibody (Transduction Laboratories, Lexington, UK) as described above.
The membrane was then stripped in 6.25 mmol/L Tris/Cl (pH 6.8) SDS, 100 mmol/L β mercaptoethanol for 30 minutes at 50°C and then washed in TBS-Tween 20 three times and incubated in blocking buffer for 1 hour. The membrane was incubated with the anti-EpoR rabbit antiserum for 1 hour at room temperature, washed again, and then incubated with antirabbit antibody horseradish peroxidase-conjugate for 1 hour. The filter was washed in TBS 0.1% Tween 20 and incubated with enhanced chemiluminescence (NEN). The signal was shown by exposure to Kodak biomax film (Eastman Kodak, Rochester, NY). After one more stripping, the same procedure was applied to the same membrane for the anti–JAK-2 antiserum.
Cell proliferation assay.
EA.hy926 cells were plated at 2 × 103 cells per well in 96-well plates precoated with 1% gelatin. After 24 hours, medium was removed and replaced every other day with fresh DMEM containing 0.25% FCS and supplemented 1:1 (vol:vol) with RPMI-1640 medium alone or containing increasing concentrations (from 0.5 to 40 U/mL) of rHuEpo. Experiments were performed in quadruplicate. The cell number was measured at day 6 of growth34 by the colorimetric method of Kueng et al.35 Briefly, cells were fixed for 20 minutes at room temperature with 2.5% glutaraldehyde, stained with 0.1% crystal violet in 20% methanol, and solubilized with 10% acetic acid. Wells were read at 595 nm with a microplate reader (Model 3550; Bio-Rad Laboratories, Richmond, CA) and the cell number was calculated from an appropriate calibration curve. Values are expressed as mean ± 1 standard deviation (SD).
Matrix metalloproteinase (MMP) SDS-PAGE zymography.
EA.hy926 cells at 80% confluence were cultured for 24 hours in serum-free medium in the absence or in the presence of 0.5, 1.0, or 2.0 U/mL of rHuEpo. After incubation, the conditioned medium was collected, sequentially centrifuged at 1,200 and 12,000 rpm for 10 minutes, filtered through 0.54-μm pore-size filters (Costar, Cambridge, MA), and stored at −80°C until use. Gelatin-zymography was performed to visualize the MMP activity present in the samples.36 Five micrograms of protein were applied to 7.5% SDS-PAGE gels copolymerized with type A gelatin from porcine skin (Sigma Chemical Co) at a final concentration of 0.6 mg/mL. After electrophoresis, gels were washed in 2.5% Triton-X100 for 1 hour to remove SDS, incubated for 18 hours at 37°C in collagenase buffer, and stained in 0.1% Coomassie brilliant blue. Gelatinolytic activity was visualized as a transparent band against a blue background and quantified by computerized image analysis of the band.
Matrigel morphogenetic assay.
This was performed as described previously.36 Briefly, EA.hy926 cells were plated at 2 × 105cells per well in 24-well plates precoated with 300 μL of Matrigel (8.1 mg/mL; Becton Dickinson Italia, Milan, Italy) in DMEM added with 0.1% BSA in the absence or in the presence of 0.5, 1.0, or 2.0 U of rHuEpo. After 6 hours of incubation in 5% CO2humidified atmosphere at 37°C, the cell three-dimensional organization was examined under an inverted phase contrast photomicroscope. Each treatment was performed in triplicate wells.
Chick embryo chorioallantoic membrane (CAM) assay.
Fertilized White Leghorn chick eggs were incubated under conditions of constant humidity at 37°C. On the third day of incubation, a square window was opened in the egg shell after removal of 2 to 3 mL of albumen so as to detach the developing CAM from the shell. The window was sealed with a glass of the same size and the eggs were returned to the incubator. At day 8, 1mm3 sterilized gelatin sponges (Gelfoam; Upjohn Co, Kalamazoo, MI) adsorbed with rHuEpo or ET-1 (Peninsula Laboratories, Belmont, CA) dissolved in 2 μL of PBS were implanted on the top of growing CAMs under sterile conditions within a laminar flow hood.37 rHuEpo was delivered at 1.0 to 10.0 U per implant and ET-1 at 10−6 to 10−8 mol/L per implant, whereas sponges containing vehicle alone or 1 μg of FGF2 were used as negative and positive controls, respectively. CAMs were examined daily until day 12 and photographed in ovo under a Zeiss stereomicroscope SR equipped with the MC 63 Camera System (Zeiss, Oberkochen, Germany). At day 12, CAMs were processed for light microscopy. Briefly, embryos and their membranes were fixed in ovo in Bouin’s fluid, and then sponges and the underlying and immediately adjacent CAM portions were removed and processed for embedding in paraffin. Eight-micrometer serial sections, cut according to a plane parallel to the surface of the CAM, were stained with 0.5% aqueous solution of toluidine blue (Merck Biochemica, Darmstadt, Germany) and observed under a Leitz-Dialux 20 light microscope (Leitz, Wetzlar, Germany). At day 12, some CAMs were also processed for electron microscopy. Briefly, the embryos and their membranes were fixed in ovo in 3% phosphate-buffered glutaraldehyde, dehydrated in serial alcohols, postfixed in 1% phosphate-buffered OsO4, and embedded in Epon 812. Ultrathin sections were cut on an LKB V ultramicrotome (LKB, Bromma, Sweden) according to a plane perpendicular to the surface of the CAM. The sections were stained with uranyl acetate followed by lead citrate and examined under a 9A Zeiss electron microscopy.
The angiogenic response and the infiltration of mononuclear cells were assessed by a planimetric method of point counting.38 39Briefly, every third section within 30 serial slides from an individual specimen was analyzed by a 144-point mesh inserted in the eyepiece of the Leitz-Dialux 20 photomicroscope. Six randomly chosen 250× fields of each section were used to count the total number of the intersection points that were occupied by vessels transversally cut (diameter ranging from 3 to 10 μm). Mean values ± SD were determined for each analysis. The vascular density was indicated by the final mean number of the occupied intersection points. The statistical significance of differences between the mean values of the intersection points in the experimental and control CAMs was determined by the Student’s t-test for unpaired data.
Immunohistochemistry.
The antibodies used in this study were (1) anti-factor VIII polyclonal rabbit antibody (Dako) and (2) polyclonal rabbit anti-EpoR antibody (Santa Cruz Biotechnology). Eight-micrometer acetone-fixed cryostat CAM sections, treated with 7.5% H2O2 to destroy endogenous peroxidase, were stained with a three-step avidin-biotin-immunoperoxidase, as described elsewhere.40 Briefly, incubation with primary antibodies and then biotin-labeled swine antirabbit Ig (Dako) and avidin-horseradish-peroxidase conjugate (Vector Inc, Burlingame, CA) was followed by red-staining with a 3-amino-9-ethylcarbazole (Sigma Chemical Co) solution and counterstaining with Gill’s hematoxylin no. 2 (Polysciences Inc, Warrington, PA) and was mounted in buffered glycerin. A preimmune rabbit serum (Dako) replacing the antibodies served as negative control.
RESULTS
EA.hy926 endothelial cells express the biologically active EpoR.
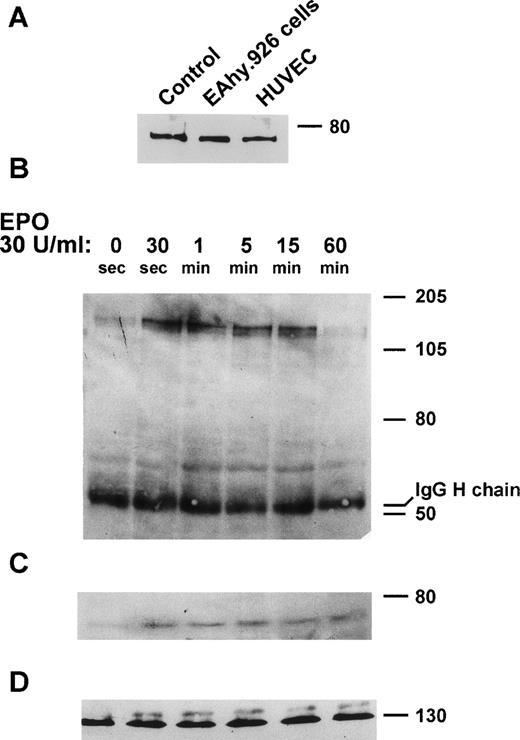
The immortalized EA.hy926 endothelial cell line retains several endothelial characteristics.33 As a preliminary experiment, the ability of EA.hy926 cells to express EpoR was investigated. To this purpose, an EA.hy926 cell extract was probed with anti-EpoR antibodies. As shown in Fig 1A, these antibodies recognize a molecular weight (Mr) 78,000 immunoreactive protein in the extract of EA.hy926 cells as well as HUVEC extracts used as an additional positive control.22 To assess whether the EpoR expressed by EA.hy926 cells is functionally active, the ability of Epo to stimulate JAK-2 phosphorylation41 was investigated. EA.hy926 cell cultures were treated with 0.5 to 30 U/mL rHuEpo for periods ranging from 30 seconds to 1 hour. Cell extracts were immunoprecipitated with anti–JAK-2 antibodies and sequentially probed with anti-phosphotyrosine, anti-EpoR, and anti–JAK-2 antibodies in a Western blot (Fig 1B through D). rHuEpo (30 U/mL) causes a transient increase in JAK-2 phosphorylation, with a maximal effect being observed 5 minutes after stimulation. Similar results were obtained when cells were stimulated with 5, 10, and 15 U/mL rHuEpo (data not shown). Moreover, EpoR coprecipitates with phosphorylated JAK-2, thus demonstrating the formation of a EpoR/phospho-JAK-2 complex in rHuEpo-treated cells (Fig 1C).
EpoR expression and rHuEpo-dependent JAK-2 activation in EA.hy926 cells. (A) Twenty-five–microgram aliquots of the extracts of confluent EA.hy926 cells and HUVECs were run on 8% SDS-PAGE gel and probed with anti-EpoR rabbit antiserum. EpoR synthetic peptide was used as a positive control. (B through D) EA.hy926 cells were incubated with 30 U/mL rHuEpo in serum-free conditions for the indicated periods of time. Cell extracts were then immunoprecipitated with anti–JAK-2 antibody. Immunoprecipitates were subjected to 8% SDS-PAGE and probed with anti-phosphotyrosine antibody in a Western blot (B). After stripping of the membrane, immunoprecipitates were probed with anti-EpoR antibody (C). Note that EpoR coprecipitates with phosphorylated JAK-2. Uniform loading of the gel was shown by probing the membrane with anti–JAK-2 antibody (D).
EpoR expression and rHuEpo-dependent JAK-2 activation in EA.hy926 cells. (A) Twenty-five–microgram aliquots of the extracts of confluent EA.hy926 cells and HUVECs were run on 8% SDS-PAGE gel and probed with anti-EpoR rabbit antiserum. EpoR synthetic peptide was used as a positive control. (B through D) EA.hy926 cells were incubated with 30 U/mL rHuEpo in serum-free conditions for the indicated periods of time. Cell extracts were then immunoprecipitated with anti–JAK-2 antibody. Immunoprecipitates were subjected to 8% SDS-PAGE and probed with anti-phosphotyrosine antibody in a Western blot (B). After stripping of the membrane, immunoprecipitates were probed with anti-EpoR antibody (C). Note that EpoR coprecipitates with phosphorylated JAK-2. Uniform loading of the gel was shown by probing the membrane with anti–JAK-2 antibody (D).
rHuEpo induces a pro-angiogenic phenotype in EA.hy926 endothelial cells.
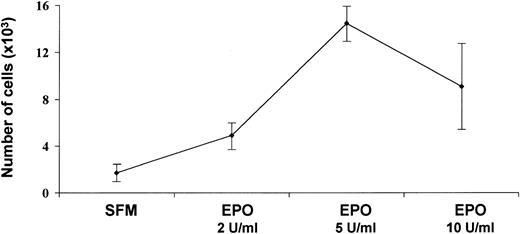
Several angiogenic growth factors induce a pro-angiogenic phenotype in endothelial cell cultures. This phenotype includes, among other responses, endothelial cell proliferation and protease production.42 To evaluate the mitogenic capacity of Epo, EA.hy926 cells were seeded at 2.5 × 103 per well in 96-well plates and treated every other day with fresh medium containing 0.25% FCS in the absence or in the presence of 0.5 to 40 U/mL rHuEpo. On day 6, cells were counted. In agreement with previous studies23 and our own observations on HUVECs and bovine adrenal capillary endothelial cells, rHuEpo exerted a significant increase in EA.hy926 cell proliferation (Fig 2). Maximal stimulation, corresponding to an eightfold increase in cell number, was observed at 5 U/mL rHuEpo. No further increase in cell number was observed at higher rHuEpo concentrations.
Effects of rHuEpo on EA.hy926 cell proliferation. Cells were seeded at 2 × 103 cells per well. After 24 hours, medium was removed and replaced every other day with fresh medium containing 0.25% FCS and supplemented 1:1 (vol:vol) with RPMI-1640 medium containing increasing concentrations of rHuEpo. The cell number was counted at day 6 of growth. The experiment was performed in quadruplicate and values are shown as the mean ± SD.
Effects of rHuEpo on EA.hy926 cell proliferation. Cells were seeded at 2 × 103 cells per well. After 24 hours, medium was removed and replaced every other day with fresh medium containing 0.25% FCS and supplemented 1:1 (vol:vol) with RPMI-1640 medium containing increasing concentrations of rHuEpo. The cell number was counted at day 6 of growth. The experiment was performed in quadruplicate and values are shown as the mean ± SD.
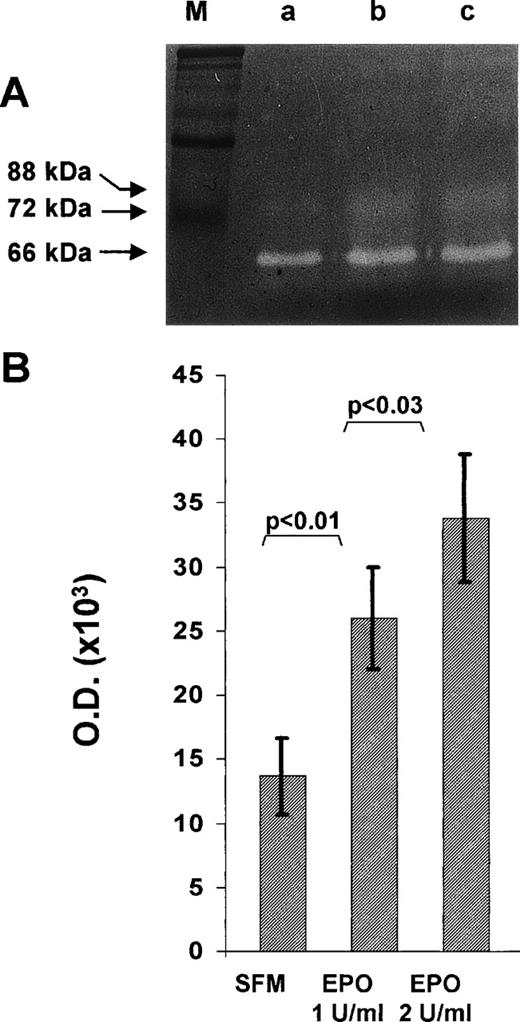
EA.hy926 cell cultures maintained in serum-free medium for 24 hours secreted significant amounts of the cleaved, activated form of MMP-2, as shown by the presence of a gelatinolytic band with an apparent Mr equal to 62,000 when their conditioned medium is analyzed by gelatin SDS-PAGE zymography (Fig 3A). When the cells were grown for 24 hours in the same medium supplemented with rHuEpo, MMP-2 activity increased. Soft laser scanning of the band, in fact, showed a threefold increase in the conditioned medium of EA.hy926 cells treated with 2 U/mL rHuEpo when compared with nonstimulated cultures (Fig 3B).
Effect of rHuEpo on MMP-2 production in EA.hy926 cells. Subconfluent EA.hy926 cells were cultured for 24 hours in serum free medium in the absence (SFM) or in the presence of the indicated concentrations of rHuEpo. After incubation, the conditioned medium was analyzed by gelatin-zymography as described in Materials and Methods. (A) One representative experiment showing the presence of a Mr 62,000 gelatinolytic band corresponding to activated MMP-2 in the conditioned medium of control (a), 1 U/mL rHuEpo (b), and 2 U/mL rHuEpo (c) treated cells. M, molecular weight markers. (B) Quantitation of MMP-2 activity by computerized image analysis of the gelatinolytic bands. Data are the mean ± SD of eight independent experiments (statistical analysis by Student’s t-test).
Effect of rHuEpo on MMP-2 production in EA.hy926 cells. Subconfluent EA.hy926 cells were cultured for 24 hours in serum free medium in the absence (SFM) or in the presence of the indicated concentrations of rHuEpo. After incubation, the conditioned medium was analyzed by gelatin-zymography as described in Materials and Methods. (A) One representative experiment showing the presence of a Mr 62,000 gelatinolytic band corresponding to activated MMP-2 in the conditioned medium of control (a), 1 U/mL rHuEpo (b), and 2 U/mL rHuEpo (c) treated cells. M, molecular weight markers. (B) Quantitation of MMP-2 activity by computerized image analysis of the gelatinolytic bands. Data are the mean ± SD of eight independent experiments (statistical analysis by Student’s t-test).
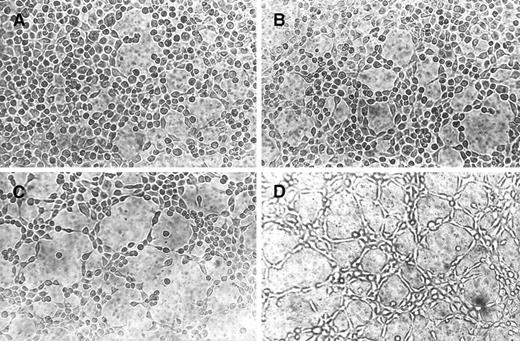
Cultures of endothelial cells on Matrigel resulted in the formation of vascular tubes and cord-like structures connecting cellular nodes, a phenomenon known as angiogenesis in vitro.43-47 This assay has been extensively used to study positive and negative regulators of angiogenesis.48 As shown in Fig4A, EA.hy926 cells remained spherical and isolated when seeded on Matrigel in the presence of serum-free medium, with small cellular nests and short tubes being rarely observed. By contrast, rHuEpo caused a dose-dependent morphogenetic effect (Fig 4B through D). At 2 U/mL rHuEpo, EA.hy926 cells migrated throughout the Matrigel surface and formed branching, anastomosing tubes with multicentric junctions, originating a meshwork of capillary-like structures (Fig 4D).
Morphogenic activity of rHuEpo. EA.hy926 cells were seeded on Matrigel and incubated in DMEM added with 0.1% BSA (A) or with 0.5 U/mL (B), 1.0 U/mL (C), or 2.0 U/mL (D) rHuEpo. After 6 hours, cells were photographed using an inverted phase contrast photomicroscope. A dose-dependent morphogenetic effect of rHuEpo was observed.
Morphogenic activity of rHuEpo. EA.hy926 cells were seeded on Matrigel and incubated in DMEM added with 0.1% BSA (A) or with 0.5 U/mL (B), 1.0 U/mL (C), or 2.0 U/mL (D) rHuEpo. After 6 hours, cells were photographed using an inverted phase contrast photomicroscope. A dose-dependent morphogenetic effect of rHuEpo was observed.
rHuEpo induces an angiogenic response in the chick embryo CAM.
The ability of rHuEpo to stimulate both early and late responses of the angiogenic cascade in EA.hy926 cells in vitro prompted us to assess its angiogenic capacity in vivo. To this purpose, chick embryo CAMs at day 8 of incubation were implanted with gelatin sponges adsorbed with rHuEpo dissolved in PBS. Sponges adsorbed with vehicle alone or with FGF2 were used as negative and positive controls, respectively.
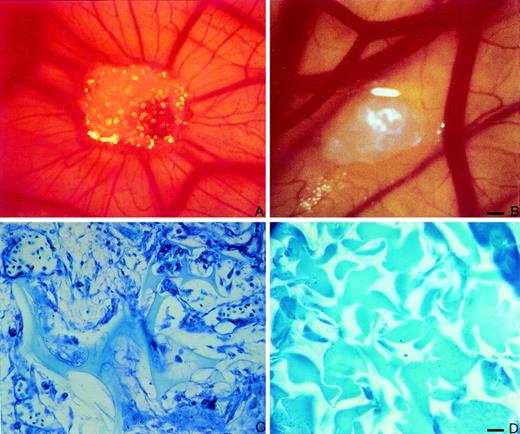
At day 12 of incubation, macroscopic observation of the CAMs showed that rHuEpo induced an angiogenic response characterized by the presence of allantoic vessels spreading radially towards the sponge in a spoked wheel pattern (Fig5A). The effect of rHuEpo was dose-dependent, with the number of positive implants (of a total of 30 embryos per group) being equal to 0, 6, 9, 27, and 27 implants for 1.0 U, 2.0 U, 5.0 U, 10.0 U, and 20.0 U of rHuEpo per sponge, respectively. A similar macroscopic angiogenic response was observed in 16 of 20 implants treated with 1 μg of FGF2 (data not shown), whereas no vascular reaction was detectable around the sponge in the 20 specimens treated with PBS alone (Fig 5B).
rHuEpo stimulates angiogenesis in the chick embryo CAM. CAM of 12-day-old chick embryo incubated for 4 days with a gelatin sponge adsorbed with 10 U of rHuEpo. Note the presence of an increased number of blood vessels with a radially arranged spoked wheel pattern around the implant. (B) CAM of 12-day-old chick embryo incubated for 4 days with a sponge adsorbed with vehicle alone (PBS) used as negative control. No vascular response is detectable around the sponge. (C) Histological section of a gelatin sponge treated with 10 U of rHuEpo. Note the collagenous matrix containing numerous capillaries among the sponge trabeculae and a cellular infiltrate prevalently formed by fibroblasts. (D) Histological section of a sponge treated with PBS. No collagenous matrix, blood vessels, or fibroblasts are detectable. Bar for (A) and (B) is 25 μm and for (C) and (D) is 12.5 μm.
rHuEpo stimulates angiogenesis in the chick embryo CAM. CAM of 12-day-old chick embryo incubated for 4 days with a gelatin sponge adsorbed with 10 U of rHuEpo. Note the presence of an increased number of blood vessels with a radially arranged spoked wheel pattern around the implant. (B) CAM of 12-day-old chick embryo incubated for 4 days with a sponge adsorbed with vehicle alone (PBS) used as negative control. No vascular response is detectable around the sponge. (C) Histological section of a gelatin sponge treated with 10 U of rHuEpo. Note the collagenous matrix containing numerous capillaries among the sponge trabeculae and a cellular infiltrate prevalently formed by fibroblasts. (D) Histological section of a sponge treated with PBS. No collagenous matrix, blood vessels, or fibroblasts are detectable. Bar for (A) and (B) is 25 μm and for (C) and (D) is 12.5 μm.
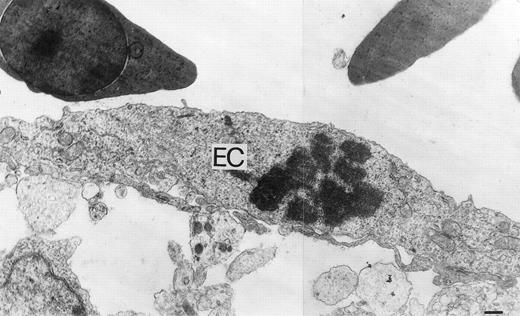
Microscopically, the sponges adsorbed with rHuEpo showed a collagenous matrix containing numerous small blood vessels and fibroblasts localized among the sponge trabeculae (Fig 5C). Numerous host capillaries piercing the sponge in some points were also recognizable at the boundary between the sponge and the CAM mesenchyme. A very scarce mononuclear cell infiltrate was present among the sponge trabeculae. Similar findings had been reported previously for FGF2-treated sponges.37 No collagenous matrix, blood vessels, or fibroblasts were instead present among the sponge trabeculae in the samples treated with PBS (Fig 5D). In keeping with the strong angiogenic response elicited by rHuEpo, numerous mitotic figures of the endothelial cells were recognizable at ultrastructural level in CAMs treated with rHuEpo but not in control, PBS-treated CAMs (Fig 6).
Ultrastructural features of rHuEpo-treated CAM vasculature. An ultrathin section showing a mitotic endothelial cell (EC) in rHuEpo-treated CAM. Bar is 0.3 μm.
Ultrastructural features of rHuEpo-treated CAM vasculature. An ultrathin section showing a mitotic endothelial cell (EC) in rHuEpo-treated CAM. Bar is 0.3 μm.
In agreement with the observations listed above, evaluation of the microvessel density of the CAM at day 12 of incubation demonstrated that rHuEpo exerted an angiogenic response in the chick embryo quantitatively similar to that elicited by FGF2 (Table 1). It must be pointed out that no correlation was observed between microvessel density and the number of infiltrating mononuclear cells in histological sections of rHuEpo-treated CAMs from 8 independent experiments (Pearson’sr = .53; P = .17).
Quantitation of the Angiogenic Response at Day 12 of Incubation
| Treatment . | No. . | No. of Intersection Points (mean ± SD) . | Microvessel Density (%) . |
|---|---|---|---|
| PBS | 20 | 0 | 0 |
| FGF2 | 20 | 30 ± 4.2 | 23.6 |
| rHuEpo | 20 | 28 ± 3.0 | 21.5 |
| Treatment . | No. . | No. of Intersection Points (mean ± SD) . | Microvessel Density (%) . |
|---|---|---|---|
| PBS | 20 | 0 | 0 |
| FGF2 | 20 | 30 ± 4.2 | 23.6 |
| rHuEpo | 20 | 28 ± 3.0 | 21.5 |
In vitro studies had shown that rHuEpo stimulates ET-1 production in endothelial cells and that rHuEpo-induced endothelial cell sprouting from rat aorta rings is partially blocked by neutralizing anti–ET-1 antibodies, thus suggesting rHuEpo activity can be partially modulated in vitro by an autocrine action of ET-1.25 However, no angiogenic response was observed in the CAM when sponges were adsorbed with ET-1 at 10−6 to 10−8 mol/L per implant, ruling out the possibility that ET-1 may play a significant role in mediating the angiogenic activity exerted by rHuEpo in this in vivo model (data not shown).
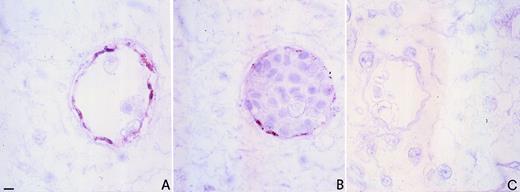
The capacity of rHuEpo to exert an angiogenic response in the chick CAM prompted us to assess this tissue for the presence of EpoRs. As shown in Fig 7B, a marked immunoreactivity to anti-EpoR antibody was observed in the microvessels of the CAM of untreated embryos starting from day 8 of incubation. EpoR immunostaining colocalized with factor VIII positivity (Fig 7A), thus confirming that EpoR expression is limited to endothelial cells of the CAM.
Immunohistochemical localization of EpoR in CAM. Immunoperoxidase staining of an 8-day-old chick embryo CAM using polyclonal antibodies to factor VIII (A) and anti-EpoR (B). Note the coexpression of factor VIII and EpoR on vascular endothelial cells. The negative control (preimmune serum replacing the primary antibody) is shown in (C). Bar for (A) through (C) is 5 μm.
Immunohistochemical localization of EpoR in CAM. Immunoperoxidase staining of an 8-day-old chick embryo CAM using polyclonal antibodies to factor VIII (A) and anti-EpoR (B). Note the coexpression of factor VIII and EpoR on vascular endothelial cells. The negative control (preimmune serum replacing the primary antibody) is shown in (C). Bar for (A) through (C) is 5 μm.
DISCUSSION
Previous observations have shown the capacity of endothelial cells to express EpoR and to respond to this cytokine with an increase in cell proliferation and chemotaxis.22,23 rHuEpo has also been shown to stimulate endothelial cell sprouting in the in vitro rat aorta ring assay.25 These findings were taken as a suggestion that Epo might be endowed with an angiogenic activity. However, the ability of rHuEpo to stimulate neovascularization in vivo was not assessed.
We have demonstrated here that rHuEpo induces a pro-angiogenic phenotype in human endothelial EA.hy926 cells. This phenotype includes both early (ie, increase in cell proliferation and MMP-2 production) and late angiogenic events (differentiation into vascular tubes). Accordingly, EA.hy926 cells express EpoR that binds to JAK-2 and induces its transient activation after rHuEpo exposure. An in vivo chick embryo CAM assay showed that rHuEpo elicits an angiogenic response quantitatively and qualitatively similar to that exerted by the prototypic angiogenic factor FGF2 in the absence of a significant mononuclear cell infiltrate. Accordingly, endothelial cells of the CAM express EpoRs that colocalize with factor VIII positivity. Taken together, the data demonstrate that Epo can act as a bona fide direct angiogenic factor.
Previous observations had shown that blood vessel outgrowth induced in vitro in the rat aorta ring assay by rHuEpo is partially dependent on ET-1 production by endothelial cells.25 Our data show that ET-1 is unable to stimulate new blood vessel growth in the CAM and agree with other in vivo studies demonstrating that ET-1 is not angiogenic.49 This suggests that the role of ET-1 upregulation in mediating Epo activity on endothelial cells may be restricted to some experimental systems.
EpoR is a member of the cytokine receptor superfamily and lacks a kinase domain. Epo induces tyrosine phosphorylation in EpoR-expressing cells and this is correlated with gene transcription and mitogenesis.41 Experimental evidence indicates that JAK-2 serves as a signaling molecule for EpoR. Indeed, JAK-2 is tyrosine phosphorylated and associates to EpoR after stimulation with rHuEpo.41 EpoR intracellular signaling has been poorly investigated in endothelial cells. rHuEpo induces an increase in cytosolic free calcium concentration26 and causes tyrosine phosphorylation of various proteins, including the transcription factor STAT-5, in endothelial cell cultures.50 We have shown here that rHuEpo causes a rapid and transient phosphorylation of JAK-2 and its association with EpoR in endothelial EA.hy926 cells. It is interesting to note that JAK-2 is involved in the intracellular signaling of receptors for various cytokines, including the angiogenic G-CSF and GM-CSF (see Witthuhn et al41 for discussion of this issue). Recent observations have shown that GM-CSF induces JAK-2 activation in EA.hy926 cells51 and in HUVECs.52Taken together, these results suggest a possible role for JAK-2/STAT-5 signaling pathway in cytokine-mediated angiogenesis. Further experiments are required to elucidate this point.
MMP-2 is a major extracellular matrix proteolytic enzyme that degrades various constituents of the interstitial stroma and basement membrane, including type IV, type V, type VII, and type X collagens; fibronectin; laminin; and elastin.42 It is secreted when endothelial sprouting takes place, thus enhancing endothelial cell migration across the matrix.42 Indeed, MMP inhibitors prevent endothelial cell invasion and angiogenesis.53 The ability of rHuEpo to induce a significant increase of MMP-2 activity released by EA.hy926 cells may thus represent an important step in Epo-mediated neovascularization. Accordingly, rHuEpo has been demonstrated to increase vessel outgrowth from rat aortic rings embedded in a reconstituted basement matrix.25
The culture of certain populations of endothelial cells on Matrigel results in the formation of vascular tubes and cord-like structures.43-47 EA.hy926 cells exposed to rHuEpo migrate throughout the Matrigel surface and align to form branching and anastomosing tubes. This originates a meshwork of capillary-like structures, indicating that Epo is able to stimulate morphogenesis in cultured endothelium. Thus, as demonstrated for FGF2 and VEGF,29 30 Epo is able to stimulate the early invasive phase of the angiogenic process that leads to endothelial sprouting (characterized by an increase in cell motility, matrix degradation, and cell proliferation) and the late differentiation phase required for the formation of hollow vascular structures.
Overall, our observations suggest a role of Epo in vasoproliferative processes and emphasize its direct interaction with endothelial cells. These data are in agreement with increasing experimental evidence on the role of various hematopoietic cytokines in angiogenesis. As stated above, receptors for G-CSF and GM-CSF have been detected on the surface of endothelial cells.1,6,8 These cytokines induce endothelial cells to migrate and proliferate and are angiogenic in vivo in the rabbit cornea.1,2 GM-CSF also induces angiogenesis in rat connective tissue by a direct effect on endothelial cells and/or by the recruitment and activation of macrophages that release their own angiogenic factors.4 Human endothelial cells express receptors for IL-3.3,8 IL-4, a lymphocyte growth and differentiation factor, stimulates the growth of microvascular endothelial cells5 and exerts an anti-angiogenic effect in vivo.31 IL-6, a B-cell differentiation factor, regulates the growth of vascular cells and its overexpression in the central nervous system of transgenic mice correlates with neovascularization.7,9 IL-6 mRNA is expressed in endothelial cells during the angiogenesis that accompanies folliculogenesis and formation of decidua.54 Furthermore, IL-6 participates in an autocrine manner to the growth of Kaposi’s sarcoma55 and exerts an autocrine activity in middle T-antigen–transformed endothelial cells.56 IL-6 is also secreted by non-Hodgkin’s lymphoma, lymphoblastic leukemia, and multiple myeloma,57 tumors in which the extent of angiogenesis is related to neoplastic progression.40,58,59Finally, IL-8, which regulates the functions of mature myeloid cells, is an angiogenic factor that induces proliferation and chemotaxis of human endothelial cells.60-63
Differentiation of vascular endothelium is closely linked to the appearance of primitive hematopoietic cells, suggesting that both cell lineages share a common progenitor, the hemangioblast.10,11Experimental studies of the angiogenic growth factor receptor VEGFR2, which was initially thought to be expressed specifically in cells of endothelial lineage,64 supported this hypothesis. Endothelial and hematopoietic cells are, in fact, absent in homozygous VEGFR2 knock-out mice,16 both cell types share a VEGFR2+ common precursor in the chick embryo,18and VEGFR2 expression defines a population of early embryonic hematopoietic precursors.17 In addition, putative endothelial cell progenitors have been isolated from human peripheral blood.19 Epo functions as the primary humoral regulator of erythropoiesis. Our data suggest that its full action and hence the production of erythrocytes and their release into the blood are rendered possible by the convergence of two phenomena, namely (1) proliferation and differentiation of progenitor erythroid cells and (2) bone marrow angiogenesis.
ACKNOWLEDGMENT
The authors thank F. Bussolino (University of Torino, Torino, Italy) for critical reading of the manuscript and Janssen-Cilag (Cologno Monzese, Milan, Italy) for the generous supply of rHuEpo.
Supported in part by a grant from Ministero dell’Università e della Ricerca Scientifica e Tecnologica (MURST, 60%), Rome, Italy, to D.R.; by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy, to D.R, F.D., and M.P.; and by Istituto Superiore di Sanità (AIDS Project), Rome, Italy, to M.P.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Domenico Ribatti, MD, Institute of Human Anatomy, Histology and Embryology, University of Bari, School of Medicine, Piazza G. Cesare, 11, Policlinico, I-70124 Bari, Italy; e-mail: ribatti@anatomia.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal