Abstract
Laminins are extracellular matrix glycoproteins that influence the phenotype and functions of many types of cells. Laminins are heterotrimers composed of , β, and γ polypeptides. So far five , three β, and two γ polypeptide chains, and 11 variants of laminins have been proposed. Laminins interact in vitro with mature blood cells and malignant hematopoietic cells. Most studies have been performed with laminin-1 (1β1γ1), and its expression in bone marrow is unclear. Employing an antiserum reacting with most laminin isoforms, we found laminins widely expressed in mouse bone marrow. However, no laminin 1 chain but rather laminin 2, 4, and 5 polypeptides were found in bone marrow. Our data suggest presence of laminin-2 (2β1γ1), laminin-8 (4β1γ1), and laminin-10 (5β1γ1) in bone marrow. Northern blot analysis showed expression of laminin 1, 2, 4, and 5 chains in long-term bone marrow cultures, indicating upregulation of laminin 1 chain expression in vitro. Laminins containing 5 chain, in contrast to laminin-1, were strongly adhesive for multipotent hematopoietic FDCP-mix cells. Integrin 6 and β1 chains mediated this adhesion, as shown by antibody perturbation experiments. Our findings indicate that laminins other than laminin-1 are functional in adhesive interactions in bone marrow.
STROMAL MICROENVIRONMENTS in the bone marrow regulate proliferation and differentiation of hematopoietic stem and progenitor cells. Extracellular matrix molecules play an important role in this regulation by binding and stabilizing growth factors, and by colocalizing primitive stem cells and developing hematopoietic cells to specific microenvironmental niches.1,2 Adhesion to stromal cells and extracellular matrix molecules also directly influences functions of hematopoietic cells by signaling via cell surface receptors.3-5
Laminins are extracellular matrix proteins found in all basement membranes, but also in the embryonic mesenchyme and loose connective tissue. Laminins interact with other extracellular proteins and adhere to cells via integrins and other receptors, thereby influencing cell motility, proliferation, and differentiation.6,7 Laminins are heterotrimers composed of variants of α, β, and γ polypeptide chains. So far five α, three β, and 2γ chains have been characterized, and 11 different heterotrimers have been proposed.7 Laminin-1, the first characterized laminin, composed of α1, β1, and γ1 chains, is easily isolated from a transplantable mouse tumor.8 Therefore cellular interactions of laminin-1 in vitro are well known. Less is known about the functions of other laminin isoforms. However, in vitro studies, gene targeting experiments, and studies of mutated genes indicate different functional roles for different laminins.7Different laminins show varying binding specificity to cellular receptors, also indicating that laminin isoform diversity is functionally significant.9,10 Dystroglycan, for example, is a major receptor for laminin α1 and α2 chains.9 Several integrins (α1β1, α2β1, α3β1, α6β1, α6β4, α7β1, α9β1, αvβ3) bind to laminins, but with varying affinities.7 10 The cellular interactions of the newly characterized laminins containing α5 and α4 polypeptides are still largely unknown.
Blood cells of several lineages have been found to interact with laminins. Mature granulocytes,11,12lymphocytes,13 mononuclear phagocytes,14activated macrophages,15 and eosinophils16adhere to laminins in vitro. Adhesion to laminins influences survival and maturation of eosinophils17 and proliferation of macrophages,15 and facilitates CD3-mediated T-cell proliferation.5 Bone marrow progenitor cells have not been found to adhere to laminin-1,18 even though laminin binding integrins have found on murine stem cells.19 Malignant progenitor cells from chronic myelogenous leukemia have been reported to adhere less to fibronectin and more to laminin-1 and have increased integrin α6 chain expression, as compared with normal progenitor cells.18 It has been suggested that such altered adhesive interactions may render the leukemic cells insensitive to the normal regulatory stimuli from the stroma,20 and facilitate premature release of the leukemic cells into the circulation.18,21 Laminins have been found to promote differentiation of a promyelocytic leukemia cell line with all-trans retinoic acid22 and to promote chemotaxis of malignant plasma cell lines mediated by integrin α3 and α6 subunits.23 It is therefore of interest to study which laminin isoforms are expressed in the bone marrow.
Immunofluorescence staining using antisera against laminin-1 has shown that laminins are abundantly expressed in long-term bone marrow cultures24 and in the native bone marrow.25However, 10 out of 11 of the laminin isoforms so far characterized contain at least one of the three chains present in laminin-17 and are recognized by anti–laminin-1 antisera. Our previous findings showed the absence of laminin α1 polypeptide and consequently laminin-1 in the bone marrow.25 Here, we have analyzed the expression of other laminin isoforms in the bone marrow. The myeloid long-term bone marrow culture system is a widely used model for hematopoiesis,26 and therefore expression of laminin isoforms was analyzed both in authentic bone marrow and in culture conditions. Furthermore, we studied whether some of the laminin isoforms expressed in the bone marrow are functional in adhesive interactions with hematopoetic cells.
MATERIALS AND METHODS
Cell cultures.
The C57Bl/6 mouse strain (BK Stockholm, Sweden) was used for long-term bone marrow cultures. Myeloid long-term bone marrow cultures were generated essentially as described.26 Bone marrow cells derived from 3-month-old mice were cultured at a density of 1.3 to 2 × 106 cells/mL in 6 mL in 25 cm2 Falcon tissue culture flasks in Fischer’s medium (GIBCO BRL, Täby, Sweden), supplemented with 20% horse serum (GIBCO) with or without 1 × 10−6 mol/L hydrocortisone sodium succinate (Sigma Chemical Co, Stockholm Sweden). The cells were grown at 33°C in 5% CO2 in humidified air. After 1 week 4 mL culture medium was added, and thereafter half of the medium was replaced with fresh medium weekly. Human bone marrow obtained during orthopedic surgery was a gift from Dr Östen Ljunggren (Uppsala, Sweden). For human myeloid long-term bone marrow culture, mononuclear cells were separated by density gradient centrifugation through Ficoll (Pharmacia, Uppsala, Sweden). The cells were grown in Iscove’s modified Dulbecco’s medium (IMDM) (GIBCO) with 10% horse serum, 10% fetal calf serum (FCS) and 5 × 10−7 M hydrocortisone sodium succinate at 33°C in 5% CO2humidified air. MC3T3-G2/PA6 preadipocyte37 and 3T3 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO) supplemented with 10% FCS at 37°C in 5% CO2. The mouse multipotent hematopoietic FDCP-mix cell line28 was maintained in IMDM (GIBCO) supplemented with 20% horse serum and recombinant interleukin-3 (PeproTech EC, London, UK).
Antisera and antibodies.
Polyclonal rabbit antiserum against laminin-1,29 from mouse transplantable Engelbreth-Holm-Swarm (EHS) tumor,8 or affinity purified polyclonal antilaminin antiserum against EHS-laminin (Sigma) were used to detect laminin α1, β1, and γ1 polypeptide chains. Rat monoclonal antibodies (MoAb) employed were 198 and 200 against the E3 carboxyterminal fragment of laminin α1 polypeptide,30 4H8-2 and 8G11-D10 against laminin α2 polypeptide,31 4G6 against the laminin α5 chain,32 and MECA-32 against an endothelial cell surface marker.33 Mouse MoAb D5 was used to study expression of laminin β2 chain34 in rat bone marrow. Rabbit antiserum against the human laminin α4 chain was from Dr Robert Burgeson, and rabbit antiserum raised against a synthetic peptide KPPVKRPELT located at the beginning of the carboxyterminal G-domain of human laminin α4 chain35 was from Dr Alan Richards. Monoclonal rat antibody GoH3 (IgG2a, κ) against integrin α6 chain36was from Dr Arnoud Sonnenberg, monoclonal rat antimouse antibody 9EG7 (IgG2a, κ) and monoclonal hamster antirat antibody Ha2/5 (IgM) against integrin β1 chain were from Pharmingen. Mouse MoAb 4C7, previously thought to detect human laminin α1 chain,37but now shown to be specific for human laminin α5 chain,38 was also employed. Cy3 and fluorescein-conjugated sheep antibodies against rat, rabbit, or mouse immunoglobulins were purchased from Jackson Immunoresearch Laboratory (West Grove, PA).
Immunofluorescence.
Tissues were frozen in Tissue Tek (Miles, Naperville, IL). 5 to 7 μm cryostat sections from adult and newborn mouse (NMRI and C57 Bl/6) bone marrow, newborn rat bone marrow, and human bone marrow were fixed in methanol at −20°C for 2 to 5 minutes or 4% paraformaldehyde at room temperature for 10 minutes. Adherent cells from myeloid long-term bone marrow cultures grown on coverslips were fixed with methanol at −20°C. The cells or tissue sections were treated with 2% to 5% bovine serum albumin (BSA) (Sigma) or 5% goat serum in phosphate-buffered saline (PBS), and thereafter incubated with antisera or antibodies diluted in 2% goat serum in PBS.
Immunoblotting.
Adherent cells from bone marrow cultures were washed two times with PBS. Proteins were extracted in Tris-buffered saline (TBS), pH 7.4 containing 10 mmol/L EDTA (TBS-EDTA), or sequentially in triple detergent buffer39 followed by TBS-EDTA and 6 mol/L urea in TBS pH 7.4, all containing protease inhibitors. Proteins from 4-weeks-old rat bone marrow and newborn rat kidney were extracted in TBS-EDTA. For immunoblots, the samples were reduced by boiling 5 minutes in Laemmli buffer containing dithiotreitol or β-mercaptoethanol (Sigma) and separated on 6% or 3% to 12% (wt/vol) continuous sodium dodecyl sulfate (SDS)-polyacrylamide gel. EHS tumor extract or laminin-1 purified from the EHS tumor40 were used as controls. Prestained Rainbow (BioRad, Hercules, CA) or Caleidoscope (Amersham, Buckinghamshire, UK) high molecular weight markers were run in parallel. Nonspecific binding was blocked with 5% goat serum in PBS, 0.1% Tween-20 (USB) (PBS-T). Membranes were incubated with the antibodies in 2% goat serum, PBS-T. Control stainings were performed by omitting the first antibody. Bound antibodies were detected with a peroxidase-conjugated goat antirabbit, or antirat or antimouse antiserum (Amersham) at a dilution of 1:3000, and by 4-chloro-1-naphthol and H2O2, or by chemiluminescence using the ECL Western blotting protocol (Amersham Int, Buckinghamshire, England) and Hyperfilm ECL (Amersham).
Immunoprecipitation.
Bone marrow cells from tibiae and femora of 1-week-old NMRI mice were incubated overnight in cysteine- and methionine-free RPMI, 5% FCS, 30 μL/mL 35S-cysteine-methionine (Pro-mix, Amersham) at 37°C. The culture medium was collected after centrifugation. C57Bl/6 mouse bone marrow cells were cultured for 3 weeks in myeloid long-term culture conditions. Thereafter, adherent cells were washed free of nonadherent cells and 4 mL of culture medium consisting of cysteine- and methionine-free RPMI 1640 (GIBCO), 10% horse serum and 1 × 10−6 mol/L hydrocortisone sodium succinate and 25 μL 35S-cysteine-methionine was added. After 24-hours incubation in 33°C 5% CO2 in a humidified atmosphere the nonadherent cells were removed by centrifugation and the culture medium was collected. In case of long-term bone marrow cultures, the same volume of labeled medium was used for each immunoprecipitation. For immunoprecipitation, the labeled culture media were precleared by incubation with rabbit serum and Protein A Sepharose (Pharmacia). Thereafter they were incubated with the primary antiserum and Protein A Sepharose alone or, in case of monoclonal rat antibody, together with rabbit antirat antiserum. Control immunoprecipitations were performed by using rat IgG and nonimmune rabbit serum instead of the primary antibodies. After washing three times with a high salt buffer (0.5 mol/L NaCl, 1 mmol/L MgCl2, 1mmol/L CaCl2, 20 mmol/L TRIS-HCl pH 7.4, 0.1% SDS), two times with a low-salt buffer (the same as above except for 0.15 mol/L NaCl) and once with TBS, the immunoprecipitated proteins were incubated 5 minutes in 95°C in Laemmli loading buffer containing dithiotreitol (Sigma) and electrophoresed on a 6% or 3% to 12% SDS polyacrylamide gel. 14C labeled or prestained molecular mass markers (Bio-Rad, Amersham) were run in parallel. After electrophoresis the gels were fixed, treated with Amplify (Amersham), dried, and exposed to a Kodak XAR film (Rochester, NY).
Complementary DNA (cDNA) probes.
A 1.5 kb Sph1-Sma1 fragment of a cDNA clone corresponding to the 3′ end of the coding region of mouse laminin α1 messenger RNA (mRNA)41 was used. To detect laminin α2 mRNA, a 765 bp cDNA probe corresponding to a BamHI/KpnI restriction fragment (position 5969-6732)42 was used. A 601 bp cDNA fragment43 corresponding to laminin α5 mRNA,44 a 795 bp cDNA corresponding to laminin α4 mRNA,45 and a 564 bp cDNA fragment46corresponding to laminin α3A and α3B mRNAs were used. A 1.1 kb human G3PDH cDNA probe (Clontech, Palo Alto, CA) was used as a control for the amount of mRNA loaded.
Northern blot analysis.
Total RNA from bone marrow cells of 1-week-old and adult mice, adherent layers of long-term bone marrow cultures grown without hydrocortisone, 3T3, and MC3T3-G2/PA6 cells were isolated by ultracentrifugation or by phenol extraction.39 RNA was denatured with glyoxal, electrophoresed on a 1% agarose gel, transferred to a Zeta-Probe membrane (BioRad, Solna, Sweden) and fixed by UV cross-linking with a Stratalinker (Stratagene, AH Diagnostics AB, Skarholmen, Sweden). Membranes were prehybridized in 0.25 mol/L Na2HPO4, 7% SDS, for 60 minutes at 65°C and hybridized overnight in the same solution at 65°C. cDNA probes were labeled with 32P-dCTP (Amersham) using RediPrime labeling system (Amersham). Free nucleotides were removed by Push Columns (Stratagene). After hybridization membranes were washed two times for 1 hour in 20 mmol/L Na2HPO4, 5% SDS at 65°C, and two times for 1 hour in 20 mmol/L Na2HPO4, 1% SDS at 65°C. Membranes were exposed to a Kodak XAR film.
Cell attachment assay.
96-well nontissue culture microtiter plates (Greiner, Sigma) were coated for 1 hour at 37°C with extracellular matrix proteins diluted in Dulbeccos’s PBS (GIBCO) at concentrations of 10 to 50 μg/mL in volumes of 50 to 100 μL. As negative controls, wells were incubated with Dulbecco’s PBS only. After coating, wells were washed three times with PBS and incubated with 2% heat-denatured fatty acid free BSA (Sigma) in PBS for 1 hour at 37°C. After 3 washes with PBS, cells (1.5 to 3.1 × 105 cells/mL) in Dulbecco’s minimal essential medium (DMEM, GIBCO) in a volume of 50 to 100 μL/well were added and incubated at 37°C for 1 hour. After two to three washes with PBS, adherent cells were fixed with 96% ethanol for 10 minutes, washed two times with PBS and stained with 0.1% chrystal violet in H2O for 30 minutes. Thereafter, plates were washed three times with large volumes of deionized water, and adherent cells were lyzed with 0.2% Triton X-100. Absorbance was measured at 595 nm with a microtiter plate reader (Multiskan PLUS, Labsystems, Stockholm, Sweden). All samples were analyzed in triplicates. Proteins employed in adhesion assays were plasma fibronectin (from Dr Staffan Johansson, Uppsala, Sweden), laminin-1 purified from the EHS tumor40 (from Dr Mats Paulsson, Cologne, FRG), and laminin from human placenta, affinity purified with the MoAb 4C7 (GIBCO). For the antibody perturbation assays, the plates were coated with 30 μg/mL protein as described above. The FDCP-mix cells were resuspended at +4°C in DMEM containing 2% BSA and the antibodies. The cells were incubated in the wells for 1 hour on ice. Thereafter, the plates were warmed to +37°C for 10 minutes, washed, fixed, stained, and analyzed as described above.
RESULTS
Widespread distribution of laminins but absence of laminin-1 in the bone marrow.
The expression of laminin α1, β1 and γ1 polypeptides in the bone marrow was studied using an antiserum against laminin-1 from the EHS tumor. This antiserum reacts well with both the 400 kD α1 polypeptide and the 200 to 210 kD β1 and γ1 polypeptides, as shown in immunoblotting of protein extracts from the EHS tumor (Fig 1). Immunostaining of adult mouse bone marrow with the antilaminin-1 antiserum showed widespread distribution of laminins in the arteriolar walls and in the sinusoidal subendothelial basement membranes, but also in the intersinusoidal interstitial connective tissue. This was shown by double immunofluorescence for laminin, and an endothelial-specific antigen identified with the MoAb MECA-32 (Fig 2a and 2b).
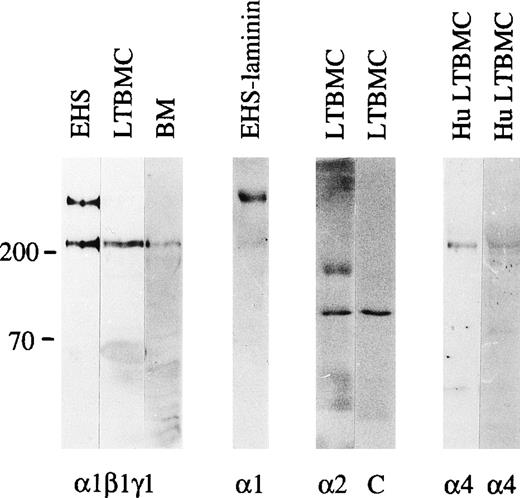
Immunoblotting of laminin polypeptides in the bone marrow stroma. Immunoblotting of protein extracts from an EHS tumor (EHS), adherent cells from mouse long-term bone marrow cultures (LTBMC), adult mouse bone marrow (BM), human long-term bone marrow cultures (HuLTBMC), and laminin-1 isolated from EHS tumor (EHS-laminin) was performed. Antibodies used were: (1β1γ1), a polyclonal antiserum against the three chains of laminin-1; 1, MoAb 200 against laminin 1 chain; 2, MoAb 8G11-D10 against laminin 2 chain; 4, two polyclonal antibodies against a fragment of human laminin 4 protein (left), or a synthetic peptide corresponding to human laminin 4 chain (right) as an immunogen. C, control immunoblotting in the absence of primary antibody. The positions of a 200 and 70 kD molecular mass markers run in parallel are shown to the left of each blot.
Immunoblotting of laminin polypeptides in the bone marrow stroma. Immunoblotting of protein extracts from an EHS tumor (EHS), adherent cells from mouse long-term bone marrow cultures (LTBMC), adult mouse bone marrow (BM), human long-term bone marrow cultures (HuLTBMC), and laminin-1 isolated from EHS tumor (EHS-laminin) was performed. Antibodies used were: (1β1γ1), a polyclonal antiserum against the three chains of laminin-1; 1, MoAb 200 against laminin 1 chain; 2, MoAb 8G11-D10 against laminin 2 chain; 4, two polyclonal antibodies against a fragment of human laminin 4 protein (left), or a synthetic peptide corresponding to human laminin 4 chain (right) as an immunogen. C, control immunoblotting in the absence of primary antibody. The positions of a 200 and 70 kD molecular mass markers run in parallel are shown to the left of each blot.
Expression of laminin polypeptides in the adult mouse bone marrow (a-f), and human bone marrow (g-j). Double immunofluorescence staining with an antiserum reacting with laminin 1, β1, and γ1 polypeptides (a) and with an endothelial cell marker MECA-32 (b) shows expression of either laminin 1, β1, or γ1 chains in the artery, sinusoidal subendothelial basement membranes, and in the intersinusoidal spaces. Immunostaining with MoAb 4G6 (d) shows expression of laminin 5 chain in the arteriolar walls and in the subendothelial basement membrane of the sinusoids (arrows). Immunostaining with antibody 200 specific for laminin 1 chain (e) is negative. Laminin 2 chain, identified with antibody 8G11-D10 (f) is present only in the arteriolar walls. Laminin 4, identified with a polyclonal antiserum against a synthetic peptide corresponding to laminin 4 chain, is localized in the intersinusoidal spaces (h,i), and in the walls of arteries (i). Double immunofluorescence staining with antibody 4C7 against human laminin 5 chain (g) and with the antiserum against synthetic peptide of laminin 4 chain (h) shows expression of 5 and 4 chains in the arteriolar walls (arrows), and 4 chain also in the intersinusoidal connective tissue. Control immunostainings with nonimmune rabbit serum (NRS) (c, j) are negative. Laminin polypeptide chains detected by each antiserum or antibody are indicated in the lower left corners of the figures.
Expression of laminin polypeptides in the adult mouse bone marrow (a-f), and human bone marrow (g-j). Double immunofluorescence staining with an antiserum reacting with laminin 1, β1, and γ1 polypeptides (a) and with an endothelial cell marker MECA-32 (b) shows expression of either laminin 1, β1, or γ1 chains in the artery, sinusoidal subendothelial basement membranes, and in the intersinusoidal spaces. Immunostaining with MoAb 4G6 (d) shows expression of laminin 5 chain in the arteriolar walls and in the subendothelial basement membrane of the sinusoids (arrows). Immunostaining with antibody 200 specific for laminin 1 chain (e) is negative. Laminin 2 chain, identified with antibody 8G11-D10 (f) is present only in the arteriolar walls. Laminin 4, identified with a polyclonal antiserum against a synthetic peptide corresponding to laminin 4 chain, is localized in the intersinusoidal spaces (h,i), and in the walls of arteries (i). Double immunofluorescence staining with antibody 4C7 against human laminin 5 chain (g) and with the antiserum against synthetic peptide of laminin 4 chain (h) shows expression of 5 and 4 chains in the arteriolar walls (arrows), and 4 chain also in the intersinusoidal connective tissue. Control immunostainings with nonimmune rabbit serum (NRS) (c, j) are negative. Laminin polypeptide chains detected by each antiserum or antibody are indicated in the lower left corners of the figures.
In immunoblotting of protein lysates of adult mouse bone marrow cells, laminin β1, and γ1 polypeptide chains were well expressed, but the 400 kD laminin α1 polypeptide was not detectable. This was further analyzed by immunofluorescent staining using MoAb 200 specific for laminin α1 polypeptide (Fig 1). This antibody did not stain any structures in adult bone marrow (Fig 2e), indicating that the laminin α1 polypeptide is not expressed in the bone marrow. In the adherent stromal cells of myeloid long-term bone marrow cultures a very weak reaction for the α1 chain was detected in immunoblotting after a long exposure (not shown), suggesting an upregulation of laminin α1 in stromal cells in culture.
Expression of laminin α polypeptides other than laminin α1 chain in bone marrow.
The low level of laminin α1 chain expression in the bone marrow stroma implies that other α chains are assembled to bone marrow laminins. In immunoblotting of the adherent layer of bone marrow cultures the MoAb 8G11-D10 against α2 chain reacted with a 150 kD proteolytic fragment of laminin α2 polypeptide31 (Fig 1). In immunofluorescent staining laminin α2 polypeptides were found in the arteriolar walls in adult bone marrow (Fig 2f). Laminin α5 polypeptides were localized by immunofluorescent staining with MoAb 4G6 also in arteriolar walls in adult mouse bone marrow, but in addition in subendothelial basement membranes in the sinusoids (Fig 2d). However, in the intersinusoidal connective tissue neither laminin α2 nor α5 chains were expressed. Polyclonal antibodies against either the human laminin α4 polypeptide or against a synthetic peptide corresponding to laminin α4 polypeptide sequences were tested. The specificity of the antibodies was shown by immunoblotting. Both antibodies reacted with a polypeptide of slightly over 200 kD in adherent stromal layer of human bone marrow cultures (Fig 1), corresponding to the described molecular mass for laminin α4 chain.47 In immunostainings, laminin α4 chain was localized in the intersinusoidal spaces, in large arteries, and in smaller arterioles in adult human bone marrow (Fig 2h,i). In agreement with the findings from mouse bone marrow, the vasculature in the human bone marrow expressed laminin α5 chain, as shown by immunostaining with antibody 4C7 (Fig 2g).
Developmentally regulated expression of laminin α polypeptides in the bone marrow.
Laminins were found well expressed in newborn mouse bone marrow and showed a widespread distribution, similar to that observed in adult bone marrow. Double immunofluorescence staining with the antilaminin-1 antiserum and MECA-32 showed laminins in sinusoidal basement membranes, arteriolar walls, and in the intersinusoidal spaces (Fig 3a,b). As in adult bone marrow, in newborn bone marrow laminin α1 polypeptide was not found (Fig 3e), and faint immunostaining for laminin α2 chain was observed in some arterioles (Fig 3f). Laminin α5 polypeptide was also immunolocalized in arteriolar walls, but in contrast to adult bone marrow, laminin α5 chain was not expressed in sinusoidal basement membranes in newborn bone marrow (Fig 3d), indicating a postnatal shift in the expression of laminin isoforms in the sinusoids. The sinusoidal basement membranes and the intersinusoidal connective tissue in newborn mouse bone marrow, therefore, do not contain laminin α1, α2, or α5 polypeptides.
Expression of laminin polypeptides in newborn mouse bone marrow. Immunostaining with an antiserum reacting with laminin 1, β1, and γ1 chains (a) shows widespread expression of some of these chains in the arteriolar walls (arrow), in the sinusoids, and in the intersinusoidal spaces, as shown by a double immunofluorescence with MECA-32 to stain endothelial cells in the sinusoids and in the arteriole (arrow) (b). Antibodies 4G6 reacting with laminin 5 polypeptide (d) and 8G11-D10 recognizing laminin 2 chain (f) and show a reaction in some arterioles. Laminin 1 chain, identified with MoAb 198, (e) is not expressed in the newborn bone marrow. Control staining (c) with nonimmune rabbit serum (NRS) is negative. Laminin polypeptide chains detected by each antiserum or antibody are indicated in the lower left corners of the figures.
Expression of laminin polypeptides in newborn mouse bone marrow. Immunostaining with an antiserum reacting with laminin 1, β1, and γ1 chains (a) shows widespread expression of some of these chains in the arteriolar walls (arrow), in the sinusoids, and in the intersinusoidal spaces, as shown by a double immunofluorescence with MECA-32 to stain endothelial cells in the sinusoids and in the arteriole (arrow) (b). Antibodies 4G6 reacting with laminin 5 polypeptide (d) and 8G11-D10 recognizing laminin 2 chain (f) and show a reaction in some arterioles. Laminin 1 chain, identified with MoAb 198, (e) is not expressed in the newborn bone marrow. Control staining (c) with nonimmune rabbit serum (NRS) is negative. Laminin polypeptide chains detected by each antiserum or antibody are indicated in the lower left corners of the figures.
The molecular masses of laminin α polypeptides expressed in the early postnatal bone marrow was studied by immunoprecipitation with the antiserum against laminin-1 (Fig 4), resulting in a precipitation of proteins with an apparent molecular mass slightly above 200 kD, corresponding to the sizes of α4, β1, and γ1 polypeptides. No polypeptides of higher molecular mass were detected. Because laminin α1, α2, α3B, and α5 chains have molecular masses of 300 to 400 kD,7 the finding is in agreement with the observed absence of laminin α1 chain and low level of expression of laminin α2 and α5 chains in the immunostainings. It suggests that laminin α4 chain is the major laminin α chain in the early postnatal bone marrow. This was supported by Northern blot analysis that showed expression of laminin α4 mRNA in total RNA from bone marrow of 1-week-old mice, whereas mRNAs for laminin α2, α5 (Fig 5), α3A, or α3B chains (not shown) could not be detected.
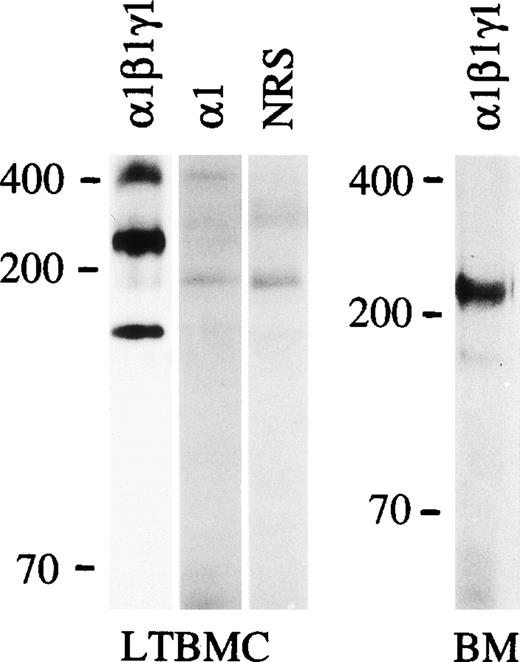
Immunoprecipitation of the proteins synthesized in the culture medium by adherent cells of mouse long-term bone marrow culture (LTBMC) or cells from 1 week postnatal mouse bone marrow (BM) with an antiserum against laminin-1 (1β1γ1), laminin 1 chain (1; MoAb 198) or with a nonimmune rabbit serum (NRS). Positions of a prestained 200 and 70 kD molecular mass markers and 400 kD laminin 1 polypeptide from EHS extract stained by Coomassie blue after electrophoresis are shown to the left. LTBMC: 6% gel; BM: 3% to 12% gradient gel.
Immunoprecipitation of the proteins synthesized in the culture medium by adherent cells of mouse long-term bone marrow culture (LTBMC) or cells from 1 week postnatal mouse bone marrow (BM) with an antiserum against laminin-1 (1β1γ1), laminin 1 chain (1; MoAb 198) or with a nonimmune rabbit serum (NRS). Positions of a prestained 200 and 70 kD molecular mass markers and 400 kD laminin 1 polypeptide from EHS extract stained by Coomassie blue after electrophoresis are shown to the left. LTBMC: 6% gel; BM: 3% to 12% gradient gel.
Expression of mRNAs for laminin 1, 2, 4, and 5 chains in 1-week postnatal mouse bone marrow (BM), adherent cells from mouse long-term bone marrow cultures (LTBMC), 3T3, and MC3T3-G2/PA6 (PA6M) cell lines. Northern hybridization of total RNA was performed with the labeled cDNA probes corresponding to laminin 1, 2, 4, and 5 mRNA. Hybridizations with cDNA fragments corresponding to laminin 2, 4, and 5 mRNAs were performed consecutively on the same membrane. The amount of mRNA loaded was analyzed by hybridizations of the membranes with a cDNA probe corresponding to G3PDH mRNA (G3PDH). 28S; localization of 28S rRNA on the membranes. The blots were exposed to radiograph films; exposure time for a cDNA fragment corresponding to laminin 1 mRNA was 17 days, to laminin 2 mRNA 19 days, to laminin 4 mRNA 21 days, and to laminin 5 mRNA 28 days.
Expression of mRNAs for laminin 1, 2, 4, and 5 chains in 1-week postnatal mouse bone marrow (BM), adherent cells from mouse long-term bone marrow cultures (LTBMC), 3T3, and MC3T3-G2/PA6 (PA6M) cell lines. Northern hybridization of total RNA was performed with the labeled cDNA probes corresponding to laminin 1, 2, 4, and 5 mRNA. Hybridizations with cDNA fragments corresponding to laminin 2, 4, and 5 mRNAs were performed consecutively on the same membrane. The amount of mRNA loaded was analyzed by hybridizations of the membranes with a cDNA probe corresponding to G3PDH mRNA (G3PDH). 28S; localization of 28S rRNA on the membranes. The blots were exposed to radiograph films; exposure time for a cDNA fragment corresponding to laminin 1 mRNA was 17 days, to laminin 2 mRNA 19 days, to laminin 4 mRNA 21 days, and to laminin 5 mRNA 28 days.
Expression of laminin α chain mRNAs in the adherent stroma of long-term bone marrow cultures, and in stromal cell lines.
Laminin polypeptides with a molecular mass of about 400 kD were expressed in the adherent stromal layer of long-term bone marrow cultures (Fig 4), as shown by immunoprecipitation with the antilaminin-1 antiserum. The proteins precipitated have a molecular mass of slightly above 200 kD, corresponding to laminin α4, β1, and γ1 polypeptides (Fig 4), whereas the precipitated 150 kD band has an electrophoretic mobility identical to the laminin-associated polypeptide nidogen, as judged from immunoprecipitations of bone marrow culture media with an antiserum against nidogen (not shown). Immunoprecipitation with MoAb 200 (not shown) and 198 against the laminin α1 polypeptide showed a very faint band of about 400 kD (Fig4). Northern blot analysis showed that mRNAs for laminin α1, α2, α4, and α5 chains were expressed in adherent cells from bone marrow cultures (Fig 5), whereas mRNAs for laminin α3A or α3B chains were not detected (not shown).
In adult bone marrow no signals for laminin α mRNAs were obtained in Northern hybridization to total RNA (not shown). In the studied cell lines, mRNA for laminin α5 chain was detected in 3T3 cells, whereas mRNAs for laminin α2 and α4 chains were detected in preadipocytic MC3T3-G2/PA6 cells (Fig 5).
Absence of laminin β2 polypeptide in bone marrow.
Laminin β2 polypeptide was not found in rat bone marrow in immunoblotting and immunofluorescence by using MoAb D5, even though the antibody detected β2 chains in other anatomical areas (Fig 6).
Absence of laminin β2 polypeptide in the bone marrow. (a); immunoblotting of proteins from 4-weeks-old rat bone marrow (BM) and newborn rat kidney with the MoAb, D5, shows a band of about 190 kD corresponding to laminin β2 in kidney lysates but not in bone marrow. Position of 200 and 97 kD molecular mass markers run in parallel are shown on the left. Immunofluorescent staining with the antibody D5 does not stain any structures in newborn rat bone marrow (b), whereas the antibody stains an artery in the dermis in the same section (c).
Absence of laminin β2 polypeptide in the bone marrow. (a); immunoblotting of proteins from 4-weeks-old rat bone marrow (BM) and newborn rat kidney with the MoAb, D5, shows a band of about 190 kD corresponding to laminin β2 in kidney lysates but not in bone marrow. Position of 200 and 97 kD molecular mass markers run in parallel are shown on the left. Immunofluorescent staining with the antibody D5 does not stain any structures in newborn rat bone marrow (b), whereas the antibody stains an artery in the dermis in the same section (c).
Adhesion of hematopoietic FDCP-mix and stromal cell lines to laminins containing α1 and α5 polypeptides.
The FDCP-mix cells adhered to the laminin-containing α5 chain (laminin-10/11) isolated from human placenta, but to a much lesser degree to laminin-1 (Fig 7A). FDCP-mix cells also adhere to fibronectin.57 Adhesion of FDCP-mix cells to laminins containing the α5 chain was inhibited by antibody GoH3 against α6 integrin subunit. Of two MoAbs against integrin β1 chains employed, Ha2/5 inhibited adhesion of FDCP-mix cells to laminin-10/11, whereas 9EG7 did not (Fig 7B). The fibroblastic 3T3 cells and preadipocytic MC3T3-G2/PA6 cells adhered equally well to laminin 10/11, laminin-1, and fibronectin, with the exception that higher concentrations of laminin-1 were required (Fig 8).
(A) Adhesion of the FDCP-mix cells to fibronectin, laminin-1 purified from the EHS-tumor, and human placental laminin containing the 5 chain (Laminin-10/11). The results are from four experiments performed in triplicate (Mean ± SD). The mean absorbance values at 595 nm were 0.099 to 0.264 for laminin 10/11, 0.034 to 0.133 for fibronectin, and 0.028 to 0.055 for laminin-1, each coated at 30 μg/mL, and 0.012 to 0.023 for controls. (B) Effect of MoAbs GoH3 against integrin 6 chain, and Ha2/5 and 9EG7 against integrin β1 chain on the adhesion of FDCP-mix cells to laminins containing 5 chain. (Mean ± SD, GoH3, and 9EG7, two experiments performed in triplicate; Ha2/5, two experiments with a total of nine measurements). The mean absorbance values for controls without antibodies were 0.482 and 0.081 for the two experiments.
(A) Adhesion of the FDCP-mix cells to fibronectin, laminin-1 purified from the EHS-tumor, and human placental laminin containing the 5 chain (Laminin-10/11). The results are from four experiments performed in triplicate (Mean ± SD). The mean absorbance values at 595 nm were 0.099 to 0.264 for laminin 10/11, 0.034 to 0.133 for fibronectin, and 0.028 to 0.055 for laminin-1, each coated at 30 μg/mL, and 0.012 to 0.023 for controls. (B) Effect of MoAbs GoH3 against integrin 6 chain, and Ha2/5 and 9EG7 against integrin β1 chain on the adhesion of FDCP-mix cells to laminins containing 5 chain. (Mean ± SD, GoH3, and 9EG7, two experiments performed in triplicate; Ha2/5, two experiments with a total of nine measurements). The mean absorbance values for controls without antibodies were 0.482 and 0.081 for the two experiments.
Adhesion of MC3T3-G2/PA6 cells (A) and 3T3 cells (B) to fibronectin, laminin-1, and laminin containing 5 chain (Laminin-10/11) (Mean ± SD, 3 experiments).
Adhesion of MC3T3-G2/PA6 cells (A) and 3T3 cells (B) to fibronectin, laminin-1, and laminin containing 5 chain (Laminin-10/11) (Mean ± SD, 3 experiments).
Expression of the integrin α 6 chain in the cultured bone marrow stromal cells.
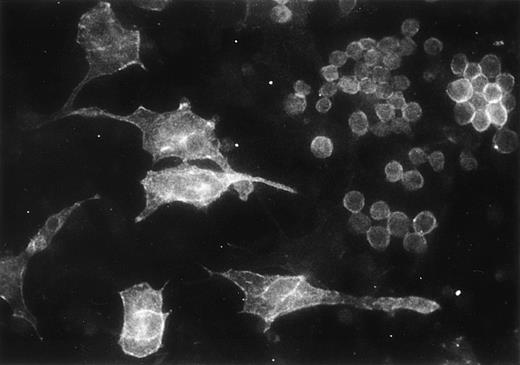
We analyzed by immunofluorescence the expression of the integrin α6 subunit in the adherent cells from myeloid long-term bone marrow cultures. The GoH3 antibody stained a subset of hematopoietic cells in the adherent cell layer, but also some large stromal cells (Fig 9).
Immunofluorescent localization of integrin 6 chain in the adherent cells of mouse myeloid long-term bone marrow culture, studied with GoH3 antibody.
Immunofluorescent localization of integrin 6 chain in the adherent cells of mouse myeloid long-term bone marrow culture, studied with GoH3 antibody.
DISCUSSION
We show here that laminin α1 chain, and consequently laminin-1, is not detectable in the bone marrow. Rather, we have identified laminin α2, α4, and α5 chains apparently assembled into laminins -2, -8, and -10 as the isoforms in the bone marrow. We also show that laminins containing the α5 chain are more adhesive to multipotent hematopoietic FDCP-mix cells than laminin-1 and fibronectin, suggesting that bone marrow laminins are functional in adhesive interactions with hematopoietic cells.
The finding that bone marrow does not contain laminin α1 chain is in agreement with our previous findings that laminin α1 chain is restricted mainly to epithelial basement membranes.48,49Recent studies by us and others have confirmed this finding both in mouse and human tissues.38,43,50,51 Other laminin isoforms have been localized in the mesenchyme and in nonepithelial basement membranes.43,50,51 We show here that laminin α2 chain is present in the arteriolar walls of bone marrow. In other tissues, the laminin α2 polypeptide is expressed in the basement membranes of muscle fibers and Schwann cells.31 It is likely that laminin α2 chain in the bone marrow is also produced by smooth muscle cells in the arteriolar walls. Recently, laminin α5 polypeptide has been found widely expressed in adult and embryonic tissues,32,43,50,51 with expression increasing during postnatal development. This chain has been suggested to be a major laminin α chain in adult blood vessels.32 We found laminin α5 polypeptides in arteriolar walls and in sinusoidal endothelial linings in the adult bone marrow, but only in the arteriolar walls in newborn bone marrow, indicating a postnatal shift in the laminins in the sinusoidal basement membranes. Laminin α4 chain was expressed in the intersinusoidal loose connective tissue and in arteries in adult bone marrow.
Because laminin α polypeptides have considerable size variations, it was to some degree possible to analyze by immunoprecipitation followed by gel electrophoresis, which α polypeptides form authentic laminin heterotrimers with the β1 and γ1 polypeptides in the bone marrow stroma. Immunoprecipitation of laminins synthesized by 1-week postnatal bone marrow cells showed polypeptides with a molecular mass slightly above 200 kD, corresponding to the molecular mass of laminin α4, β1, and γ1 polypeptides, but no proteins of higher molecular mass. The data indicate that laminin α4 chain, assembled with β1 and γ1 chains to form laminin-8, is the major laminin isoform in the developing bone marrow. This finding was further supported by Northern blot analysis, which showed expression of laminin α4 chain but no other α chain mRNAs in bone marrow from 1-week-old mice.
Immunoprecipitation from long-term bone marrow cultures with polyclonal antilaminin-1 antiserum showed a 400 kD α polypeptide and 200 kD polypeptides. The 400 kD α polypeptide band could consist of laminin α1, α2, and α5 polypeptides, because their mRNA was found by Northern blot analysis. Expression of laminin α1 chain in adherent cells from long-term bone marrow cultures but not in bone marrow in vivo suggests that the expression of laminin α chains is altered in bone marrow cells during in vitro culture. As shown by immunoblots and by Northern hybridization, laminin α4 chain was likewise well expressed in the stromal layer of human and mouse bone marrow cultures. Laminin β2 chain, which forms laminin heterotrimers with α chains and γ1 chain,7 was not found in bone marrow. Accordingly, our findings indicate that laminin-2 (α2β1γ1), laminin-8 (α4β1γ1), and laminin-10 (α5β1γ1) are present in the hematopoietic tissue. The laminin isoforms in arteriolar walls are laminin-2, laminin-8, and laminin-10, whereas in sinusoidal endothelial linings laminin-10 is the only isoform expressed, and the laminin in the intersinusoidal spaces is laminin-8.
Northern hybridization to total RNA did not show any expression of laminin α chain mRNA in adult bone marrow. Because the laminin polypeptides were nevertheless well expressed in adult bone marrow as shown by immunofluorescence and immunoblotting, the result indicates a low level of synthesis and a low turnover of the laminins in the fully differentiated bone marrow. This low rate of synthesis despite abundant expression of the proteins has also been noted in other adult organs.49
Laminin β3 and γ2 chains, the other known laminin β and γ polypeptide variants, are associated with laminin α3 to form laminin-5, which is present in epithelia.52 Because mRNAs for the α3 splice variants α3A and α3B were not detectable in the early postnatal or adult bone marrow or in bone marrow cultures, it is unlikely that laminin-5 is expressed in the bone marrow. However, low expression of mRNA at some developmental stage does not necessarily exclude expression of the corresponding protein, because the turnover and rate of protein synthesis may be low. Therefore, expression of laminins containing α3A and α3B polypeptides in the bone marrow should be studied with specific antibodies.
Because of its localization in the adult bone marrow adjacent to the hematopoietic cells, laminin containing the α4 chain is the most likely isoform to have biologically relevant interactions with developing hematopoietic cells, whereas laminin containing the α5 polypeptide might be involved both in adhesive interactions with the bone marrow cells within the intersinusoidal spaces and during the trafficking of the mature blood cells across the sinusoidal linings.53 Indeed, we have here shown that laminins containing the α5 chain are more adhesive for hematopoietic cells than laminin-1. Several laminin-binding integrin receptors, including the integrin α6 subunit, have been found in hematopoietic stem cells.19 We show here that integrin α6 chain is found also in a subset of bone marrow stromal cells, suggesting interaction of laminins not only with hematopoietic cells but also with bone marrow stromal cells. Moreover, we show that the multipotent hematopoetic FDCP-mix cells use α6 and β1 integrin chains as receptors for the α5 containing laminins, laminin-10/11. This was shown by antibody perturbation experiments by using blocking antibodies GoH3 and Ha2/5 against integrin α6 and β1 chains. In contrast, another antibody 9EG7, which binds to an inducible epitope in the β1 integrin chain and can both inhibit and stimulate ligand binding,54 55 did not inhibit adhesion of FDCP-mix to laminin-10/11.
The laminin preparation shown here to be adhesive for FDCP-mix cells was purified with 4C7 antibody, which is specific for the laminin α5 chain.38 The preparation contains laminin-10 and some laminin-11.56 Of these only laminin-10 is expressed in the bone marrow. So far, very limited information is available on the cell adhesive properties of the laminin isoforms containing the α5 chain,57 as well as of those containing the laminin α4 chain. Our current findings emphasize the need to test the adhesive role of laminins other than laminin-1 for hematopoietic cells. Proteins purified from cell cultures or tissues, or recombinant fragments corresponding to specific domains of the individual polypeptides may be used. Because of the heterogeneity of laminins in tissues, many of the newly discovered laminin isoforms are difficult to purify in large amounts. Studies with recombinant fragments are complicated by the findings that an intact structure composed of all three polypeptides may be required for receptor binding.58 Furthermore, fragments may contain cryptic binding sites normally not exposed in the intact molecule,59,60 and analyses using recombinant trimeric domains or mutated proteins may be necessary to confirm the results obtained by synthetic peptides or proteolytic fragments.7
The finding that laminin-1 is not present in bone marrow significantly alters the interpretation of previous studies concerning interactions of normal and malignant hematopoietic cells with laminins. Because several isoforms of laminins are able to bind to the same cellular receptors, the interactions observed with laminin-1 with hematopoietic cells11,12,14,15,18 may reflect cellular interactions with the laminin isoforms present in the bone marrow. Alternatively, the observations11,12,14,15 18 may reflect in vivo interactions of circulating cells in tissues other than bone marrow. Because we show here adhesion of hematopoietic stem cells to a laminin isoform present in the bone marrow, we suggest that further functional studies on the role of laminins within the bone marrow should focus on the isoforms containing α2, α4, and α5 chains.
ACKNOWLEDGMENT
We thank Robert Burgeson, Alan Richards, and Arnoud Sonnenberg for the antisera and antibodies, Staffan Johansson and Mats Paulsson for proteins, Östen Ljunggren for human bone marrow specimens, and Anne-Mari Olofsson for skillful technical assistance.
Supported by the Swedish Cancer Fund, Lions’ Cancer Foundation, Academic Hospital, Uppsala.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal