Tumor cells that survive initial courses of chemotherapy may do so by acquiring a multidrug-resistant phenotype. This particular mechanism of drug resistance may also confer resistance to physiological effectors of apoptosis that could potentially reduce the efficacy of immune therapies that use these pathways of cell death. We have previously demonstrated high efficacy for a cytokine-based tumor cell vaccine in a murine MPC11 myeloma model. In the present study, the effects of this vaccination were compared in MPC11 cells and their isogenic sublines selected for mdr1/P-glycoprotein (Pgp)-mediated multidrug resistance (MDR). Immunization with MPC11 cells expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-12 (IL-12) led to long-lasting protection of mice against subcutaneous (sc) challenge with both parental cells or their MDR variants. Similarly, immunization with GM-CSF/IL-12–transfected MDR sublines caused rejection of transplantation of both parental cells and the MDR sublines. Whereas MPC11 cells and their MDR variants were resistant to APO-1/CD95/Fas ligand, the immunization generated potent granzyme B/perforin-secreting cytotoxic T lymphocytes (CTLs) that were similarly effective against both parental and isogenic MDR cells. We conclude that MDR mediated bymdr1/Pgp did not interfere with lysis by pore-forming CTLs. Immunotherapy based on pore-forming CTLs may be an attractive approach to the treatment of drug-resistant myeloma.
THE RESISTANCE OF tumor cells to various therapeutic regimens remains a major obstacle for cure of patients with multiple myeloma. Recurrence of the disease after initial courses of chemotherapy indicates that a population of myeloma cells emerges due to the acquisition of multidrug resistance (MDR). This type of resistance is commonly mediated by P-glycoprotein (Pgp), a transmembrane pump capable of effluxing many agents out of the cell.1 The role of Pgp overexpression as a factor of poor prognosis in patients with multiple myeloma is widely recognized.2-4 Thus, the cells that acquired MDR in the course of chemotherapy may constitute a significant component of minimal residual disease in patients with multiple myeloma. This, in turn, raises the necessity to develop novel therapeutic approaches capable of eliminating the drug-resistant population of cells.
In recent years, immunotherapy has been gaining popularity as an adjunct to cancer chemotherapy. The expression of Igs that contain the unique antigenic determinants (idiotypes) in myeloma cells provides a possibility to generate anti-idiotypic immunity. The idiotype-specific T-cell–mediated immune responses against B-cell lymphomas have been demonstrated in a number of experimental reports and clinical trials.5-8 Tumor cell-associated antigens other than idiotypic Ig may also be recognized by the immune system and processed to evoke proliferation of specific clones of cytotoxic T lymphocytes (CTLs). The high therapeutic efficacy of vaccination of mice with irradiated tumor cells transfected with cytokine genes9-11demonstrates the potential value of using multiple tumor-associated antigens for generation of antitumor immunity. We have demonstrated effective CTL-mediated antitumor immunity in animals immunized with poorly immunogenic MPC11 myeloma cells transfected with granulocyte-macrophage colony-stimulating factor (GM-CSF) cDNA.11 These data underscore the possibility of using immunotherapy in combination with conventional therapeutic modalities in multiple myeloma.
Using immunotherapy as an approach to eliminating minimal residual disease requires that MDR tumor cells be sensitive to the effector mechanisms of the immune system. However, the susceptibility of drug-resistant tumor cells to these mechanisms has been questioned. It has been shown that several tumor cell lines selected for resistance to different chemotherapeutic drugs were also resistant to lymphokine-activated killers (LAK) or natural killer (NK) cells.12,13 Kimmig et al14 have found that two of three independently selected MDR sublines of the T-cell leukemia line, CCRF-CEM, were significantly less susceptible to LAK-mediated lysis, whereas one subline was as sensitive as the parental cells. In contrast, Scheper et al15 have demonstrated that the selection of RPMI-8226 myeloma cells for resistance to doxorubicin (Dox; Pgp-mediated MDR) or mitoxantrone (Pgp-unrelated resistance) did not alter the sensitivity to in vitro lysis by LAK or NK. Given that T-cell response against human myeloma has been demonstrated16-18 and that CTL-mediated killing of myeloma cells in mice has been shown to be highly effective,11 we examined the question of whether the selection for Pgp-mediated MDR affects susceptibility of myeloma cells to a CTL-based vaccine.
The granzyme B/perforin and APO-1/CD95/Fas ligand (FasL) pathways are two main mechanisms mediating the lytic effects of CTLs.19,20 However, it has been shown that myeloma cells can be resistant to Fas receptor cross-linking before chemotherapy.21 Moreover, the selection of myeloma cells for drug resistance may result in coselection for cross-resistance to Fas-mediated apoptosis.22 Furthermore, Pgp may confer an additional protection of tumor cells from caspase-dependent apoptotic signals, including Fas-induced death.23 Consequently, the functionality of signaling pathways involved in lysis of MDR myeloma cells by granzyme B/perforin is likely to be an important prerequisite for the efficacy of CTL-based immunotherapy.
In the present study, we examined the effects of myeloma cell-based vaccine against isogenic tumor cells selected in vitro for low levels of Pgp-mediated MDR. In particular, we investigated whether CTLs generated in response to immunization with cytokine-expressing myeloma cells were capable of killing isogenic MDR variants. Our results provide evidence that immunization with GM-CSF/interleukin-12 (IL-12)–transfected myeloma cells caused highly efficient and prolonged cross-protection of mice from challenge with either parental or isogenic MDR myeloma cells and that this effect was mediated by granzyme B/perforin-secreting CTLs.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female Balb/c mice (National Cancer Institute, Bethesda, MD) were used for all experiments. Mice were housed in the animal facility located at H. Lee Moffitt Cancer Center and Research Institute.
Drugs.
Dox, vincristine, and melphalan (all from Sigma Chemical Co, St Louis, MO) were reconstituted in distilled water or acidified 70% aqueous methanol (melphalan) as 100× to 1,000× stock solutions and kept at −20°C. Aqueous solution of dexamethasone was purchased from Genisa Pharmaceuticals, Inc (Irvine, CA). Recombinant soluble human FasL (Alexis Corp, San Diego, CA) was reconstituted in sterile phosphate-buffered saline (PBS), pH 7.4, and stored at less than −20°C. Concanamycin A (CMA; follimycin; Calbiochem, San Diego, CA) was dissolved in dimethyl sulfoxide at 100 μmol/L immediately before the experiments.
Cell lines and selection for MDR.
Mouse myeloma cell line MPC11 and T-cell leukemia line S49.1 (both derived from Balb/c mice) were purchased from American Type Culture Collection (Manassas, VA) and propagated according to the vendor’s instructions. Cells were routinely tested and found to be free ofMycoplasma. Cells in logarithmic phase of growth were used for all experiments. For selection of Dox-resistant sublines, MPC11 cells were cultured in the presence of increasing concentrations of Dox starting from 20 nmol/L. After 8 to 10 weeks, two independently selected sublines, MPC11Dox10-1 and MPC11Dox10-2, were established and propagated in the presence of 100 nmol/L Dox. For cytotoxicity assays, cells (2 × 104 in 100 μL of culture medium) were plated into a 96-well plate. Increasing concentrations of cytotoxic agents were added, and cells were incubated for 72 hours at 37°C, 5% CO2. The percentage of survival was determined in a colorimetric CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega Corp, Madison, WI).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of the mdr1 gene expression.
Isolation of total RNA and RT were performed as described.24 PCR was performed with amounts of cDNA corresponding to 50 ng of total RNA, 1.5 mmol/L MgCl2, 200 μmol/L each of deoxynucleotide triphosphate, 1 U Taq polymerase (Boehringer Mannheim Co, Indianapolis, IN), and 10 ng/μL primers specific for murine mdr125 and β-actin (internal standard; R&D Systems, Minneapolis, MN) cDNAs. The reaction conditions were 94°C for 45 seconds, 55°C for 1 minute, and 72°C for 1 minute. Each cDNA was amplified in separate tubes in duplicate in a Peltier Thermal Cycler (Watertown, MA). In preliminary experiments we found that 29 cycles of PCR for mdr1 and 31 cycles for β-actin cDNAs, respectively, yielded clearly detectable mdr1- (395 bp) and β-actin (528 bp)-specific products within the exponential range. PCR products were resolved in a 1.5% agarose gel electrophoresis, stained with ethidium bromide, and photographed.
Pgp-mediated transport.
The intracellular accumulation of calcein was used to analyze Pgp-mediated transport.26 MPC11 cells or Dox-selected sublines (106 cells in 1 mL of PBS) were loaded with 50 nmol/L calcein/acetoxymethyl ester (AM; Molecular Probes, Eugene, OR) for 20 minutes at 37°C in the presence or absence of 40 μmol/L verapamil (Sigma). Cells were preincubated with verapamil for 10 minutes before the addition of calcein/AM. Cell-associated green fluorescence was detected on a FACScan (Becton Dickinson, San Jose, CA; excitation 488 nm, emission 524 nm). Ten thousand events were acquired for each treatment.
Topoisomerase II activity.
A kinetoplast DNA decatenation assay was performed using Topoisomerase II assay kit (TopoGEN, Inc, Columbus, OH) as recommended by the manufacturer. Nuclear extracts were prepared as described.27
Transfection, immunization, and tumor cell challenge.
The vectors expressing GM-CSF or IL-12 cDNA and transfection with the Accell gene gun (Agracetus/PowderJect, Middleton, WI) have been described previously.28 The MPC11, MPC11Dox10-1, or MPC11Dox10-2 cells were γ-irradiated (40 Gy), transfected with the vector expressing GM-CSF cDNA28 or mock-transfected (also referred to as irradiated cells), and injected (1.5 × 106 cells) subcutaneously (sc) into the abdominal area of mice. Seven days later, an equal number of irradiated, IL-12 cDNA-transfected (or mock-transfected) cells were injected sc into the adjacent area. In all experiments, the amounts of GM-CSF and IL-12 in the cell supernatants 24 hours posttransfection were 40 to 60 ng and 200 to 250 ng per 106 cells, respectively (as determined by enzyme-linked immunosorbent assay11). At day 14, 106 nonirradiated cells were inoculated sc into the right flank. In the cross-immunization experiments, mice were immunized either with irradiated, GM-CSF/IL-12–transfected parental MPC11 cells followed by engraftment of Dox-resistant cells or with irradiated, GM-CSF/IL-12–transfected Dox-resistant cells and then challenged with parental MPC11 cells. For challenge, 106 cells/animal, the fivefold TD100 (the number of tumor cells that induce tumors in 100% of injected mice), was used. Tumor growth was monitored 3 times/week for 5 weeks. In preliminary experiments, we determined that tumorigenicity and tumor growth rates in parental cells and MDR sublines were indistinguishable (data not shown).
CTL activity assay.
Mice were immunized as described above. Single-cell suspensions of splenocytes pooled from 3 mice were obtained 21 days after the injection of IL-12–transfected cells. The splenocytes were stimulated in vitro with irradiated cells used for immunization (ie, MPC11 or their Dox-resistant counterparts) for 5 days at 37°C, 5% CO2. Fresh target cells (MPC11 or MDR variants) were labeled with 100 μCi sodium chromate-51 (1 mCi/mL; New England Nuclear Life Science Products, Boston, MA) for 1 hour at 37°C, followed by three washings. The activity of CTLs was tested in a 5-hour51Cr release assay.29 In some experiments, the cocultures of splenocytes and stimulator cells after 5 days of incubation were pretreated with 250 nmol/L CMA for 2 hours before the addition of target cells.30
RESULTS
Mechanisms of MDR in MPC11-derived sublines selected in vitro for Dox resistance.
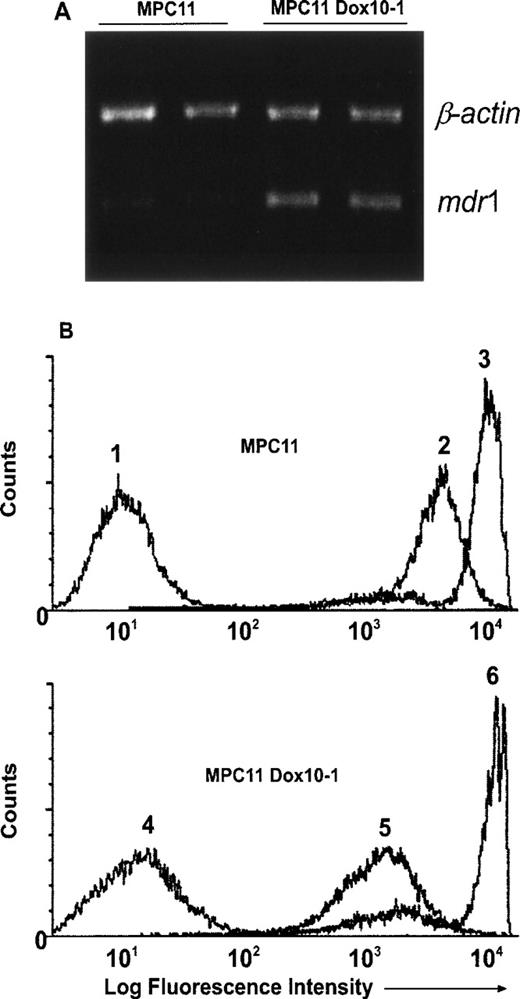
We have previously shown that immunization of mice with irradiated MPC11 cells transfected with GM-CSF expression vector resulted in the rejection of subsequent engraftment of intact MPC11 cells.11 We sought to test whether this vaccination was also effective in protecting mice from MPC11 cells selected in vitro for MDR. The stepwise selection of parental MPC11 cells with increasing concentrations of Dox resulted in the establishment of MPC11Dox10-1 subline. We found that the MPC11Dox10-1 cells were resistant to Dox (IC50, 460 nmol/L v 50 nmol/L in MPC11 cells; 9.2-fold resistance) and cross-resistant to vincristine (IC50, 60 nmol/L v 8 nmol/L in MPC11 cells; 7.5-fold resistance). No significant resistance to melphalan (IC50, 10 μmol/L in MPC11Dox10-1 cells v 9 μmol/L in MPC11 cells) or dexamethasone (0.3 μmol/L v 0.3 μmol/L, respectively) was observed in MPC11Dox10-1 cells, suggesting typical Pgp-mediated MDR. Indeed, the increased levels of mdr1 mRNA were detected in MPC11Dox10-1 subline by RT-PCR (Fig 1A). In addition, accumulation of calcein, a measure of Pgp-mediated transport,26 was decreased in the selectants, indicating that MPC11Dox10-1 cells expressed functional Pgp (Fig 1B; compare profiles 2 and 5). We also examined the expression of the mrp and lrp genes as well as the activity of topoisomerase II, mechanisms known to be involved in the resistance to Dox,31-34 in MPC11 cells and in Dox-selected variants. No changes in the steady-state levels of the mrp or lrp mRNAs (by RT-PCR) or topoisomerase II activity (by decatenation assay) were found in MPC11Dox10-1 cells as compared with their parental counterparts (data not shown). Finally, blocking of Pgp transport with verapamil completely abrogated the resistance of MPC11Dox10-1 cells to Dox (data not shown). Together, these results indicate that the overexpression ofmdr1/Pgp is the main mechanism of acquired MDR in MPC11Dox10-1 cells.
MDR in MPC11Dox10-1 cells is conferred bymdr1/Pgp. (A) Detection of increased mdr1 mRNA in MPC11Dox10-1 cells by RT-PCR. Total RNA was isolated from MPC11 and MPC11Dox10-1 cells, reverse transcribed, and amplified with primers specific for the mdr1 and β-actin genes as described in Materials and Methods. The experiments were repeated twice with the same results. (B) Calcein accumulation is decreased in MPC11Dox10-1 cells. MPC11 (upper panel, profiles 1 through 3) and MPC11Dox10-1 (lower panel, profiles 4 through 6) cells were left untreated (1 and 4) or treated with 50 nmol/L calcein/AM in the absence (2 and 5) or presence (3 and 6) of 40 μmol/L verapamil. After washing, cellular fluorescence was measured on a FACScan (excitation 488 nm, emission 524 nm) on FL1. The experiments were performed twice with comparable results.
MDR in MPC11Dox10-1 cells is conferred bymdr1/Pgp. (A) Detection of increased mdr1 mRNA in MPC11Dox10-1 cells by RT-PCR. Total RNA was isolated from MPC11 and MPC11Dox10-1 cells, reverse transcribed, and amplified with primers specific for the mdr1 and β-actin genes as described in Materials and Methods. The experiments were repeated twice with the same results. (B) Calcein accumulation is decreased in MPC11Dox10-1 cells. MPC11 (upper panel, profiles 1 through 3) and MPC11Dox10-1 (lower panel, profiles 4 through 6) cells were left untreated (1 and 4) or treated with 50 nmol/L calcein/AM in the absence (2 and 5) or presence (3 and 6) of 40 μmol/L verapamil. After washing, cellular fluorescence was measured on a FACScan (excitation 488 nm, emission 524 nm) on FL1. The experiments were performed twice with comparable results.
Provided that the selection procedure may upregulate different mechanisms of MDR and also change the malignant potential of selectants,35 36 we performed another independent selection of MPC11 cells for resistance to Dox. This selection resulted in the establishment of MPC11Dox10-2 subline propagated in the presence of 100 nmol/L Dox. The levels of cross-resistance to chemotherapeutic drugs in this subline were essentially the same as in MPC11Dox10-1 variant. Also, the overexpression of the mdr1 gene and the decreased calcein accumulation were detected in MPC11Dox10-2 cells (data not shown) indicating typical Pgp-mediated MDR. Each Dox-selected subline was tested separately in the following in vivo experiments (see below).
Cross-protection from challenge with MPC11 cells and MDR derivatives in mice vaccinated with irradiated, GM-CSF/IL-12–expressing myeloma cells.
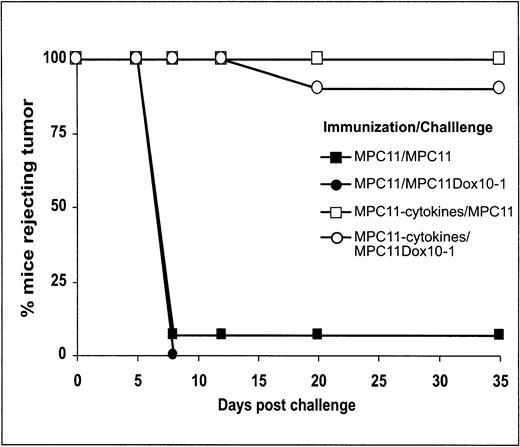
Despite the fact that GM-CSF cDNA-based vaccination resulted in potent protection against MPC11 cells, under more stringent conditions in which tumor challenge was performed 1 week after immunization, only approximately 60% of mice were protected.11 An additional immunization with IL-12 cDNA-transfected MPC11 cells at least 1 day before tumor cell challenge improved the antitumor protection to greater than 90% (manuscript in preparation). Based on these data, we used the combination of GM-CSF– and IL-12–transfected tumor cells (MPC11 or their MDR variants) for vaccination experiments. Figure 2 shows the kinetics of tumor take in control animals (naı̈ve or immunized with irradiated cells) and mice immunized with GM-CSF/IL-12–transfected cells (both MPC11 and MPC11Dox10-1). Typically, in control cohorts tumors were registered by days 8 to 12 after the engraftment of tumor cells. In contrast, mice immunized with GM-CSF/IL-12–transfected cells remained tumor-free for at least 35 days postchallenge.
Time course of initiation of tumor growth in mock-immunized mice and mice vaccinated with irradiated, GM-CSF/IL-12–transfected MPC11 cells. Mice were vaccinated with irradiated MPC11 cells with or without cytokine gene transfection. Both MPC11 and MPC11Dox10-1 cells were used for tumor challenge. Tumor growth was monitored for up to 35 days. Data shown represent cumulative results of three independent experiments (n = 15).
Time course of initiation of tumor growth in mock-immunized mice and mice vaccinated with irradiated, GM-CSF/IL-12–transfected MPC11 cells. Mice were vaccinated with irradiated MPC11 cells with or without cytokine gene transfection. Both MPC11 and MPC11Dox10-1 cells were used for tumor challenge. Tumor growth was monitored for up to 35 days. Data shown represent cumulative results of three independent experiments (n = 15).
The results presented in Table 1 show that both the parental MPC11 and the MDR variants were capable of producing tumors in nonimmunized (naı̈ve) animals. Only 1 mouse (of 15) vaccinated with irradiated MPC11 cells rejected subsequent tumor cell challenge, whereas all animals vaccinated with cytokine-expressing cells were tumor-free. Importantly, 90% of mice immunized with GM-CSF/IL-12–expressing MPC11 cells were protected from the engraftment of MDR variants. Furthermore, all animals vaccinated with cytokine-expressing MPC11Dox10 sublines rejected the challenge with parental cells (Table 1).
Cross-Protection of Mice Immunized With GM-CSF/IL-12–Transfected Tumor Cells From Challenge With MPC11 Cells or MDR Variants
| Mice . | Challenge . | |
|---|---|---|
| MPC11 . | MPC11Dox10* . | |
| Naive | 15/15† | 10/10 |
| Immunized with | ||
| MPC11 | ||
| Irradiated cells | 14/15 | 10/10 |
| Irradiated, GM-CSF/IL-12–transfected cells | 0/15‡ | 1/10‡ |
| MPC11 Dox 10 | ||
| Irradiated cells | 13/15 | 15/15 |
| Irradiated, GM-CSF/IL-12–transfected cells | 1/15‡ | 0/15‡ |
| Mice . | Challenge . | |
|---|---|---|
| MPC11 . | MPC11Dox10* . | |
| Naive | 15/15† | 10/10 |
| Immunized with | ||
| MPC11 | ||
| Irradiated cells | 14/15 | 10/10 |
| Irradiated, GM-CSF/IL-12–transfected cells | 0/15‡ | 1/10‡ |
| MPC11 Dox 10 | ||
| Irradiated cells | 13/15 | 15/15 |
| Irradiated, GM-CSF/IL-12–transfected cells | 1/15‡ | 0/15‡ |
The results with MPC11Dox10-1 and MPC11Dox10-2 sublines were similar; therefore, cumulative data of three experiments obtained with both sublines (designated as MPC11Dox10) are presented.
Number of mice that developed tumor/number of mice challenged.
P < .001 (by χ2 criterion) as compared with naive mice or animals immunized with irradiated cells.
The cytotoxic effect of vaccination is mediated by granzyme B/perforin-secreting CTLs.
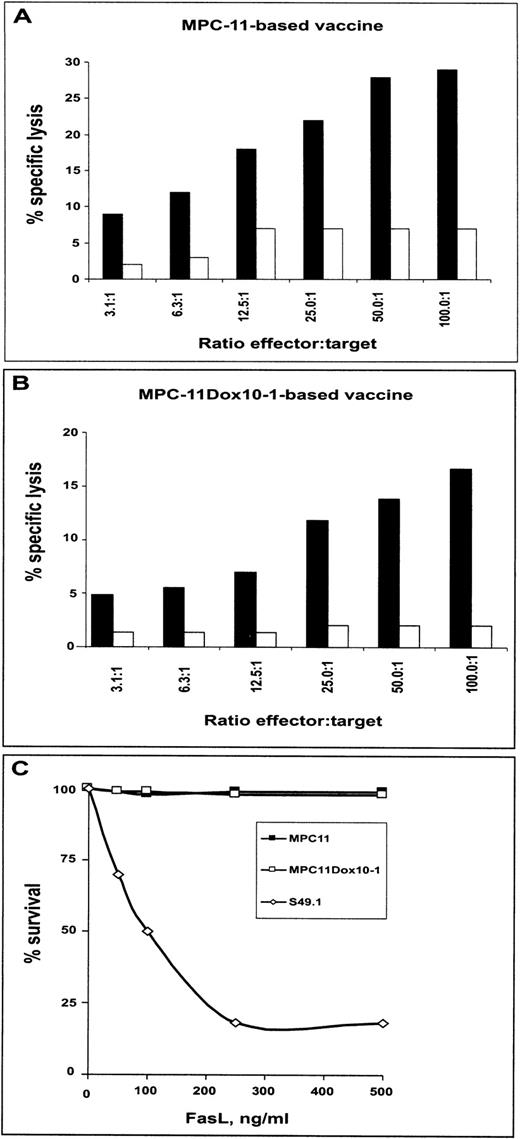
Previous studies have demonstrated that immunization with cytokine-based tumor cell-based vaccines generated T-cell–mediated antitumor immunity.9-11 We investigated whether CTLs generated by vaccination with cytokine gene-based MPC11 cells were capable of lysing MPC11Dox10 sublines. After in vitro stimulation with respective myeloma cells for 5 days, the splenocytes from mice immunized with GM-CSF/IL-12–transfected MPC11 cells lysed both MPC11 cells and isogenic MDR cell variants (Fig3A). Also, CTLs from mice vaccinated with GM-CSF/IL-12–transfected MPC11Dox10 cells effectively lysed both parental cells and MDR variants (Fig 3B). The results indicate similar sensitivity to CTL-mediated killing of both parental cells and their isogenic sublines with acquired MDR.
CTL activity in mice immunized with irradiated cells and GM-CSF/IL-12–expressing vaccine. Mice were immunized with irradiated only or irradiated, GM-CSF/IL-12–transfected cells (MPC11 [A] or MPC11Dox10-1 [B]). At day 21 after the initial vaccine injection, animals were killed, and CTL activity was tested as described in Materials and Methods. CTL activity is expressed as the percentage of specific lysis of 51Cr-labeled target cells. Each value represents mean of duplicate samples. The error between duplicates was less than 10%. The experiments were repeated twice with similar results.
CTL activity in mice immunized with irradiated cells and GM-CSF/IL-12–expressing vaccine. Mice were immunized with irradiated only or irradiated, GM-CSF/IL-12–transfected cells (MPC11 [A] or MPC11Dox10-1 [B]). At day 21 after the initial vaccine injection, animals were killed, and CTL activity was tested as described in Materials and Methods. CTL activity is expressed as the percentage of specific lysis of 51Cr-labeled target cells. Each value represents mean of duplicate samples. The error between duplicates was less than 10%. The experiments were repeated twice with similar results.
To specify which mechanism(s) was involved in rejection of engraftment of MPC11 and MPC11Dox10 cells, two sets of experiments were performed. We first tested whether inhibition of perforin secretion would attenuate CTL activity. It has been previously shown that CMA at submicromolar concentrations caused degradation of perforin and abrogated perforin-mediated lytic activity of CTLs.30 37Figures 4A and B show that pretreatment of cocultures of splenocytes and stimulator cells with 250 nmol/L CMA for 2 hours significantly decreased the ability of splenocytes from mice vaccinated with GM-CSF/IL-12–expressing cells (parental or MDR sublines) to lyse both MPC11 cells and MDR variants. Furthermore, treatment with recombinant FasL showed that MPC11 and MPC11Dox10-1 cells were resistant to cross-linking of Fas receptor (Fig 4C). The MPC11Dox10-2 subline was also resistant to FasL (data not shown). Together, the results suggest that immunization with cytokine-based tumor cell vaccine generates CTLs that are capable of killing both MPC11 cells and their MDR variants via a granzyme B/perforin-sensitive and Fas-independent mechanism.
CTLs from mice immunized with GM-CSF/IL-12–expressing vaccines lyse MPC11 cells and their MDR variants via granzyme B/perforin- but not Fas-dependent mechanism. Mice were immunized with GM-CSF/IL-12–expressing MPC11 cells (A) or MPC11Dox10-1 cells (B) as described. Splenocytes isolated at day 21 after the initial injection of the vaccine were stimulated with irradiated MPC11 (A) or irradiated MPC11Dox10-1 (B) cells for 5 days, and CTL activity in the absence (▪) or presence (□) of 250 nmol/L CMA was determined. (C) Cells were incubated with recombinant FasL for 17 hours. The cytotoxicity was determined in a colorimetric test (see Materials and Methods). The experiments were repeated twice with similar results. The S49.1 cell line was included as a positive control for FasL-mediated apoptosis.
CTLs from mice immunized with GM-CSF/IL-12–expressing vaccines lyse MPC11 cells and their MDR variants via granzyme B/perforin- but not Fas-dependent mechanism. Mice were immunized with GM-CSF/IL-12–expressing MPC11 cells (A) or MPC11Dox10-1 cells (B) as described. Splenocytes isolated at day 21 after the initial injection of the vaccine were stimulated with irradiated MPC11 (A) or irradiated MPC11Dox10-1 (B) cells for 5 days, and CTL activity in the absence (▪) or presence (□) of 250 nmol/L CMA was determined. (C) Cells were incubated with recombinant FasL for 17 hours. The cytotoxicity was determined in a colorimetric test (see Materials and Methods). The experiments were repeated twice with similar results. The S49.1 cell line was included as a positive control for FasL-mediated apoptosis.
DISCUSSION
The major findings of this study are (1) vaccination with myeloma cells expressing transfected GM-CSF/IL-12 confers cross-protection of mice from challenge with poorly immunogenic MPC11 cells as well as from their MDR counterparts and (2) granzyme B/perforin-secreting CTLs can efficiently eliminate myeloma cells that are resistant to Pgp-transported chemotherapeutic drugs and FasL.
In accordance with the data from in vivo experiments, CTLs from immune mice showed high in vitro toxicity against MPC11 cells or MDR sublines regardless of which cell line was used for immunization. CTLs exert cytolytic effect on tumor cells by two mechanisms, namely, via soluble or CTL membrane-bound FasL, and/or by exocytosis of 70-kD protein perforin that forms pores in the plasma membrane of tumor cells.38 The latter event allows granzyme B, a serine protease produced by CTLs, to enter the target cell and initiate apoptotic cascade.38 It has been shown that perforin is stored in acidic compartments of the cell38 and that the increase of pH in these organelles by blocking vacuolar type H+-ATPase by CMA leads to degradation of perforin.37 In our model, the FasL-mediated lytic mechanism was eliminated because neither MPC11 cells nor MPC11Dox10 variants were sensitive to FasL. In contrast, pretreating of splenocytes derived from immunized mice with CMA significantly attenuated the CTL lytic activity against MPC11 cells and their MDR variants. These results demonstrate that immunization with GM-CSF/IL-12–secreting myeloma cells can generate potent CTLs that are capable of killing both parental and isogenic MDR myeloma cells via perforin/granzyme B-mediated mechanism.
The fact that the lysis of myeloma cells by CTLs was observed in a short-term (5-hour) 51Cr release assay suggests perforin-induced necrosis a mechanism of CTL cytotoxicity. However, we cannot rule out the possibility that granzyme B acted as an apoptosis-initiating agent in MPC11 and MPC11Dox10 cells. Several proenzyme caspases, including caspase 3, are the substrates for granzyme B.39 Moreover, caspase 3 may be directly cleaved by granzyme B in vivo.40 These data imply that apoptosis triggered by granzyme B requires functional activity of signaling mechanisms downstream of caspase 3. It remains to be determined whether these mechanisms execute granzyme B-initiated programmed death in MPC11 cells and whether the selection for MDR impairs these pathways. If that is the case, perforin and granzyme B may act in concert to ensure killing of parental and MDR myeloma cells. Taking into consideration that the selection for resistance to chemotherapeutic drugs may render tumor cells less sensitive to several caspase-dependent apoptotic stimuli, including Fas cross-linking,22 23 the lysis via perforin should be sufficient to effect antitumor immunity in MDR myeloma.
Our data indicate that myeloma cells that acquire MDR in the course of selection with Dox are still sensitive to lysis by CTLs. This implies potential therapeutic value for CTL-based immunotherapy in patients with minimal residual disease, given that proliferative T-cell responses are not compromised by preceding treatment.18Collectively, we provide experimental evidence that immunization with cytokine-based tumor cell vaccine is an efficient adjunct to conventional therapeutic protocols in MDR myeloma.
ACKNOWLEDGMENT
The authors are grateful to Agracetus/PowderJect (Middleton, WI) for providing us with the gene gun, to M. Oshiro for selecting the MPC11Dox10-1 subline, to Dr T. Landowski for critical discussion of the manuscript, and to J. Kroeger (Flow Cytometry Core, H. Lee Moffitt Cancer Center and Research Institute) and J. Szucs (Multimedia Educational Resource Center, H. Lee Moffitt Cancer Center and Research Institute) for computer graphic assistance.
A.A.S. and J.G.T. contributed equally to this work.
Supported by grants from Kathy Guisti Myeloma Research Foundation and National Institutes of Health (CA 75243 and CA 77859) and by the Dr. Tsai-Fan Yu Cancer Research Endowment.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hua Yu, PhD, Immunology Program, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612-9497.

![Fig. 3. CTL activity in mice immunized with irradiated cells and GM-CSF/IL-12–expressing vaccine. Mice were immunized with irradiated only or irradiated, GM-CSF/IL-12–transfected cells (MPC11 [A] or MPC11Dox10-1 [B]). At day 21 after the initial vaccine injection, animals were killed, and CTL activity was tested as described in Materials and Methods. CTL activity is expressed as the percentage of specific lysis of 51Cr-labeled target cells. Each value represents mean of duplicate samples. The error between duplicates was less than 10%. The experiments were repeated twice with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1831.406k38_1831_1837/5/m_blod40638003x.jpeg?Expires=1766010579&Signature=EptWpjZoDIZ3CfPIYViJDJWS1TTLLOhys9BvEm7j7b-Bm59BAVLh9b4jwWSJN5Q3PoHinZqrC-UMbI88-fxpK78QMUzo7-8th0vgGK3tm~QxyKEN1j7jBEuRpNMVQkbpTOv5sw5JoaYu1YmlcuXQ14Iz7bt15b07LrylNAgISuSZO~GPHRDzI5SL5gJ37RVLaaIW2xdLRAtgF-hoCdY-zaWrcvRmL25naqCrrSTT28pOfbS4qvpFffgACA9nEwoXf3AQBYRdIjp3pazSaHE6lozeRXfy4hXHOYZtiZhEp00LgfVtj1fq6Yk6DCQBTMAJe7aIXSxXkEJqhU-D58T7OA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal