Abstract
The receptor for the gene product of the obesity gene, leptin, was recently reported to be expressed on murine and human hematopoietic progenitor cells. Therefore, we studied the expression of the leptin receptor, OB-R, in normal myeloid precursors, human leukemia cell lines, and primary leukemic cells using reverse-transcriptase polymerase chain reaction. In normal hematopoiesis, OB-R was expressed in CD34+ cells. Normal promyelocytes (CD34−33+ and CD34−13+) expressed only very low levels of the short, presumably nonsignaling isoform. Both the long and short isoforms of OB-R were expressed in 10 of 22 samples from patients with newly diagnosed primary or secondary acute myeloid leukemia (AML), with a higher incidence of the long isoform in primary AML (87.6% v28.6%; P = .01). The incidence of OB-R expression was higher in recurrent than in newly diagnosed AML (P < .001), and samples from four patients with refractory AML showed strong expression of both isoforms. Both OB-R isoforms were also expressed in newly diagnosed and recurrent acute promyelocytic leukemia cells but were essentially absent in samples of chronic or acute lymphocytic leukemia. In vitro growth of myeloid leukemic cell lines and of blasts from 14 primary AMLs demonstrated that recombinant human leptin alone induced low level proliferation, significantly (P < .05) increased proliferation induced by recombinant human granulocyte colony-stimulating factor, interleukin 3, and stem cell factor in a subset of AML and increased colony formation (P < .005). Also, leptin reduced apoptosis induced by cytokine withdrawal in MO7E and TF-1 cells. Serum leptin levels correlated only with body mass index (P < .001) and gender (P = .03). Results confirm the reported expression of leptin receptor in normal CD34+ cells and demonstrate the frequent expression of leptin receptors in AML blasts. While normal promyelocytes lack receptor expression, leukemic promyelocytes express both isoforms. We also demonstrate proliferative effects of leptin alone and in combination with other physiologic cytokines, and anti-apoptotic properties of leptin. These findings could have implications for the pathophysiology of AML.
MANY CYTOKINES have been shown to regulate the survival, proliferation, differentiation, and function of normal hematopoietic1 and leukemic cells.2 Two groups recently cloned the leptin receptor from CD34-expressing (CD34+) human progenitor cells3 and from murine fetal liver and yolk sac-derived cell lines.4 The receptor is expressed in primitive hematopoietic cells and transduces signals from the obese (Ob) gene product, leptin.5Leptin is involved in the regulation of fat metabolism in mammals.6,7 It is expressed primarily in white adipose tissue and is secreted as a nonglycosylated protein of 16 kD into the circulation. The amount of leptin mRNA and serum levels of leptin are highly correlated with body fat volume.8,9 The human leptin receptor gene (OB-R) is localized on chromosome 1.10 Interestingly, the primary structure of OB-Rshows homologies to the signaling subunits of the interleukin (IL)-6–type cytokine receptors, including gp130, and to receptors for the leukemia inhibitory factor and the granulocyte colony-stimulatory factor (G-CSF).5 mRNAs for four human and six murine splice variants of OB-R have been identified, although the exact significance of the various isoforms has yet to be determined.4,6,7 The predominant OB-R mRNA found in most tissues encodes a transmembrane protein with a short cytoplasmic domain of 34 amino acid residues,5 referred to hereafter as the short isoform. In the hypothalamus, an OB-R mRNA exists that encodes a protein with an extracellular domain identical to that of the short isoform, but with a longer cytoplasmic domain of 302 amino acids.4,6,7 The long isoform was expressed in hematopoietic tissues (murine and human fetal livers and hematopoietic cell lines).4 The db mutation leads to the production of an aberrant splice variant of the long isoforms transcript, resulting in a protein with a truncated cytoplasmic domain.7,11 The loss of the carboxy-terminal region in the mutated protein has been proposed to render OB-R inactive and generates the obese phenotype in db/db mice.11
Recent studies of the physiologic expression of OB-R and the type of normal cells responsive to leptin suggest that leptin andOB-R may function as a growth factor/receptor-ligand system in hematopoietic stem and/or progenitor cells.3,4,12,13 The potential role of OB-R in primary hematopoietic malignancies has not been investigated. However, considering the pattern of receptor expression in normal hematopoietic progenitor cells, we hypothesized that the leptin gene product could be an important, and perhaps a distinct, regulator of leukemia cell proliferation. This hypothesis is supported by the reported presence ofOB-R transcripts in myeloid and some lymphoid T-cell cell lines.3 A rise in the level of fetal leptin has recently been reported in cord blood,14 suggesting that theOB-R has a possible role in fetal development. Because leptin is thought to regulate pluripotent stem cells, and because some leukemias might be derived from the transformation of these cells, we thought it of interest to analyze the expression of the OB-Rgene and its isoforms in human leukemic cells. Because leptin is produced by adipocytes and stromal cells,3 15 which make up a significant part of the bone marrow microenvironment, it could also stimulate leukemic progenitors in a paracrine fashion. No data has been reported on the effects of leptin on proliferation and clonal growth of leukemic progenitors from patients with acute myeloid leukemia (AML). Therefore, the aims of this study were (1) to investigate the expression patterns of the different receptor isoforms in purified myeloid progenitors from normal bone marrow, myeloid leukemic cell lines, and primary AML; (2) to investigate the mitogenic potential of recombinant leptin on the proliferation and clonal growth of human acute leukemia cells of myeloid origin, alone and in combination with other hematopoietic growth factors; and (3) to assess whether the biologic effects of leptin could be associated with the expression of distinct OB-R isoforms. Results indicate the presence of a functional leptin receptor capable of transducing proliferative and survival signals in the majority of patients with AML.
MATERIALS AND METHODS
Cell lines.
The OCI/AML3 and OCI/AML2 cell lines, originally established from AML patients,16 were kindly provided by Dr M.D. Minden (Ontario Cancer Institute, Toronto, Canada). HL-60, KG-1, K562, BV173, Raji, Jurkat, and TF-1 cell lines were obtained from the American Type Culture Collection (Rockville, MD). NB4 cells were kindly provided by Dr M. Lanotte.17 In addition, HL-60-doxorubicin resistant cells (HL-60-DOX)18 were also used. Cell lines were maintained in RPMI-1640 medium containing 10% fetal calf serum, 1% L-glutamine, and penicillin-streptomycin. Cultures for MO7E cells (provided by Dr H. Broxmeyer, Indiana University, Indianapolis, IN) were supplemented with 10 U/mL of recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (Schering-Plough, Kenilworth, NJ) and were maintained under conditions similar to those described for the other cell lines. Cell density was adjusted to a starting concentration of 0.5 × 106cells/mL, and cells were cultured in 96-well well plates at 37°C for 48 hours in 5% CO2 with and without leptin in increasing concentrations (10 to 300 ng/mL) for 72 hours. For costimulation experiments, cells were cultured for 72 hours with or without leptin (100 ng/mL) in combination with G-CSF, IL-3, and stem cell factor (SCF).
Human subjects.
Bone marrow or peripheral blood cells for in vitro studies were obtained from patients with newly diagnosed and recurrent AML, and from patients with advanced myelodysplastic syndrome (MDS) after obtaining informed consent according to institutional policy. The mononuclear cells were separated by Ficoll-Hypaque (Sigma, St Louis, MO) density gradient centrifugation. For proliferation experiments, only samples that contained more than 80% blasts were used.
The morphological subtypes of AML were defined according to the French-American-British (FAB) classification. Patients with a history of MDS or chemotherapy-induced AML were classified as having secondary AML.
Reagents.
Highly purified human recombinant leptin was purchased from R&D Systems (Minneapolis, MN). Recombinant human (rH) G-CSF (Schering-Plough) was added to cultures in concentrations of 100 U/mL. Recombinant IL-3 (Sandoz, East Hanover, NJ) and SCF (Amgen, Thousand Oaks, CA) were added at 50 and 100 ng/mL, respectively.19
[3H]Thymidine uptake.
Triplicate samples of 1 × 105 cells suspended in 200 μL RPMI were cultured in the presence or absence of recombinant leptin (100 ng/mL) in 96-well flat-bottom microtiter well plates (Costar, Cambridge, MA) in a humidified atmosphere of 5% CO2 in air at 37°C. After 2 days, 0.1 μCi of [3H]thymidine (specific activity, 6.7 Ci/mmol), (DuPont, Wilmington, DE) was added to each well, and the cultures were incubated overnight. For costimulation experiments, rHG-CSF or GM-CSF was added to cultures at 100 U/mL; IL-3 and SCF were added at 50 and 100 ng/mL, respectively. The cells were then deposited onto harvester filters using a semiautomatic cell harvester. Radioactivity was measured in a scintillation counter and expressed as counts per minute. The stimulation index (SI) was calculated as the ratio of the leptin-treated and control samples in counts per minute.
Clonogenic assay.
The clonogenic assay was performed as previously described.20 Briefly, OCI/AML2 cells were cultured in 0.8% methylcellulose (Fluka Chemical Corp, Ronkoncoma, NY), 10% fetal bovine serum, and Iscove’s modified Dulbecco medium (IMDM). Leptin was added in increasing concentrations (10 to 300 ng/mL) at the initiation of culture. Triplicate culture mixtures were placed in 35-mm Petri dishes (Nunc Inc, Naperville, IL) and maintained at 37°C with 5% CO2 in air in a humidified atmosphere. Colonies were counted after 7 days using an inverted microscope. A colony was defined as a cluster of more than 40 cells.
AML blast colony assay.
A previously described method was used to assay AML blast colony formation.21,22 Briefly, 1 × 105T-cell–depleted nonadherent low-density bone marrow cells were plated in 0.8% methylcellulose in IMDM supplemented with 10% fetal bovine serum and 15 ng/mL rHGM-CSF. Leptin was added at the initiation of cultures at concentrations ranging from 10 to 300 ng/mL. Duplicate cultures were incubated in 35-mm Petri dishes for 7 days at 37°C in a humidified atmosphere of 5% CO2 in air. AML blast colonies were microscopically evaluated on day 7 of culture. A blast colony was defined as a cluster of 20 or more cells. The leukemic origin of these colonies was previously shown by cytogenetic analysis.23
Apoptosis assays.
Apoptosis was analyzed in cytokine-dependent MO7E and TF1 cell lines after 48 and 72 hours of GM-CSF withdrawal in the presence or absence of leptin using the acridine orange DNA/RNA technique.24Samples were measured in a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Briefly, aliquots (80 μL) of cell suspension were mixed with 100 μL of a solution containing 0.1% (vol/vol) Triton X-100 (Sigma Chemical Co), 0.05 mol/L HCl, 0.15 mol/L NaCl, and 8 μg/mL acridine orange (Polysciences, Warrington, PA). DNA and RNA fluorescence was measured within 5 minutes of staining. The percentage of cells in the subG1 peak defined the proportion of apoptotic cells.
RNA isolation and reverse transcription.
RNA was isolated according to the single-step acid guanidinium thiocyanate-phenol-chloroform method.25 Purified RNA samples were quantitated using a DU-640 spectrophotometer (Beckman Instruments, Fullerton, CA). A reverse-transcription kit was used to synthesize cDNA according to the manufacturer’s instructions (Boehringer-Mannheim, Indianapolis, IN). One microgram of the total RNA template was used per 10 μL of reverse transcriptase (RT) reaction. Primers for the long and short isoforms of OB-R and primers for β2-microglobulin were synthesized on an oligonucleotide synthesizer (Model 392; Applied Biosystems, Foster City, CA). Oligonucleotide primers (F, forward; R, reverse) used for expression analysis by RT polymerase chain reaction (PCR) were as follows: primers that recognize all forms amplify a conserved sequence in the extracellular domain of the receptor. The human OB-R: F 5′-GTCAGAAGATGTGGGAAA-3′ (nucleotides 2266-2283) and R 5′-GTGCCCAGGAACAATTCTT-3′ (nucleotides 2828-2846). The long isoform F 5′-GCTATTTTGGGAAGATGT-3′ (nucleotides 2800-2817) and R 5′-TGCCTGGGCCTCTATCTC-3′ (nucleotides 3281-3298); and the short isoform (6.4 sequence) F 5′-GACTCATTGTGCAGTGTTCAG-3′ (nucleotides 2204-2221) and R 5′-TGGCACATTGGGTTCATC-3′ (nucleotides 2905-2923) are shown in Fig 1. The expected PCR products are 579 base pairs (bp) for OB-R, 501 bp for the OB-Rlong isoform, and 720 bp for the short isoform. The primers for β2-microglobulin were F 5′-ACCCCCACTGAAAAAGATGA-3′ and R 5′-TATCTTCAAACCTCCATGATG-3′. In all experiments, PCR was performed for 35 cycles. A32P-labeling PCR method was used to detect the radioactive products.26 The radioactive products were detected on the Betascope 603 (Betagen, Waltham, MA) after exposing the gel to Kodak film. The quality of each cDNA was determined by amplification of β2-microglobulin.
OB-R map: (OB-Rl), long form of leptin receptor; (OB-Rs), short (6.4 variant) form. The short form of the receptor represents a splice variant of the receptor, which has a unique cytoplasmic domain following the lysine residue at position 891 (stops 5 amino acids after Lys891). The reverse primers for the long and short isoforms of the receptor were designed to anneal to the unique sequences in the cytoplasmic portion of the receptor. → forward primer; ← reverse primer; SF, short form; LF, long form; ECD, extracellular domain; TM, transmembrane domain.
OB-R map: (OB-Rl), long form of leptin receptor; (OB-Rs), short (6.4 variant) form. The short form of the receptor represents a splice variant of the receptor, which has a unique cytoplasmic domain following the lysine residue at position 891 (stops 5 amino acids after Lys891). The reverse primers for the long and short isoforms of the receptor were designed to anneal to the unique sequences in the cytoplasmic portion of the receptor. → forward primer; ← reverse primer; SF, short form; LF, long form; ECD, extracellular domain; TM, transmembrane domain.
Flow cytometry and FACS-sorting.
Mononuclear cells from normal bone marrow donors were isolated by density gradient centrifugation (Sigma), followed by two washes in phosphate-buffered saline. Cells were incubated with HPCA-2/CD34, Leu-M9/CD33, and Leu-M7/CD13 and IgG controls (Becton Dickinson, San Jose, CA), respectively, for 30 minutes on ice, as recommended by the manufacturer, and were subsequently washed twice with phosphate-buffered saline. Cells displaying greater fluorescence intensity than their controls were considered positive. Promyelocytic cells were sorted based on forward and side scatter characteristics and CD33 positivity. Other cells were sorted according to their specific immunophenotype on a FACS Vantage (Becton Dickinson) equipped with an argon-ion laser (Spectra Physics, San Jose, CA) operated at 488 nm and 300 mW. Fluorescent signals were detected using 530/30-nm and 585/40-nm bandpass filters. Sorting was performed using R mode and Lysis II software (Becton Dickinson) as previously described.27 All cells were kept on ice during the sorting procedure. An aliquot of sorted cells was reanalyzed for purity.
Serum leptin levels.
Serum leptin levels were measured in 56 patients with MDS or AML and in five normal controls. Serum samples were drawn between 8 and 9am after an overnight (12-hour) fast. Leptin levels were determined by radioimmunoassay for human leptin (Linko Research Inc, St Charles, MO) as previously described.28 Briefly, patient samples were assayed in duplicate by a completely homogeneous assay using a rabbit antihuman leptin antibody; human leptin was used for both the standard and the tracer. The assay is sensitive down to 0.5 ng/mL; its limit of linearity is 100 ng/mL. Random samples that showed leptin immunoreactivity by radioimmunoassay were confirmed to contain bioactive leptin by assaying them on engineered leptin sensitive cell lines (data not shown). The degree of adiposity was determined as the body mass index (BMI), calculated as weight in kilograms divided by height in meters squared.29
Statistical analysis.
The statistical significance of differences in measured qualities was determined with the two-tailed Student’s t-test. A Pfactor of .05 or less was considered statistically significant. Unless otherwise indicated, average values were expressed as means ± SEM. The chi-square test was used to compare the expression of OB-Rwith clinical features.
The leptin levels in serum were analyzed after logarithmic transformation to eliminate the impact of skewing and variance heterogeneity. Multiple regression analysis was used to examine whether diagnosis (MDS and primary or secondary AML), patient status (new diagnosis, recurrence, resistance, complete remission, or partial remission), treatment, age, sex, and blood counts influenced the relationship between leptin and BMI.
RESULTS
Expression of OB-R in normal hematopoiesis.
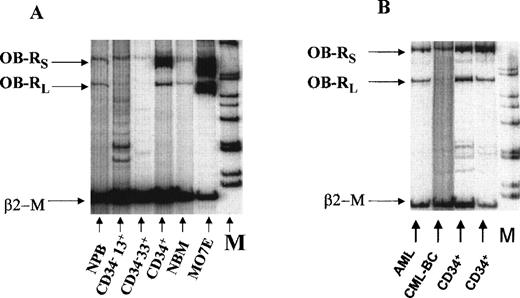
Because it was shown that leptin can stimulate normal myeloid progenitors, we first analyzed the expression of OB-R isoforms in purified myeloid subsets from adult human bone marrow. Both long and short OB-R isoforms were detected in bone marrow mononuclear (n = 3) and FACS-sorted CD34+ progenitor cells (n = 5) (Table 1). Both isoforms were expressed in purified CD34+ cells with perhaps a higher expression of the short isoform (Fig 2A and B). However, sorted CD34−CD33+ and CD34−CD13+ cells did not have the long isoform and showed only weak expression for the short isoform by RT-PCR (n = 3, Fig 2A), suggesting rapid downregulation of OB-Rexpression during myeloid differentiation. Cord blood mononuclear cells (n = 3) had lower expression of OB-R than normal adult bone marrow, and normal peripheral blood samples (n = 3) showed low levels of expression for both isoforms (Table 1).
Expression of OB-R in Normal Myeloid Progenitors
| . | Long Isoform . | Short Isoform . |
|---|---|---|
| Adult bone marrow MNCs | + | + |
| BM CD34+ | + | ++ |
| BM CD34−33+ | ND | + |
| BM CD34− 13+ | ND | + |
| Peripheral blood MNCs | + | + |
| Cord blood MNCs | + | + |
| . | Long Isoform . | Short Isoform . |
|---|---|---|
| Adult bone marrow MNCs | + | + |
| BM CD34+ | + | ++ |
| BM CD34−33+ | ND | + |
| BM CD34− 13+ | ND | + |
| Peripheral blood MNCs | + | + |
| Cord blood MNCs | + | + |
Myeloid cells were sorted based on their specific immunophenotypes and scatter characteristics. Intensity of bands on polyacrylamide gel: positive (+), strongly positive (++) and ND, not detected. The radioactive products were detected using the Betascope 603 (see Materials and Methods). Equal loading of lanes was verified byβ2-microglobulin amplification band.
(A) Expression of OB-R in normal bone marrow (NBM), normal peripheral blood (NPB) and sorted myeloid progenitors (CD34+, CD34−33+, CD34−13+) from adult bone marrow. The MO7E cell line served as a positive control. (B) OB-Rexpression in FACS-sorted CD34+ cells from normal bone marrow, AML, and chronic myeloid leukemia in blast crisis (CML-BC). β2-M, β2-microglobulin; OB-Rl, long isoform (501 bp); OB-Rs, short isoform (720 bp); M, molecular-weight marker.
(A) Expression of OB-R in normal bone marrow (NBM), normal peripheral blood (NPB) and sorted myeloid progenitors (CD34+, CD34−33+, CD34−13+) from adult bone marrow. The MO7E cell line served as a positive control. (B) OB-Rexpression in FACS-sorted CD34+ cells from normal bone marrow, AML, and chronic myeloid leukemia in blast crisis (CML-BC). β2-M, β2-microglobulin; OB-Rl, long isoform (501 bp); OB-Rs, short isoform (720 bp); M, molecular-weight marker.
Expression of leptin receptor in human leukemia cells.
We then studied the expression of OB-R mRNA in leukemia cell lines and primary leukemia cells. OB-R transcripts were detectable at significant levels in all myeloid and lymphoid cell lines studied (Table 2). The highest levels were detected in MO7E (Fig 2A) and K562 cell lines. Interestingly, both isoforms were expressed in the majority of cell lines studied.
OB-R Expression in Hematopoietic Cell Lines
| Cell Type . | LongOB-R Isoform . | Short OB-R Isoform . |
|---|---|---|
| K562 | ++ | ++ |
| MO7E | ++ | ++ |
| HL-60 | + | + |
| HL-60-DOX | + | + |
| NB4 | + | + |
| KG1A | + | + |
| OCI-AML 2 | + | + |
| OCI-AML 3 | + | + |
| BV173 | + | + |
| Jurkat | + | ++ |
| Raji | + | ++ |
| Cell Type . | LongOB-R Isoform . | Short OB-R Isoform . |
|---|---|---|
| K562 | ++ | ++ |
| MO7E | ++ | ++ |
| HL-60 | + | + |
| HL-60-DOX | + | + |
| NB4 | + | + |
| KG1A | + | + |
| OCI-AML 2 | + | + |
| OCI-AML 3 | + | + |
| BV173 | + | + |
| Jurkat | + | ++ |
| Raji | + | ++ |
OB-R expression was determined by PCR using primers specific for the long and short isoforms. Estimates of OB-Rexpression were evaluated by visual comparison withβ2-microglobulin amplification.
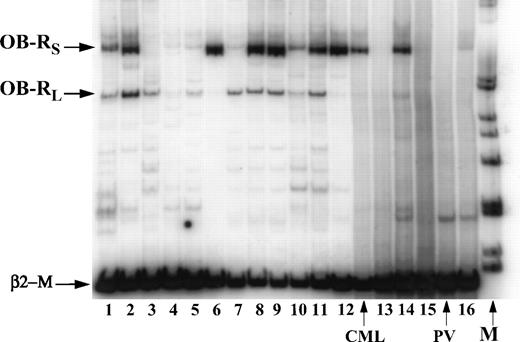
OB-R mRNA expression was analyzed in 49 AML and 6 MDS samples. Examples of mRNA expression are shown in Fig3. Overall, both isoforms were detected in 10 of 22 samples of newly diagnosed AML. The long isoform was detected in 15 of 22 (57.7%), and the short isoform in 13 of 22 (59.1%) samples. Newly diagnosed cases of AML were classified as primary (n = 15) and secondary (after MDS or chemo-/radiotherapy for the primary tumor, n = 7). The OB-R long isoform was more frequently expressed in primary rather than in secondary AML (13/15 v 2/7;P = .01); no significant difference in the expression of the short isoform was found (9/15 v 4/7). The observed difference could not be attributed to the blast count, which did not differ significantly between the two groups (78.3 ± 3.4 v82.1 ± 3.9, respectively; P = .2). OB-Rtranscripts were not limited to a specific AML FAB subtype (Fig4). In MDS, expression of OB-R was similar to that seen in secondary AML (long isoform 3/6; short isoform 4/6) (Table3). Only the short isoform was detected in three AML and two MDS cases, which showed no particular pattern with regard to morphology, immunophenotype, or chromosomal abnormalities compared with other samples. In five samples, the short isoform was not detected and the level of expression of the long isoform was consistently low.OB-R expression was observed in both CD34+ and CD34− (n = 2) AML samples.
Expression of long and short isoforms of OB-R by RT-PCR in AML (N 2,3,5-9,11,13, 15,16) and MDS (N 1,4,10,12,14) samples. PV, polycytemia vera.
Expression of long and short isoforms of OB-R by RT-PCR in AML (N 2,3,5-9,11,13, 15,16) and MDS (N 1,4,10,12,14) samples. PV, polycytemia vera.
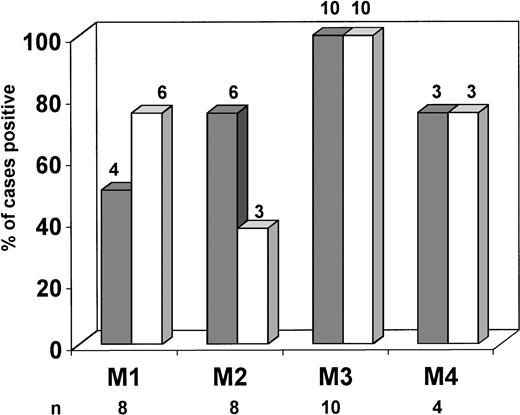
OB-R expression in different FAB subtypes of newly diagnosed AML. Long (▩) and short (□) OB-Risoforms were identified using specific primers as described in Materials and Methods. Amplification and detection was performed using32P-dCTP incorporation. Results are shown as percentages of positive cases in each category. The long isoform only was detected in one case classified as FAB M0, and both isoforms were present in one case classified as FAB M5 (not shown).
OB-R expression in different FAB subtypes of newly diagnosed AML. Long (▩) and short (□) OB-Risoforms were identified using specific primers as described in Materials and Methods. Amplification and detection was performed using32P-dCTP incorporation. Results are shown as percentages of positive cases in each category. The long isoform only was detected in one case classified as FAB M0, and both isoforms were present in one case classified as FAB M5 (not shown).
Level of Expression of OB-R Short and Long Isoforms in AML and MDS
| Group . | Total Cases . | Long Isoform (No. of Positive Cases) . | Short Isoform (No. of Positive Cases) . |
|---|---|---|---|
| Newly diagnosed AML | 22 | 15 | 13 |
| Primary AML | 15 | 13 | 9 |
| Secondary AML3-150 | 7 | 2 | 4 |
| Refractory AML | 4 | 4 | 4 |
| APL | 10 | 10 | 10 |
| Recurrent AML | 13 | 13 | 13 |
| MDS | 6 | 3 | 4 |
| Group . | Total Cases . | Long Isoform (No. of Positive Cases) . | Short Isoform (No. of Positive Cases) . |
|---|---|---|---|
| Newly diagnosed AML | 22 | 15 | 13 |
| Primary AML | 15 | 13 | 9 |
| Secondary AML3-150 | 7 | 2 | 4 |
| Refractory AML | 4 | 4 | 4 |
| APL | 10 | 10 | 10 |
| Recurrent AML | 13 | 13 | 13 |
| MDS | 6 | 3 | 4 |
OB-R expression was determined by PCR using primers for the mRNAs of the long and short OB-R isoforms (see Materials and Methods).
Abbreviations: MDS, myelodysplastic syndrome; APL, acute promyelocytic leukemia.
Secondary AML includes patients with a history of MDS or chemotherapy-induced AML.
No correlation was found with cytogenetics, blast count, or response to chemotherapy with regard to OB-R expression. In 39 AML samples tested (excluding M3), samples from 26 patients had more than 80% blasts, 11 had 50% to 80% blasts, and two had 46% and 31% blasts, respectively, before density gradient separation. Of nine AML samples tested in which the blast count exceeded 90% (before gradient separation), the long isoform of OB-R was expressed in seven samples, and the short isoform in eight samples. Four samples from patients with refractory AML who were studied after initial chemotherapy were found to express high levels of OB-R. OB-Rwas also expressed in all 13 recurrent AML samples tested (Table 3).
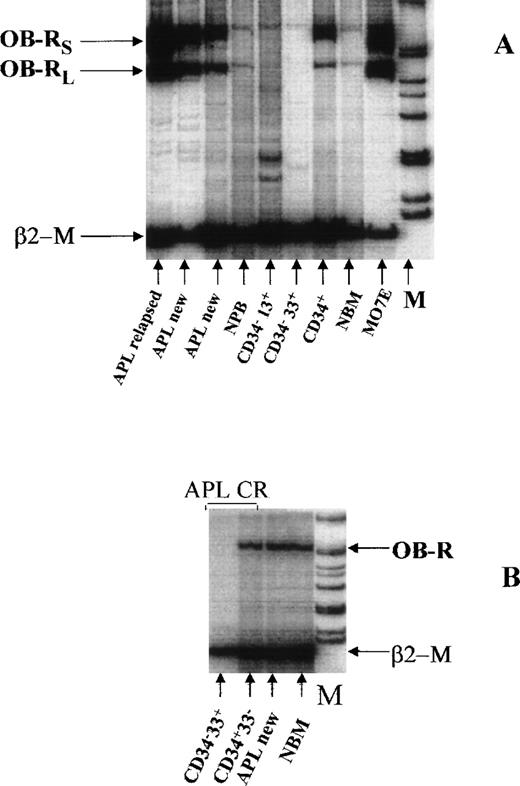
Of interest, OB-R was expressed in all samples analyzed from 10 patients with acute promyelocytic leukemia (APL), five of whom were in relapse (Fig5A, Table 3). In seven cases, both the long and short isoforms were expressed, and in three additional cases, OB-R was detected by primers that did not discriminate between the short and long isoforms. In two APL cases, no detectable CD34+ cells were identified by FACS analysis, but OB-R was detected. In three cases, a high expression of OB-R in peripheral blood promyelocytes was found. Both OB-R isoforms were detected in sorted CD34−33+ promyelocytes of one newly diagnosed APL sample (data not shown). In contrast, in sorted myeloid cells from patients in complete remission, OB-R was expressed only in the bone marrow CD34+ compartment, but not in CD34−33+ promyelocytes (Fig 5B).
(A) Expression of both isoforms of OB-R in two patients newly diagnosed with APL and one patient with recurrent APL compared with expression in normal bone marrow (NBM), FACS-sorted normal myeloid progenitors, peripheral blood (PB), and MO7E cells (as positive control). In one APL sample (lane 3) OB-R expression was detected in peripheral blood containing 37% promyelocytes. (B) OB-R expression of FACS-sorted CD34+33− and CD34−33+ APL cells in complete remission (CR). OB-R was identified only in the CD34+compartment of NBM. In contrast, in a sample from a patient with newly diagnosed APL containing 90% promyelocytes and no CD34+cells, the level of OB-R expression corresponded to that seen in NBM, which was used as a control.
(A) Expression of both isoforms of OB-R in two patients newly diagnosed with APL and one patient with recurrent APL compared with expression in normal bone marrow (NBM), FACS-sorted normal myeloid progenitors, peripheral blood (PB), and MO7E cells (as positive control). In one APL sample (lane 3) OB-R expression was detected in peripheral blood containing 37% promyelocytes. (B) OB-R expression of FACS-sorted CD34+33− and CD34−33+ APL cells in complete remission (CR). OB-R was identified only in the CD34+compartment of NBM. In contrast, in a sample from a patient with newly diagnosed APL containing 90% promyelocytes and no CD34+cells, the level of OB-R expression corresponded to that seen in NBM, which was used as a control.
We also studied the expression of OB-R in some other samples from hematologic malignancies. Only the short isoform was found in three samples of CD34+ cells from patients with chronic myeloid leukemia in blast crisis (Fig 2B). Leptin receptor expression was detected in none of five peripheral blood samples from patients with advanced chronic lymphocytic leukemia. In samples from four patients with acute lymphocytic leukemia and one with APL, only very low-level expression of the short isoform was detected.
Effect of rH leptin on human leukemia cell proliferation.
The effect of rH leptin on proliferation of leukemia cell lines and fresh AML samples by [3H]thymidine incorporation and colony-forming ability was tested. Among six myeloid growth factor-independent cell lines tested, only OCI/AML2 displayed a significant proliferative response to leptin alone (P = .01; SI = 1.7). Low enhancement of proliferative activity (SI = 1.3) was found in NB4 (P < .05). No synergistic response was found in the cell lines tested when leptin was combined with G-CSF, IL-3, or SCF. In OCI-AML2 cells, the response to leptin was dose-dependent over concentrations ranging from 10 to 100 ng/mL (Fig6). Leptin also enhanced the colony-forming ability of OCI/AML2 cells in a dose-dependent fashion (data not shown), with greater than 50% increase observed in colony-forming units in leptin-treated cultures at 50 ng/mL (control, 323.5 ± 6.4; leptin, 519.5 ± 23.3 CFUs). The proliferative response in cell lines did not correlate with the level of OB-R expression; eg, K562 cells exhibited no proliferative response in spite of the high levels ofOB-R mRNA. Cytokine-dependent MO7E cells consistently responded to leptin with increased proliferation (SI = 1.7) (Fig 6) but showed no additive response when leptin was combined with other cytokines. The maximal proliferative response was observed at a higher leptin concentration than was required for OCI/AML2 cells.
Effect of leptin on proliferation of the OCI/AML2 (⧫) and MO7E (▩) cell line. MO7E cells were growth-factor starved for 18 hours. OCI/AML2 or MO7E cells were adjusted to the starting concentration of 100,000 cells/mL and cultured with increasing concentrations of rH leptin. After 48 hours of incubation, cultures were pulsed for 12 hours with [3H]thymidine (0.1 μCi/well), harvested onto filter paper, immersed in scintillation fluid, and quantitated in counts per minute. Each data point was sampled in triplicate. Data from one of two experiments that yielded similar results are shown.
Effect of leptin on proliferation of the OCI/AML2 (⧫) and MO7E (▩) cell line. MO7E cells were growth-factor starved for 18 hours. OCI/AML2 or MO7E cells were adjusted to the starting concentration of 100,000 cells/mL and cultured with increasing concentrations of rH leptin. After 48 hours of incubation, cultures were pulsed for 12 hours with [3H]thymidine (0.1 μCi/well), harvested onto filter paper, immersed in scintillation fluid, and quantitated in counts per minute. Each data point was sampled in triplicate. Data from one of two experiments that yielded similar results are shown.
The proliferative response to leptin was also investigated in 14 fresh samples from patients with AML with a high blast count. Leptin alone at 100 ng/mL induced proliferation in three samples (SI > 1.5) (Table4). Costimulatory effects of leptin and other cytokines (G-CSF, IL-3, and SCF) were also evaluated. Leptin additively enhanced IL-3–induced proliferation of AML cells in four samples and enhanced the effect of SCF in three. The combination of leptin and G-CSF yielded variable responses; three samples showed a synergistic increase and four showed inhibition of the proliferative response induced by G-CSF. Cytokine-stimulated proliferation was also augmented by leptin in some samples that showed no proliferative response to leptin alone. As in the cell lines tested, the proliferative response to leptin did not correlate with the intensity of expression of OB-R isoforms. AML cells from patient no. 5, in which only the long OB-R isoform was expressed, exhibited a proliferative response to leptin combined with G-CSF, IL-3, and SCF and enhancement of colony-forming ability. Despite high expression of bothOB-R isoforms, only one of four samples from patients with recurrent AML responded to leptin alone, and one showed a synergistic response when leptin was combined with G-CSF.
Stimulation of [3H]Thymidine Uptake by AML Cells in Response to Leptin
| Patient . | Diagnosis . | Source Blasts, % . | rhL . | IL-3 . | G-SCF . | SCF . | IL-3 + rhL . | G-CSF + rhL . | SCF + rhL . | Long Isoform . | Short Isoform . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M5 | BM 85 | 1.65 | 3.58 | 0.9 | 1.29 | 4.2 | 1.1 | 1.57 | + | + |
| 2 | M1 | BM 87 | 0.67 | 1.02 | 0.85 | 4.2 | 4.6 | 1.02 | 4.12 | ++ | + |
| 3 | M1 | BM 88 | 0.65 | 0.43 | 0.95 | 0.7 | 0.65 | 0.9 | 0.7 | + | ND |
| 4 | M0 | BM 81 | 0.8 | 1.62 | 0.61 | 0.78 | 1.73 | 0.53 | 0.94 | + | ND |
| 5 | M2 | BM 88 | 1 | 73.1 | 1.2 | 23.07 | 85.9 | 3.9 | 29.5 | + | ND |
| 6 | M1 | BM 94 | 0.96 | 1.28 | 1.32 | 8.05 | 1.6 | 1.02 | 7.19 | + | ++ |
| 7 | M2 | BM 88 | 1.2 | 3.1 | 1 | 9.4 | 3.28 | 1.23 | 9 | ++ | ++ |
| 8 | M2 | PB 84 | 1.14 | 43.3 | 2.78 | 7.5 | 43.8 | 5.27 | 43.8 | ++ | ++ |
| 9 | Secondary AML | BM 82 | 1.8 | 24 | 2.4 | 22.3 | 25.5 | 0.85 | 20.4 | + | + |
| 10 | Secondary AML | BM 81 | 1.1 | 4.3 | 0.9 | 29.3 | 3.5 | 0.7 | 32.8 | + | + |
| 11 | M1, relapse | PB 93 | 1.5 | 2.06 | 1.1 | 1.14 | 1.42 | 0.14 | 1.15 | + | ++ |
| 12 | M1, relapse | BM 90 | 1.07 | 1.17 | 2.54 | 11.9 | 0.9 | 2.78 | 11.48 | ++ | ++ |
| 13 | M4, relapse | BM 90 | 1.3 | 4.3 | 1.15 | 6.2 | 3 | 1.7 | 6.4 | + | + |
| 14 | M1, relapse | BM 89 | 1.01 | 0.72 | 0.9 | 3.05 | 0.9 | 0.27 | 3.36 | + | + |
| Patient . | Diagnosis . | Source Blasts, % . | rhL . | IL-3 . | G-SCF . | SCF . | IL-3 + rhL . | G-CSF + rhL . | SCF + rhL . | Long Isoform . | Short Isoform . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M5 | BM 85 | 1.65 | 3.58 | 0.9 | 1.29 | 4.2 | 1.1 | 1.57 | + | + |
| 2 | M1 | BM 87 | 0.67 | 1.02 | 0.85 | 4.2 | 4.6 | 1.02 | 4.12 | ++ | + |
| 3 | M1 | BM 88 | 0.65 | 0.43 | 0.95 | 0.7 | 0.65 | 0.9 | 0.7 | + | ND |
| 4 | M0 | BM 81 | 0.8 | 1.62 | 0.61 | 0.78 | 1.73 | 0.53 | 0.94 | + | ND |
| 5 | M2 | BM 88 | 1 | 73.1 | 1.2 | 23.07 | 85.9 | 3.9 | 29.5 | + | ND |
| 6 | M1 | BM 94 | 0.96 | 1.28 | 1.32 | 8.05 | 1.6 | 1.02 | 7.19 | + | ++ |
| 7 | M2 | BM 88 | 1.2 | 3.1 | 1 | 9.4 | 3.28 | 1.23 | 9 | ++ | ++ |
| 8 | M2 | PB 84 | 1.14 | 43.3 | 2.78 | 7.5 | 43.8 | 5.27 | 43.8 | ++ | ++ |
| 9 | Secondary AML | BM 82 | 1.8 | 24 | 2.4 | 22.3 | 25.5 | 0.85 | 20.4 | + | + |
| 10 | Secondary AML | BM 81 | 1.1 | 4.3 | 0.9 | 29.3 | 3.5 | 0.7 | 32.8 | + | + |
| 11 | M1, relapse | PB 93 | 1.5 | 2.06 | 1.1 | 1.14 | 1.42 | 0.14 | 1.15 | + | ++ |
| 12 | M1, relapse | BM 90 | 1.07 | 1.17 | 2.54 | 11.9 | 0.9 | 2.78 | 11.48 | ++ | ++ |
| 13 | M4, relapse | BM 90 | 1.3 | 4.3 | 1.15 | 6.2 | 3 | 1.7 | 6.4 | + | + |
| 14 | M1, relapse | BM 89 | 1.01 | 0.72 | 0.9 | 3.05 | 0.9 | 0.27 | 3.36 | + | + |
Mononuclear cells from patient samples were cultured in triplicate in the presence or absence of recombinant human leptin (100 ng/mL) in 96-well flat-bottom microtiter well plates (2.5 × 104cells/0.2 mL/well). For costimulation experiments, rHG-CSF was added to cultures at concentrations of 100 U/mL; IL-3 and SCF were added at 50 and 100 ng/mL, respectively. After 2 days of incubation, 0.1 μCi of [3H]thymidine was added to each well, and the cultures were continued overnight. The cells were then deposited onto harvester filters, and radioactivity was determined in a scintillation counter. The stimulation index was calculated as the ratio of [3H]thymidine incorporated in leptin-treated and untreated samples in counts per minute. Underscoring indicatesP < .05.
Leptin suppresses growth factor withdrawal-induced apoptosis of MO7E and TF-1 cell lines.
Because various cytokines were found to promote cell survival, we tested the effect of leptin on apoptosis of cytokine-dependent cell lines after cytokine withdrawal. We incubated MO7E and TF-1 cells in the presence or absence of 10% fetal calf serum and leptin (200 ng/mL) for 3 days and then detected DNA fragmentation by flow cytometry. Leptin-treated cells had a markedly decreased population of apoptotic cells in both cell lines tested in serum-free and serum-containing conditions. Results shown in Table 5 are representative of the two independent experiments conducted. No significant difference in proliferating cell fraction was documented (data not shown), which suggests a primarily anti-apoptotic, rather than proliferation-inducing, role for leptin in these cell lines.
Effect of Leptin on Apoptosis of MO7E and TF-1 Cells
| Medium . | Day 2 . | Day 3 . | |||
|---|---|---|---|---|---|
| FCS . | Leptin . | MO7E . | TF1 . | MO7E . | TF1 . |
| + | − | 6.4 | 2.9 | 44.8 | 47.9 |
| + | + | 4.5 | 2.0 | 11.2 | 17.5 |
| − | − | 60.3 | 7.5 | 78.1 | 68.9 |
| − | + | 27.7 | 3.5 | 54.6 | 31.8 |
| Medium . | Day 2 . | Day 3 . | |||
|---|---|---|---|---|---|
| FCS . | Leptin . | MO7E . | TF1 . | MO7E . | TF1 . |
| + | − | 6.4 | 2.9 | 44.8 | 47.9 |
| + | + | 4.5 | 2.0 | 11.2 | 17.5 |
| − | − | 60.3 | 7.5 | 78.1 | 68.9 |
| − | + | 27.7 | 3.5 | 54.6 | 31.8 |
Cytokine-deprived MO7E and TF-1 cells were cultured with or without fetal calf serum in the presence or absence of rH leptin. After 2 and 3 days, the cells were stained with acridine orange for cellular DNA and RNA content and analyzed on a FACScan flow cytometer. The percentage of cells in the subG1 region defined the proportion of apoptotic cells. Results represent the data of one of two independent experiments.
Effect of leptin on the clonogenic leukemic cells from AML patients.
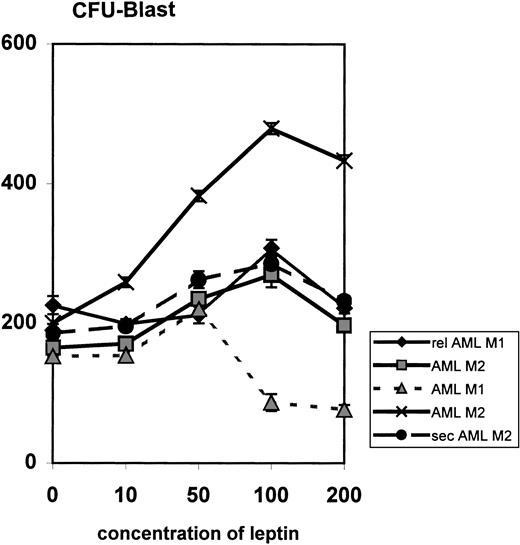
The effect of leptin on the proliferation of AML progenitor cells was examined by colony-forming unit blast assays using fresh bone marrow cells from five patients. In all patients, leptin treatment caused a significant (P < .005) dose-dependent increase in the proliferation of AML colony-forming cells; the optimum effect was observed at plateau concentrations of leptin (50 to 100 ng/mL) (Fig7). At higher concentrations (200 and 300 ng/mL), the effect of leptin was inhibitory.
Influence of leptin on myeloid leukemia clonogenic progenitor growth of primary AML cells. Data represent results from five different samples. FAB subtypes are indicated in the legend. Results are expressed as the mean number of colonies in the presence of increasing concentrations of leptin (10 to 200 ng/mL) compared with control. Rel, relapsed; sec, secondary.
Influence of leptin on myeloid leukemia clonogenic progenitor growth of primary AML cells. Data represent results from five different samples. FAB subtypes are indicated in the legend. Results are expressed as the mean number of colonies in the presence of increasing concentrations of leptin (10 to 200 ng/mL) compared with control. Rel, relapsed; sec, secondary.
Serum leptin levels in patients with AML and MDS.
The serum leptin levels were tested in 56 patients with MDS and AML. The mean of the logarithmic leptin levels in patients with AML and MDS (1.633 ± 0.147) was not significantly different from that in normal controls (1.89 ± 0.169). Serum samples studied showed significant correlations between patients’ BMIs and serum leptin levels (r2 = 0.303; P < .001). Plasma levels were higher in women (2.203 ± 0.255, n = 15) than in men (1.424 ± 0.168, n = 41; P = .03). In multivariate analysis, only BMI and gender were found to correlate with leptin serum levels; no correlation with current treatment, patient status, blast count, or blood counts was noted. In four patients with resistant AML, serum levels were lower than in other patients, but the differences were not statistically significant (P = .184).
DISCUSSION
Many cytokines are capable of stimulating a variety of biologic responses in a wide spectrum of cell types. It is known that the primary physiologic role of leptin is to control adipose tissue mass. This is presumably mediated, at least in part, by signal transduction through the leptin receptor in the hypothalamus.5 However, because the leptin receptor exhibits functional pleiotropy, as do many other cytokines, the leptin signaling pathway is also functional in hematopoiesis. Leptin and the leptin receptor were recently shown to play a role in normal myeloid development by exerting a stimulatory effect on the GM precursors in adult human and murine bone marrow.13,30 Leptin production was demonstrated by hematopoietic stromal cell lines and bone marrow adipocytes, as well as by extramedullary fat cells.3,15 30 It is possible that fat cell content of the bone marrow microenvironment reflects the requirement for leptin in hematopoietic development.
We compared the expression of the long and short isoforms of the leptin receptor in normal and leukemic myeloid cell compartments of bone marrow. As described previously, OB-R expression was detected in adult bone marrow and to a lesser degree in cord blood cells.3 In purified myeloid progenitors from normal adult bone marrow, both OB-R isoforms were strongly expressed in CD34+ cells, perhaps with the short isoform being predominant, consistent with published results.4 12 In more mature CD34−33+ (promyelocytes) and CD34−13+ cells, we detected only low level expression of the short isoform and no expression of the long isoform, which suggests that OB-R expression is rapidly downregulated in normal myeloid progenitors during differentiation.
Analysis of OB-R isoform expression in samples from patients with hematologic malignancies suggests that OB-R has a possible role in the pathogenesis of some myeloid leukemia. Both OB-Risoforms were expressed in the majority of samples from AML patients, whereas all samples from patients with chronic lymphocytic leukemia were negative, and those from patients with acute lymphocytic leukemia showed only low-level expression of the short isoform. BothOB-R isoforms were expressed in both peripheral blood and bone marrow cells from AML cases with a high (>90%) blast count indicating that the observed OB-R expression originates in the leukemic cells. Expression of the long, but not the short isoform was found to occur significantly more often in primary AML than in secondary AML or MDS. OB-R was found to be expressed in all recurrent AML samples studied. Surprisingly, only the short isoform was detected in three samples of CD34+ cells from patients with chronic myeloid leukemia in blast crisis, which might suggest a different function of OB-R in this disease.
Previous results showed that leptin is capable of functioning as a proliferation factor in primitive murine and normal human hematopoietic cells.11,12,30 Our studies demonstrate that among seven myeloid growth-factor independent cell lines tested, only OCI/AML2 cells showed a significant proliferative response to leptin, a result confirmed by an increase in the colony-forming ability of these cells. However, leptin stimulated growth of cytokine-dependent cell lines in a dose-dependent manner. A cohort of fresh AML leukemic cells (3 of 14 samples tested) exhibited a proliferative response to leptin alone, and combinations of leptin with other hematopoietic growth factors induced an additive proliferative response in 7 of 14 AML cases. SCF was shown previously to act synergistically with leptin to stimulate GM precursors in adult human and murine bone marrow.12,30 This effect could be attributable to the upregulation of specific receptors so that leukemic precursors become more responsive to the additional factor. Alternatively, leptin could prevent apoptosis of leukemic progenitor cells as was shown by us for growth-factor–dependent cell lines. Leptin also exerted stimulatory effects on leukemic clonogenic cells when concentrations of 50 and 100 ng/mL were used in combination with SCF and GM-CSF. At higher, perhaps nonphysiologic concentrations, leptin inhibited colony formation, as was demonstrated previously for murine bone marrow cells.13
In other experimental systems, it was shown that the long OB-Risoform has signaling capabilities of the IL-6–type cytokine receptors and uses STAT proteins in the signaling cascade.31 The truncated short form exerts a reduced signaling repertoire.32 33 The ability to proliferate after stimulation with leptin or leptin in combination with cytokines was observed in eight of nine samples expressing both receptor isoforms and in one sample in which only the long isoform was expressed. This data does not allow a correlation between receptor isoforms and signaling in AML.
The documentation of leptin production by placenta during pregnancy and by gestational trophoblastic neoplasms suggests a pathophysiologic role of leptin in leptin-producing tumors.34 We analyzed serum leptin levels in 56 patients with AML and MDS. The positive correlation with BMI and gender, which was established earlier for normal individuals,8 9 was confirmed in the leukemia patients tested here. Multivariate analysis showed no correlation between leptin levels and clinical parameters (diagnosis, treatment, patient status, blast count, or peripheral blood counts). However, these data do not necessarily rule out the possibility of leptin production by AML blasts or bone marrow stroma, which would create a local high concentration of leptin within the bone marrow microenvironment. This hypothesis requires further investigation.
The recently reported interesting observation of increased BMI in patients with APL35 suggests a possible role for leptin in this disease. Our data demonstrate high OB-R long isoform expression in leukemic, but not in normal promyelocytes. The expression was also found in CD34−33+ FACS sorted cells in newly diagnosed APL, but was absent in normal promyelocytes and in promyelocytes from a patient with APL in remission. Hence, it is conceivable that the proliferation of leukemic promyelocytes is driven, in part, by increased leptin levels of obese APL patients. Alternatively, it may reflect the abnormal expression pattern of leptin receptor on leukemic cells, and the observation that OB-R is expressed in CD34− blasts from two non-APL cases is consistent with this notion.
In conclusion, we found expression of the leptin receptors in normal progenitor cells and in the majority of AML. Aberrant expression was seen in leukemic promyelocytes. Leptin induced a proliferative signal and stimulated the colony-forming ability of leukemic progenitors, presumably through the long isoform of OB-R. Leptin acts as an inhibitor of apoptosis in cytokine-dependent leukemia cell lines. Our understanding of the biologic and pathophysiologic role of leptin thus far indicates that it may be part of a redundant network of cytokines that regulates growth and viability of normal and leukemic progenitor cells.
Supported by Grants No. CA 55164, CA 49639, and CA 16672 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael Andreeff, MD, PhD, Department of Molecular Hematology and Therapy, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 81, Houston, TX 77030; e-mail:mandreef@notes.mdacc.tmc.edu.


![Fig. 6. Effect of leptin on proliferation of the OCI/AML2 (⧫) and MO7E (▩) cell line. MO7E cells were growth-factor starved for 18 hours. OCI/AML2 or MO7E cells were adjusted to the starting concentration of 100,000 cells/mL and cultured with increasing concentrations of rH leptin. After 48 hours of incubation, cultures were pulsed for 12 hours with [3H]thymidine (0.1 μCi/well), harvested onto filter paper, immersed in scintillation fluid, and quantitated in counts per minute. Each data point was sampled in triplicate. Data from one of two experiments that yielded similar results are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1668/4/m_blod40515006x.jpeg?Expires=1767733845&Signature=0qla-EGypgIEPVT6AKO6XdSNTei0jtRB~KL18643hQtwqZRtqteiQuetvpj3RTQt-yBKqv6i96xW4qWJZHGn2~BAvuF7QaOPIj846EKeanmEfwSrVQV2eHbU7q6Z8UCUSmhWL6WkiLyZo1SIBGxeP6Ig3pZkhct95Pv4OE3rSIkXiCRMGCNNxaT6DPR264gZtNmmV6kdE2u08KJUwz5cZuVsNJwoWExTlqNxjTnmA~INHBFdSCrEGYH6GHsEKPKinxqKgH7LA7WXpMgV3nffL0vTqSEG0~bCve~Rut-5UM9PNvu5QBi7d1zitKbnpdkWyafemQVp4se6Uzxs8PbYaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal