Abstract
Erythropoietin (EPO) and its cell surface receptor (EPOR) play a central role in proliferation, differentiation, and survival of erythroid progenitors. Signals induced by EPO have been studied extensively by using erythroid as well as nonerythroid cell lines, and various controversial results have been reported as to the role of signaling molecules in erythroid differentiation. Here we describe a novel approach to analyze the EPO signaling by using primary mouse fetal liver hematopoietic cells to avoid possible artifacts due to established cell lines. Our strategy is based on high-titer retrovirus vectors with a bicistronic expression system consisting of an internal ribosome entry site (IRES) and green fluorescent protein (GFP). By placing the cDNA for a signaling molecule in front of IRES-GFP, virus-infected cells can be viably sorted by fluorescence-activated cell sorter, and the effect of expression of the signaling molecule can be assessed. By using this system, expression of cell-survival genes such as Bcl-2 and Bcl-XL was found to enhance erythroid colony formation from colony-forming unit–erythroid (CFU-E) in response to EPO. However, their expression was not sufficient for erythroid colony formation from CFU-E alone, indicating that EPO induces signals for erythroid differentiation. To examine the role of EPOR tyrosine residues in erythroid differentiation, we introduced a chimeric EGFR-EPOR receptor, which has the extracellular domain of the EGF receptor and the intracellular domain of the EPOR, as well as a mutant EGFR-EPOR in which all the cytoplasmic tyrosine residues are replaced with phenylalanine, and found that tyrosine residues of EPOR are essential for erythroid colony formation from CFU-E. We further analyzed the function of the downstream signaling molecules by expressing modified signaling molecules and found that both JAK2/STAT5 and Ras, two major signaling pathways activated by EPOR, are involved in full erythroid differentiation.
TWO TYPES OF erythropoiesis are known. Primitive erythropoiesis occurs first in the yolk sac blood islands, and definitive erythropoiesis begins at the aorta-gonad-mesonephros (AGM) region and shifts to the fetal liver, spleen, and bone marrow. Primitive erythropoiesis does not require erythropoietin (EPO), but definitive erythropoiesis depends on EPO.1 Mature definitive erythrocytes are generated from stem cells through several steps of differentiation, and progenitors of a distinct stage have been defined by their ability to make colonies in methylcellulose, ie, burst-forming unit–erythroid (BFU-E), colony-forming unit–erythroid (CFU-E), and mature erythrocyte. The progenitors proliferate and differentiate in vitro into erythrocytes in the presence of growth factors. BFU-E cells are the earliest progenitor of erythropoiesis and give rise to a large colony (burst) of greater than 500 erythrocytes by 7 to 10 days in methylcellulose culture. BFU-E is stimulated by interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and stem cell factor (SCF), which are not specific to erythropoiesis. As they differentiate, the BFU-E cells become sensitive to EPO. CFU-E cells represent a late stage of erythroid progenitor, which corresponds to proerythroblasts, and are sensitive to EPO in vitro and in vivo. CFU-E gives rise to an erythrocyte colony of 8 to 64 cells in 2 days.2 3
EPO is a lineage-restricted cytokine required for survival, proliferation, and differentiation of committed erythroid progenitor cells.4 EPO exerts its function through the EPO receptor (EPOR), a member of the class I cytokine receptor family. Both EPO and EPOR are essential for the production of red blood cells, and knock out of either EPO or EPOR genes results in embryonic lethality at around 13.5 days postcoitum (dpc) due to the lack of definitive erythropoiesis. Interestingly, even in the absence of EPO or EPOR, almost normal numbers of BFU-E are generated, indicating that EPO and EPOR are not required for the commitment of hematopoietic stem cells to the erythroid lineage but are required for the terminal differentiation of these committed progenitors.5-7 Therefore, downstream signaling pathways from EPOR are important for the terminal differentiation. Janus kinases (JAKs) play an important role in signal transduction via cytokine and growth factor receptors. JAK2-deficient embryos are anemic and die at around 12.5 dpc. Although primitive erythrocytes are found normally, definitive erythropoiesis is absent in the mutant mice. Compared with EPOR-deficient mice, phenotype of JAK2 deficiency is more severe. BFU-E and CFU-E colonies from fetal liver cells of JAK2-deficient embryos are completely absent.8 9
It has been a central question in hematopoiesis whether the commitment for differentiation is instructive or stochastic, ie, whether extracellular signals instruct the cells to commit to a particular cell lineage or the commitment is a stochastic process and cytokines simply serve as growth/survival factors for the committed cells that express the cognate receptor. Although it is still a question whether or not novel molecules yet to be identified initiate the commitment of hematopoietic stem cells to differentiation, it is clear that EPO plays a major role in the late stages of erythroid differentiation as described above. A number of different experiments were performed to address the signaling mechanism for cell survival and differentiation. However, the results were rather controversial. To test the role of cytokine on the commitment, Fairbairn et al10constitutively expressed Bcl-2 in an IL-3–dependent multipotential hematopoietic cell line, FDCP-Mix. Bcl-2 not only suppressed apoptosis, but it also induced multilineage hematopoietic differentiation in the absence of IL-3,10 indicating that the cells undergo multilineage differentiation in the absence of any cytokines if a cell-survival signal is provided. In contrast, targeted expression of Bcl-2 in the erythroid lineage in transgenic mice did not induce erythroid differentiation in the absence of EPO.11
Previously, we and others have analyzed the signaling pathways from the EPOR that lead to erythroid differentiation of EPO-responsive cell lines and found that STAT5 is involved in the EPO-dependent erythroid differentiation12-14; however, Chretien et al15and Pless et al16 described that STAT5 is not required for the erythroid differentiation. One reason for such a major difference could be the cell lines that were used by different investigators. As the nature of cell lines can be variable, it would be ideal to use a more physiological experimental system to address the role of signaling molecules in erythroid differentiation. By using high-titer retrovirus vectors, it is possible to introduce genes of interest to the progenitors in the primary culture. In fact, retrovirus-mediated gene transfer of EPOR into EPOR-deficient fetal liver cells restored EPO responsiveness.6
Here we describe a role of signaling molecules activated by the EPOR in the erythroid differentiation of fetal liver cells. We have used a bicistronic expression system in which the gene to be expressed is placed upstream of the internal ribosome entry site (IRES) and the green fluorescent protein (GFP) gene. Virus-infected cells are collected by fluorescence-activated cell sorter (FACS) as a GFP-positive cell population and analyzed their potential for erythroid differentiation. We show that both JAK2-STAT5 and Ras pathways contribute to the erythroid differentiation from CFU-E.
MATERIALS AND METHODS
Cells.
The retrovirus packaging cell line BOSC23 for the production of ecotropic retrovirus was maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing the GPT selection reagent as described previously.17 Ba/F3 cells stably expressing the β-casein 344 promoter Luciferase construct were established by transfecting the β-casein 344 promoter Luciferase construct18 into Ba/F3 cells (H. Wakao and A. Miyajima, unpublished observation, October 1996). Fetal livers from Balb/c mouse (Sankyo Laboratory, Tokyo, Japan) embryos at 13.5 dpc were dissociated mechanically by pipetting. The cells were strained through a 70-μm cell filter (Becton Dickinson, San Jose, CA).
Plasmids.
pBabe plasmids containing the EGFR-EPOR wild-type or Null mutant were constructed by inserting the EGFR-EPOR fragments into the pBabeXc vector. pMX EGFR-JAK2 was constructed by inserting the EGFR-JAK2 fragment19 into the pMX vector. The pMX/IRES-GFP vector (pMIG) was constructed by inserting polioma virus IRES and EGFP (Clontech, Palo Alto, CA) into pMX vector (M. Takeuchi and A.Miyajima, unpublished observation, April 1997). pMIG containing Bcl-2, Bcl-XL, ΔJAK2, JAB, RasV12, RasN17, STAT5b, ΔSTAT5, or ΔSTAT3 was constructed by inserting cDNA in front of the IRES in pMIG. The junction sequences between the retrovirus vector and the cDNA inserts were verified by sequencing using the Taq Dye Deoxy Terminator Cycle Sequencing kit (Perkin Elmer, Norwalk, CT).
Production of retrovirus stock.
BOSC23 cells were seeded onto 100-mm dishes 1 day before transfection. Transfection was performed using the LipofectAmine Plus reagent (GIBCO-BRL, Rockville, MD) according to the manufacturer’s protocol. Cells were cultured for 48 hours, and the supernatant was used for infection of target cells.
Infection of recombinant retrovirus.
For the infection of Ba/F3 cells, 2 × 105 cells were incubated with 1 mL of the retrovirus stock for 6 hours in the presence of 10 μg/mL Polybrene (Sigma Chemical Co, St Louis, MO) and IL-3. Then, 2 mL of fresh DMEM/10% fetal calf serum (FCS) containing 10 μg/mL Polybrene and IL-3 was added to the culture and incubation was continued overnight. Medium was changed to RPMI/10% FCS containing 10 ng/mL IL-3 on the next day. For the infection of fetal liver cells, 2 × 106 cells were incubated with 10 mL of retrovirus stock solution for 24 hours in the presence of 4 μg/mL Polybrene, 10 ng/mL IL-3, 100 ng/mL IL-6 (provided by Ajinomoto, Tokyo, Japan), and 100 ng/mL SCF (provided by Kirin Brewery, Tokyo, Japan). Then the medium was changed to RPMI/15% FCS containing IL-3, IL-6, and SCF. After a 24-hour incubation, the cells were collected and analyzed for receptor expression or GFP fluorescence. The positive cells (30% to 50%) were sorted by FACS Vantage (Becton Dickinson).
CFU-E assay.
One milliliter of the culture mixture containing 2 × 104 cells GFP-positive cells, α-minimum essential medium (MEM) (GIBCO BRL), 1.2% (1,500 centipoieses) methylcellulose (Nacalai Tesque, Kyoto, Japan), 1% bovine serum albumin (BSA) fraction V (Sigma), 30% FCS (Hyclone, Logan, UT), 10−4 mol/L mercaptoethanol (Sigma), and 2 U/mL human EPO (provided by Kirin Brewery) and 10 ng/mL IL-3 were incubated at 37°C in a humidified atmosphere with 5% CO2 in air. The optimal concentration of EPO for CFU-E formation was determined to be 2 U/mL, and we used EPO at 2 U/mL throughout our studies. CFU-E colonies were counted after 2 days of incubation using an inverted microscope. About 40 CFU-E colonies were observed from 2 × 104virus-vector–infected GFP-positive cells.
RESULTS
Gene transfer into primary fetal liver cells by retrovirus.
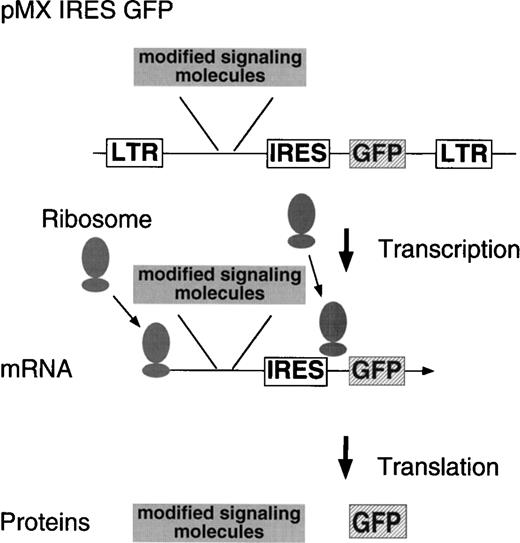
Previously, we used SKT6 cells established from the spleen cells of the spleen focus-forming virus-infected mouse and showed that the tyrosine residues in the cytoplasmic domain of the EPOR are essential for the EPO-dependent erythroid differentiation and that activation of STAT5 is involved in the differentiation.14 To analyze EPOR signaling in erythroid terminal differentiation in a more physiological system, we expressed various signaling molecules in a primary culture of the fetal liver cells by retrovirus. Retrovirus with cDNA encoding a signaling molecule is prepared by packaging the retrovirus construct in BOSC23 cells and is infected to the fetal liver cells isolated from embryos at 13.5 dpc. IL-3, IL-6, and SCF are included in the culture during infection to support immature hematopoietic progenitors. Selection of the retrovirus-infected cell population can be achieved by FACS using the antireceptor antibody in the case of receptor gene transduction. To select the cells infected with the retrovirus encoding intracellular signaling molecules, in which no such antibodies are available for selection, we have used a bicistronic expression system (Fig 1). By placing an IRES and a selection marker gene (GFP) downstream of the gene to be expressed, both genes are cotranscribed as a single mRNA. GFP, which is downstream of the gene to be expressed, is translated by reentry of ribosomes at the IRES. Thus, GF-positive cells are expected to express virus-encoded genes as well. Our vector encodes the GFP gene derived from the jellyAequorea vibtoria.20 GFP emits bright green light when it is exposed to ultraviolet or blue light without additional proteins, substrates, or cofactors, and GFP expression can be monitored in living cells. After sorting the virus-infected cells, the cells are plated in the presence or absence of a cytokine, and erythroid colony formation from CFU-E is evaluated.
Structure of the modified retrovirus vector containing IRES GFP. The cDNA to be expressed is inserted in front of the IRES, allowing it to be transcribed as a single mRNA together with GFP by the 5′ LTR.
Structure of the modified retrovirus vector containing IRES GFP. The cDNA to be expressed is inserted in front of the IRES, allowing it to be transcribed as a single mRNA together with GFP by the 5′ LTR.
Suppression of apoptosis is not the only function of EPOR leading to erythroid colony formation.
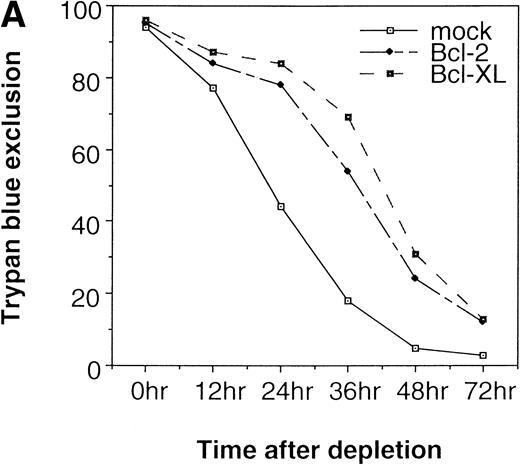
EPO maintains cell survival of erythroid progenitors by inducing expression of cell-survival genes including Bcl-2 and Bcl-XL.21 If EPO simply serves as a growth/survival factor for the committed cells that express EPOR, a survival signal may be sufficient for the induction of terminal erythroid differentiation. To test this possibility, we expressed anti-apoptotic genes, Bcl-2 and Bcl-XL, in fetal liver cells by the bicistronic expression system described above and examined the effect on erythroid colony formation from CFU-E. First, the activity of Bcl-2 or Bcl-XL on cell survival was confirmed in Ba/F3 cells. Ba/F3 cells were infected with retrovirus containing either Bcl-2 or Bcl-XL cDNA, and the virus-infected GFP-positive cells were sorted by FACS. The GFP-positive cells were cultured in the absence of any cytokine, and the cell viability was monitored by the Trypan blue exclusion assay. Ba/F3 cells expressing Bcl-2 or Bcl-XL survived longer than the control cells in the absence of IL-3 (Fig 2A).
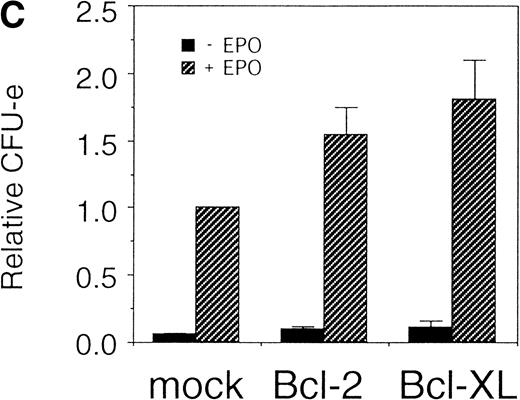
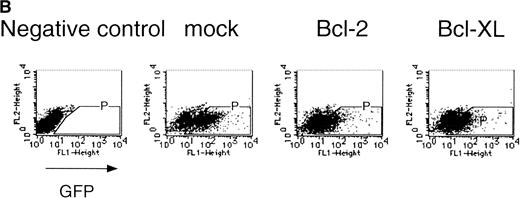
Expression of cell-survival genes, Bcl-2 and Bcl-XL, is not sufficient for the erythroid colony formation from CFU-E in fetal liver cells but enhances erythroid colony formation from CFU-E in response to EPO. (A) Expression of Bcl-2 or Bcl-XL in Ba/F3 cells prolongs cell survival. Ba/F3 cells were infected with retrovirus encoding Bcl-2 or Bcl-XL or the empty virus vector. GFP-positive cells were collected by FACS and cultured in the absence of cytokine, and their viability was monitored by the Trypan blue exclusion assay. (B) Fetal liver cells were infected with retrovirus encoding Bcl-2 or Bcl-XL or the empty virus vector. GFP-positive cells were collected by FACS. P shows the GFP-positive cell population subjected to the CFU-E assays. (C) Sorted GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as the ratio of CFU-E colony numbers of fetal liver cells infected with retrovirus vector without any cDNA insert. The mean ± SEM for five independent experiments are shown.
Expression of cell-survival genes, Bcl-2 and Bcl-XL, is not sufficient for the erythroid colony formation from CFU-E in fetal liver cells but enhances erythroid colony formation from CFU-E in response to EPO. (A) Expression of Bcl-2 or Bcl-XL in Ba/F3 cells prolongs cell survival. Ba/F3 cells were infected with retrovirus encoding Bcl-2 or Bcl-XL or the empty virus vector. GFP-positive cells were collected by FACS and cultured in the absence of cytokine, and their viability was monitored by the Trypan blue exclusion assay. (B) Fetal liver cells were infected with retrovirus encoding Bcl-2 or Bcl-XL or the empty virus vector. GFP-positive cells were collected by FACS. P shows the GFP-positive cell population subjected to the CFU-E assays. (C) Sorted GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as the ratio of CFU-E colony numbers of fetal liver cells infected with retrovirus vector without any cDNA insert. The mean ± SEM for five independent experiments are shown.
Fetal liver cells were infected with the retrovirus encoding either Bcl-2 or Bcl-XL or with the empty virus vector, and GFP-positive cells (P fraction in Fig 2B) were collected by FACS (Fig 2B). The sorted cells were cultured in the presence or absence of EPO, and the number of CFU-E colonies was counted. As shown in Fig 2C, although the expression of Bcl-2 or Bcl-XL enhanced colony formation in response to EPO, the number of spontaneously differentiated CFU-E colonies was not increased compared with that from the vector-infected cells, indicating that expression of the survival genes is not sufficient for erythroid colony formation from CFU-E and additional signaling molecules activated by EPOR are required for erythroid colony formation from CFU-E in fetal liver cells.
Tyrosine residues of EPOR are required for erythroid colony formation from CFU-E.
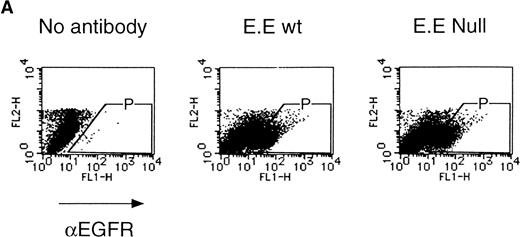
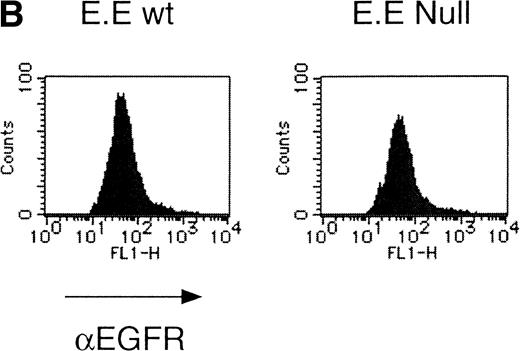
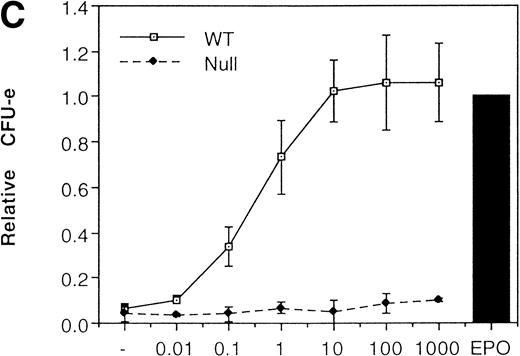
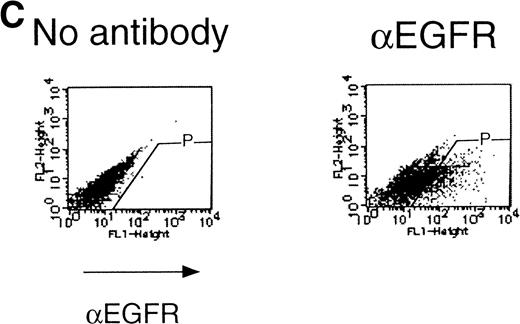
We then analyzed EPOR signaling leading to erythroid differentiation using mutant EPOR in fetal liver cells. To distinguish the exogenous EPOR from the endogenous EPOR, we used the chimeric EGFR-EPOR in which the extracellular domain of the EGF receptor (EGFR) was fused to the intracellular domain of the EPOR.14 The fetal liver cells infected with the retrovirus encoding the chimeric receptor were examined for EGFR expression using anti-EGFR antibody, and virus-infected cells (P fraction in Fig 3A) were sorted by FACS. There was no difference in fluorescein isothiocyanate (FITC) fluorescence between the wild-type chimeric receptor–expressing cells and the Null chimeric receptor–expressing cells (Fig 3B), suggesting that there is no significant difference in receptor numbers between these cell populations. The collected cells were cultured in the presence of EPO or EGF, and the number of CFU-E colonies was counted. As shown in Fig 3C, EGF induced CFU-E as efficiently as EPO, indicating that the chimeric receptor is capable of inducing erythroid colony formation from CFU-E in fetal liver cells. In contrast, the chimeric receptor EGFR-EPOR Null, in which all tyrosine residues within the cytoplasmic domain of the EPOR are substituted with phenylalanine, failed to form an erythroid colony in response to EGF (Fig 3C). The results clearly indicate that the tyrosine residues of the EPOR are essential for EPO-induced erythroid differentiation.
Erythroid colony formation from CFU-E by chimeric receptors. (A) Fetal liver cells were infected with retrovirus encoding the EGFR-EPOR chimeric receptor and stained with anti-EGFR antibody (Amersham, Uppsala, Sweden). The EGFR-positive cells were sorted by FACS. P shows EGFR-positive cell populations used for the CFU-E assays. (B) The EGFR-positive cells shown as P in (A) were reanalyzed. The vertical axis is cell numbers and the horizontal axis is FITC fluorescence. (C) Sorted EGFR-positive cells were subjected to in vitro colony assays without cytokine or with EPO or EGF at a concentration indicated. The relative CFU-E colony numbers were calculated by dividing the CFU-E colony numbers obtained with the EGF stimulation by that of EPO stimulation. The means ± SEM for three experiments are shown.
Erythroid colony formation from CFU-E by chimeric receptors. (A) Fetal liver cells were infected with retrovirus encoding the EGFR-EPOR chimeric receptor and stained with anti-EGFR antibody (Amersham, Uppsala, Sweden). The EGFR-positive cells were sorted by FACS. P shows EGFR-positive cell populations used for the CFU-E assays. (B) The EGFR-positive cells shown as P in (A) were reanalyzed. The vertical axis is cell numbers and the horizontal axis is FITC fluorescence. (C) Sorted EGFR-positive cells were subjected to in vitro colony assays without cytokine or with EPO or EGF at a concentration indicated. The relative CFU-E colony numbers were calculated by dividing the CFU-E colony numbers obtained with the EGF stimulation by that of EPO stimulation. The means ± SEM for three experiments are shown.
Essential role of JAK2 in the formation of erythroid colonies from CFU-E.
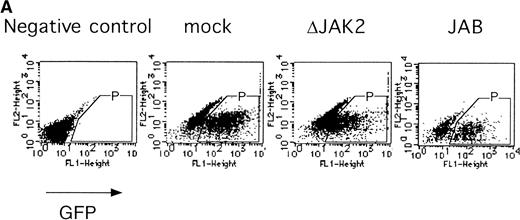
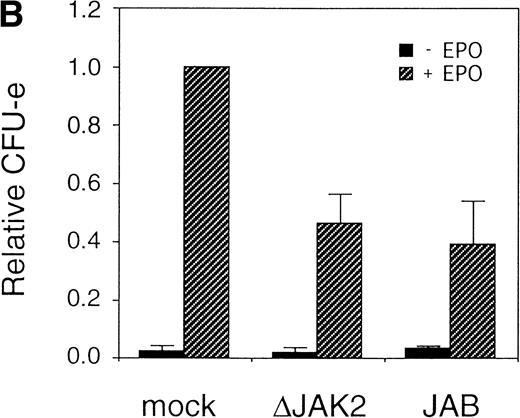
It was previously shown that JAK2 is essential for EPOR-mediated cell proliferation.22 We asked whether the activation of JAK2 is also essential for the erythroid colony formation from CFU-E. By using the bicistronic expression system, we expressed a dominant negative JAK2, ΔJAK2, which is a kinase negative JAK2 molecule that was previously shown to function as a dominant negative molecule in Ba/F3 cells.23 Expression of ΔJAK2 inhibited activation of STAT5 as well as Ras and the induction of c-myc transcription, leading to defective proliferation of Ba/F3 cells.23 In addition, we also expressed JAB, also known as SSI and SOCS, which is a JAK2-binding protein and was shown to inactivate JAK2 by direct binding.24-26 The fetal liver cells were infected with the retrovirus encoding ΔJAK2 or JAB, or the empty vector, and the virus-infected GFP-positive cells (P fraction in Fig 4A) were sorted by FACS. The sorted cells were cultured in the presence of EPO, and the number of CFU-E colonies was counted. As shown in Fig 4B, expression of ΔJAK2 and JAB resulted in the reduction of CFU-E colony numbers, indicating that the activation of JAK2 is required for erythroid colony formation from CFU-E. Although the inhibition by ΔJAK2 and JAB was partial, this is probably due to the expression level of these molecules achieved by retrovirus.
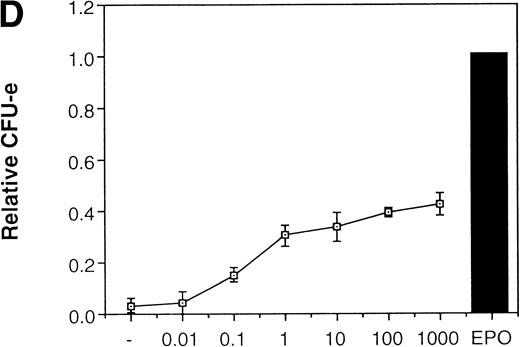
JAK2 plays an essential role in EPO-mediated erythroid differentiation. (A) Fetal liver cells infected with retrovirus encoding ▵JAK2 or JAB or the virus vector and GFP-positive cells were collected by FACS. P shows GFP-positive cell populations used for the CFU-E assays. (B) Sorted GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as in Fig 2C. (C) Fetal liver cells infected with retrovirus encoding EGFR-JAK2 were stained with anti-EGFR antibody, and the EGFR-positive cells were sorted by FACS. P shows EGFR-positive cell populations used for the CFU-E assays. (D) The EGFR-positive cells were subjected to in vitro colony assays without cytokine or with EPO or EGF at a concentration indicated. The relative CFU-E colony numbers were calculated as in Fig 3B.
JAK2 plays an essential role in EPO-mediated erythroid differentiation. (A) Fetal liver cells infected with retrovirus encoding ▵JAK2 or JAB or the virus vector and GFP-positive cells were collected by FACS. P shows GFP-positive cell populations used for the CFU-E assays. (B) Sorted GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as in Fig 2C. (C) Fetal liver cells infected with retrovirus encoding EGFR-JAK2 were stained with anti-EGFR antibody, and the EGFR-positive cells were sorted by FACS. P shows EGFR-positive cell populations used for the CFU-E assays. (D) The EGFR-positive cells were subjected to in vitro colony assays without cytokine or with EPO or EGF at a concentration indicated. The relative CFU-E colony numbers were calculated as in Fig 3B.
Because JAK2 is activated by dimerization of the cytokine receptors, forced dimerization of JAK2 can result in the activation of the kinase without any cytokine. It was previously shown that the chimeric EGFR-JAK2,19 in which the entire cytoplasmic domain of the EGFR is replaced with the tyrosine kinase domain of JAK2, is capable of inducing proliferation of 32D cells in response to EGF. Presumably EGF induces dimerization as well as activation of JAK2. We therefore examined if the activation of JAK2 is sufficient for erythroid colony formation from CFU-E by expressing EGFR-JAK2 in fetal liver cells. The fetal liver cells were infected with the retrovirus encoding EGFR-JAK2, and EGFR-expressing cells were sorted by FACS using anti-EGFR antibody (P fraction in Fig 4C). As shown in Fig 4D, activation of EGFR-JAK2 by EGF in the virus-infected cells led to the erythroid colony formation from CFU-E in response to EGF, although the colony number was about a half of that induced by EPO (Fig 4D). The low level of erythroid colony formation from CFU-E by EGF could be due to the incomplete activation of Ras by EGFR-JAK2, as previously shown in 32D cells.19Therefore, we analyzed the role of Ras in erythroid colony formation from CFU-E.
Role of Ras in erythroid colony formation from CFU-E.
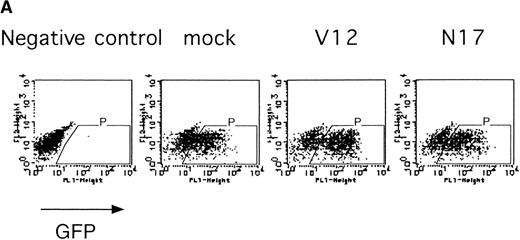
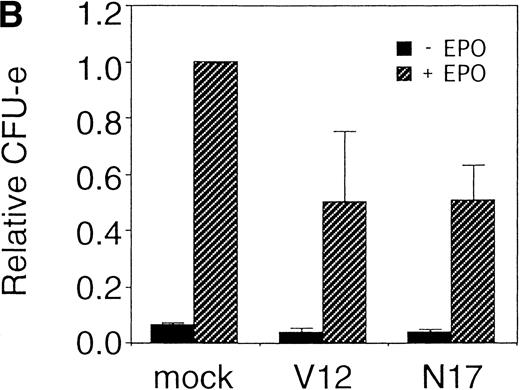
The Ras-Raf-MAPK pathway is activated by binding of EPO to the EPOR and is known to be involved in cytokine-mediated cell proliferation, differentiation, and cell survival depending on the cell type.27 We examined the role of Ras in the erythroid colony formation from CFU-E by expressing an active form of Ras (RasV12) or a dominant negative form of Ras (RasN17) using our bicistronic expression system. Fetal liver cells were infected with the retrovirus encoding either RasV12 or RasN17, or with the empty virus vector, and the virus-infected GFP-positive cells (P fraction in Fig 5A) were isolated by FACS. The sorted cells were cultured in the presence or absence of EPO, and the number of CFU-E colonies was counted. As shown in Fig 5B, both the active form and the dominant negative form of Ras inhibited erythroid colony formation from CFU-E. These results will be discussed below.
Expression of active and dominant negative forms of Ras inhibits erythroid colony formation from CFU-E in fetal liver cells. (A) Fetal liver cells were infected with retrovirus encoding RasV12 or RasN17 or the virus vector, and GFP-positive cells were collected by FACS. P shows GFP-positive cell populations used for the CFU-E assays. (B) The GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as in Fig 2C.
Expression of active and dominant negative forms of Ras inhibits erythroid colony formation from CFU-E in fetal liver cells. (A) Fetal liver cells were infected with retrovirus encoding RasV12 or RasN17 or the virus vector, and GFP-positive cells were collected by FACS. P shows GFP-positive cell populations used for the CFU-E assays. (B) The GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as in Fig 2C.
Role of STAT5 in erythroid colony formation from CFU-E.
Our previous results obtained with EPO-responsive SKT6 cells suggest that STAT5 plays an important role in erythroid differentiation.14 To evaluate the role of STAT5 in erythroid colony formation from CFU-E, we expressed a dominant negative form of STAT5 in the fetal liver cells and examined its effect on the erythroid colony formation from CFU-E.
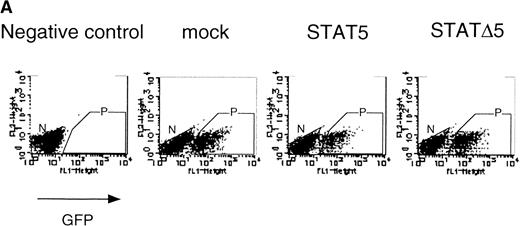
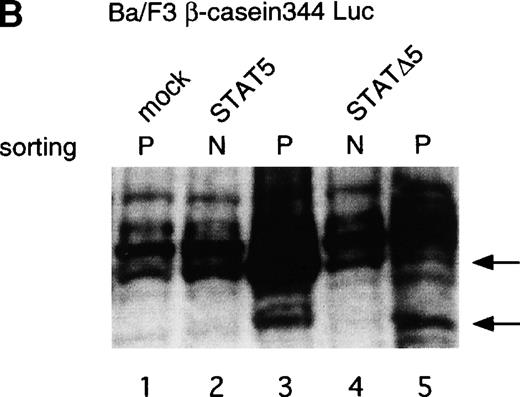
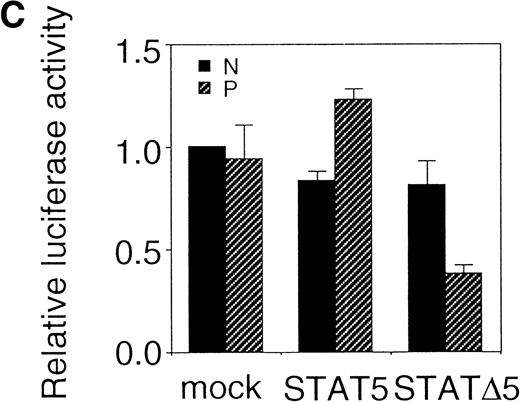
Using the bicistronic expression system, we expressed STAT5b and C-terminal truncation mutants of STAT5 (ΔSTAT5) and of STAT3 (ΔSTAT3). Because these C-terminal truncations eliminate the tyrosine residue essential for phosphorylation and dimerization of the STATs, they function as a dominant negative mutant.28,29 To confirm the dominant negative action of the mutant STATs, we infected Ba/F3 cells that were stably transfected with the β-casein 344 Luciferase construct, which has a STAT5-responsive element. The GFP-positive and -negative Ba/F3 cells were sorted by FACS (Fig 6A), and expression of the exogenous STATs was evaluated by Western blotting. As shown in Fig 6B, GFP-positive cells from wild-type STAT5b virus-infected cells showed an increased level of STAT5b and a processed form of STAT5b,30whereas GFP-negative cells showed the same level of STAT5 as the empty virus vector-infected cells. Likewise, GFP-positive cells, but not GFP-negative cells, from the ΔSTAT5-infected cells expressed the truncated STAT5b. These cells were cultured in the presence of 1 ng/mL IL-3 for 1 day and subjected to luciferase assays (Fig 6C). Luciferase activity driven by the β-casein 344 promoter was slightly increased in the GFP-positive STAT5b-infected cells, whereas it was significantly decreased in the GFP-positive ΔSTAT5-expressing cells. Expression of ΔSTAT3 did not affect the luciferase activity (data not shown). These results indicate that retrovirus-mediated expression of ΔSTAT5 inhibits the endogenous STAT5 activity.
C-terminal truncation mutant of STAT5 (▵STAT5) inhibited STAT5 activation in Ba/F3 cells. (A) Ba/F3 cells were infected with retrovirus encoding STAT5, ▵STAT5, or the virus vector, and GFP-positive cells were sorted by FACS. P shows the GFP-positive cell populations, whereas N shows the GFP-negative cell populations used for the analysis. (B) GFP-positive and -negative cells were examined for the expression of STAT5b and ▵STAT5 by Western blotting. Sorted cells were lysed and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with anti-STAT5 monoclonal antibody (Transduction Laboratory, Lexington, KY). (C) Effect of ▵STAT5 on the transactivation of β-casein Luciferase reporter. GFP-positive and -negative cells were cultured in the presence of 1 ng/mL IL-3 for 24 hours and subjected to the luciferase assays. The relative Luciferase activity was calculated as the ratio of the Luciferase activity of GFP-positive cells infected with the retrovirus vector stimulated with IL-3. The means ± SEM for three experiments are shown.
C-terminal truncation mutant of STAT5 (▵STAT5) inhibited STAT5 activation in Ba/F3 cells. (A) Ba/F3 cells were infected with retrovirus encoding STAT5, ▵STAT5, or the virus vector, and GFP-positive cells were sorted by FACS. P shows the GFP-positive cell populations, whereas N shows the GFP-negative cell populations used for the analysis. (B) GFP-positive and -negative cells were examined for the expression of STAT5b and ▵STAT5 by Western blotting. Sorted cells were lysed and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with anti-STAT5 monoclonal antibody (Transduction Laboratory, Lexington, KY). (C) Effect of ▵STAT5 on the transactivation of β-casein Luciferase reporter. GFP-positive and -negative cells were cultured in the presence of 1 ng/mL IL-3 for 24 hours and subjected to the luciferase assays. The relative Luciferase activity was calculated as the ratio of the Luciferase activity of GFP-positive cells infected with the retrovirus vector stimulated with IL-3. The means ± SEM for three experiments are shown.
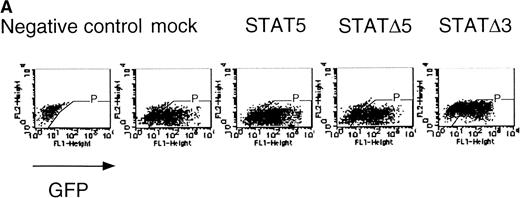
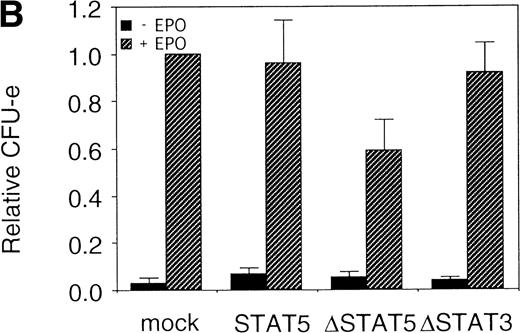
The fetal liver cells were infected with these retroviruses, and GFP-positive cells (P fraction in Fig 7A) were sorted. The collected cells were subjected to the in vitro colony assay in the presence or absence of EPO (Fig 7B). Although the CFU-E colony numbers from fetal liver cells infected with retrovirus encoding STAT5 as well as those of the empty virus vector-infected cells were almost the same, the number of CFU-E colonies from fetal liver cells infected with retrovirus encoding ΔSTAT5 was significantly reduced. In contrast, the CFU-E colony numbers of the GFP-positive fetal liver cells infected with retrovirus encoding ΔSTAT3 were almost the same as those of the control cells. Thus, the effect of the dominant negative STAT5 was specific.
▵STAT5 inhibited the erythroid colony formation from CFU-E in fetal liver cells. (A) Fetal liver cells were infected with retrovirus encoding STAT5, ▵STAT5, or ▵STAT3, or the vector alone, and GFP-positive cells were collected by FACS. P shows the GFP-positive cell populations used for the CFU-E assays. (B) The GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as in Fig 2C.
▵STAT5 inhibited the erythroid colony formation from CFU-E in fetal liver cells. (A) Fetal liver cells were infected with retrovirus encoding STAT5, ▵STAT5, or ▵STAT3, or the vector alone, and GFP-positive cells were collected by FACS. P shows the GFP-positive cell populations used for the CFU-E assays. (B) The GFP-positive cells were subjected to in vitro colony assays. The relative CFU-E colony numbers were calculated as in Fig 2C.
DISCUSSION
Analysis of signaling pathways is generally conducted by using cell lines, and numerous signaling molecules have been identified. However, studies using cell lines often provide controversial results as to the physiological role of each signaling molecule. Such controversial results are most likely reflecting the difference of cell lines used for each experiment. In principle, because cell lines are generated as a result of alteration of the cellular program of proliferation and/or differentiation, each cell line may have acquired an abnormal characteristic during a process of establishing the cell line. Moreover, phenotype of a cell line can be variable during the culture, and descendants of the same original cell sometimes exhibit quite different phenotype. Erythroid progenitor cell lines are not the exception, and each cell line might have stopped the differentiation at a specific step. This may be a major reason for the discrepancy among various investigators as to the role of cytokines and their signaling molecules in erythroid differentiation. To overcome these potential problems intrinsic to cell lines, it is ideal to use an in vivo experimental system to address the physiological role of signaling molecules. In this study, we have used primary fetal liver cells and a high-efficiency gene transfer system using retrovirus. As described above, a significant fraction of the fetal liver cells can be infected with the recombinant retrovirus, and the effect of expression of the virus-encoded gene can be assessed by colony-forming assays. This type of approach was already applied for dissecting EPOR signaling to erythroid colony formation from CFU-E,31,32 though the analysis was limited to EPOR mutants. By using the IRES-GFP bicistronic expression system, we have extended this type of approach to the functional analysis of signaling molecules, which lacks any selection reagents such as specific antibody. 33 34 Although in this study we have analyzed only the erythroid colony-formation from CFU-E, it should be also applicable for evaluation of the role of such signaling molecules in CFU-C, CFU-S, or even long-term reconstitution of the entire hematopoietic cells. We have successfully detected a GFP-positive virus-infected cell population in spleens of lethally irradiated mice after reconstituting with fetal liver cells infected with retrovirus encoding GFP (D.C. and A.M., unpublished results, April 1997). Because a CFU-E colony is formed as a result of the proliferation and differentiation of a CFU-E, and because we could not detect difference in CFU-E colony size, our current results are unable to discriminate the possibilities of whether the signaling molecules expressed by retrovirus affect proliferation or differentiation, or both.
It remains unclear whether EPO induces a specific signal for differentiation or if it simply serves as a survival factor for committed erythroid cells. It is generally accepted that survival of hematopoietic progenitors is supported by antiapoptotic proteins such as Bcl-2 and Bcl-XL. Bcl-XL–deficient mice develop massive apoptosis of neuronal and hematopoietic progenitor cells and die at day 13 or 14 of embryonic development.35 Whereas Bcl-2–deficient mice exhibit increased spontaneous apoptosis of lymphoid cells after birth, they show no apparent abnormalities of the erythroid compartment.36 Thus, it is likely that Bcl-XL is a critical factor for survival of erythroid progenitors. If EPO simply serves as a growth/survival factor for the committed cells with the EPOR, a survival signal could induce terminal differentiation of erythroid progenitors. Consistent with this hypothesis, expression of Bcl-2 in FDCP-mix cells led to not only cell survival in the absence of IL-3, but also multilineage hematopoietic differentiation.10 In addition, expression of Bcl-XL in HCD-57 cells resulted in erythroid differentiation in the absence of EPO in response to hemin.21 We therefore evaluated the role of anti-apoptotic genes, Bcl-2 and Bcl-XL, on erythroid colony formation from CFU-E. Consistent with the previous reports using transgenic mice expressing Bcl-2,11 no CFU-E colonies were formed from Bcl-2– or Bcl-XL–infected fetal liver cells in the absence of cytokine. Our observation that expression of Bcl-2 or Bcl-XL enhanced erythroid colony formation from CFU-E in response to EPO suggests the possibility that the erythroid progenitors yet to be sensitive to EPO could survive and make colonies in response to EPO. Because the expression of Bcl-2 or Bcl-XL is not sufficient for CFU-E colony formation in the absence of EPO, suppression of apoptosis is not the sole function of EPOR signaling that leads to the erythroid colony formation from CFU-E.
Using EGFR-EPOR chimeric receptors, we have shown that tyrosine residues of the cytoplasmic domain of EPOR are essential for erythroid colony formation from CFU-E. The results are consistent with the previous reports by Wu et al37 using EPOR-deficient fetal liver cells, but they are inconsistent with the report by Longmore et al31 in which they show phosphotyrosine-independent development of erythrocytes using an active form of EPOR. This discrepancy could be due to the constitutive activation of mutant receptor EPOR(R129C) they used. It is possible that signals from EPOR(R129C) might be different from cytokine-induced activation of EPOR.
JAKs play an central role in signal transduction via cytokine receptors. Our results that a dominant negative form of JAK2 as well as a JAK2 inhibitor JAB inhibit the erythroid colony formation from CFU-E are consistent with JAK2-deficient mice.8,9 The result that JAB inhibits erythroid colony formation is the first demonstration of JAB functions in vivo as a negative regulator of EPO signaling. Previously, cytokine-independent activation of JAK2 by the chromosomal translocations was shown in a patient of T-cell childhood acute lymphoblastic leukemia.38 Here, we show that activation of JAK2 kinase independent of EPOR followed by the activation of STAT5 could induce erythroid colony formation from CFU-E. Thus, activation of JAK2 is sufficient not only for T-cell proliferation but also for differentiation of erythroid progenitors.
Although Ras is known to be activated by EPOR, the role of Ras in EPO-mediated signaling for cell proliferation, survival, or differentiation is still not clear. Mutation of the Ras genes is frequently found in patients with hematological malignancies. Myelodysplatic patients exhibit a disorder of hematopoietic lineages, including the erythroid lineage.39 The role of Ras activation in erythroid differentiation has been examined by various approaches. The receptor mutants (EPORH) defective in Ras activation have been used to show that Ras is not required for the differentiation of erythroid cell lines40 and fetal liver cells.32 In contrast, Damen et al41 recently showed that Ba/F3 cells expressing the truncated EPOR (EPORH) proliferate in response to EPO only if FCS is present. As serum factors such as insulin-like growth factor (IGF) activate the Ras pathway, FCS seems to complement the defective signaling by the EPORH.41 Thus, Ras appears to be involved in EPO-mediated response. In addition, the observations that K-Ras−/− ES cells failed to contribute to hematopoietic cell populations indicate that K-Ras plays a role in hematopoietic development.42 Our results that a dominant negative form of Ras inhibits erythroid colony formation from CFU-E is consistent with the knock-out mice.
Our results that an active form of Ras inhibits the erythroid colony formation from CFU-E are consistent with the report by Darley et al39 that expression of constitutively active N-Ras in human CD34+ cells interferes with erythroid differentiation by inhibiting cell proliferation. In contrast, the results that both active and dominant negative forms of Ras inhibit erythroid colony formation from CFU-E are apparently controversial. However, the discrepancies may be explained by the level of Ras activation, ie, modest and temporal activation of Ras is required for erythroid colony formation from CFU-E, but constitutive and strong activation induced by RasV12 inhibits erythroid colony formation from CFU-E. This interpretation is supported by previous reports described above.
Whether or not STAT is involved in erythroid differentiation has been a subject of dispute for the last few years. We and others have shown that STAT5 is involved in the differentiation12-14 using SKT6 and ELM-I-1 cells, respectively, whereas Chretien et al13 and Pless et al16 reported that STAT5 is not necessary for the differentiation using TF-1 and Ba/F3, respectively. STATs have been proposed to be a molecular switch for differentiation in many hematopoietic43-49 and neuronal systems50 as well as Dictyostelium differentiation.51 We have confirmed the importance of STAT5 in erythroid differentiation in fetal liver cells. However, as the inhibition by dominant negative STAT5 is rather weak compared to the inhibitory effects of ΔJAK2 and Ras, the contribution of STAT5 on erythroid colony formation from CFU-E may be limited.
Recently, double knock-out mice lacking both STAT5a and STAT5b were generated.52 Bone marrow cells from these mice did not show any defects, indicating that STAT5 is not essential for myelopoiesis and erythropoiesis. These observations are apparently inconsistent with our present studies. Several explanations may be possible for the discrepancies. First, the fundamental difference between the knock-out experiment and the primary culture system we describe here should be considered. As knock-out mice lack the STAT5 molecules from the onset of the embryonic development, the mutant embryo may develop an alternative pathway that can compensate for the lack of STAT5 during the development. Interestingly, as many as one third of STAT5a/b mutant mice died within 48 hours after birth. These mice might have failed to develop an alternative pathway. If the hypothesis is correct, STAT5 expression in the STAT5a/b-deficient erythroid progenitors may enhance CFU-E colony formation. Second, it is also possible that in the absence of STAT5 another STAT molecule may be recruited to the phosphotyrosine residues of the EPOR and compensate the lack of STAT5. We have previously shown that STAT6 could bind to the STAT5 binding sites and induce transcription of the STAT5 target genes such as β-casein, Cis, and Osm.53 Third, in the absence of STAT5 the other signaling pathways, eg, the Ras pathway, may compensate for the STAT5 deficiency and lead to erythroid colony formation from CFU-E.
In conclusion, retrovirus-mediated gene transfer of various signaling molecules into fetal liver hematopoietic cells indicates that JAK2 plays an essential role for erythroid terminal differentiation, and both Ras and STAT5 contribute to the erythroid colony formation from CFU-E.
ACKNOWLEDGMENT
We thank T. Sekiguchi for cell sorting; Dr H. Wakao for Ba/F3 cells expressing β-casein 344 promoter, Dr G.P. Nolan, Dr S. Watanabe, Dr Y. Tsujimoto, A. Kamiya, and M. Takeuchi for proving plasmids; Dr M. Takagi and Dr T. Nakahata for CFU-E assays; and Kirin Brewery and Ajinomoto for providing us with recombinant cytokines.
Supported in part by the grants from the Ministry of Education, Culture, Sports, and Science (Monbushou); Core Research for Evolutional Science and Technology (CREST) of Japan Science and Technology Corporation, Riken; the Toray Research Foundation; and Uehara Research Foundation. D.C. is supported by Fellowships in Cancer Research of Japan Society for the Promotion of Science for Young Scientists.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Atsushi Miyajima, PhD, Institute of Molecular and Cellular Biosciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-0032, Japan; e-mail:miyajima@ims.u-tokyo.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal