Abstract
Hematopoietic reconstitution of ablated recipients requires that intravenously (IV) transplanted stem and progenitor cells “home” to organs that support their proliferation and differentiation. To examine the possible relationship between homing properties and subsequent engraftment potential, murine bone marrow (BM) cells were labeled with fluorescent PKH26 dye and injected into lethally irradiated hosts. PKH26+ cells homing to marrow or spleen were then isolated by fluorescence-activated cell sorting and assayed for in vitro colony-forming cells (CFCs). Progenitors accumulated rapidly in the spleen, but declined to only 6% of input numbers after 24 hours. Although egress from this organ was accompanied by a simultaneous accumulation of CFCs in the BM (plateauing at 6% to 8% of input after 3 hours), spleen cells remained enriched in donor CFCs compared with marrow during this time. To determine whether this differential homing of clonogenic cells to the marrow and spleen influenced their contribution to short-term or long-term hematopoiesis in vivo, PKH26+ cells were sorted from each organ 3 hours after transplantation and injected into lethally irradiated Ly-5 congenic mice. Cells that had homed initially to the spleen regenerated circulating leukocytes (20% of normal counts) approximately 2 weeks faster than cells that had homed to the marrow, or PKH26-labeled cells that had not been selected by a prior homing step. Both primary (17 weeks) and secondary (10 weeks) recipients of “spleen-homed” cells also contained approximately 50% higher numbers of CFCs per femur than recipients of “BM-homed” cells. To examine whether progenitor homing was altered upon ex vivo expansion, highly enriched Sca-1+c-kit+Lin−cells were cultured for 9 days in serum-free medium containing interleukin (IL)-6, IL-11, granulocyte colony-stimulating factor, stem cell factor, flk-2/flt3 ligand, and thrombopoietin. Expanded cells were then stained with PKH26 and assayed as above. Strikingly, CFCs generated in vitro exhibited a 10-fold reduction in homing capacity compared with fresh progenitors. These studies demonstrate that clonogenic cells with differential homing properties contribute variably to early and late hematopoiesis in vivo. The dramatic decline in the homing capacity of progenitors generated in vitro underscores critical qualitative changes that may compromise their biologic function and potential clinical utility, despite their efficient numerical expansion.
THE MOST PRIMITIVE HEMATOPOIETIC stem cells (HSCS), identified by their capacity to repopulate lymphoid and myeloid lineages upon transplantation into myeloablated or immunocompromised hosts, reside in the bone marrow (BM) and are closely associated with mesenchymal cells lining the bone endosteum.1 2 Within this microenvironment, HSC self-renewal, proliferation, and differentiation are thought to be regulated through specific interactions with a heterogeneous population of stromal cells, and the extracellular matrix components and cytokines they produce. When HSCs are collected from BM or peripheral blood for hematopoietic rescue of patients with hematologic disease, efficient and timely reconstitution requires that at least some intravenously transplanted stem and progenitor cells reseed niches that have been ablated by radiation or chemotherapy to eliminate malignant cells. This migration of stem cells through the circulation and back to a supportive hematopoietic microenvironment is referred to as “homing.”
Classical experiments by Wolf and Trentin have long implicated the role of the hematopoietic microenvironment in influencing the lineage commitment of HSCs in vivo.3 Recently, the existence of unique niches that promote either short-term or long-term hematopoiesis has been affirmed by the isolation of murine stromal cell lines that differ markedly in their ability to maintain enriched populations of repopulating stem cells in vitro.4,5 Some stromal cells sustain clonogenic cells only transiently, while others support high levels of primitive cell proliferation, but only after prolonged culture.4,6 These data suggest that clonogenic cells with early hematopoietic reconstitution potential may home to different niches than HSCs which sustain long-term hematopoiesis in vivo. This concept is supported by studies demonstrating that the precursors of murine spleen colony-forming units (pre–CFU-S) and stem cells with marrow repopulating ability migrate first to the BM after transplantation, where they generate progeny CFU-S that emigrate to the spleen.7,8 Since most CFU-S are separable from more primitive stem cells with long-term repopulating ability,9it seems reasonable to speculate that the observed differences in the repopulating potential of these two classes of cells might be due to differences in their localization in vivo.
Manipulations directed at improving the homing of HSCs to sites that support their rapid proliferation may provide a means to overcome the delayed engraftment that compromises the safe recovery of patients following stem cell transplantation. However, a potential relationship between stem cell homing and engraftment kinetics has not previously been examined, due in part to the lack of a suitable assay to monitor the fate of limited numbers of transplanted stem cells in the whole animal. To address this problem, Hendrikx et al exploited the availability of brightly fluorescent aliphatic dyes, such as PKH26, to label murine hematopoietic cells enriched for CFU-S.10 The homing of fluorescent cells to different hematopoietic organs following transplantation was then monitored by flow cytometry. These studies established the feasibility of this approach to track HSCs in vivo, but no functional assays were performed to compare the biology of hematopoietic cells that had homed to different organs. Recently, two groups have reported such studies. Oostendorp and Eaves studied murine BM cells labeled with 5-(and 6-) carboxyfluorescein diacetate succinimydl ester (CFSE) and demonstrated that progenitors identified by their potential to generate colonies of mature blood cells in vitro homed predominantly to the marrow by 24 hours after transplantation, where they appeared to divide more slowly than in the spleen.11 Primitive stem cells enriched by counterflow centrifugal elutriation have also been stained with PKH26 and tracked to the marrow of recipient animals up to 96 hours after transplantation. PKH26+ cells that were sorted and retransplanted into secondary hosts could give rise to stable hematopoietic engraftment up to 3 months later, demonstrating the preservation of their initial potential after the labeling and homing procedure.12
We now report the refinement of the general technique of fluorescently labeling cells before transplantation in the development of a quantitative assay to measure HSC and progenitor cell homing in vivo. Temporal analyses indicate a preferential concentration of colony-forming cells (CFCs) in the spleen compared with marrow within the first few hours after transplantation. To determine whether this differential accumulation of CFCs in these organs influenced their subsequent contribution to early and sustained hematopoiesis, labeled cells were sorted from marrow and spleen and retransplanted into lethally irradiated Ly-5 congenic mice. Interestingly, cells that had homed initially to the spleen regenerated circulating leukocytes almost 2 weeks faster than cells that homed to marrow. Both primary and secondary recipients of “spleen-homed” cells also recovered higher numbers of CFCs per femur than recipients of “BM-homed” cells. Thus, clonogenic cells with differential homing properties appear to contribute variably to early and late hematopoiesis in vivo.
In a practical application of this assay, we also evaluated the homing properties of progenitor cells generated by ex vivo expansion. It has been suggested that by culturing HSCs in a mixture of hematopoietic growth factors in vitro, their expanded and partially differentiated progeny may regenerate circulating neutrophils and/or platelets more quickly than unmanipulated HSCs in a clinical setting.13-16 Support for this hypothesis was provided initially by Muench et al, who demonstrated that lethally irradiated mice transplanted with BM cells that had been cultured for 1 week in interleukin (IL)-1 plus stem cell factor (SCF) regenerated twofold to fivefold higher peripheral blood cell counts 4 to 10 days after transplantation than recipients of fresh marrow.17 We have also recently shown that the delayed engraftment of myeloablated mice injected with 103 highly enriched Thy-1loSca-1+Lin− stem cells is accelerated by cotransplantation of their partially differentiated progeny generated after 1 week of culture in IL-3, IL-6, granulocyte colony-stimulating factor (G-CSF), and SCF.18 However, despite the fact that expanded cells contained several hundred–fold more day 12 CFU-S and CFCs than noncultured stem cells, they were not as effective at hematopoietic reconstitution as might be predicted from their composition. HSCs and progenitor cells that have been cultured in vitro typically exhibit a diminished capacity for long-term reconstitution, although the severity of this defect is clearly related to the source of HSCs and the culture conditions used.19-23This has led some investigators to speculate that changes in adhesion molecule expression in response to pancytopenia or cytokine elaboration may alter HSC homing properties and reduce engraftment efficiency by mechanisms that may include (1) conferring a propensity to migrate to tissues not permissive for hematopoiesis, or (2) reducing the localization of progenitors to, or extravasation of their progeny from, a permissive microenvironment once inside the marrow. As a first step to address these questions, we now show using the homing assay described herein that progenitor cells generated by the in vitro expansion of HSCs exhibit an approximately 10-fold reduction in homing efficiency compared with unmanipulated cells. Our data suggest that qualitative changes in transplantation potential may pose significant obstacles to the clinical application of cultured hematopoietic cells, despite their successful numerical expansion.
MATERIALS AND METHODS
Animals.
Five- to 8-week-old C57BL/6 (B6) mice (Ptprcb[Ly-5.2]) were used as marrow donors. Age-matched B6 or B6.SJL (Ptprca [Ly-5.1]) mice were used as recipients as indicated. Mice were purchased from commercial suppliers and maintained under specific pathogen-free conditions at the animal facility of the Chandler Medical Center until use.
Enrichment of HSCs.
Donor B6 mice were injected intravenously (IV) with a sterile solution of 5-fluorouracil (5-FU; Roche Laboratories, Nutley, NJ) in phosphate-buffered saline at a dose of 150 mg/kg body weight. Twenty-four hours later, 5-FU–treated BM (FUBM) cells were flushed into Hank’s balanced salt solution (HBSS) containing 2% fetal calf serum (FCS) (HF medium) using a 21-gauge needle. Erythrocytes were eliminated by hypotonic lysis, and the remaining leukocytes incubated with saturating amounts of biotinylated rat monoclonal antibodies (MoAbs) specific for murine CD3 (clone KT3.1), CD5 (clone 53-7.3), CD8 (clone 53-6.72), CD11b/Mac-1 (clone M1/70), CD45R/B220 (clone RA3-6B2), and Ly-6G/Gr-1 (clone RB6-8C5). The labeled cells were then incubated with goat antirat IgG paramagnetic beads (Dynal Inc, Lake Success, NY) at a bead:cell ratio of approximately 4:1, and mature lymphoid and myeloid cells were removed by exposure to a magnetic field. Lineage-depleted cells (∼6% of initial numbers) were blocked with anti-CD16/32 (clone 2.4G2, Fc Block) MoAb, and then stained with phycoerythrin (PE)-conjugated anti–Ly-6A/E (Sca-1; clone E13-161.7) and allophycocyanin (APC)-conjugated anti-CD117 (c-kit; clone 2B8) MoAbs and streptavidin∼fluorescein isothiocyanate (FITC) (all from Pharmingen, San Diego, CA). Stained cells were washed and resuspended in HF containing 5 μg/mL propidium iodide (PI). Unstained FUBM cells or lineage-depleted cells stained with appropriate fluorochrome-conjugated isotypic control MoAbs (Pharmingen) or streptavidin∼FITC were used as background controls. PI-negative Sca-1+c-kit+Lin− FUBM cells, representing 0.013% ± 0.003% of whole FUBM, were sorted using a dual-laser FACS Vantage instrument (Becton Dickinson Immunocytometry Systems, San Jose, CA). Reanalysis of the sorted cells immediately after sorting indicated a purity typically greater than 90%.
Ex vivo expansion of unfractionated marrow or sorted stem cells.
A quantity of 106 unfractionated normal BM or 103 sorted Sca-1+c-kit+Lin−cells were cultured per milliliter of StemPro-34 serum-free medium (Life Technologies, Gaithersburg, MD) containing 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mmol/L L-glutamine, 0.05 mmol/L 2-mercaptoethanol (2-ME), supplemented with the following recombinant cytokines: 10 ng/mL murine IL-3, IL-6 and human G-CSF, and 100 ng/mL murine SCF for cultures initiated with whole BM cells; 10 ng/mL murine IL-6 and human G-CSF, 50 ng/mL murine IL-11, and 100 ng/mL murine SCF, murine thrombopoietin (TPO), and human FLK for cultures initiated with Sca-1+c-kit+Lin−cells. All cytokines were purchased from R&D Systems (Minneapolis, MN), except G-CSF (Amgen Inc, Thousand Oaks, CA). Cultures were incubated undisturbed at 37°C for 7 days. From this time onward, half of the medium was replaced daily and all cells harvested and counted after 9 days.
In vivo homing assay.
Fresh or cultured BM cells were labeled with PKH26 dye according to the manufacturer’s (Sigma Chemical Co, St Louis, MO) instructions with some modifications. Briefly, cells were resuspended in Diluent C at a concentration of 2 to 8 × 107/mL, combined with an equal volume of PKH26 dye freshly prepared at 10 μmol/L in Diluent C, and incubated at room temperature for approximately 10 minutes with periodic gentle mixing. Staining was terminated by addition of an equal volume of FCS for 1 minute, and the labeled cells then washed thoroughly in HF. The average cell recovery after this procedure was approximately 65%. An aliquot of cells before and after PKH26 staining was assayed for CFCs as described below. The remaining cells were then injected IV into lethally irradiated syngeneic B6 recipients (106 to 108 cells per mouse). Total body γ-irradiation (9 Gy) was administered approximately 19 hours before transplant in a single dose at a rate of 2.2 Gy/min from a137Cs source (J.L. Shepard & Assoc, San Fernando, CA). One to 24 hours after transplantation, all of the cells in each spleen and both femora and tibiae were procured in HF. After washing and red blood cell lysis, BM and spleen cells were suspended in HF containing PI for flow cytometric analysis or sorting of the originally transplanted PKH26+ population. Cell counts performed to determine whole-organ cellularity were based on the assumption that two femora and two tibiae represent 25% of total marrow.24 After PKH26+ cells were sorted, an aliquot was always reanalyzed and the percent purity used to correct PKH26+ cell counts before assay.
CFC assays.
Unseparated, sorted or ex vivo–expanded BM cells were assayed for CFCs as previously described.18
Measurement of in vivo hematopoietic reconstitution kinetics.
B6.SJL mice were exposed to 9 Gy total-body γ-irradiation (administered in two doses of 4.5 Gy ∼3 hours apart just before transplantation) and injected with 106 PKH26-labeled but otherwise unmanipulated B6 BM cells, or 106PKH26+ BM cells that had been isolated by fluorescence-activated cell sorting (FACS) from the marrow or spleen of lethally irradiated B6 mice following a 3-hour homing step. Mice were bled from the retro-orbital sinus at 6, 9, 12, 15, 18, 25, and 32 days after transplantation. Until day 25, only half of the mice in each cohort were analyzed alternately at each time so that no individual animal was bled more frequently than every 7 days. Circulating leukocyte, erythrocyte, and platelet counts were measured by analysis of 40 μL of blood using a System 9118+ Hematology Series Cell Counter (BioChem ImmunoSystems Inc, Allentown, PA). At selected times, blood samples were also stained with a donor-specific anti–Ly-5.2∼FITC MoAb (clone ALI4A2) and PE-conjugated MoAbs specific for B (anti-CD45R/B220; clone RA3-6B2) or T lymphocytes (anti–Thy-1.2; clone 30H12), or granulocytes (anti-Ly6G/Gr-1; clone RB6-8C5) and macrophages (anti-CD11b/Mac-1; clone M1/70). Multilineage progeny of transplanted stem cells were quantitated using a FACScan instrument (Becton Dickinson). After 120 days, all mice were euthanized and marrow cells were assayed for CFCs, and injected into lethally irradiated B6.SJL mice (0.5 femurs per mouse) for secondary repopulation. Secondary mice were analyzed 10 weeks later for donor-derived peripheral blood leukocytes and BM CFCs as described earlier.
Statistical analysis.
The statistical significance of differences between means was assessed using the two-tailed t-test.
RESULTS
Development of a homing assay for transplanted hematopoietic cells.
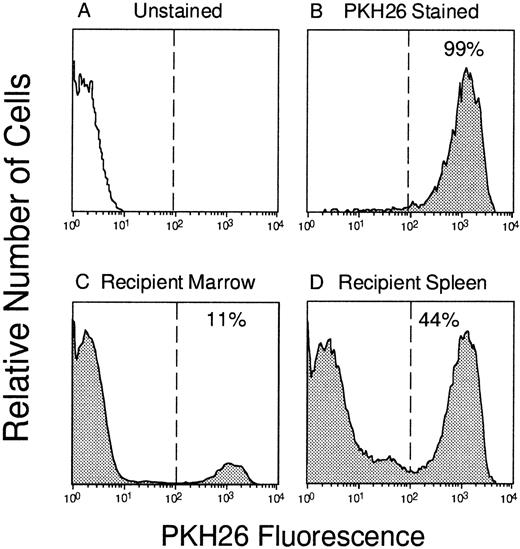
To track the localization of IV-transplanted BM cells to various hematopoietic organs in vivo, we used a well-established procedure for fluorescently labeling cells with the fluorescent dye PKH26. Using the staining procedure described in Materials and Methods, 99% of BM cells could be brightly stained with a median fluorescence intensity approximately 1,000-fold higher than that of unlabeled control cells (Fig 1). This degree of separation was found to be critical for the sorting of pure populations of labeled cells from hematopoietic organs after transplantation (see later), and homing assays were only performed when this bright staining was achieved. As reported previously,10 PKH26 staining did not have any deleterious effect on the cloning efficiency of hematopoietic progenitors detected by their capacity to generate myeloid, erythroid, or mixed colonies in vitro. Labeled BM cells generated comparable numbers of total colonies as unstained control cells; 75 ± 4 (n = 16) and 60 ± 5 (n = 14) per 3 × 104 cells, respectively. This indicates that PKH26 does not alter the functional activity of hematopoietic progenitors and is suitable to track their homing in vivo.
Tracking of PKH26-labeled BM cells in vivo. Murine marrow cells (A) were labeled with PKH26 (B) and 108 cells were injected into a lethally irradiated syngeneic mouse. Three hours later, PKH26+ cells that had “homed” to the bone marrow (C; 11% labeled) or spleen (D; 44% labeled) were identified by flow cytometry and sorted according to the gates depicted by the vertical lines.
Tracking of PKH26-labeled BM cells in vivo. Murine marrow cells (A) were labeled with PKH26 (B) and 108 cells were injected into a lethally irradiated syngeneic mouse. Three hours later, PKH26+ cells that had “homed” to the bone marrow (C; 11% labeled) or spleen (D; 44% labeled) were identified by flow cytometry and sorted according to the gates depicted by the vertical lines.
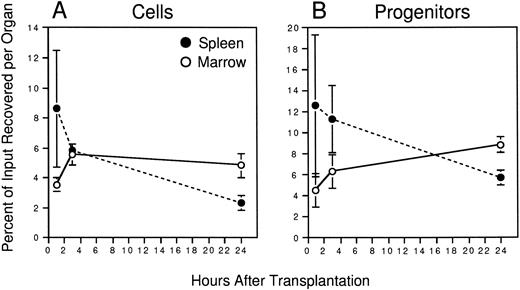
Initial experiments were conducted to establish the time after transplantation required to recover maximal numbers of PKH26+ cells in the two primary hematopoietic organs of the adult mouse: the BM and spleen. Irradiated animals were injected with approximately 108 PKH26-labeled marrow cells. BM and spleen cells were harvested from different recipients 1, 3, or 24 hours later and the frequency and absolute number of PKH26+ cells in each organ determined by flow cytometry. Marrow recoveries were calculated based on the assumption that two femora and two tibiae represent 25% of total BM.24 Figure2A shows that transplanted cells homed rapidly to the spleen (8.6% ± 3.9% of input recovered after 1 hour), but their numbers declined thereafter to 5.9% ± 0.4% and only 2.3% ± 0.5% of input after 3 and 24 hours, respectively. Although egress from this organ was accompanied initially by an approximately equivalent accumulation of PKH26+ cells in the marrow, the recovery of transplanted cells in marrow plateaued at approximately 5% of input numbers after 3 hours despite continued migration out of the spleen. Overall, it was notable that a maximum of only approximately 12% of all injected cells were recovered in these two organs, the remainder likely having accumulated in highly perfused nonhematopoietic organs such as the lungs and liver, where they are presumably removed by cells of the reticuloendothelial system.
Homing of murine BM cells. 108 PKH26-labeled BM cells were injected into lethally irradiated hosts. At the indicated times, recipient BM and spleen cells were counted to determine whole-organ cellularity, and the frequency of PKH26+cells in each determined by FACS. Labeled cells were sorted and assayed for CFCs together with an aliquot of labeled cells from the pretransplant suspension. Shown are the mean ± SEM percent recoveries of total cells (A) and CFCs (B) in BM (○) and spleen (•) for pooled data from 3 experiments (3 mice/time point). Values are derived from the formulae: % Cell Recovery = (% PKH26+ by FACS × whole-organ cellularity)/108, and % CFC Recovery = (CFC frequency in sorted PKH26+ cells × No. PKH26+ cells per organ)/(CFC frequency in pretransplant BM × 108). Differences in the recovery of total cells and CFCs is significant at 24 hours (P < .05); differences in the recovery of CFCs at 3 hours is significant to P = .1.
Homing of murine BM cells. 108 PKH26-labeled BM cells were injected into lethally irradiated hosts. At the indicated times, recipient BM and spleen cells were counted to determine whole-organ cellularity, and the frequency of PKH26+cells in each determined by FACS. Labeled cells were sorted and assayed for CFCs together with an aliquot of labeled cells from the pretransplant suspension. Shown are the mean ± SEM percent recoveries of total cells (A) and CFCs (B) in BM (○) and spleen (•) for pooled data from 3 experiments (3 mice/time point). Values are derived from the formulae: % Cell Recovery = (% PKH26+ by FACS × whole-organ cellularity)/108, and % CFC Recovery = (CFC frequency in sorted PKH26+ cells × No. PKH26+ cells per organ)/(CFC frequency in pretransplant BM × 108). Differences in the recovery of total cells and CFCs is significant at 24 hours (P < .05); differences in the recovery of CFCs at 3 hours is significant to P = .1.
To directly quantitate the homing of functional hematopoietic progenitors to the marrow and spleen, PKH26+ cells were sorted from each organ by FACS and assayed for their potential to generate colonies of mature blood cells in semisolid medium. Figure 1shows the gates used for donor cell selection from one mouse injected 3 hours previously with 108 PKH26-stained BM cells. Transplanted donor cells were readily detectable in the marrow (11% PKH26+) and spleen (44% PKH26+) and could be sorted to greater than 98% homogeneity for subsequent assays. In some experiments, PKH26− cells of lethally irradiated host origin were also sorted as a negative control, but, as expected, failed to generate any colonies in vitro (data not shown). Similar to total nucleated cells described earlier, CFCs homed rapidly to the spleen (∼13% of input recovered after 1 hour), but declined thereafter to approximately 11% and only 6% of input numbers after 3 and 24 hours, respectively (Fig 2B). Progenitors accumulated simultaneously in the BM plateauing at 6% to 8% of input after 3 hours. When the number of PKH26+ CFCs per organ was divided by the total number of PKH26+ cells, it was found that CFCs were moderately concentrated among cells homing to the spleen compared with marrow at 3 hours after transplant: 390 ± 50 versus 230 ± 20 CFCs per 105 PKH26+ cells, respectively. No such differences were noted at the 1-hour time point (260 ± 50 CFCs per 105 PKH26+ cells in both organs), but by 24 hours the relative difference in CFC concentration in the spleen versus BM was still 1.4-fold. Thus, more clonogenic progenitors were recovered in the spleen than in the BM within the first few hours after transplantation, and appeared preferentially concentrated in the former after 3 hours.
Linearity of the homing assay.
In order for the homing assay to be quantitative, it was necessary to establish (1) that the frequency and absolute number of transplanted cells measured in the BM and spleen were directly proportional to the number of labeled cells injected, and (2) that transplanted cell dose did not influence progenitor homing within the range of cell doses normally infused. To define these assay parameters, irradiated mice were injected with increasing numbers of PKH26-labeled BM cells (106 to 108 per mouse) and marrow and spleen cells analyzed 3 hours later to determine the frequency of PKH26+ cells in each organ. Figure3A shows that labeled cells homed to both organs in linear proportion to the number transplanted (correlation coefficient, r = .91 for BM and .92 for spleen), and their frequency was maintained at a constant ratio of approximately 4:1 in spleen versus marrow at all cell doses tested. The overall recovery of transplanted cells in marrow or spleen was also not significantly different and was unaffected by transplanted cell dose; an average of 5% to 10% of the injected cells were recovered in each organ 3 hours after transplantation irrespective of the number of cells infused (Fig3B). As an additional means to exclude the possibility that stem cell homing might be competitively inhibited at high cell doses, irradiated mice were cotransplanted with 106 or 107PKH26-labeled BM cells with or without a ninefold excess of unlabeled BM cells (107 and 108 total cells injected, respectively). No difference was observed in the frequency of PKH26+ cells in BM (0.08%) or spleen (0.3%) of mice injected with 106 labeled cells with or without unlabeled competitors. Consistent with the results in Fig 3A, mice injected with 107 PKH26+ cells contained approximately 10-fold more fluorescent cells in BM (0.74%) and spleen (3.9%), but again no differences in homing were observed in animals transplanted with 9 × 107 unstained BM cells. Taken together, these data demonstrate that even after infusing up to 108cells per mouse, putative niches available to sequester IV-transplanted hematopoietic cells are not yet saturated, a condition that may lead to increased nonspecific localization at these or secondary sites.
Linearity of the homing assay. Lethally irradiated mice were injected with 106 to 1.2 × 108PKH26-stained BM cells. Three hours later, BM (○) and spleen (•) cells were analyzed by flow cytometry. (A) The percent of labeled cells in each organ is shown for 32 individual mice from 18 experiments. Correlation coefficients >0.9 demonstrate a linear relationship between hematopoietic cell homing and transplanted cell dose. (B) The values in A were multiplied by organ cellularity in each case to calculate the fraction of cells homing to BM or spleen relative to the number transplanted. The essentially flat scatter of points over the x-axis demonstrates that hematopoietic cell homing is largely independent of graft size over the range of cell doses normally transplanted.
Linearity of the homing assay. Lethally irradiated mice were injected with 106 to 1.2 × 108PKH26-stained BM cells. Three hours later, BM (○) and spleen (•) cells were analyzed by flow cytometry. (A) The percent of labeled cells in each organ is shown for 32 individual mice from 18 experiments. Correlation coefficients >0.9 demonstrate a linear relationship between hematopoietic cell homing and transplanted cell dose. (B) The values in A were multiplied by organ cellularity in each case to calculate the fraction of cells homing to BM or spleen relative to the number transplanted. The essentially flat scatter of points over the x-axis demonstrates that hematopoietic cell homing is largely independent of graft size over the range of cell doses normally transplanted.
Organ-selective homing of early engrafting hematopoietic cells.
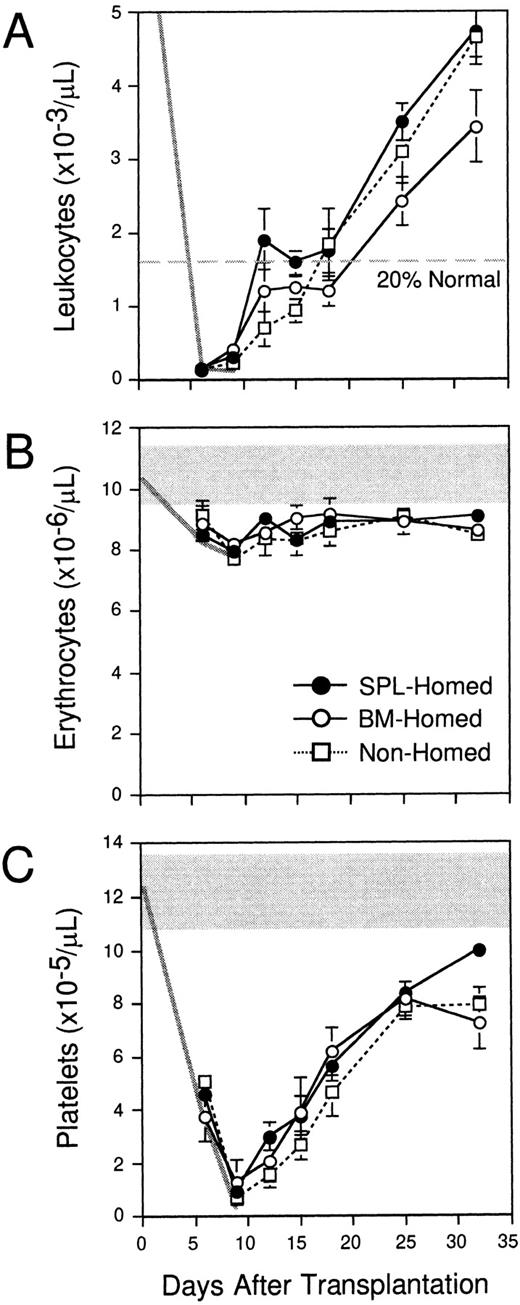
The data in Fig 2 indicate that more clonogenic progenitors home to the spleen than BM within 3 hours after IV transplantation, and that CFCs are almost twofold more concentrated within the donor compartment in spleen at this time. To compare the early and long-term reconstitution potential of these populations with differential homing properties, PKH26+ cells that had homed for 3 hours to the BM (“BM-homed”) or spleen (“SPL-homed”) of irradiated mice were sorted and retransplanted into lethally irradiated Ly-5 congenic hosts (106 cells per mouse). A third group of control B6.SJL mice were each transplanted with 106 B6 BM cells that had been labeled with PKH26, but not subsequently selected with an in vivo homing step (“nonhomed”). Peripheral blood was counted once or twice per week from 6 to 32 days after transplantation to measure early hematopoietic reconstitution kinetics. The levels of leukocytes (6 to 9.2 × 103/μL), erythrocytes (9.6 to 11.4 × 106/μL), and platelets (10.9 to 13.8 × 105/μL) in normal B6.SJL mice analyzed in parallel are shown by the shaded areas in the upper portion of Fig4B and C (normal range for leukocytes in Fig 4A is off scale). The decline in blood cell counts after irradiation is indicated by the thick gray lines in each panel. In contrast to 11 irradiated but untransplanted control animals, which all died by day 12 after transplantation, all recipients of 106BM-homed (five mice), SPL-homed (14 mice), or nonhomed (eight mice) cells survived for the entire period of analysis. Interestingly, mice that were transplanted with BM cells that had homed initially to the spleen exhibited approximately 30% higher white blood cell counts at all times during the first month after transplantation compared with recipients of 106 BM-homed cells (Fig 4A). As a result, SPL-homed cells regenerated circulating leukocytes to 20% of normal counts approximately 13 days faster (ie, in 12 days) than cells that had homed initially to the marrow (∼25 days), a finding that correlates with the approximately 1.7-fold higher CFC content of the former noted above (3,900 ± 500 v 2,300 ± 200, respectively). Nonhomed control BM cells required 18 days to engraft to this level. No differences in the rate of platelet or erythrocyte regeneration were observed in mice transplanted with cells that had homed to the BM versus the spleen (Fig 4B and C).
Early hematopoietic reconstitution kinetics of BM cells selected pretransplantation on the basis of marrow or splenic homing. Lethally irradiated mice were injected with 108PKH26-stained syngeneic BM cells. Three hours later, PKH26+ cells that had homed to the marrow (○) or spleen (•) were isolated by FACS and transplanted into lethally irradiated Ly-5 congenic mice (106 cells/mouse). Control animals were injected with 106 PKH26-stained BM cells that had not been selected by a prior homing step (□). Peripheral blood leukocytes (A), erythrocytes (B), and platelets (C) were counted on the indicated days. The normal range of blood cell counts (except leukocytes, which are off scale) and their decline after irradiation are defined by the shaded areas and thick gray lines. Shown are the mean ± SEM values for pooled data from 3 experiments (5 to 14 mice/population). Errors not shown are too small for the scale used. Differences in leukocyte counts in recipients of BM-homed or SPL-homed cells are significant on days 25 and 32 (P < .05).
Early hematopoietic reconstitution kinetics of BM cells selected pretransplantation on the basis of marrow or splenic homing. Lethally irradiated mice were injected with 108PKH26-stained syngeneic BM cells. Three hours later, PKH26+ cells that had homed to the marrow (○) or spleen (•) were isolated by FACS and transplanted into lethally irradiated Ly-5 congenic mice (106 cells/mouse). Control animals were injected with 106 PKH26-stained BM cells that had not been selected by a prior homing step (□). Peripheral blood leukocytes (A), erythrocytes (B), and platelets (C) were counted on the indicated days. The normal range of blood cell counts (except leukocytes, which are off scale) and their decline after irradiation are defined by the shaded areas and thick gray lines. Shown are the mean ± SEM values for pooled data from 3 experiments (5 to 14 mice/population). Errors not shown are too small for the scale used. Differences in leukocyte counts in recipients of BM-homed or SPL-homed cells are significant on days 25 and 32 (P < .05).
To confirm that leukocytes were regenerated by donor-derived progenitors in these mice, and to determine whether there might be differences in the distribution of the progeny of SPL-homed versus BM-homed cells to lymphoid and myeloid lineages, blood cells were also stained on days 15 and 18 with an Ly-5.2–specific MoAb together with MoAbs reactive to B cells, T cells, granulocytes, or monocytes. As expected, white blood cells were predominantly of donor type (87% ± 6%) in all mice from the three groups, and were distributed at normal ratios of 73% ± 2% to the B lineage, 18% ± 1% to the T lineage, and 13% ± 1% to the myeloid lineage (data not shown). Therefore, no differences in the differentiation potential of BM-homed versus SPL-homed cells were noted that could explain their differential leukocyte reconstitution kinetics.
Superior long-term hematopoiesis in mice transplanted with BM cells selected on the basis of splenic homing.
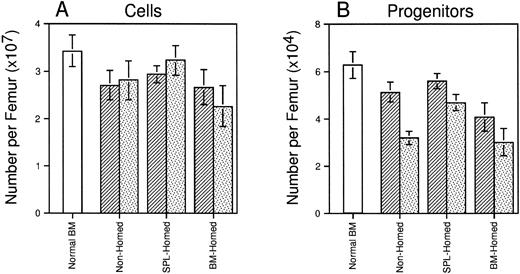
Four months after transplant, all animals recovered normal levels of circulating blood cells that were virtually completely regenerated from donor stem cells (blood 92% to 95% Ly-5.2+ in all three groups, data not shown). Marrow cellularity had recovered to approximately 80% of that of age-matched normal control mice and did not differ among recipients of 106 BM-homed, SPL-homed, or nonhomed marrow cells (Fig 5A). However, mice transplanted with SPL-homed cells contained slightly more (∼38%; P < .05) CFCs per femur than mice injected with BM-homed cells (Fig 5B). Marrow cells from these primary animals were also retransplanted into lethally irradiated secondary B6.SJL mice (0.5 femurs per mouse). Ten weeks later, SPL-homed cells had regenerated approximately 43% more cells per secondary femur than BM-homed cells (Fig 5A). Although all three groups of secondary recipients declined in femoral CFC content relative to the primary marrow’s, recipients of SPL-homed cells contained 46% (P < .05) and 55% (P < .05) more CFCs per femur than secondary recipients of nonhomed and BM-homed cells, respectively (Fig 5B). Taken together, the data presented in Figs 4 and 5 suggest that BM cells that home initially to the spleen are, as a population, enriched for HSCs and progenitor cells with early leukocyte and long-term multilineage repopulating potential in myeloablated primary and secondary hosts. In contrast, BM cells that home to the marrow within 3 hours after transplantation do not behave significantly differently than PKH26-stained cells that had not been selected by an in vivo homing step before assay.
Long-term regeneration of marrow cellularity and CFCs in primary and secondary recipients of cells selected on the basis of marrow or splenic homing. Shown are the mean ± SEM number of cells (A) and CFCs (B) per femur in lethally irradiated primary mice (▨) injected 120 days previously with BM-homed, SPL-homed, or nonhomed cells. Lethally irradiated secondary mice were transplanted with 0.5 femur/mouse and analyzed 10 weeks later (). Hematologic parameters for age-matched normal B6.SJL mice are shown for comparison (□). Pooled data from 5 to 14 mice/population. Differences in femoral cellularity between secondary recipients of BM-homed and SPL-homed cells are significant to P = .1; differences in femoral CFC content between primary recipients of BM-homed and SPL-homed cells are significant to P < .05, and between secondary recipients of SPL-homed cells and either BM-homed or nonhomed cells are significant to P < .05.
Long-term regeneration of marrow cellularity and CFCs in primary and secondary recipients of cells selected on the basis of marrow or splenic homing. Shown are the mean ± SEM number of cells (A) and CFCs (B) per femur in lethally irradiated primary mice (▨) injected 120 days previously with BM-homed, SPL-homed, or nonhomed cells. Lethally irradiated secondary mice were transplanted with 0.5 femur/mouse and analyzed 10 weeks later (). Hematologic parameters for age-matched normal B6.SJL mice are shown for comparison (□). Pooled data from 5 to 14 mice/population. Differences in femoral cellularity between secondary recipients of BM-homed and SPL-homed cells are significant to P = .1; differences in femoral CFC content between primary recipients of BM-homed and SPL-homed cells are significant to P < .05, and between secondary recipients of SPL-homed cells and either BM-homed or nonhomed cells are significant to P < .05.
Ex vivo expansion of HSCs generates progenitors with diminished in vivo homing capacity.
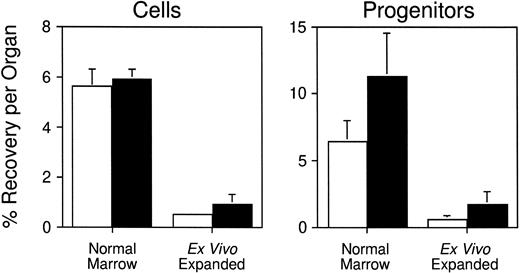
Having developed a quantitative assay to measure stem/progenitor cell homing in vivo, we next sought to employ it to measure the homing of CFCs generated by ex vivo expansion. Several investigators have demonstrated that highly enriched HSCs can be partially differentiated ex vivo to generate large numbers of functional hematopoietic progenitors.18,25-27 Although expanded cells can regenerate circulating blood cells approximately 1 to 2 weeks faster (depending on the lineage) than freshly isolated HSCs when transplanted into myeloablated mice, the rate of hematopoietic reconstitution is not as rapid as might be predicted from their clonogenic cell content.17 18 This finding suggests that the homing properties of primitive cells may be altered following their expansion in potent combinations of hematopoietic growth factors in vitro. To test this hypothesis, serum-free suspension cultures containing IL-3, IL-6, G-CSF, and SCF were initiated with 20 × 106normal BM cells (106/mL; one experiment). In two additional experiments, cultures containing IL-6, IL-11, G-CSF, SCF, FLK, and TPO were seeded with 16 to 18 × 103Sca-1+c-kit+Lin−cells (103/mL) enriched by FACS from the BM of B6 mice injected 1 day previously with 5-FU. This combination of cytokines was determined in preliminary studies to support optimal expansion of total nucleated cells and CFCs from enriched stem cells (S.J.S., unpublished data, July 1997). Limiting dilution competitive repopulation assays established that Sca-1+c-kit+Lin−cells are highly enriched in stem cells with long-term lymphomyeloid repopulating potential and contain 1 competitive repopulating unit (CRU) per 15 (95% confidence limits, 1 per 12 to 1 per 23) cells (S.J.S., unpublished data, October 1997). In contrast, more mature progenitor cells such as in vitro CFCs (59 ± 6 per 103 cells; five experiments) and CFU-S (2.6 ± 0.4 day 12 and 0 day 8 CFU-S per 103 cells; three experiments) are relatively depleted. After 9 days of culture, cells and CFCs were expanded 1.2-fold (P > .05) and 7.5-fold (P < .05), respectively, in the experiment initiated with unfractionated BM cells, and 900 ± 100-fold (P < .05) and 420 ± 230-fold (P < .05), respectively, in the two experiments initiated with purified Sca-1+c-kit+Lin−cells. Expanded cells were stained with PKH26 as described earlier, and injected into lethally irradiated syngeneic mice. Three hours later, an average of 0.53% ± 0.13% and 0.91% ± 0.43% of the cultured cells had homed to the BM and spleen, respectively (Table1; data pooled from all three experiments). Similar fractions of the input number of CFCs were recovered in each organ: 0.56% ± 0.27% in marrow and 1.67%± 0.98% in spleen (Table 1). When these data are compared with the results of experiments performed above with freshly isolated BM cells, it is clear that the homing capacity of clonogenic progenitors generated in vitro is reduced approximately 10-fold (P < .05) compared with normal (Fig6). The data are consistent with historic observations of the diminished engraftment potential of cultured hematopoietic cells in both mouse and humans.
Homing of Ex Vivo–Expanded Progenitor Cells In Vivo
| Experiment No. . | No. Transplanted (mean ± SEM) . | No. Recovered per Organ . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PKH26+ Cells . | PKH26+ CFCs (mean ± SEM) . | |||||||||
| PKH26+ Cells . | PKH26+ CFCs . | Marrow . | % Input . | Spleen . | % Input . | Marrow . | % Input . | Spleen . | % Input . | |
| 1 | 1.2 × 107 | 356,000 ± 92,000 | 91,800 | 0.77 | 37,400 | 0.31 | 190 ± 100 | 0.054 | 120 ± 90 | 0.032 |
| 2 | 8.8 × 106 | 433,130 ± 35,000 | 27,500 | 0.31 | 58,800 | 0.67 | 2,870 ± 650 | 0.66 | 6,730 ± 270 | 1.55 |
| 3 | 9.7 × 106 | 503,360 ± 43,560 | 50,400 | 0.52 | 169,520 | 1.75 | 4,870 ± 220 | 0.97 | 17,220 ± 140 | 3.42 |
| Pooled | 0.53 ± 0.13 | 0.91 ± 0.43 | 0.56 ± 0.27 | 1.67 ± 0.98 | ||||||
| Experiment No. . | No. Transplanted (mean ± SEM) . | No. Recovered per Organ . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PKH26+ Cells . | PKH26+ CFCs (mean ± SEM) . | |||||||||
| PKH26+ Cells . | PKH26+ CFCs . | Marrow . | % Input . | Spleen . | % Input . | Marrow . | % Input . | Spleen . | % Input . | |
| 1 | 1.2 × 107 | 356,000 ± 92,000 | 91,800 | 0.77 | 37,400 | 0.31 | 190 ± 100 | 0.054 | 120 ± 90 | 0.032 |
| 2 | 8.8 × 106 | 433,130 ± 35,000 | 27,500 | 0.31 | 58,800 | 0.67 | 2,870 ± 650 | 0.66 | 6,730 ± 270 | 1.55 |
| 3 | 9.7 × 106 | 503,360 ± 43,560 | 50,400 | 0.52 | 169,520 | 1.75 | 4,870 ± 220 | 0.97 | 17,220 ± 140 | 3.42 |
| Pooled | 0.53 ± 0.13 | 0.91 ± 0.43 | 0.56 ± 0.27 | 1.67 ± 0.98 | ||||||
Unfractionated (experiment 1) or Sca-1+c-kit+Lin−(experiments 2 and 3) BM cells were cultured for 9 days in serum-free medium supplemented with IL-3, IL-6, G-CSF, and SCF, or IL-6, IL-11, G-CSF, SCF, FLK, and TPO, respectively. Expanded cells were stained with PKH26, assayed for CFCs, and then injected IV into lethally irradiated syngeneic mice. Three hours later, marrow and spleen cells were procured and counted to determine total-organ cellularity. PKH26+ cells were quantitated by FACS and then sorted and assayed for CFCs. The percent recovery of input cells and CFCs in each organ was calculated as described in the legend to Fig 2.
Reduced homing capacity of hematopoietic progenitors generated in vitro. Lethally irradiated mice were transplanted with ∼108 PKH26-labeled normal BM cells or ∼107PKH26-labeled progeny of unfractionated or Sca-1+c-kit+Lin- BM cells generated after 9 days of expansion in hematopoietic growth factors (see Table 1 for details). Three hours later, PKH26+ cells that had homed to the BM (□) or spleen (▪) were quantitated by flow cytometry and isolated by FACS for CFC assays. Shown are the mean ± SEM percent recovery of donor-derived cells (left) and CFCs (right) per organ.
Reduced homing capacity of hematopoietic progenitors generated in vitro. Lethally irradiated mice were transplanted with ∼108 PKH26-labeled normal BM cells or ∼107PKH26-labeled progeny of unfractionated or Sca-1+c-kit+Lin- BM cells generated after 9 days of expansion in hematopoietic growth factors (see Table 1 for details). Three hours later, PKH26+ cells that had homed to the BM (□) or spleen (▪) were quantitated by flow cytometry and isolated by FACS for CFC assays. Shown are the mean ± SEM percent recovery of donor-derived cells (left) and CFCs (right) per organ.
DISCUSSION
In this report, we describe a quantitative assay to monitor the homing of transplanted hematopoietic cells in vivo, and more importantly, which facilitates their isolation from various hematopoietic organs for subsequent functional analysis. Quantitation of progenitor homing is achieved by multiplying the percentage of cells in a particular organ that bear the fluorescent marker of the transplanted population (PKH26+) by whole-organ cellularity at the time of assay. This calculation provides the absolute number of donor cells in each organ of interest; the absolute number of a particular class of stem or progenitor cells within the donor population is then determined by functionally assaying their frequency among PKH26+ cells sorted by FACS. As is important for any quantitative assay, we have established that the frequency of donor cells in BM and spleen is linearly related to the number of cells transplanted between 106 and 1.2 × 108 cells per mouse (Fig3). The fraction of input cells recovered in these organs is also essentially constant at these cell doses (∼5% to 10% at 3 hours), and is not diminished by the cotransplantation of a ninefold excess of unlabeled competitor cells. Taken together, the data suggest that the number of niches available for seeding by transplanted hematopoietic cells is not limiting in our model.
We have used this assay to demonstrate that in vitro CFCs derived from normal adult mouse BM accumulate in the marrow and spleen of irradiated recipients within 1 hour after IV injection. Thereafter, the number of CFCs in the spleen declines for at least 24 hours and progenitors accumulate in the BM until their numbers are largely stabilized at approximately 3 hours after transplant. Interestingly, CFCs are moderately concentrated among donor cells that home to the spleen compared with BM, so that after 3 hours PKH26+ cells sorted from each organ differ by almost twofold in CFC content. When 106 SPL-homed and BM-homed cells, containing a mean of 3,900 ± 500 or 2,300 ± 200 CFCs, respectively, were assayed for their potential to rapidly reconstitute hematopoiesis in lethally irradiated Ly-5 congenic hosts, we observed that SPL-homed cells regenerated 20% normal numbers of circulating leukocytes almost 2 weeks faster than BM-homed cells (Fig 4). Primary recipients of SPL-homed cells also recovered 38% more CFCs per femur by 17 weeks after transplant than recipients of BM-homed cells, which resulted in a 55% higher recovery of clonogenic progenitors in secondary mice injected 10 weeks previously with a 0.5 femur equivalent of primary marrow. These differences in transplantation potential may be due simply to differences in the number of in vitro CFCs present in each fraction. However, this conclusion is not supported by recent studies that suggest such cells are probably not responsible for early hematopoietic reconstitution in vivo.28,29 Instead, our data suggest either (1) that BM-homed and SPL-homed cells differ in their content of some other class of clonogenic cells able to regenerate and maintain hematopoiesis upon transplantation, or (2) that 3-hour homing to spleen segregates cells with functional properties that differ from those homing to the marrow. In the latter case, it would be important to determine whether these characteristics preexist in the marrow population before transplant, or are imposed upon the cells during their short residence in the organ to which they homed. These alternative possibilities could be resolved by normalizing PKH26+ cells isolated from each organ after homing for equivalent numbers of some functionally defined class of clonogenic cells (ie, CFCs or more primitive CRU), instead of total nucleated cells pretransplant. If the differences we have observed between BM-homed and SPL-homed cells are due to differences in their content of otherwise functionally equivalent stem/progenitor cells, then the two homed populations should perform similarly. If, however, differential homing selects for cells that contribute variably to hematopoiesis, then the present differences observed at the level of the whole populations should be maintained. Studies to test these possibilities are currently in progress. Furthermore, it remains to be demonstrated whether the differences we have documented in the activity of hematopoietic cells homing to the spleen or BM 3 hours after transplantation are reproduced at later times. We and others11 have shown that in contrast to the 3-hour time point, after 24 hours a greater proportion of injected cells (including CFCs) are recovered in the BM than the spleen. However, because the relative frequency of CFCs among PKH26+ cells in the spleen was still 1.4-fold higher in spleen than BM at this time, we would not predict that extending the assay end point at least to 24 hours should alter our results. Nonetheless, the present assay appears well suited to further exploring the dynamics of HSC localization in vivo during the first few days after transplantation.
In a useful experimental application of the homing assay, we evaluated the homing properties of hematopoietic progenitors generated in vitro by the serum-free expansion of either unfractionated normal BM cells in IL-3, IL-6, G-CSF, and SCF, or highly enriched Sca-1+c-kit+Lin−cells in IL-6, IL-11, G-CSF, SCF, FLK, and TPO. After 9 days, CFCs were expanded 7.5-fold and up to 644-fold, respectively, thereby providing very large numbers of clonogenic cells for transplantation studies. However, when expanded CFCs were labeled with PKH26 and injected into lethally irradiated mice, we found that only approximately 0.6% and 1.7% of injected progenitors localized to the marrow and spleen, respectively, after 3 hours. This represents an approximate 10-fold reduction in the homing capacity of CFCs compared with freshly isolated marrow. Sca-1+Lin− BM cells that were expanded in vitro for 7 days in serum-containing cultures supplemented with IL-1α , IL-3, IL-6, and SCF have previously been shown to generate classes of progenitor cells with different repopulating potentials depending on their replicative history.21 Cells that had divided fewer than one or two times retained the highest frequency of repopulating cells, and repopulating potential was significantly diminished after four cell divisions in vitro. These data suggest that proliferation of transplantable cells may affect their ability to engraft myeloablated hosts. A similar decline in the seeding efficiency of murine CFU-S isolated from regenerating marrow following hydroxyurea treatment was noted more than 10 years ago.30More recently, it was reported that preincubation of murine BM cells with IL-3, or IL-3 plus IL-12 and SCF, for as short as 2 hours led to a substantial decrease in the seeding of all subsets of cobblestone area-forming cells (CAFC) to both marrow and spleen.31Taken together with our present results, these data suggest that qualitative changes in the homing properties of stem and progenitor cells that have been stimulated to proliferate in response to cytokine exposure may nullify the potential clinical benefits of their quantitative expansion in vitro. Although we have not yet examined whether the loss of CFC homing that we have observed correlates with changes in their expression of particular adhesion molecules, a functional association between the expression of CD43, CD44, and L-selectin and both rapid hematopoietic reconstitution potential and long-term marrow repopulating ability has previously been noted.32,33 The challenge for the future will clearly be to overcome the decline in homing capacity of stem and progenitor cells manipulated ex vivo through continued exploration of alternative culture conditions that promote their growth. In this regard, more serious consideration should probably be paid to the importance of various integrins and extracellular matrix molecules in stem cell cultures. These may facilitate the selective expansion of transplantable cells expressing adhesion molecules critical for in vivo homing. Alternatively, efforts to improve stem cell homing through treatment of transplant recipients with small peptides that promote the attachment of clonogenic cells to stroma may also provide a route to improving the engraftment properties of ex vivo expanded hematopoietic cells.34 Regardless of the strategies to be adopted, the present model system should prove useful for future studies of the homing and engraftment properties of hematopoietic cells from a variety of sources.
ACKNOWLEDGMENT
The authors thank Dr Craig Jordan (University of Kentucky) for stimulating discussions and for critically reviewing the manuscript.
Supported by the University of Kentucky Hospital and the Department of Internal Medicine, and by Grant No. 85-001-12-IRG to S.J.S. from the American Cancer Society. S.J.S. is the recipient of a Junior Faculty Scholar Award from The American Society of Hematology.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephen J. Szilvassy, PhD, Hematology/Oncology-BMT, Lucille P. Markey Cancer Center, Room CC414, 800 Rose St, Lexington, KY 40536-0093.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal