Abstract
The proto-oncogene product, p21ras, has been implicated in the cellular mechanism of adhesion, although its precise role has been controversial. Numerous cytokines and growth-factors activate Ras, which is an important component of their growth-promoting signaling pathways. On the other hand, the role of Ras in cytokine-induced adhesion has not been elucidated. We therefore investigated the function of H-Ras in the inside-out signaling pathway of interleukin-3 (IL-3)–induced integrin activation in the murine Baf3 cell line after transfection of cells with either constitutively active, dominant-negative, or wild-type H-Ras cDNAs. Adhesion of Baf3 cells to fibronectin was induced by IL-3 in a dose-dependent manner via very late antigen-4 (VLA-4; 4β1 integrins) and VLA-5 (5β1 integrins) activation. On the other hand, IL-4 did not induce the adhesion of Baf3 cells to fibronectin, although IL-4 did stimulate the cell proliferation of Baf3 cells. Constitutively active H-Ras–transfected Baf3 cells adhered to fibronectin without IL-3 stimulation through VLA-4 and VLA-5, whereas dominant-negative H-Ras–transfected Baf3 cells showed significantly less adhesion induced by IL-3 compared with wild-type and constitutively active H-Ras–transfected Baf3 cells. Anti-β1 integrin antibody (clone; 9EG7), which is known to change integrin conformation and activate integrins, induced the adhesion of dominant-negative H-Ras–transfected Baf3 cells as much as the other types of H-Ras–transfected Baf3 cells. 8-Br-cAMP, Dibutyryl-cAMP, Ras-Raf-1 pathway inhibitors, and PD98059, a MAPK kinase inhibitor, suppressed proliferation and phosphorylation of MAPK detected by Western blotting with anti–phospho-MAPK antibody, but not adhesion of any type of H-Ras–transfected Baf3 cells, whereas U-73122, a phospholipase C (PLC) inhibitor, suppressed adhesion of these cells completely. These data indicate that H-Ras and PLC, but not Raf-1, MAPK kinase, or the MAPK pathway, are involved in the inside-out signaling pathway of IL-3–induced VLA-4 and VLA-5 activation in Baf3 cells.
ADHESION OF CELLS TO extracellular matrix proteins, such as fibronectin (FN), collagen, and laminin, has profound effects on cell growth, differentiation, and gene expression. Adhesion of cells to these extracellular matrix proteins is mediated by integrins. At least 20 different integrins have been characterized to date, all of which are heterodimeric transmembrane proteins composed of an α subunit that is noncovalently associated with a β subunit at the cell surface.1 The integrins are capable of transducing biochemical signals across the plasma membrane to regulate cellular functions; this signaling pathway initiated by cell-matrix interactions is called outside-in signaling. There are many reports regarding outside-in signaling, and protein tyrosine phosphorylation has been implicated as playing a central role in this pathway.2-5
To achieve correct cellular function through cell-matrix interactions, the interactions between integrins and their ligands need to be regulated in a number of ways. One way is regulation of expression levels of integrins at the cell surface. Another is regulation of the activity of integrins. Integrins are not always able to bind to their ligands and must be activated by intracellular signals to bind to their ligands. The cytoplasmic domains of integrins are important for their activation from inside the cell.6 Stimulating agents such as phorbol esters, calcium ionophores, chemoattractants, and aggregation of surface receptors induce activation of integrins. This signaling pathway is called inside-out signaling.7,8Recently, some cytokines have been demonstrated to activate integrins. For example, steel factor induces mast cell adhesion to FN9; interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and steel factor activate very late antigen-4 (VLA-4) and VLA-5 on human hematopoietic cell lines, MO7e and TF-110; and thrombopoietin activates VLA-4 and VLA-5 on MO7e cells.11
Protein kinase C (PKC), phospholipase C (PLC), and phosphoinositide 3-OH kinase (PI 3-kinase) are thought to be involved in the inside-out signaling pathways upstream of the cytoplasmic domains of integrins.7,8 However, the precise mechanism for these effects has not been elucidated, and conflicting reports have recently been published. It has been reported that a constitutively active R-Ras activates integrins.12 Conversely, it has been reported that a constitutively active H-Ras inactivates integrins.13Because Ras is activated via many cytokines, there is a possibility that it may have some role in inside-out signaling induced by these cytokines. No information about Ras involvement in cytokine induced inside-out signaling pathway has thus far been reported. For this reason, we investigated the function of H-Ras in the inside-out signaling pathway of cytokine-induced integrin activation. In a previous study,14 we found that IL-3 activated VLA-4 and VLA-5 on the murine hematopoietic cell line, Baf3, and induced adhesion of these cells to FN. In this study, we used constitutively active, dominant-negative, and wild-type H-Ras–transfected Baf3 cells to examine the effects of H-Ras on the adhesion of these cells to FN.
MATERIALS AND METHODS
Antibodies, cytokines, chemicals, and plasmids.
Anti-β1 integrin antibodies (Ha2/5 and 9EG7), anti-α4 integrin (R1-2), anti-α5 integrin (5H10-27), anti-αv integrin (H9.2B8), their isotype-matched control antibodies, fluorescein isothiocyanate (FITC)-conjugated goat antirat IgG, and FITC-conjugated mouse antihamster IgG were purchased from Pharmingen (San Diego, CA). Anti–H-Ras antibody (F235) was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (6C5) was purchased from Biodesign Int (Kennebunk, ME). Anti–phospho-p44/42 MAPK antibody and anti-p44/42 MAPK antibody were purchased from New England Biolabs, Inc (Beverly, MA). Recombinant mouse IL-3 and mouse IL-4 were purchased from R&D Systems, Inc (Minneapolis, MN). Human fibronectin was purchased from Collaborative Biochemical (Bedford, MA). 8-bromo adenosine 3′:5′-cyclic monophosphate (8-Br-cAMP), N6, 2′-O-dibutyryl adenosine 3′:5′-cyclic monophosphate (Dibutyryl-cAMP), and wortmannin were purchased from Sigma Chemical Co (St Louis, MO). H-7, U-73122, and U-73343 were purchased from Calbiochem (San Diego, CA). PD98059 was purchased from New England BioLabs Inc. pUSE H-Ras (a constitutively active type, a dominant-negative type, and the wild-type), eukaryotic expression vector containing H-Ras (a constitutively active type, a dominant-negative type, and the wild-type) cDNA under the control of the cytomegalovirus (CMV) promotor, and the neomycin resistance gene with the SV40 promotor were purchased from Upstate Biotechnology (Lake Placid, NY). The constitutively active type H-Ras mutation is a substitution of leucine for glutamine at position 61.15 The dominant-negative type H-Ras mutation is a substitution of asparagine for serine at position 17.16
Cells and cell culture.
The murine hematopoietic cell line, Baf3,17 a factor-dependent cell line requiring IL-3 or IL-4 for both growth and survival was used. Baf3 cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and 100 pg/mL mouse IL-3 or 1 ng/mL mouse IL-4. The optimal concentrations of these cytokines were determined in preliminary experiments. The plasmids were transfected into Baf3 cells by electroporation. Stable transfectants were selected by antibiotic G418 (1 mg/mL; GIBCO BRL, Grand Island, NY) and analyzed. These cells were washed and incubated in RPMI-1640 medium with 1% bovine serum albumin (BSA; Sigma) without growth factors for 16 to 18 hours to growth factor-starve the cells and were used for the following experiments.
[3H]-thymidine incorporation assay.
The growth factor-starved Baf3 and all transfected Baf3 cells in RPMI-1640 medium with 10% FCS were plated (0.2 mL/well) at a density of 2 × 104 cells/well in 96-well tissue culture flat-bottom plates (Corning-Costar, Ann Arbor, MI) with or without IL-3 or IL-4 and cultured for 2 days. DNA synthesis was determined by the addition of [3H]-thymidine (0.5 μCi/well; Amersham, Arlington Heights, IL) for the final 6 hours of the culture. The cells were harvested onto glass-fiber filters and the amount of incorporated radioactivity was determined by liquid scintillation counting.
Adhesion assay.
Human FN and BSA were diluted in phosphate-buffered saline (PBS). One hundred microliters of 20 μg/mL FN was distributed in 96-well tissue culture flat-bottom plates. After overnight incubation at 4°C, the coated wells were washed twice with PBS and 100 μL of PBS with 1% BSA was added and plates were incubated at 37°C for 1 hour to block nonspecific binding. At the same time, 100 μL of PBS with 1% BSA was distributed in other wells and incubated at 37°C for 1 hour to act as control wells. The wells were then washed twice with PBS. The growth factor-starved Baf3 and all transfected Baf3 cells were used for the adhesion assay. The cells were labeled with 51Cr (100 μCi/3 × 107 cells; Amersham) for 1 hour at 37°C with RPMI-1640 medium containing 1% BSA. Cells were washed twice and resuspended at 3 × 106 cells/mL in the same conditions. A total of 100 μL of the cell suspension was added to each of the FN-coated wells and BSA-coated wells, which were centrifuged at 600 rpm for 1 minute to allow attachment of cells to the bottom of the wells, followed by incubation for 30 minutes at 37°C. Unattached cells were removed by washing twice with prewarmed RPMI-1640 medium containing 1% BSA. Adherent cells were solubilized with 1% sodium dodecyl sulfate, and radioactivity was quantitated in a γ counter. The percentage of adherent cells (percentage of input) was determined by dividing the activity in the adherent fraction by the radioactivity contained in 100 μL of the initial labeled cell suspension as previously described.18
Cell lysis and immunoblotting.
Cells were lysed in lysis buffer (20 mmol/L Tris-HCl, pH 8.0, 137 mmol/L NaCl, 10% glycerol, 1% NP-40, 1 mmol/L phenylmethylsulfonyl fluoride, 0.15 U/mL aprotinin, 10 mmol/L EDTA, 10 μg/mL leupeptin, 100 mmol/L sodium fluoride, and 2 mmol/L sodium orthovanadate). After 30 minutes on ice, insoluble fractions were removed by centrifugation at 14,000 rpm for 10 minutes (whole cell lysate). Whole cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore Corp, Bedford, MA). Membranes were blocked in Tris-buffered saline containing 0.5% Tween 20 and 2% BSA at room temperature for 1.5 hours and incubated with appropriate primary antibodies for 1.5 hours. Blots were visualized using horseradish peroxidase (HRP)-conjugated secondary antibodies and an enhanced chemiluminescent system (ECL; Amersham). To reprobe with another primary antibody, membranes were incubated in stripping buffer (62.5 mmol/L Tris, pH 6.7, 100 mmol/L 2-mercaptoethanol, 2% SDS) at 70°C for 1 hour, washed, and then used for further study.
Flow cytometric analysis.
Cells (5 × 105) were incubated with 2 μg of antibody at 4°C for 30 minutes and then washed twice in PBS. Cells were incubated with 0.4 μL of FITC-conjugated goat antirat IgG or FITC-conjugated mouse antihamster IgG at 4°C for 30 minutes, washed twice in PBS, and analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, Sunnyvale, CA).
Inhibition of adhesion by anti-integrin antibodies.
Using anti-integrin antibodies (Abs; anti-β1 integrin [clone: Ha2/5], anti-α4 integrin, anti-α5 integrin, and anti-αv integrin), we evaluated the role of each FN receptor for cell adhesion by the ability of each antibody to inhibit adhesion. A concentration of 10 μg/mL of each antibody, which was found to be an effective amount, was used for the inhibition assays. The growth factor-starved transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 30 minutes in the absence or presence of these Abs or their isotype-matched control Abs. Cells were subsequently transferred to FN-coated wells and incubated for an additional 30 minutes at 37°C with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured as noted above.
Inhibition of proliferation and adhesion by the inhibitors of Ras, Raf-1, MAPK kinase, and MAPK pathway.
To investigate downstream events of H-Ras in the inside-out signaling pathway of IL-3–induced integrin activation, the effects of inhibitory compounds of Ras, Raf-1, MAPK kinase, and MAPK pathway were evaulated. We selected 8-Br-cAMP and Dibutyryl-cAMP as inhibitors of the Ras-Raf-1 pathway19,20 and PD98059 as an inhibitor of MAPK kinase.21 To examine the effects of these inhibitors on proliferation, the growth factor-starved transfected Baf3 cells were precultured with or without 1 mmol/L 8-Br-cAMP or 1 mmol/L Dibutyryl-cAMP or 10 and 50 μmol/L PD98059 for 1 hour, and the cells were used for [3H]-thymidine incorporation assay as described above. To examine the effects of these inhibitors on adhesion, the growth factor-starved transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 30 minutes to 3 hours in the absence or presence of 1 mmol/L 8-Br-cAMP or 1 mmol/L Dibutyryl-cAMP, or 10 and 50 μmol/L PD98059. Cells were subsequently transferred to FN-coated wells and incubated for an additional 30 minutes at 37°C with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured as noted above. The optimal concentrations of each inhibitor compound tested were determined in preliminary experiments.
Inhibition of adhesion by inhibitor compounds.
To examine the potential mechanisms of inside-out signaling of IL-3–induced cell adhesion to FN, the effects of different inhibitor drugs on adhesion were tested. PI-3 kinase inhibitor, wortmannin (100 nmol/L), PKC inhibitor, H-7 (50 μmol/L), and PLC inhibitor, U-7312222 (1 μmol/L), were used as inhibitor compounds. U-73433 (1 μmol/L), a close analog of U-73122, was also tested. The growth factor-starved transfected Baf3 cells were labeled with51Cr and incubated at 37°C for 30 minutes in the absence or presence of those inhibitor compounds. Cells were subsequently transferred to FN-coated wells and incubated for an additional 30 minutes at 37°C with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured as noted above. The optimal concentrations of each inhibitor compound tested were determined in preliminary experiments.
Adhesion by anti-β1 integrin antibody (clone; 9EG7).
Anti-β1 integrin antibody (clone; 9EG7) is known to change the integrin conformation from outside of cells and induce the cells’ adhesion.23 Using this antibody, we evaluated effects of H-Ras on cell cytoskeleton or signal transduction pathways from the integrins that need to be activated for cells to adhere to FN. A concentration of 5 μg/mL of this antibody, which was found to be an effective amount, was used for the adhesion assay. The growth factor-starved Baf3 and all transfected Baf3 cells were labeled with51Cr and incubated at 37°C for 30 minutes in the presence of this antibody or isotyped-matched control antibody. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes, and the percentage of adherent cells was measured as noted above.
Statistical analysis.
Statistical analysis was performed using a two-sample t-test.
RESULTS
H-Ras expression.
As shown in Fig 1, overexpression of H-Ras was seen in all types of H-Ras–transfected Baf3 cells compared with nontransfected Baf3 cells.
H-Ras expression in nontransfected Baf3 cells (designated as Baf3/c) and wild-type, constitutively active type, and dominant-negative type H-Ras–transfected Baf3 cells (designated as wild-type, constitutively active, and dominant-negative, respectively). Cells were lysed in lysis buffer, separated by 15% SDS-PAGE, immunoblotted with anti–H-Ras antibody, and then stripped and reblotted with anti-GAPDH antibody (as an internal control). This is a representative result of three independent experiments.
H-Ras expression in nontransfected Baf3 cells (designated as Baf3/c) and wild-type, constitutively active type, and dominant-negative type H-Ras–transfected Baf3 cells (designated as wild-type, constitutively active, and dominant-negative, respectively). Cells were lysed in lysis buffer, separated by 15% SDS-PAGE, immunoblotted with anti–H-Ras antibody, and then stripped and reblotted with anti-GAPDH antibody (as an internal control). This is a representative result of three independent experiments.
Proliferation capacity.
As shown in Fig 2A, the wild-type H-Ras–transfected Baf3 cells proliferated in the same IL-3 dose-dependent manner as nontransfected Baf3 cells. The constitutively active type H-Ras–transfected Baf3 cells proliferated without IL-3 stimulation. IL-3 induced additional proliferation of the constitutively active type H-Ras–transfected Baf3 cells. In high concentrations of IL-3 (>0.1 ng/mL), the proliferation capacity of the constitutively active type H-Ras–transfected Baf3 cells was the same as the other types of Baf3 cells. The proliferation capacity of the dominant-negative type H-Ras–transfected Baf3 cells was the same as the wild-type H-Ras–transfected Baf3 cells and the nontransfected Baf3 cells. These data are consistent with the data of others showing that this same dominant-negative type H-Ras exhibited no inhibitory effect on IL-3–dependent proliferation of Baf3 cells, as assessed by an increase in cell numbers and mitochondrial enzyme activity.24 We also investigated the proliferation effects of IL-4 in these cell lines. As shown in Fig 3A, the nontransfected Baf3 cells and all types of H-Ras–transfected Baf3 cells had a proliferative response to IL-4 stimulation. This result serves as control for studies reported in the next section.
[3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of various concentrations of IL-3. Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion with those of wild-type H-Ras–transfected Baf3 cells.
[3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of various concentrations of IL-3. Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion with those of wild-type H-Ras–transfected Baf3 cells.
[3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of IL-4 or IL-3 (as a control). Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion induced by cytokines with those of no cytokine stimulation.
[3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of IL-4 or IL-3 (as a control). Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion induced by cytokines with those of no cytokine stimulation.
Adhesion capacity.
As shown in Fig 2B, the wild-type H-Ras–transfected Baf3 cells adhered to FN in the same manner as nontransfected Baf3 cells in low concentrations of IL-3 (<0.01 ng/mL), whereas in high concentrations of IL-3 (>0.1 ng/mL), the adhesion of the wild-type H-Ras–transfected Baf3 cells increased significantly compared with nontransfected Baf3 cells. The constitutively active type H-Ras–transfected Baf3 cells adhered to FN without IL-3 stimulation. IL-3 induced additional adhesion to FN of the constitutively active type H-Ras–transfected Baf3 cells, and in high concentrations of IL-3 (>0.01 ng/mL), the adhesion capacity of the constitutively active type H-Ras–transfected Baf3 cells was same as the wild-type H-Ras–transfected Baf3 cells. The adhesion capacity of the dominant-negative type H-Ras–transfected Baf3 cells was significantly less than that of the other types of Baf3 cells. As shown in Fig 3B, neither nontransfected Baf3 cells nor any type of H-Ras–transfected Baf3 cells showed any significant adhesion to FN in response to IL-4 stimulation. However, Baf3 cells, which proliferate in medium containing IL-4 (see Fig 3A), did show adhesion to FN induced by IL-3 in the same manner as the Baf3 cells maintained in medium containing IL-3.
Integrin expression and function.
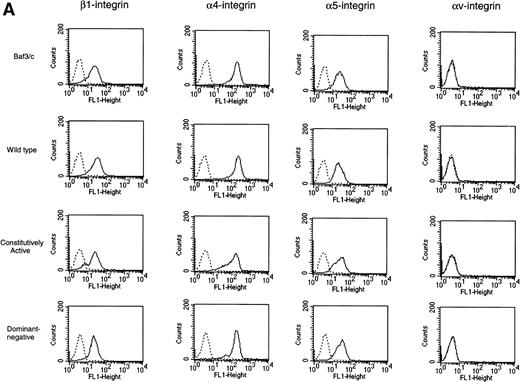
Expression of integrins (β1, α4, α5, and αv) was analyzed by flow cytometry. Baf3 cells expressed integrin β1, α4, and α5, whereas there was no expression of αv integrin on the cells. As shown in Fig 4A, there were no remarkable differences in expression of any of these integrins between nontransfected Baf3 cells and any type of H-Ras–transfected Baf3 cells. We have previously shown using blocking Abs against integrin-β1, α4, α5, and αv that IL-3 activated VLA-4 and VLA-5 on the Baf3 cells and induced the adhesion of these cells to FN.14 To analyze the role of integrins in adhesion of transfected Baf3 cells to FN, inhibition of transfected Baf3 cells adhesion to FN was analyzed using blocking Abs against integrin-β1, α4, α5, and αv. As shown in Fig 4B, anti-β1 integrin antibody blocked the constitutively active type H-Ras–transfected Baf3 cells adhesion to FN and also completely blocked any type of H-Ras–transfected Baf3 cells adhesion to FN induced by IL-3. Whereas anti-α4 integrin antibody or anti-α5 integrin antibody blocked adhesion only partially, simultaneous addition of both anti-α4 integrin and anti-α5 integrin antibodies blocked adhesion completely. Anti-αv integrin antibody showed no inhibitory effect on adhesion. These results suggest that adhesion of any type of H-Ras–transfected Baf3 cells is mediated by activation of VLA-4 (α4β1) and VLA-5 (α5β1) integrins.
Integrin expression and function. In (A), expression of integrins (β1, 4, 5, and v) was analyzed by flow cytometry. Nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) expressed integrin β1, 4, and 5, whereas there was no expression of v integrin on the cells. There were no remarkable differences in any of these integrins between nontransfected Baf3 cells and any type of H-Ras–transfected Baf3 cells. In (B), inhibition of constitutively active type H-Ras–transfected Baf3 cells adhesion to FN without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells, and dominant-negative type H-Ras–transfected Baf3 cells adhesion to FN induced by IL-3 by blocking Abs against integrin-4, 5, and β1 was examined. Data represent the means (±SD) of triplicate samples from a representative experiment of three. None of the Abs had an effect on viability of the cells. *P value (P < .01) comparing adhesion blocked by each anti-integrin antibody with that of a control antibody.
Integrin expression and function. In (A), expression of integrins (β1, 4, 5, and v) was analyzed by flow cytometry. Nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) expressed integrin β1, 4, and 5, whereas there was no expression of v integrin on the cells. There were no remarkable differences in any of these integrins between nontransfected Baf3 cells and any type of H-Ras–transfected Baf3 cells. In (B), inhibition of constitutively active type H-Ras–transfected Baf3 cells adhesion to FN without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells, and dominant-negative type H-Ras–transfected Baf3 cells adhesion to FN induced by IL-3 by blocking Abs against integrin-4, 5, and β1 was examined. Data represent the means (±SD) of triplicate samples from a representative experiment of three. None of the Abs had an effect on viability of the cells. *P value (P < .01) comparing adhesion blocked by each anti-integrin antibody with that of a control antibody.
8-Br-cAMP, Dibutyryl-cAMP (cAMP), and PD98059 inhibit proliferation and phosphorylation of MAPK but not adhesion of H-Ras–transfected Baf3 cells.
As shown in Fig 5A, both cAMP and PD98059 inhibited proliferation of the constitutively active type H-Ras–transfected Baf3 cells. We also examined effects of these compounds on phosphorylation of MAPK by Western blotting with anti–phospho-MAPK antibody, which only recognizes phosphorylated MAPK. As shown in Fig 5B, cAMP and PD98059 suppressed phosphorylation of MAPK of the constitutively active type H-Ras–transfected Baf3 cells and all types of H-Ras–transfected Baf3 cells stimulated by IL-3. These results strongly suggest that cAMP and PD98059 had inhibitory effects on Ras, Raf-1, MAPK kinase, and the MAPK pathway. On the other hand, interestingly, cAMP, but not PD98059, inhibited IL-3–induced proliferation of any type of H-Ras–transfected Baf3 cells. However, cAMP and PD98059 showed no inhibitory effects on adhesion to FN of any type of H-Ras–transfected Baf3 cells (Fig 5C). These results indicate that Raf-1, MAPK kinase, and the MAPK pathway might not be involved in inside-out signaling of IL-3–induced integrins’ activation in Baf3 cells.
Effects of Raf-1, MAPK kinase, and MAPK pathway inhibitor compounds on the proliferation (A), the phosphorylation of MAPK (B), and the adhesion (C) of constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active) without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells (designated as wild-type), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) induced by IL-3. In (A), [3H]-thymidine incorporation of those transfected Baf3 cells in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. In (B), the factor-starved transfected Baf3 cells were incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 and incubated for an additional 15 minutes with or without 0.1 ng/mL IL-3. Cells were lysed in lysis buffer, separated by 11.25% SDS-PAGE, immunoblotted with anti–phospho-MAPK antibody, and reblotted with anti-MAPK antibody to detect the total MAPK levels. Anti-MAPK antibody used here recognized only p42 MAPK of Baf3 cells. This is a representative result of three independent experiments. In (C), transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. *P value (P < .01) comparing each treatment with the control.
Effects of Raf-1, MAPK kinase, and MAPK pathway inhibitor compounds on the proliferation (A), the phosphorylation of MAPK (B), and the adhesion (C) of constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active) without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells (designated as wild-type), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) induced by IL-3. In (A), [3H]-thymidine incorporation of those transfected Baf3 cells in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. In (B), the factor-starved transfected Baf3 cells were incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 and incubated for an additional 15 minutes with or without 0.1 ng/mL IL-3. Cells were lysed in lysis buffer, separated by 11.25% SDS-PAGE, immunoblotted with anti–phospho-MAPK antibody, and reblotted with anti-MAPK antibody to detect the total MAPK levels. Anti-MAPK antibody used here recognized only p42 MAPK of Baf3 cells. This is a representative result of three independent experiments. In (C), transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. *P value (P < .01) comparing each treatment with the control.
U-73122 (a PLC inhibitor) suppresses adhesion of transfected cells to FN.
In our previous study,14 neither PI-3 kinase inhibitor, wortmannin, nor PKC inhibitor, H-7, inhibited IL-3–induced adhesion of Baf3 cells to FN. However, PLC inhibitor, U-73122, did suppress this adhesion. The effects of these same inhibitory compounds were tested on the adhesion of the transfected Baf3 cells. As shown in Fig 6, wortmannin and H-7 did not suppress constitutively active type H-Ras–transfected Baf3 cells’ adhesion or adhesion of any type of H-Ras–transfected Baf3 cells induced by IL-3. The wortmannin used here showed inhibitory activity on another cytokine-induced cell adhesion system. In contrast, U-73122 did suppress this adhesion. U-73433, a closely related analog of U-73122 that does not inhibit PLC, showed no inhibitory effect on adhesion. This suggests that PLC, but not PI3-kinase or PKC, is involved in inside-out signaling of IL-3–induced integrin activation in Baf3 cells. We examined the effects of U-73122 on IL-3–induced cell survival using merocyanine 540 (MC540) staining, which can detect the early apoptotic state of cells,25 and found that there was no difference in the percentage of apoptotic cells with and without U-73122.
Effects of inhibitor compounds on constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active) adhesion to FN without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells (designated as wild-type), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) adhesion to FN induced by IL-3. Those transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 30 minutes in the absence or presence of 100 nmol/L wortmannin, 1 μmol/L U-73122, 1 μmol/L U-73433, or 50 μmol/L H-7. Cells were subsequently transferred to FN-coated wells and incubated for an additional 30 minutes at 37°C with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. None of the inhibitor compounds had an effect on viability of the cells. *P value (P < .01) comparing adhesion blocked by U-73122 with control adhesion.
Effects of inhibitor compounds on constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active) adhesion to FN without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells (designated as wild-type), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) adhesion to FN induced by IL-3. Those transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 30 minutes in the absence or presence of 100 nmol/L wortmannin, 1 μmol/L U-73122, 1 μmol/L U-73433, or 50 μmol/L H-7. Cells were subsequently transferred to FN-coated wells and incubated for an additional 30 minutes at 37°C with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. None of the inhibitor compounds had an effect on viability of the cells. *P value (P < .01) comparing adhesion blocked by U-73122 with control adhesion.
Anti-β1 integrin antibody (clone; 9EG7) induces adhesion of any type of Baf3 cells to FN.
As shown in Fig 7, the anti-β1 integrin antibody induced the adhesion to FN of the dominant-negative type H-Ras–transfected Baf3 cells as efficiently as nontransfected Baf3 cells as well as the other types of H-Ras–transfected Baf3 cells. These data suggest that H-Ras may not affect cell cytoskeleton or the signal transduction pathways from the integrins that need to be activated for these cells to adhere to FN. However, this does support a role of H-Ras in inside-out signals.
Induction of the adhesion to FN of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) by anti-β1 integrin antibody (clone; 9EG7). These Baf3 cells were labeled with 51Cr and incubated at 37°C for 30 minutes in the presence of 5 μg/mL of anti-β1 integrin antibody (clone; 9EG7) or isotype-matched control antibody. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. *P value (P < .01) comparing adhesion induced by anti-β1 integrin antibody with the control antibody.
Induction of the adhesion to FN of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) by anti-β1 integrin antibody (clone; 9EG7). These Baf3 cells were labeled with 51Cr and incubated at 37°C for 30 minutes in the presence of 5 μg/mL of anti-β1 integrin antibody (clone; 9EG7) or isotype-matched control antibody. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. *P value (P < .01) comparing adhesion induced by anti-β1 integrin antibody with the control antibody.
DISCUSSION
In the present study, we investigated the function of H-Ras in the cytokine-induced integrin activation. IL-3, but not IL-4, induced the adhesion of Baf3 cells to FN. H-Ras is known to be activated by IL-3, but not IL-4.26 27 We also examined H-Ras activity with IL-3 stimulation using a Ras-GTP binding assay and found that H-Ras was activated by IL-3 in Baf3 cells (data not shown). Furthermore, the dominant-negative type H-Ras–transfected Baf3 cells showed significantly less adhesion induced by IL-3 compared with the wild-type and the constitutively active type H-Ras–transfected Baf3 cells. On the other hand, anti-β1 integrin antibody (clone; 9EG7), which is known to change integrin conformation from outside of the cells and activate, induced the adhesion of the dominant-negative type H-Ras–transfected Baf3 cells as much as the other types of H-Ras–transfected Baf3 cells. From these data, it is demonstrated that H-Ras is involved in the inside-out signaling pathway of IL-3–induced integrin activation in Baf3 cells.
To examine downstream events of H-Ras in the inside-out signaling pathway of IL-3–induced integrin activation, we focused on the roles of Ras, Raf-1, MAPK kinase, and the MAPK pathway. We used 8-Br-cAMP and Dibutyryl-cAMP as inhibitors of the Ras-Raf-1 pathway and PD98059 as an MAPK kinase inhibitor and tested the effects of these inhibitors on the adhesion of the transfected Baf3 cell lines. These inhibitors showed no suppressive effects on adhesion, which suggests that Ras, Raf-1, MAPK kinase, and the MAPK pathway might not be involved in the downstream events of H-Ras in the inside-out signaling pathway of IL-3–induced integrin activation in Baf3 cells. Our data suggest that H-Ras, but not Ras, Raf-1, MAPK kinase, and the MAPK pathway, is involved in the inside-out signaling pathway of IL-3–induced integrin activation in Baf3 cells.
On the other hand, the constitutively active type H-Ras–transfected Baf3 cell line was able to adhere to FN without IL-3 stimulation via activation of VLA-4 and VLA-5. This suggests that H-Ras can activate integrins of Baf3 cells. Recently, it has been reported that H-Ras suppresses integrin activation,13 data opposite from our results. One possibility for this conflict in results in the function of H-Ras might reflect differences in the cells tested. The other investigators13 used CHO cells transfected with integrin cDNAs, which expressed active forms of integrins. Our study used the hematopoietic cell line Baf3, which expresses inactive forms of integrins without IL-3 stimulation. Another possibility is the difference of targeted integrins of H-Ras. The other investigators examined the effect of H-Ras on αIIbβ3integrins, whereas we focused on the effect of H-Ras on α4β1 (VLA-4) and α5β1 (VLA-5) integrins. Still other investigators reported that R-Ras activates integrins.12 In that report, they showed that constitutively active type H-Ras, as a control study, could not activate integrins. The difference between their data12 and ours might also depend on difference in the types of cells tested. Another difference might be the mutation of H-Ras used. Both groups used constitutively active type H-Ras, which is a mutation in which a substitution of valine for glycine is made at position 12. We used the constitutively active type H-Ras, in which the mutation is a substitution of leucine for glutamine at position 61. H-Ras and R-Ras are highly homologous to one another,28 and they are known to have some of the same functions, such as antiapoptotic effects induced by cytokine deprivation.29Our data demonstrated that H-Ras has another function similar to that of R-Ras, which is the activation of integrins.
PI-3 kinase is thought to be involved in the inside-out signaling pathway.30,31 This was demonstrated by the inhibition of integrin activation by a PI-3 kinase inhibitor, wortmannin.32 In other integrin activation models, wortmannin was shown to inhibit adhesion. In our previous study, wortmannin showed no inhibitory effect on Baf3 cell adhesion to FN induced by IL-3.14 From these data, PI-3 kinase might not be involved in this inside-out signaling model of IL-3–induced integrin activation in Baf3 cells. However, because there is a report that PI-3 kinase is downstream of Ras,33 we examined the effect of wortmannin on the adhesion of the constitutively active type H-Ras–transfected Baf3 cells and found that wortmannin showed no inhibitory effect. Wortmannin also showed no inhibitory effects on the adhesion of the wild-type and the dominant-negative type H-Ras–transfected Baf3 cell lines induced by IL-3. Based on these data, we believe that PI-3 kinase is not involved in the inside-out signaling of IL-3–induced integrin activation in the Baf3 cell line we are using. Because our findings about PI-3 kinase, both previous and present, are very clear, it is difficult to reconcile this contradiction to some previous reports at this time. Further study of this discrepancy within the context of other model systems could be revealing.
On the other hand, PLC is also thought to be involved in the inside-out signaling pathway.11 34 In our previous study, a PLC inhibitor, U-73122, completely inhibited Baf3 cell adhesion to FN induced by IL-3. Thus, we decided to examine the effect of U-73122 on the adhesion of transfected Baf3 cells. We found that U-73122 suppressed the constitutively active type H-Ras–transfected Baf3 cell adhesion and adhesion of wild-type and dominant-negative type H-Ras–transfected Baf3 cells induced by IL-3. The result that U-73122 inhibited the adhesion of any type of H-Ras–transfected Baf3 cells suggests that PLC might be downstream of H-Ras in the inside-out signaling pathway of IL-3–induced integrin activation in Baf3 cells.
Ras has been reported to be involved in outside-in signaling pathways initiated by cell-matrix interactions.4 35 Taking this and our own data into consideration, Ras appears to be involved in both inside-out signaling and outside-in signaling pathways of cell-matrix interactions and might play a central role in cellular functions regulated by cell-matrix interactions.
Supported by US Public Health Service Grants No. R01 DK53674, R01 HL56416, and R01 HL54037; by a project in P01 HL53586 from the National Institutes of Health to H.E.B.; and by the scholarship for young investigator travel grant for 1997 Osaka University Medical School Fund for International Exchange.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hal E. Broxmeyer, PhD, Walther Oncology Center, Indiana University School of Medicine, 1044 W Walnut St, Room 302, Indianapolis, IN 46202; e-mail: hbroxmey@iupui.edu.


![Fig. 2. [3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of various concentrations of IL-3. Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion with those of wild-type H-Ras–transfected Baf3 cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1540/4/m_blod40510002ax.jpeg?Expires=1766179520&Signature=h5RY4O-Dk4cPZBWHuEx6sUOfxJvHQRv-bNUocyH0uSO5R0tFdBuP~NSYyif1KFESlRQLP0Py6dUgv~4Zomg5F9xDS1B5h7Vd~X8-INC8HcD-NQ9H1FUTBVzK1ellkcstQBZCx1G9wv~FFiSPYlHDU3LjRUgz0Os-w-ALhZ1RggWfWFcEdwwi8f67f0MAoPXgGX90-DmM7tNXBoUajoa4oJcYehm46~cQzuAT0bKEyuqwtdfpbujdA1zWH81Q-REhjqaUSPk-cy5wmIWu9JcLZJMIAxd0vGzPjcAhi8AHQzUS5ujWeraonogfOn1YR9NEZsSFZduF43M8DZ3pACEZGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. [3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of various concentrations of IL-3. Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion with those of wild-type H-Ras–transfected Baf3 cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1540/4/m_blod40510002bx.jpeg?Expires=1766179520&Signature=vWzh6CYlpJGFdNCbFTWeIfovYTxyBwfbl~0tF5gW6Z1W4TzL0B1pCG5fgUTgaAGF6yFnrzGZ-wUHgSMVXdQuAz6JbClX87CmlkOlBN-0Q7imdbVqGgzz90VJykyvCz8phuyWKbhY3kjpI7rfTv9Sef9nUOkWWBmzwFmhr9riNRyleMAeKSXy3-avRqonL0wchv2I8Dhr3jmbD7fJPol7BZm4xwQgf1UyjR0tcQrrThvtEqz7czHonBnyXSM5KHXUFUgbslNaEqS-nP0dz0j9E2uBPz1hA2cpGMr8iLfR62ecT3soSXo3v5ATd-OlLvKvRNNlnQnaj11X6xK2LOkHBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. [3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of IL-4 or IL-3 (as a control). Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion induced by cytokines with those of no cytokine stimulation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1540/4/m_blod40510003ax.jpeg?Expires=1766179520&Signature=c0-iCY~rxi3MFdtfW2N4RfK72WELF~U2HusTPQOSGvLXkOr~~RNhbkuIyh3m~wo2RK8clAiler6DXpx~ocbLVv3E3oE7z1tcNNLpDwX6SdhnQA~sQLVqYp7Cn6E09A7DJdH8sFF3ujr-m~f09K68HGY0URdHCLkmc4r~5sr8tcnHEIaZXS~m9nDKmYJ00Mfg7gXzS-z7~Qo3CEACGe1wqcjvYRNnQUObQJZTfK5ilBg1n8hSm7W~YNNhW89-xIAc1XrNV9SnpN0wSqkadZHJCVLOyB4kBp61Qx3C~rURimAAr7OGAjRpKVw8MSlPMj48iqSRQnvHB1Me6DJgMzwq7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. [3H]-thymidine incorporation (A) and adhesion to FN (B) of nontransfected Baf3 cells (designated as Baf3/c), wild-type H-Ras–transfected Baf3 cells (designated as wild-type), constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) in the presence or absence of IL-4 or IL-3 (as a control). Data represent the mean (±SD) of triplicate samples from one of three representative experiments. *P value (P < .01) comparing proliferation or adhesion induced by cytokines with those of no cytokine stimulation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1540/4/m_blod40510003bx.jpeg?Expires=1766179520&Signature=RSFXPbXdYSaxTd6m5z2JrWGfjGNq2jCqn~46UTNYzlOvmcOwoqpC6sbptfGGL1gX246skaTwMib~~DYeCfH-kHUZcytbwpxTbVfHkthhYXI70l-2CGzgWp2bcjBS9HbLRiWbsvYDeeqwK4WVoBQWXS1mYCTnP~ciAvwkEJJTjbczYua6qDzUy5vU2pWvAbwHVz7~aC~xjqMlcLW9MX0t12IOrJiHS4lVoPDcR6~2i7TZzIXOPEhcbemnCpvLGs~LXWpDxJMbGxZwuA8ex1YZ1W1kzpF6nsK0nBd1K6OXjEqp7l5Cd1OgLQYM3DBiG-j6NwuAcui2Cm5KK55KEtkD-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Effects of Raf-1, MAPK kinase, and MAPK pathway inhibitor compounds on the proliferation (A), the phosphorylation of MAPK (B), and the adhesion (C) of constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active) without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells (designated as wild-type), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) induced by IL-3. In (A), [3H]-thymidine incorporation of those transfected Baf3 cells in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. In (B), the factor-starved transfected Baf3 cells were incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 and incubated for an additional 15 minutes with or without 0.1 ng/mL IL-3. Cells were lysed in lysis buffer, separated by 11.25% SDS-PAGE, immunoblotted with anti–phospho-MAPK antibody, and reblotted with anti-MAPK antibody to detect the total MAPK levels. Anti-MAPK antibody used here recognized only p42 MAPK of Baf3 cells. This is a representative result of three independent experiments. In (C), transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. *P value (P < .01) comparing each treatment with the control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1540/4/m_blod40510005ax.jpeg?Expires=1766179520&Signature=unQ9ccF~QJj-6WEJKS4MJykNBI3rXpP61TozqlW-ug9PawDN6-L3TyuhjEccoeuAZh7e9t03byZMF4wf~YkgTYEk5qdoMwyzQnEHhqZv5Q~QKUC009Re6FRSEzf1KN8rdtqCEHpHCh83HS-A0NJzN9cjTL4eWXicqPHrHyNKvELuM~D9K-yLY-N4dcH8mtPIy5h1U31K2s3LHm-cF-jMMW1warbmoHfsS~ah56UMk4JsfZVCds5de1kvUHjMyr1WvyA47O6PMaeR~ElhRUASX8EdBHhT7NOwd2QokWCffDoYkb7JrJSpq9toQVLMreBzz5YXVvvC8N8O8WuSI6a-nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of Raf-1, MAPK kinase, and MAPK pathway inhibitor compounds on the proliferation (A), the phosphorylation of MAPK (B), and the adhesion (C) of constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active) without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells (designated as wild-type), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) induced by IL-3. In (A), [3H]-thymidine incorporation of those transfected Baf3 cells in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. In (B), the factor-starved transfected Baf3 cells were incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 and incubated for an additional 15 minutes with or without 0.1 ng/mL IL-3. Cells were lysed in lysis buffer, separated by 11.25% SDS-PAGE, immunoblotted with anti–phospho-MAPK antibody, and reblotted with anti-MAPK antibody to detect the total MAPK levels. Anti-MAPK antibody used here recognized only p42 MAPK of Baf3 cells. This is a representative result of three independent experiments. In (C), transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. *P value (P < .01) comparing each treatment with the control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1540/4/m_blod40510005cx.jpeg?Expires=1766179520&Signature=PxZnVLnVQ99jbgCep6jyb1I5-nW4nzWrUr7ZHh5jlqsOjvKvDaZDdy28Utn6iGZYkuSXMPmItUtNbDZELFYZh5GPRJG2VbPQDZnwDtfj3C7lrcvlfPPNPdwF8Ew4k1TdqbSF4Mn~e6XS0vjWADC4fg3HZ81sGQ1rXkCy2S21FXYhdP4NQl2nxUSO0O9lImsRuC4ZKZmcbmf2RckpD7AP6Js~DwA6dvXu5s6yaayAFTGWZyG1QfM4893pIbuUKDbr~NsJ8~GPDJj~W9TElB3Br1nFImgm4XbWj8g~tChQ3rVVLyuyZWm0x1osea3kI2vRjzLqni9u3HZISP~66QqeCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of Raf-1, MAPK kinase, and MAPK pathway inhibitor compounds on the proliferation (A), the phosphorylation of MAPK (B), and the adhesion (C) of constitutively active type H-Ras–transfected Baf3 cells (designated as constitutively active) without IL-3 stimulation and constitutively active type H-Ras–transfected Baf3 cells, wild-type H-Ras–transfected Baf3 cells (designated as wild-type), and dominant-negative type H-Ras–transfected Baf3 cells (designated as dominant-negative) induced by IL-3. In (A), [3H]-thymidine incorporation of those transfected Baf3 cells in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. In (B), the factor-starved transfected Baf3 cells were incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059 and incubated for an additional 15 minutes with or without 0.1 ng/mL IL-3. Cells were lysed in lysis buffer, separated by 11.25% SDS-PAGE, immunoblotted with anti–phospho-MAPK antibody, and reblotted with anti-MAPK antibody to detect the total MAPK levels. Anti-MAPK antibody used here recognized only p42 MAPK of Baf3 cells. This is a representative result of three independent experiments. In (C), transfected Baf3 cells were labeled with 51Cr and incubated at 37°C for 3 hours in the absence or presence of either 1 mmol/L 8-Br-cAMP, 1 mmol/L Dibutyryl-cAMP, or 50 μmol/L PD98059. Cells were subsequently transferred to FN-coated wells and incubated at 37°C for an additional 30 minutes with or without 0.1 ng/mL IL-3, and the percentage of adherent cells was measured. Data represent the means (±SD) of triplicate samples from a representative experiment of three. *P value (P < .01) comparing each treatment with the control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1540/4/m_blod40510005bw.jpeg?Expires=1766179520&Signature=uZ299g4-s3ece74l7xFv0eXZ2QudzWORQdHZmzOGMAQJljLL4iY~62fJb1HZf2i7S6UDyeAJ2V1p5ZgJQ4r51T1T8aC03XXZ2l7IcqoGpN1MpTPMYIQFPSPkoY2HO2N5ar350kNXcRIFxrLtwRrGj7AzOm4l9YE3vqzqccaPuTVIt0mVeqeoRxS4WnlFyhGEdamvk6r3Susg9glBDY1t8YwewSnQcY~duqJHABk2pu6h6AZgGDjlgPDyINdKIW0jPsReShWF4V1P57eiz0RX~q3yT-nx1-J5-98mo-E9DfaJE9UewFK1fzlq1MDur5fbvUpbTM9m4UskBecpDog7qQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal