Abstract
Intensive chemotherapy given in early chronic phase of chronic myelogenous leukemia (CML) has resulted in high numbers of circulating Philadelphia (Ph) chromosome–negative hematopoietic progenitor cells (HPC). We have autografted 30 consecutive patients with CML in chronic phase with HPC collected in this way to facilitate restoration of Ph-negative hematopoiesis in bone marrow after high-dose therapy. Hematopoietic recovery to greater than 0.5 ×109/L neutrophils and to greater than 25 × 109/L platelets occurred in all patients, a median of 13 (range, 9 to 32) days and 16 (range, 6 to 106) days postautograft, respectively. Regenerating marrow cells were Ph-negative in 16 (53%) patients and greater than 66% Ph-negative in 10 (33%) patients. Twenty-eight patients are alive 6 to 76 months (median, 24 months) after autografting. Three patients have developed blast crisis from which 2 have died. Eight patients are in complete cytogenetic remission at a median of 20 (range, 6 to 44) months with a median ratio BCR-ABL/ABL of 0.002 (range, <0.001 to 0.01). Eight patients are in major cytogenetic remission at a median of 22 (range, 6 to 48) months. No patient died as a consequence of the treatment. All patients had some degree of stomatitis that was severe in 15 (50%) patients. Gastrointestinal and hepatic toxicities were observed in about one fourth of patients. Thus, autografting with Ph-negative mobilized HPC can result in prolonged restoration of Ph-negative hematopoiesis for some patients with CML; moreover, most autograft recipients report normal or near normal activity levels, suggesting that this procedure need not to be associated either with prolonged convalescence or with chronic debility.
CHRONIC MYELOGENOUS leukemia (CML) is a malignant disease of hematopoietic stem cells. Conventional treatment with chemotherapy controls signs of disease but neither postpones onset of acute phase nor prolongs survival. Except for MD Anderson,1 interferon-α can offer significant cytogenetic remission in 9% to 19% rates.2-4 Transplantation with stem cells obtained from either related or unrelated donors can result in prolonged disease-free survival, and it is curative in many patients.5-7 Unfortunately, donor stem-cell transplants may be offered to only a minority of CML patients. In these last years, several investigators have observed that diploid hematopoietic progenitor cells (HPC) coexist with their malignant counterparts in the marrow of some patients with CML,8-13 observations that led to clinical trials testing autografting after high-dose chemoradiotherapy regimens.14-20
Beginning in July 1989, our group initiated a pilot study with intensive chemotherapy to induce aplasia followed by leukaphereses of peripheral blood HPC, in an attempt to verify whether residual diploid cells have, in the repopulating phase, a proliferative advantage over Philadelphia (Ph) chromosome–positive cells and if they could be collected in quantities suitable for autografting applications. These studies showed that it was possible to collect diploid cells also in patients with advanced phases of disease cytogenetically refractory to interferon therapy.21 In a subsequent study, we confirmed that patients treated early in chronic phase with chemotherapy had a higher number of diploid cells than patients in the more advanced phase of disease.22
Here we present the results from 30 consecutive patients autografted with Ph-negative or prevalently Ph-negative (≤34% Ph-positive) HPC during their chronic phase of CML.
PATIENTS AND METHODS
Patients with Ph-positive CML who were less than 65 years old, ineligible for allogeneic stem-cell transplantation, and in morphologic chronic phase entered this study. All patients had previously received only hydroxyurea to reduce white blood cells less than or equal to 20 × 109/L. The first cohort of patients (n = 11) were mobilized with the ICE protocol consisting of idarubicin 8 mg/m2/d intravenously for 5 days; cytarabine (ARA-C) 800 mg/m2 by 2-hour infusion for 5 days; and etoposide 150 mg/m2/d by 2-hour infusion for 3 days. Subsequently, 19 patients were given a mini-ICE protocol (the same three drugs at the same doses but idarubicin and ARA-C were given for 3 days only). Beginning 8 days after the end of the chemotherapy, recombinant human granulocyte colony-stimulating factor (rhG-CSF) was administered daily at a dosage of 5 μg/kg until the total neutrophil count was more than 1.0 × 109/L for 3 consecutive days. Leukaphereses were performed using CS 3000 PLUS machine (Baxter, Deerfield, IL) or Dideco Excel (Milan, Italy), generally starting on the first day that the white blood cell count exceeded 0.8 × 109/L and the concentration of CD34+ cells was at least 5/μL on peripheral blood. The next leukapheresis was usually performed when the white blood cell count had surpassed 109/L and the circulating CD34+ cells were more than 10/μL. The collections were continued until the total number of CD34+ cells collected was at least 2 × 106/kg. Each leukapheresis product was analyzed cytogenetically and for CD34+ and colony-forming unit–granulocyte-macrophage (CFU-GM) numbers. The total leukapheresis product for each patient was frozen using a Planner R-203 (SACI, Milan, Italy). Between June 1992 and April 1998, 40 candidates had their peripheral blood HPC treated with mobilization procedure, but 10 of them could not be autografted because of high level of Ph-positive cells in the leukapheretic product (6 patients), very low level of CD34+ cells (<1 × 106/kg; 3 patients), and matched unrelated donor (MUD) transplant in the last 1 patient.
Patients.
The 30 patients were aged 22 to 62 years (median 46 years) at the time of autografting (Table 1). All patients were in first chronic phase. The treatment was approved by the local ethical committee, and patients gave informed consent before entry into the study.
Details of Patients at Autografting, Outcome, and Survival
| Patients . | Age/ Sex . | Sokal Index . | Interval Diagnosis to Autografting (mo) . | Mobilizing Therapy . | Cytogenetics on HPC Preautografting (Ph-positive/metas) . | HighDose Therapy . | Marrow Cytogenetics Ph-Positive (%) . | Outcome . | BCR-ABL/ABL Ratio . | Follow-up From ASCT (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|
| TP | 59/F | 1.05 | 6 | ICE | 0/126 | IVT | 0 | A/Ph+ 100% | 76 | |
| BG | 46/M | 1.45 | 17 | ICE | 0/119 | IVT | 0 | Died of BC disease | 29 | |
| BF1 | 44/F | 0.75 | 30 | ICE | 0/119 | BU | 0 | A/Ph+ 46% | 42 | |
| BC | 53/F | 0.71 | 10 | ICE | 0/87 | IVT | 4 | A/Ph+ 10% | 48 | |
| BP | 37/M | 0.77 | 7 | ICE | 0/75 | IVT | 0 | A/Ph+ 100% | 48 | |
| MC1 | 48/F | 0.85 | 6 | ICE | 0/30 | IVT | 15 | A/Ph+ 26% | 48 | |
| SE1 | 40/M | 0.79 | 5 | ICE | 0/178 | BU | 0 | Alive, in CCR | <0.001 | 44 |
| GR | 47/M | 0.91 | 9 | ICE | 0/114 | IVT | 30 | Died of BC disease | 18 | |
| VG1 | 39/M | 0.87 | 4 | ICE | 0/137 | BU | 0 | Alive, in CCR | 0.003 | 41 |
| GO | 46/M | 0.66 | 9 | ICE | 0/128 | BU | 5 | A/Ph+ 50% | 39 | |
| SS | 60/M | 0.84 | 6 | mini-ICE | 17/285 | BU | 0 | A/Ph+ 65% | 31 | |
| UK | 49/F | 0.65 | 5 | ICE | 14/203 | BU | 0 | A/Ph+ 85% | 36 | |
| PN | 30/M | 1.43 | 14 | mini-ICE | 0/178 | BU | 0 | A/Ph+ 100%/BC | 25 | |
| PG | 56/M | 3.03 | 4 | mini-ICE | 0/59 | BU | 0 | A/Ph+ 100% | 26 | |
| BS | 22/F | 0.51 | 8 | mini-ICE | 12/124 | BU | 100 | A/Ph+ 100% | 23 | |
| NF | 57/M | 1 | 3 | mini-ICE | 13/106 | BU | 25 | A/Ph+ 29% | 26 | |
| DG | 41/M | 0.68 | 5 | mini-ICE | 0/42 | BU | 0 | Alive, in CCR | 0.002 | 24 |
| CE | 62/M | 0.92 | 6 | mini-ICE | 0/40 | BU | 20 | A/Ph+ 20% | 23 | |
| GG | 27/M | 1.03 | 5 | mini-ICE | 0/100 | BU | 0 | Alive, in CCR | 0.002 | 21 |
| PL | 37/F | 1.35 | 6 | mini-ICE | 0/88 | BU | 5 | A/Ph+ 20% | 19 | |
| MA | 42/M | 0.59 | 4 | mini-ICE | 7/51 | BU | 30 | A/Ph+ 12% | 21 | |
| BM | 34/M | 1.69 | 4 | mini-ICE | 65/176 | BU | 95 | A/Ph+ 58% | 18 | |
| PM | 46/M | 0.614 | 3 | mini-ICE | 0/248 | BU | 0 | Alive, in CCR | 0.001 | 19 |
| CR | 48/M | 0.63 | 7 | mini-ICE | 0/69 | BU | 0 | Alive, in CCR | 0.01 | 15 |
| SP | 32/M | 1.0 | 11 | mini-ICE | 0/35 | BU | 0 | Alive, in CCR | 0.01 | 7 |
| MM | 52/M | 0.71 | 6 | mini-ICE | 0/40 | BU | 6 | A/Ph+ 6% | 13 | |
| MG | 45/M | 1.06 | 4 | mini-ICE | 1/30 | BU | 90 | A/Ph+ 90% | 13 | |
| FV | 34/M | 0.6 | 7 | mini-ICE | 0/30 | BU | 0 | Alive, in CCR | 0.01 | 6 |
| GA | 49/M | 0.72 | 14 | mini-ICE | 0/130 | BU | 40 | A/Ph+ 46% | 6 | |
| MA | 47/M | 0.61 | 3 | mini-ICE | 2/2 | BU | 10 | A/Ph+ 10% | 6 |
| Patients . | Age/ Sex . | Sokal Index . | Interval Diagnosis to Autografting (mo) . | Mobilizing Therapy . | Cytogenetics on HPC Preautografting (Ph-positive/metas) . | HighDose Therapy . | Marrow Cytogenetics Ph-Positive (%) . | Outcome . | BCR-ABL/ABL Ratio . | Follow-up From ASCT (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|
| TP | 59/F | 1.05 | 6 | ICE | 0/126 | IVT | 0 | A/Ph+ 100% | 76 | |
| BG | 46/M | 1.45 | 17 | ICE | 0/119 | IVT | 0 | Died of BC disease | 29 | |
| BF1 | 44/F | 0.75 | 30 | ICE | 0/119 | BU | 0 | A/Ph+ 46% | 42 | |
| BC | 53/F | 0.71 | 10 | ICE | 0/87 | IVT | 4 | A/Ph+ 10% | 48 | |
| BP | 37/M | 0.77 | 7 | ICE | 0/75 | IVT | 0 | A/Ph+ 100% | 48 | |
| MC1 | 48/F | 0.85 | 6 | ICE | 0/30 | IVT | 15 | A/Ph+ 26% | 48 | |
| SE1 | 40/M | 0.79 | 5 | ICE | 0/178 | BU | 0 | Alive, in CCR | <0.001 | 44 |
| GR | 47/M | 0.91 | 9 | ICE | 0/114 | IVT | 30 | Died of BC disease | 18 | |
| VG1 | 39/M | 0.87 | 4 | ICE | 0/137 | BU | 0 | Alive, in CCR | 0.003 | 41 |
| GO | 46/M | 0.66 | 9 | ICE | 0/128 | BU | 5 | A/Ph+ 50% | 39 | |
| SS | 60/M | 0.84 | 6 | mini-ICE | 17/285 | BU | 0 | A/Ph+ 65% | 31 | |
| UK | 49/F | 0.65 | 5 | ICE | 14/203 | BU | 0 | A/Ph+ 85% | 36 | |
| PN | 30/M | 1.43 | 14 | mini-ICE | 0/178 | BU | 0 | A/Ph+ 100%/BC | 25 | |
| PG | 56/M | 3.03 | 4 | mini-ICE | 0/59 | BU | 0 | A/Ph+ 100% | 26 | |
| BS | 22/F | 0.51 | 8 | mini-ICE | 12/124 | BU | 100 | A/Ph+ 100% | 23 | |
| NF | 57/M | 1 | 3 | mini-ICE | 13/106 | BU | 25 | A/Ph+ 29% | 26 | |
| DG | 41/M | 0.68 | 5 | mini-ICE | 0/42 | BU | 0 | Alive, in CCR | 0.002 | 24 |
| CE | 62/M | 0.92 | 6 | mini-ICE | 0/40 | BU | 20 | A/Ph+ 20% | 23 | |
| GG | 27/M | 1.03 | 5 | mini-ICE | 0/100 | BU | 0 | Alive, in CCR | 0.002 | 21 |
| PL | 37/F | 1.35 | 6 | mini-ICE | 0/88 | BU | 5 | A/Ph+ 20% | 19 | |
| MA | 42/M | 0.59 | 4 | mini-ICE | 7/51 | BU | 30 | A/Ph+ 12% | 21 | |
| BM | 34/M | 1.69 | 4 | mini-ICE | 65/176 | BU | 95 | A/Ph+ 58% | 18 | |
| PM | 46/M | 0.614 | 3 | mini-ICE | 0/248 | BU | 0 | Alive, in CCR | 0.001 | 19 |
| CR | 48/M | 0.63 | 7 | mini-ICE | 0/69 | BU | 0 | Alive, in CCR | 0.01 | 15 |
| SP | 32/M | 1.0 | 11 | mini-ICE | 0/35 | BU | 0 | Alive, in CCR | 0.01 | 7 |
| MM | 52/M | 0.71 | 6 | mini-ICE | 0/40 | BU | 6 | A/Ph+ 6% | 13 | |
| MG | 45/M | 1.06 | 4 | mini-ICE | 1/30 | BU | 90 | A/Ph+ 90% | 13 | |
| FV | 34/M | 0.6 | 7 | mini-ICE | 0/30 | BU | 0 | Alive, in CCR | 0.01 | 6 |
| GA | 49/M | 0.72 | 14 | mini-ICE | 0/130 | BU | 40 | A/Ph+ 46% | 6 | |
| MA | 47/M | 0.61 | 3 | mini-ICE | 2/2 | BU | 10 | A/Ph+ 10% | 6 |
Abbreviations: IVT, idarubicin, etoposide, TBI; BU, busulfan; ASCT, autografting; CCR, complete cytogenetic remission; BC, blastic crisis.
High-dose therapy.
Patients were treated with one of the two intensive therapy regimens. The median time from diagnosis and autograft was 6 months (range, 3 to 30 months). Four patients received autograft over 11 months after diagnosis. Two of them decided to wait for research of MUD, and in the meantime they were treated with hydroxyurea. MUD research was negative; therefore, they were autografted at 14 months from diagnosis. The other 2 patients did not agree to receive autograft so early, and they were given only hydroxyurea. After 17 and 30 months they underwent autografting.
Six patients received idarubicin (50 mg intravenously in single dose over 3 hours) on day −11; etoposide (800 mg/m2 as a 2-hour infusion) on days −8/−7; and total body irradiation (850 cGy in single dose) on day −2. Peripheral HPC were reinfused on day 0. The subsequent 24 patients received busulfan alone at 4 mg/kg/d orally for 4 consecutive days (total 16 mg/kg). A loading dose of 600 mg oral phenytoin was administered 1 day before the start of chemotherapy, and phenytoin was continued at a dose of 100 mg/d for 5 days to prevent epileptic seizures. All patients received hydration with urine alkalinization, allopurinol, and ranitidine. Peripheral HPC were reinfused 5 days after the end of chemotherapy.
Supportive care.
Patients were managed in conventional rooms (without high-efficiency particulate air filtration) and given intravenous antibiotics and amphotericin B, irradiated blood products, and intravenous nutrition as indicated.
Assessment for autografting.
Marrow samples were taken at regular intervals after autografting for morphologic and cytogenetic analyses. BCR-ABL/ABL ratio was performed after autografting and during the follow-up only on patients resulting Ph-negative.
Definition of response.
Hematologic remission was defined as the absence of morphologic evidence of CML in the blood and marrow. Cytogenetic remission was defined as the presence of 100% (complete) or 66% to 99% (major) Ph-negative metaphases in Giemsa-banded preparations from 24- to 48-hour marrow cultures. BCR-ABL/ABL ratio was evaluated for assessing the BCR-ABL transcript and BCR-ABL/ABL ratio on marrow cells during the follow-up as described.23
Treatment after autografting.
Therapy with interferon-α 2a or 2b was begun earlier after engraftment and given at 3 M/U daily for 5 days per week for 8 weeks together with interleukin-2 (IL-2) at 2 × 106 daily for 5 days every 9 weeks. This course was repeated for 3 times, and after that the patient received interferon therapy alone.
Statistical methods.
Actuarial curves were estimated according to the Kaplan and Meier method.24 Surviving patients were censored on the last day of follow-up.
RESULTS
Composition of the autograft.
The number of CD34+ cells infused ranged from 1.06 to 74.5 × 106/kg (median, 4.04) and the number of CFU-GM from 0.11 to 108 × 104/kg (median, 19.2). Twenty-two patients were reinfused with Ph-negative cells only and 14 (64%) achieved marrow cytogenetic remission postgraft. Six patients were infused with less than 34% Ph-positive cells and 2 (33%) of them achieved marrow cytogenetic remission postautograft. The last 2 patients were reinfused with greater than 34% Ph-positive cells, and they achieved 10% and 95% Ph-positive cells postautograft. No correlation is possible considering the very few patients reinfused with Ph-positive greater than 34%.
Hematopoietic recovery.
Recovery of neutrophils to greater than 0.5 × 109/L occurred at a median of 13 (range, 9 to 32) days and recovery of platelets to greater than or equal to 25 × 109/L at a median of 16 (range, 6 to 106) days. During the first 2 months following hematopoietic recovery after autografting, cytogenetic analysis showed complete remission in marrow cells in 16 (53%) of the 30 patients and major remission in 10 (33%) patients (Table 1). Four patients showed Ph-positive cells greater than 34% of marrow cells. In 8 patients, platelets recovery greater than 50 × 109/L did not occur during the first 8 weeks after autografting. In 1 patient this recovery occurred after 15 weeks. After this time, in all cases engraftment was achieved and there were no cases of late cytopenia.
Toxicity.
All patients had some degree of stomatitis, which was severe in 15 patients (Table 2). Gastrointestinal and hepatic toxicities were observed in about one fourth of patients. No other major toxicities were encountered.25 Specifically, no patient had busulfan-related pulmonary toxicity, hepatic veno-occlusive disease, or epileptic seizures.
Toxicity After High-Dose Therapy in 30 Patients Autografted for CML
| . | Cardiac . | Bladder . | Renal . | Respiratory . | Hepatic . | CNS . | Stomatitis . | Gastrointestinal . |
|---|---|---|---|---|---|---|---|---|
| Grade III or less | 0 | 0 | 0 | 0 | 0 | 0 | 15 (50%) | 3 (10%) |
| Grade II or less | 0 | 0 | 1 (3%) | 0 | 4 (13%) | 0 | 6 (20%) | 3 (10%) |
| Grade I only | 0 | 0 | 1 (3%) | 0 | 3 (10%) | 0 | 3 (10%) | 1 (3%) |
| Total with any degree of toxicity | 0 | 0 | 2 (6%) | 0 | 7 (23%) | 0 | 24 (80%) | 7 (23%) |
| . | Cardiac . | Bladder . | Renal . | Respiratory . | Hepatic . | CNS . | Stomatitis . | Gastrointestinal . |
|---|---|---|---|---|---|---|---|---|
| Grade III or less | 0 | 0 | 0 | 0 | 0 | 0 | 15 (50%) | 3 (10%) |
| Grade II or less | 0 | 0 | 1 (3%) | 0 | 4 (13%) | 0 | 6 (20%) | 3 (10%) |
| Grade I only | 0 | 0 | 1 (3%) | 0 | 3 (10%) | 0 | 3 (10%) | 1 (3%) |
| Total with any degree of toxicity | 0 | 0 | 2 (6%) | 0 | 7 (23%) | 0 | 24 (80%) | 7 (23%) |
Abbreviation: CNS, central nervous system.
OUTCOME
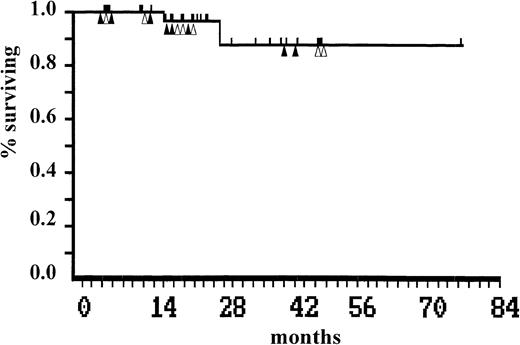
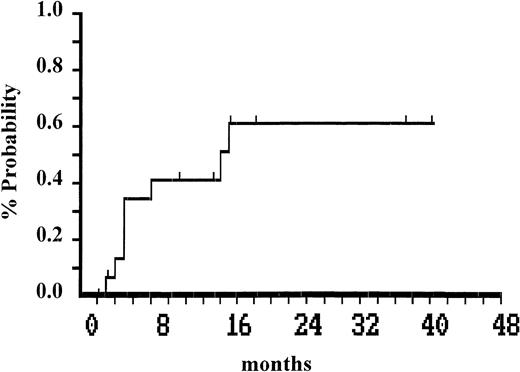
Two patients died of myeloid blastic transformation at 18 and 29 months after autografting. Twenty-eight patients are alive 6 to 76 months (median, 24 months) after autografting, with an actuarial survival of 87% (95% confidential limit [CL]: 54 to 97). Eight of these maintain complete cytogenetic remission at a median of 20 (range, 6 to 44) months after autografting. The median ratio BCR-ABL/ABL was of 0.002 (range, <0.001 to 0.01). Between 3 and 17 months (median 5 months), Ph-positive cells became detectable in 8 of the 16 patients who achieved complete cytogenetic remission (Fig2). These 8 patients are now in major cytogenetic remission at a median of 22 (range, 6 to 48) months. All patients (Ph-negative and Ph-positive) remain on interferon-α therapy. The status of each patient on the last day of follow-up is shown in Table 1. Survival plot after autografting is shown in Fig1.
Actuarial survival after autografting. Patients who remain in complete (▴) or in major (▵) cytogenetic remission are indicated.
Actuarial survival after autografting. Patients who remain in complete (▴) or in major (▵) cytogenetic remission are indicated.
Time to recurrence of greater than 1% Ph-positive cells after infusion of mobilized Ph-negative cells in 16 patients in whom complete cytogenetic remission was achieved.
Time to recurrence of greater than 1% Ph-positive cells after infusion of mobilized Ph-negative cells in 16 patients in whom complete cytogenetic remission was achieved.
DISCUSSION
The general rationale for autografting in first chronic phase is that a reduction in the target population of leukemic stem cells available for secondary mutational events will delay emergence of a blast-phase subclone and thereby prolong survival. The fact that Ph-positive cells subsequently became detectable is not surprising in view of experience with syngeneic transplants and evidence that immune effectors cells in allografting can make an important contribution to the prevention of disease recurrence after allografting. Most of the published experience in autografting for CML in chronic phase has involved the infusion of Ph-positive cells obtained from untreated patients at diagnosis.14,16,20,26 Although significant decreases in the percentage of Ph-positive marrow metaphases posttransplant have been noted in many patients, only a minority of patients have shown less than 25% Ph-positive cells, and only exceptional cases were completely Ph-negative early or at greater than or equal to 1 year posttransplantation.14,16 Several investigators suggest that total survival may be extended in autografted patients compared with historic controls, particularly in those who show some Ph-negative cells after grafting.14,16,27,28 However, it has been noted that a significant percentage of such patients may require repeated autografts because of early or late graft failure.14
There is limited experience to date using Ph-negative cells in an attempt to increase the curative potential of autografting in CML. Approaches to obtaining such cells have involved the use of repeated courses of intensive chemotherapy,29 in vitro purging,12,15,17,31-37 or long-term culture8,10,18 and in vivo purging.21,22,30,31 46-48
A total of 40 patients were treated with intensive chemotherapy and G-CSF. Of these, 10 patients could not be autografted because 1 patient underwent MUD, 6 patients collected highly contaminated Ph-positive cells, and the last 3 patients achieved a very low level of CD34+ cells (<1 × 106/kg).
In this report we have analyzed the results achieved in 30 consecutive patients autografted with Ph-negative or prevalently Ph-negative cells combined with adequate number of HPC. It was shown that the restoration of normal hematopoiesis was achieved in the majority of patients, suggesting that the chance of graft failure is minimized with cells mobilized with this procedure. The speed of hematopoietic recovery was consistent with an autograft-derived origin of predominantly diploid cells. No patient died as a consequence of the treatment. The majority of patients had stomatitis, which was severe in 50% of them, and only a minority of patients encountered some degree of hepatic and gastrointestinal toxicities. Specifically, no patient had busulfan-related pulmonary toxicity, hepatic veno-occlusive disease, or epileptic seizures. Thus, we can reasonably agree with the London data that the use of busulfan produces results at least as good as those achieved with total body irradiation (TBI)-containing regimens.38 There has been predominantly Ph-negative hematopoiesis in the majority of our patients for months postgrafting, and with the exception of 3 patients evolved in blastic crisis, there have been no hematologic relapses to date. The median follow-up postautograft was 24 (range, 6 to 76) months, and the actuarial survival at 3.5 years postautograft was 87% (95% CL: 54 to 97). Finally, most autograft recipients report normal or near-normal activity levels, suggesting that this procedure need not be associated either with prolonged convalescence or with chronic debility.
All autografted patients received postgraft immunobiological therapy with rhIL-2 and interferon-α in the attempt to delay the blastic evolution. Combination therapies of rhIL-2 and interferon-α have been shown to function synergistically in augmenting cytolytic activity in mice, both in vitro and in vivo.39-45 Both rhIL-2 and interferon-α were able to increase the suppressed natural killer cytolytic activity of lymphocytes isolated from CML patients.43,44 Recently, the efficacy of this combination has been confirmed in chronic and advanced phases of CML.45
In conclusion, our data suggest that early transplantation with mobilized Ph-negative or prevalently Ph-negative is a technique worth developing further. Because it is well tolerated, it can increase the age for investigative treatment for CML; 23% of the 30 patients we have autografted were between 52 and 62 years of age. Taken together, the results achieved by our group suggest that this approach, when applied in early chronic phase, is able to restore and to maintain major or complete cytogenetic response in greater than 50% of the initial population. This percentage seems to be superior to that obtained with interferon therapy alone; however, such a small series cannot be compared with the results of large-scale trials. Randomized trials are needed to evaluate our procedure versus interferon-α ± low-dose ARA-C. This approach is being investigated in three randomized trials under way in Germany, the United States/United Kingdom (ECOG-MRC CML trial), and European Group for Blood and Marrow Transplantation.
ACKNOWLEDGMENT
We thank Mara Capurro for manuscript preparation.
Supported by Associazione Italiana Ricerca contro il Cancro (AIRC), 1998.
Address reprint request to Angelo M. Carella, MD, Hematology/ABMT Unit, Via Acerbi, 10/22, 16148 Genoa, Italy; e-mail:amcarella@smartino.ge.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.