Abstract
PML/RAR is the leukemogenetic protein of acute promyelocytic leukemia (APL). Treatment with retinoic acid (RA) induces degradation of PML/RAR, differentiation of leukaemic blasts, and disease remission. However, RA resistance arises during RA treatment of APL patients. To investigate the phenomenon of RA resistance in APL, we generated RA-resistant sublines from APL-derived NB4 cells. The NB4.007/6 RA-resistant subline does not express the PML/RAR protein, although its mRNA is detectable at levels comparable to those of the parental cell line. In vitro degradation assays showed that the half-life of PML/RAR is less than 30 minutes in NB4.007/6 and longer than 3 hours in NB4. Treatment of NB4.007/6 cells with the proteasome inhibitors LLnL and lactacystin partially restored PML/RAR protein expression and resulted in a partial release of the RA-resistant phenotype. Similarly, forced expression of PML/RAR, but not RAR, into the NB4/007.6 cells restored sensitivity to RA treatment to levels comparable to those of the NB4 cells. These results indicate that constitutive degradation of PML/RAR protein may lead to RA resistance and that PML/RAR expression is crucial to convey RA sensitivity to APL cells.
ACUTE PROMYELOCYTIC leukemia (APL) is associated with the 15;17 chromosomal translocation which fuses the PML and retinoic receptor α (RARα) genes to yield a PML/RARα fusion protein.1,2 Transgenic mice studies showed that expression of PML/RARα results in altered myelopoiesis and development of leukemia, indicating a crucial role of the fusion protein in leukemogenesis.3-5
PML/RARα retains most of the functional domains of the RARα protein. In vitro studies showed that the DNA binding domain of the RARα component is indispensable for the property of the fusion protein to block differentiation of hematopoietic precursors.6 Altered regulation of RA target genes may, therefore, represent a fundamental mechanism of PML/RARα-mediated leukaemogenesis.
Pharmacological doses of RA induce disease remission in almost 90% APL cases by triggering terminal differentiation of leukemic blasts.7 RA induces degradation of the fusion protein through mechanisms that involve caspases8 and the proteasome pathway.9,10 Although PML/RARα degradation was initially suggested as the molecular cause of RA sensitivity, recent results suggest that PML/RARα expression is required to achieve RA sensitivity.8 11-13
Relapse and RA resistance arise after RA treatment of APL patients.1,2 Although the recent association of RA and chemotherapy resulted in a marked reduction of relapses,14RA resistance of APLs remains an unresolved biological phenomenon.
Several RA-resistant sublines have been derived from the APL NB4 cell line.15-18 The NB4.007/6 subline was selected under the selective pressure of RA and is characterized by undetectable levels of PML/RARα protein.16 Because PML/RARα mRNA is present at comparable levels in NB4.007/6 and parental NB4 cells, we investigated the possible involvement of posttranslational regulatory pathways that could determine degradation of PML/RARα protein and RA resistance in NB4.007/6.
MATERIALS AND METHODS
Cell culture and chemicals.
The APL NB4 cells, obtained from Dr M. Lanotte (INSERM, Paris, France), and NB4.007/6 RA-resistant cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). All-trans retinoic acid and N-Acetyl-Leu-Leu-Norleucinal (LLnL) were obtained from Sigma (St Louis, MO). Anti-RARα antibody was obtained from Pierre Chambon (Institut de Chimie Biologique, Faculté de Médecine, Strasbourg, France).19 The anti-PML PG-M3 antibody has been described.20
In vitro degradation assay.
Cells were washed in phophate-buffered saline (PBS) and then lysed in NP40 buffer (50 mmol/L HEPES-pH 7.0, 250 mmol/L NaCl, 0.1% NP40, 5 mmol/L EDTA, 1 mmol/L dithiothreitol (DTT), 2 mmol/L phenylmethylsulfonyl fluoride (PMSF), 2 μg/mL leupeptin, 2 μg/mL aprotinin). NB4 and NB4.007/6 lysates were mixed (1:1) and incubated at 37°C. Reactions were stopped by addition of sodium dodecyl sulfate (SDS) sample buffer.
Western blotting.
The samples were resolved by 8% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose filters. Western blots were performed as described.6
Transglutaminase activity assay.
TGase type II activity was performed as described.21
Infection of NB4 and NB4.007/6 cells.
The RARα and PML/RARα cDNAs were fused to the green fluorescent protein (GFP) cDNA sequence and then cloned in a hybrid Epstein-Barr virus (EBV)/retroviral vector.22 In the resulting construct, GFP-RARα or GFP-PML/RARα expression is driven by the LTR promoter. The packaging Phoenix cells were transfected by the calcium-phosphate/chloroquine method.22 The supernatant from the transfections, containing the viral particles, was collected after 48 hours, filtered, and then used to perform the infection of the target cells as described.22 Forty-eight hours after infection, cells were treated with 1 μmol/L RA to induce cell differentiation. After 5 days of RA treatment, cells were analyzed by fluorescent-activated cell sorter (FACS) for expression of differentiation markers. Immunophenotyping with α-cd11b and α-cd11c monoclonal antibodies was performed with formaldehyde fixation and a phycoerythrin-conjugated secondary antibody hybridization.
RESULTS
Expression of the PML/RARα protein in the NB4.007/6 RA-resistant subline.
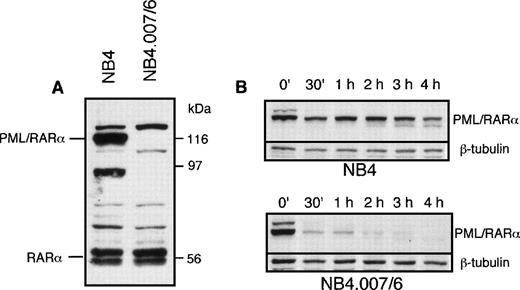
PML/RARα mRNA is detectable at comparable levels in NB4.007/6 and NB4 cells.15 16 Expression of PML/RARα protein in NB4.007/6 and parental NB4 cells was assessed by Western blot analysis. The anti-RARα antibody recognized a PML/RARα 116-kD band in NB4, but not in the NB4.007/6 cells (Fig1A). To exclude the possibility that the fusion protein could lack the carboxy-terminal RARα F-domain recognized by the anti-RARα antibody, we tested other antibodies directed against different epitopes in the PML portion of the fusion protein (PG-M3 and PG, directed against the N-terminus and α-helix of the PML protein, respectively): PML/RARα truncated polypeptides were undetectable in the NB4.007/6 cells (data not shown). These results suggest the presence of degradative activity(ies) against PML/RARα in the NB4.007/6 subline. To test this hypothesis we measured the stability of the PML/RARα protein in cell extracts from NB4 and NB4.007/6. NB4 cell lysates were used as source of PML/RARα protein and then mixed with lysates of NB4.007/6 or NB4, as control. Western blot analysis of fusion protein expression in the NB4.007/6 lysates showed complete degradation of PML/RARα after 2 hours while, at the same time point, 60% of the fusion protein was still detected in the NB4 lysates (Fig 1B). The calculated half-life of PML/RARα is less than 30 minutes in lysates from NB4.007/6 cells and longer than 3 hours in the NB4-derived lysates, suggesting the presence of a degradative activity in NB4.007/6 cells that decreases PML/RARα stability. Notably, in vitro–translated PML/RARα was not degraded by the NB4.007/6 cell lysates, suggesting that posttranslational modification(s) is necessary for the degradation of the fusion protein (data not shown).
PML/RAR expression in NB4.007/6 cells. (A) Western blot analysis of NB4 and NB4.007/6 whole-cell lysates using an anti-RAR antibody. The immunoreactive polypeptide that migrates above the indicated PML/RAR in both cell lysates is an anti-RAR cross reactive cellular protein. (B) In vitro degradation of PML/RAR in NB4 and NB4.007/6 cell lysates: NB4 cell lysate or NB4.007/6 + NB4 mixed cell lysates were incubated as indicated and 20 μg (NB4; top panel) or 40 μg (NB4.007/6 + NB4; bottom panel) of proteins were resolved by SDS-PAGE and analyzed by Western blotting using an anti-RAR antibody.
PML/RAR expression in NB4.007/6 cells. (A) Western blot analysis of NB4 and NB4.007/6 whole-cell lysates using an anti-RAR antibody. The immunoreactive polypeptide that migrates above the indicated PML/RAR in both cell lysates is an anti-RAR cross reactive cellular protein. (B) In vitro degradation of PML/RAR in NB4 and NB4.007/6 cell lysates: NB4 cell lysate or NB4.007/6 + NB4 mixed cell lysates were incubated as indicated and 20 μg (NB4; top panel) or 40 μg (NB4.007/6 + NB4; bottom panel) of proteins were resolved by SDS-PAGE and analyzed by Western blotting using an anti-RAR antibody.
Involvement of the proteasome pathway in both constitutive and RA-induced degradation of PML/RARα.
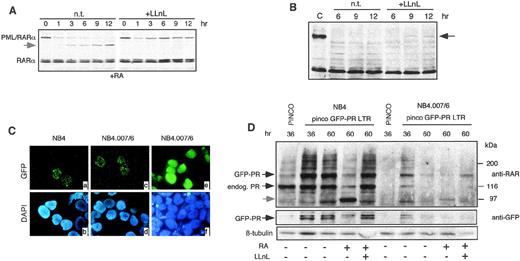
To investigate degradation of PML/RARα in vivo, we tested the effect of the proteasome inhibitors LLnL in NB4 and NB4.007/6 cells. NB4 cells were pretreated with 1 μmol/L RA for 6 hours, cultured in the presence or absence of 50 μmol/L LLnL for 12 hours, and analyzed by Western blotting at different time points. As previously described, RA treatment induced downregulation of PML/RARα protein and the appearance of an apparent degradation product of ∼97 kD (Fig2A). In contrast, RARα protein levels remained unchanged. The proteasome inhibitor LLnL blocked the RA-induced degradation of PML/RARα in NB4 cells (Fig 2A). LnLL was then tested in NB4.007/6 RA-resistant cells: a polypeptide reacting with the anti-RARα antibody and comigrating with PML/RARα was detected in NB4.007/6 upon LLnL treatment, although at a lower level than in NB4 cells (Fig 2B). Comparable results were obtained with another proteasome inhibitor (lactacystin; data not shown), suggesting that blocking of the proteasome pathway in NB4.007/6 cells partially restores PML/RARα expression. The fact that the effect of LLnL or lactacystin on PML/RARα expression was only partial, might be due to the short duration of treatment, because of its toxicity, or to the existence of additional PML/RARα degradation pathways in NB4.007/6 cells. We have recently shown that caspases are involved in RA-mediated degradation of PML/RARα in NB4 cells8 and that treatment with the caspase inhibitor ZVAD prevents this degradation. However, treatment of NB4.007/6 cells with ZVAD had no effects on PML/RARα expression, suggesting that caspases are not involved in the constitutive degradation of PML/RARα in these cells (data not shown).
Involvement of the proteasome in PML/RAR degradation. (A) NB4 cells were treated with 1 μmol/L RA in the presence (+LLnL) or in the absence (n.t.) of 50 μmol/L LLnL. The total lysates were resolved by SDS-PAGE and then analyzed by Western blotting using an anti-RAR antibody. The apparent degradation product of ∼97 kD is shown by the arrowhead. (B) NB4.007/6 cells were treated with 50 μmol/L LLnL (+LLnL) or left untreated (n.t.). Lysates were blotted with anti-RAR antibody. A polypeptide comigrating with PML/RAR appeared after 9 and 12 hours of LLnL incubation (black arrow). (C) NB4 and NB4.007/6 cells infected with retroviruses expressing GFP alone (pinco) or GFP-PML/RAR (pinco-GFP-PR LTR) and were then analyzed by fluorescence microscopy for GFP localization. After 36 hours from infection with the PINCO-GFP-P/R LTR, the NB4 and NB4.007/6 cell lines showed the typical nuclear microspeckled pattern of PML/RAR (panels a and c). Both cell lines showed a diffuse distribution of the GFP protein upon infection with the PINCO control retrovirus (only shown for the NB4.007/6 cells; panel e). Panels b, d, and f show the corresponding DAPI staining. (D) NB4 and NB4.007/6 cells were infected with the pinco or the pinco-GFP-PR LTR retroviral vectors and variably treated with RA or LLNL, as indicated. Corresponding lysates were resolved by SDS-PAGE and then analyzed by Western blotting using anti-RAR, anti-GFP, and anti-β–tubulin (to normalize for protein amounts loaded) antibodies, as indicated.
Involvement of the proteasome in PML/RAR degradation. (A) NB4 cells were treated with 1 μmol/L RA in the presence (+LLnL) or in the absence (n.t.) of 50 μmol/L LLnL. The total lysates were resolved by SDS-PAGE and then analyzed by Western blotting using an anti-RAR antibody. The apparent degradation product of ∼97 kD is shown by the arrowhead. (B) NB4.007/6 cells were treated with 50 μmol/L LLnL (+LLnL) or left untreated (n.t.). Lysates were blotted with anti-RAR antibody. A polypeptide comigrating with PML/RAR appeared after 9 and 12 hours of LLnL incubation (black arrow). (C) NB4 and NB4.007/6 cells infected with retroviruses expressing GFP alone (pinco) or GFP-PML/RAR (pinco-GFP-PR LTR) and were then analyzed by fluorescence microscopy for GFP localization. After 36 hours from infection with the PINCO-GFP-P/R LTR, the NB4 and NB4.007/6 cell lines showed the typical nuclear microspeckled pattern of PML/RAR (panels a and c). Both cell lines showed a diffuse distribution of the GFP protein upon infection with the PINCO control retrovirus (only shown for the NB4.007/6 cells; panel e). Panels b, d, and f show the corresponding DAPI staining. (D) NB4 and NB4.007/6 cells were infected with the pinco or the pinco-GFP-PR LTR retroviral vectors and variably treated with RA or LLNL, as indicated. Corresponding lysates were resolved by SDS-PAGE and then analyzed by Western blotting using anti-RAR, anti-GFP, and anti-β–tubulin (to normalize for protein amounts loaded) antibodies, as indicated.
We next investigated in vivo the stability of an exogenous source of PML/RARα in NB4 or NB4.007/6 cells. To facilitate monitoring of ectopic protein expression, PML/RARα was fused to the green fluorescent protein (GFP).22 The resulting GFP-PML/RARα recombinant protein retains the same biological activities of the parental PML/RARα when expressed into U937 or 32D cells (our unpublished results, 1998). The GFP-PML/RARα cDNA was cloned under the control of the 5′ LTR of a derivative of the hybrid EBV/retroviral PINCO vector22 (see Materials and Methods). NB4 and NB4.007/6 cells infected with the GFP-PML/RARα vector showed the typical micro-speckled expression pattern of PML/RARα (Fig2C, panels a and c), while cells infected with the control vector (PINCO), encoding GFP alone, presented a diffuse signal (Fig 2C, panel e). To examine the stability of the fusion protein, we analyzed the GFP-PML/RARα expression by fluorescence microscopy and Western blotting. The efficiency of infection and the stability of the GFP protein from the control vector were identical in the NB4 and NB4.007/6 cell lines (data not shown). Instead, at 36 hours postinfection, the expression of the GFP-PML/RARα in NB4.007/6 cells was markedly lower than in NB4, decreased afterwards, and was undetectable 60 hours after, while GFP-PML/RARα expression in NB4 cells was stable up to several days postinfection (see Fig 2D for Western blots; fluorescence data are not shown). RA treatment induced degradation of the fusion protein in both cell lines with a proteasome-dependent mechanisms because it could be blocked by LLnL treatment (Fig 2D). Taken together, these results indicate that the proteasome pathway is involved in both RA-induced degradation of PML/RARα in NB4 cells and constitutive degradation of PML/RARα in NB4.007/6 cells.
To evaluate whether proteasome-dependent constitutive degradation of PML/RARα is a common event leading to RA resistance in APL cells, we evaluated the effects of LLnL or Lactacystin in the NB4.306 cell line. This is another, independently derived, RA-resistant cell line, which also expresses PML/RARα mRNA but no detectable protein.15We did not observe any effect of the proteasome inhibitors on the steady-state levels of fusion protein expression in the NB4.306 cell line (data not shown).
Forced expression of PML/RARα restores RA sensitivity in NB4.007/6 cells.
We then tested whether restored PML/RARα expression in NB4.007/6 cells by LLNL was conducive to RA sensitivity. Since prolonged LLnL treatment leads to cell death of APL cells (data not shown), we could not evaluate terminal differentiation by examining canonical markers, such as cd11b or cd11c expression. Type II transglutaminase (TGII) activity is induced as an early event in NB4 cells during RA-mediated differentiation.21 Therefore, we examined the induction of TGII activity after RA and LLnL treatment in NB4 and NB4.007/6 cells. NB4.007/6 cells did not respond to RA, but in the presence of RA and LLnL we measured a significant increase in TGII activity (25% to 30% of the levels observed in NB4 cells; Fig3A). These results suggest that the partial recovery of PML/RARα expression in NB4.007/6 cells after LLnL treatment partially restored RA sensitivity. To further support this interpretation, we analyzed the effect of RA in NB4.007/6 cells infected with the GFP-PML/RARα retroviral vector. As controls, cells were infected with retroviruses expressing GFP-RARα or the GFP alone. Comparable levels of exogenous protein expressions were obtained, as determined by FACS analysis of GFP expression (not shown). Infected cells were treated with RA for 5 days and then assayed for expression of the differentiation markers cd11b and cd11c by FACS analysis. Cells were gated according to GFP expression and GFP-positive cells only were considered for further analysis. In cells expressing GFP alone, RA did not induce CD11b and CD11c expression. In contrast, RA induced CD11b and CD11c expression in 31% and 40% of GFP-PML/RARα expressing NB4.007/6 cells, respectively. The RA response observed in GFP-PML/RARα cells was comparable to that measured in uninfected NB4 cells or NB4 cells infected with either control or the GFP-PML/RARα vectors. A partial RA response (10% to 15%) was observed in the GFP-RARα–expressing cells (Fig 3B). These results show that expression of PML/RARα in the RA-resistant NB4.007/6 cell line is sufficient to restore RA sensitivity and that capacity of the fusion protein to mediate RA signals into these RA-resistant cells is only partially exerted by RARα.
PML/RAR expression in NB4.007/6 cells restores RA sensitivity. (A) NB4 and NB4.007/6 cells were treated with RA for 12 hours and then further cultured in RA-containing medium, either in the presence or in the absence of 50 μmol/L LLnL, as indicated. At the end of treatment, TGase II activity was measured in the cellular extracts. (B) NB4.007/6 cells were infected with retroviruses expressing GFP alone (GFP), GFP-RAR (GFP-RAR), or GFP-PML/RAR (GFP-P/R) and then treated for 5 days with RA. At the end of the treatment, GFP-positive cells were analyzed for expression of the differentiation antigens cd11b and cd11c. The white bar (NB4) refers to the uninfected, control NB4 cells after 5 days of RA treatment. These results are derived from a single experiment of three that gave comparable results.
PML/RAR expression in NB4.007/6 cells restores RA sensitivity. (A) NB4 and NB4.007/6 cells were treated with RA for 12 hours and then further cultured in RA-containing medium, either in the presence or in the absence of 50 μmol/L LLnL, as indicated. At the end of treatment, TGase II activity was measured in the cellular extracts. (B) NB4.007/6 cells were infected with retroviruses expressing GFP alone (GFP), GFP-RAR (GFP-RAR), or GFP-PML/RAR (GFP-P/R) and then treated for 5 days with RA. At the end of the treatment, GFP-positive cells were analyzed for expression of the differentiation antigens cd11b and cd11c. The white bar (NB4) refers to the uninfected, control NB4 cells after 5 days of RA treatment. These results are derived from a single experiment of three that gave comparable results.
DISCUSSION
The results presented here lead to two main conclusions: (1) in RA-resistant NB4.007/6 cells, PML/RARα expression is below detectable levels due to enhanced degradation of the fusion protein, and (2) forced expression of PML/RARα is sufficient to restore RA sensitivity in NB4.007/6 cells.
Constitutive degradation of PML/RARα in NB4.007/6 cells.
RA treatment of APL cells induces degradation of PML/RARα through mechanisms which involve both the proteasome and activated cellular caspases.8-10 Our results in NB4 and NB4.007/6 cells reinforce the view that the proteasome is a critical component of this process. NB4.007/6 cells were derived from NB4 cells under the selective pressure of continuous RA-treatment.9,10 The pathway of PML/RARα degradation in NB4.007/6 cells is RA independent and treatment with specific proteasome inhibitors increased the levels of PML/RARα, suggesting that the proteasome is responsible for the RA-independent degradation of PML/RARα in NB4.007/6 cells. Therefore, it appears that alteration(s) of the proteasome pathway leading to constitutive degradation of PML/RARα were selected in NB4.007/6 cells. However, proteasome inhibitors were unable to completely restore PML/RARα expression in the NB4.007/6 cells. Because caspases are not responsible for fusion protein degradation in these cells, a third pathway of proteolysis might be involved in the regulation of PML/RARα in the NB4.007/6 cells. Interestingly, constitutive degradation of PML/RARα did not lead to differentiation or to loss of the transformed phenotype in NB4.007/6 cells. We cannot rule out that PML/RARα is expressed below the detectable threshold in these cells, but at sufficient levels to mediate the differentiation block and/or the transformed phenotype. In this case, the resistance to RA would suggest that different levels of PML/RARα protein are required to mediate differentiation block/transformation and RA sensitivity. Alternatively, additional genetic events may have occurred during the establishment of the RA-resistant NB4.007/6 subline which resulted in a PML/RARα-independent phenotype. Indeed, NB4 cells carry additional chromosomal abnormalities with respect to those observed in fresh APL cells.23
PML/RARα expression restores RA sensitivity in the RA-resistant NB4.007/6 cells.
It has been proposed that PML/RARα degradation by RA is the key event underlying RA sensitivity of APL cells.9 The most striking result presented here is the observation that enhanced PML/RARα expression in the RA-resistant NB4.007/6 cells is sufficient to restore an RA-sensitive phenotype. This result suggests that the enhanced degradation of PML/RARα is the cause of RA resistance in NB4.007/6 cells. Notably, abnormal patterns of PML/RARα expression, including mutations, have been described in other RA-resistant sublines, as well as in the blasts from RA-resistant APL patients.16-18,24 25 NB4.007/6 cells, therefore, represent an in vitro system for the characterization of a phenomenon that may occur during the natural history of the disease; ie, appearance of RA-resistant cells as a consequence of enhanced degradation of PML/RARα.
The PML/RARα and RA-dependent differentiation observed in NB4.007/6 cells is the most direct confirmation of a recently proposed model, where PML/RARα expression is required to mediate RA sensitivity in APL cells. Because RA-induced degradation of PML/RARα is complete within 24 hours of RA treatment, PML/RARα may mediate the earliest steps required for RA-induced cell differentiation and natural RARα cannot substitute this function. Notably, NB4.007/6 cells express detectable levels of RXR protein (unpublished results, 1998).
The requirement for PML/RARα to achieve RA sensitivity in APL represents one of the paradoxes observed in this disease: supporting a dual role for the fusion protein, its expression is leukemogenetic at physiological concentrations of RA and then critical to mediate RA-induced differentiation at pharmacological doses of RA.
ACKNOWLEDGMENT
We thank Edoardo Marchesi, Giardina Giuseppina, Cristian Matteucci, and Stefania Lupo for their excellent technical assistance.
C.G.-P. and P.G.P. contributed equally to this paper.
Supported by grants from Consiglio Nazionale Ricerche (CNR) to C.G.-P. and from Associazione Italiana per la Ricerca sul Cancro (AIRC) and Fondazione Italiana per la Ricerca sul Cancro (FIRC) to P.G.P., C.G.-P., and S.M.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Pier Giuseppe Pelicci, MD, European Institute of Oncology, Department of Experimental Oncology Via Ripamonti, 435-20141, Milan, Italy; e-mail: pgpelicci@ieo.cilea.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal