THE NADPH OXIDASES are a group of plasma membrane–associated enzymes found in a variety of cells of mesodermal origin. The most thoroughly studied of these is the leukocyte NADPH oxidase, which is found in professional phagocytes and B lymphocytes. It catalyzes the production of superoxide (O2−) by the one-electron reduction of oxygen, using NADPH as the electron donor:
The O2− generated by this enzyme serves as the starting material for the production of a vast assortment of reactive oxidants, including oxidized halogens, free radicals, and singlet oxygen. These oxidants are used by phagocytes to kill invading microorganisms, but they also cause a lot of what the military would call “collateral damage” to nearby tissues, so their production has to be tightly regulated to make sure they are only generated when and where required.
In the 40 years since Sbarra and Karnovsky first reported findings suggesting the existence of such an enzyme in neutrophils, a great deal has been learned about the leukocyte oxidase. Research over this period of time has shown that the core enzyme comprises five components: p40PHOX (PHOX for PHagocyte OXidase), p47PHOX, p67PHOX, p22PHOX and gp91PHOX. In the resting cell, three of these five components—p40PHOX, p47PHOXand p67PHOX—exist in the cytosol as a complex. The other two components—p22PHOX and gp91PHOX—are located in the membranes of secretory vesicles* and specific granules, where they occur as a heterodimeric flavohemoprotein known as cytochrome b558. Separating these two groups of components by distributing them between distinct subcellular compartments guarantees that the oxidase is inactive in the resting cell.
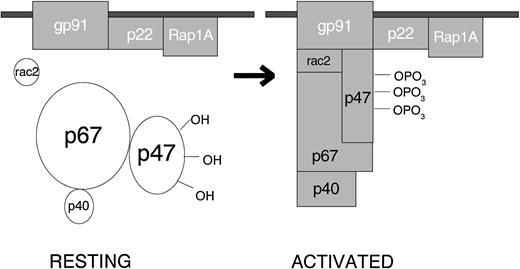
When the resting cell is exposed to any of a very wide variety of stimuli, the cytosolic component p47PHOX becomes heavily phosphorylated and the entire cytosolic complex migrates to the membrane, where it associates with cytochrome b558 to assemble the active oxidase (Fig 1). The assembled oxidase is now able to transfer electrons from the substrate to oxygen by means of its electron-carrying prosthetic groups—its flavin and then (according to most investigators, but not me) its heme group(s). Activation requires the participation, not only of the core subunits, but of two low-molecular-weight guanine nucleotide-binding proteins: Rac2, which in the resting cell is located in the cytoplasm in a dimeric complex with Rho-GDI (Guanine nucleotideDissociation Inhibitor), and Rap1A, which is located in membranes from which it can be copurified with the cytochrome. During activation, Rac2 binds guanosine triphosphate (GTP) and migrates to the membrane along with the core cytosolic complex. At the same time, cytochrome b558 and Rap1A are delivered to the cell surface by fusion of the secretory vesicle membranes and later the specific granule membranes with the plasma membrane of the cell. This fusion event also releases the contents of the organelles to the exterior. When phagocytosis takes place, the plasma membrane is internalized as the wall of the phagocytic vesicle, with what was once the outer membrane surface now facing the interior of the vesicle. From this location, the enzyme pours O2− into the vesicle, and the rapid conversion of this O2−into its successor products bathes the internalized target in a lethal mixture of corrosive oxidants.
Activation of the leukocyte NADPH oxidase. In the resting cell, the subunits of the oxidase are distributed between the cytosol (p40PHOX, p47PHOX, p67PHOX and Rac2) and the membranes (Rap1A and cytochrome b558, a p22PHOX · gp91PHOXcomplex). Rac2 and Rap1A are low-molecular-weight guanine nucleotide-binding proteins that function in other processes besides oxidase activation. The other 5 proteins are unique to the NADPH oxidase. When the cell is activated, p47PHOXbecomes heavily phosphorylated and the cytosolic subunits migrate to the membrane, where they bind to cytochrome b558 to assemble the active oxidase.
Activation of the leukocyte NADPH oxidase. In the resting cell, the subunits of the oxidase are distributed between the cytosol (p40PHOX, p47PHOX, p67PHOX and Rac2) and the membranes (Rap1A and cytochrome b558, a p22PHOX · gp91PHOXcomplex). Rac2 and Rap1A are low-molecular-weight guanine nucleotide-binding proteins that function in other processes besides oxidase activation. The other 5 proteins are unique to the NADPH oxidase. When the cell is activated, p47PHOXbecomes heavily phosphorylated and the cytosolic subunits migrate to the membrane, where they bind to cytochrome b558 to assemble the active oxidase.
The leukocyte NADPH oxidase continues to be the object of a considerable amount of investigation by scientists who are curious as to how it works. Interest in the leukocyte oxidase has increased with the growing recognition that oxidases closely related to the leukocyte oxidase can be found in a variety of cells in which the enzymes serve a variety of purposes. The following is a review of some of the new information obtained through these recent studies.
THE LEUKOCYTE NADPH OXIDASE
Properties and Functions of the Oxidase Components
Cytochrome b558.
A question that has been under study for some time has been the exact composition of the cytochrome b558 molecule: how many subunits of each kind does the cytochrome contain, and how many prosthetic groups? The most recent answers are that the cytochrome is a heterodimer containing one of each kind of subunit,1 and that it contains one FAD and two hemes.2 The two heme groups on the flavocytochrome are functionally distinct, with midpoint potentials at pH 7.0 of −225 and −265 mV.3 In the enzyme-bound FAD, a corner of the benzene ring of the electron-carrying isoalloxazine group is exposed to the solvent,2 suggesting that this corner is where the electron enters or leaves.
New information has appeared concerning the redox properties of cytochrome b558. The kinetics of electron flow through the flavocytochrome have been redetermined in a cell-free system containing purified cytochrome b558 and recombinant cytosolic components. The turnover number† for the system was 165 heme−1, s−1, while the rate of oxidation of reduced heme was 1,720 s−1.4Assuming that the concentration of cytochrome b558 in neutrophils corresponds to 10 pmol heme/106cells5 and that all the cytochrome b558participates in O2− production, the observed turnover number is equivalent to a rate of O2− production of about 0.2 nmol/min/106 cells, only 2% of the rate seen with maximally activated whole neutrophils. On the other hand, a rate of heme reduction fast enough to account for O2−production by whole cells was calculated from observed levels of reduction of the partly reduced flavin and heme in the working enzyme. However, this calculation depended on the assumption that the observed levels of reduction were steady-state levels; but it was later shown that this assumption was not in accord with experimental findings6 (see also ref 7). Moreover, earlier studies under anerobic conditions had shown that both in whole cells and in a cell-free system, the actual rate of reduction of the cytochrome b558 heme by NADPH was only about 0.1% of the rate of O2− production by the identical systems operating in air.8,9 Because a multistep reaction can go no faster than its slowest step, the rate of O2−production by neutrophils could be no greater than a few pmol/min/106 cells if the heme were an obligatory intermediate in electron transfer. Therefore, it is difficult for me to grasp the logic of the widely held belief that a heme residue of cytochrome b558 participates at all in electron transport by the leukocyte NADPH oxidase, much less that it is the terminal electron carrier. Evidence for heme participation such as the recent demonstration that exposure of the oxidase to an activating agent changes the spin state of the iron in cytochrome b558 from low-spin hexacoordinate to high-spin pentacoordinate,10though of great interest, is indirect and can be interpreted in many ways. Kinetic competence is the gold standard, and there is no escape from the fact that the rate of reduction of the heme is far too slow for it to participate in any meaningful way as an electron carrier for the oxidase.
The foregoing considerations apply only to the heme of the flavocytochrome. There is universal agreement that its flavin is an electron carrier. Oxidase activity is lost when FAD is removed from the enzyme, and restored when FAD is added back.11 The oxidase is inhibited by flavin antagonists such as deaza-FAD12 and diphenylene iodonium13 (in contrast to the lack of effect of heme antagonists such as CN−, N3−, CO, and butyl isonitrile14). In model systems, O2− is produced by the reaction between oxygen and reduced flavins. Finally, recent experiments demonstrating that under special conditions purified cytochrome b558alone catalyzes O2− production from NADPH and oxygen15 16 leaves little doubt that the flavin of cytochrome b558 is an electron carrier for the leukocyte NADPH oxidase.
The ability of the leukocyte NADPH oxidase to use artificial electron acceptors was discovered some time ago by Green and Wu.17Recently, iodonitrotetrazolium violet (INT) was added to the list of oxidants that could accept electrons from the oxidase.18,19Partial purification of the INT-reducing activity from activated neutrophil membranes showed that it contained cytochrome b558, and that its activity was dependent on cytosol and FAD.19 INT reduction persisted under anerobic conditions, indicating that the dye accepted electrons directly from the enzyme.
Chronic granulomatous disease (CGD) is an inherited disorder characterized by the failure of O2−production by phagocytes, resulting in a marked increase in the susceptibility of affected patients to bacterial and fungal infections. The disease is caused by a mutation that results in the loss or inactivation of one of the core subunits of the oxidase (CGD due to a mutation affecting p40PHOX has not yet been reported). Inactivating mutations (Table 1) have provided important information concerning the mechanism of the oxidase. Several recent reports have identified new mutations leading to inactive gp91PHOX. These include is gp91PHOX A57E,20 E309K, C537R, P339H, and ΔK31521 and gp91PHOX ΔF215 or 216.22 Cells containing gp91PHOXΔF215 or 216 have normal amounts of gp91PHOX but show no trace of the cytochrome b558 spectrum. This finding suggests that one or both of these phenylalanine residues are essential for the binding of the cofactors to the cytochrome.
Natural Mutations That Inactivate the Leukocyte NADPH Oxidase With Retention of the Mutated Protein
| Oxidase Subunit . | Mutation . |
|---|---|
| gp91PHOX | P415H |
| gp91PHOX | D500G |
| gp91PHOX | A57E |
| gp91PHOX | R54S |
| gp91PHOX | E309K |
| gp91PHOX | C537R |
| gp91PHOX | P339H |
| gp91PHOX | QKT(507-509)HIWA |
| gp91PHOX | ΔF215 or 216 |
| p22PHOX | P156Q |
| p67PHOX | ΔK58 |
| Oxidase Subunit . | Mutation . |
|---|---|
| gp91PHOX | P415H |
| gp91PHOX | D500G |
| gp91PHOX | A57E |
| gp91PHOX | R54S |
| gp91PHOX | E309K |
| gp91PHOX | C537R |
| gp91PHOX | P339H |
| gp91PHOX | QKT(507-509)HIWA |
| gp91PHOX | ΔF215 or 216 |
| p22PHOX | P156Q |
| p67PHOX | ΔK58 |
Cytosolic Components
p47PHOX is the subunit chiefly responsible for transporting the cytosolic complex from the cytosol to the membrane during oxidase activation. This is evident because neutrophils that lack p47PHOX are unable to transfer p67PHOX from the cytosol to the membrane during activation, although p67PHOX-deficient neutrophils transfer p47PHOX in a normal fashion.23Before the cytosolic oxidase components can be transferred to the membrane, however, p47PHOX must be extensively phosphorylated. This phosphorylation is one of the characteristic events of oxidase activation. p47PHOX, however, is not absolutely indispensible for oxidase activity. Even though its deficiency results in one form of CGD, two groups have now shown that in a detergent-activated cell-free system containing purified cytochrome b558 and recombinant cytosolic factors, p47PHOX can be omitted as long as the system contains high concentrations of p67PHOX and Rac2.24 25 The effect of p47PHOX is to tighten by nearly 100-fold the binding of each of the other cytosolic proteins to the assembled oxidase.
The function of p67PHOX has been a mystery. Unlike p47PHOX, p67PHOX is absolutely required for oxidase activity. When incubated with membranes in the detergent-activated cell-free system, a cytosol lacking p47PHOX but containing p67PHOXwas able to support INT reduction.18 Furthermore, p67PHOX facilitated electron transfer to the flavin of cytochrome b558 in the absence of p47PHOX 26; when p47PHOX was also present, the system was said to allow electron transfer “to proceed beyond the flavin center to the heme in cytochrome b−245‡ and thence to oxygen,” a claim with which I am obviously not in full agreement. We recently furnished some evidence as to the possible function of p67PHOX in oxidase activity by showing that this subunit contained a catalytically essential binding site for NADPH.27 However, there is evidence that an NADPH binding site also exists on cytochrome b558.28,29 The relationship between these two NADPH binding sites remains to be determined, though it should be noted that cytochrome b558alone catalyzes active O2− production using NADPH as the reductant,16 while catalysis of NADPH dehydrogenation by p67PHOX has to date not been demonstrated.
A CGD patient with a functionally significant mutation of p67PHOX has recently been described.30This mutant, p67PHOX ΔK58, loses its interaction with Rac, and when phagocytes containing the p67PHOX mutant are activated, the cytosolic complex fails to translocate to the membrane. These results imply that Rac, like p47PHOX, is involved in the translocation of the cytosolic complex during oxidase activation.
The function of p40PHOX has recently been examined.31 Oxidase activity can be established in K562 cells by transfecting them with plasmids that express recombinant p47PHOX, p67PHOX, and gp91PHOX.32 Cells that express p40PHOX along with other recombinant oxidase components produce only about half the amount of O2− generated by cells not expressing p40PHOX. Similarly, adding p40PHOX to a cell-free detergent-dependent oxidase activating system inhibits O2− production. These results suggest that p40PHOX is an inhibitory oxidase subunit. On the other hand, interfering with the binding of p40PHOX to p67PHOX reduces by 50% the production of O2− in a detergent-dependent cell-free system, a result implying that p40PHOX is a stimulatory subunit.33Clearly, more research on this question is needed.
Location of the Oxidase
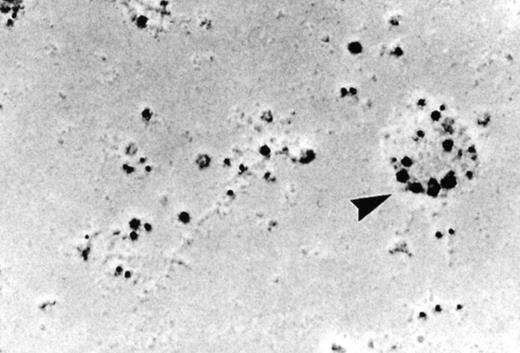
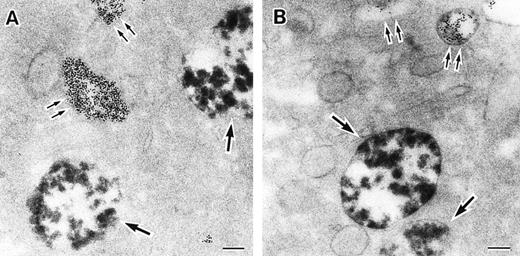
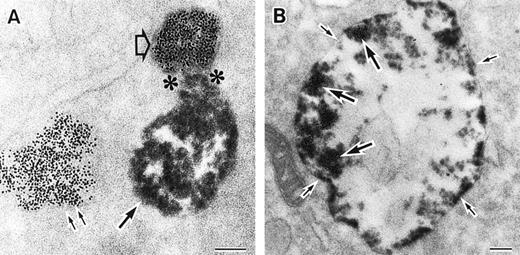
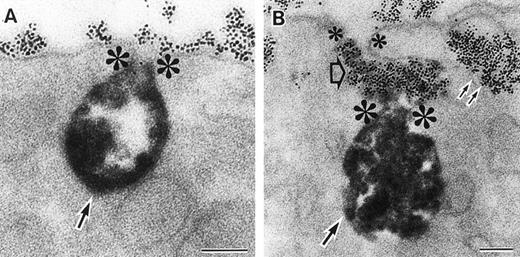
As discussed above, the activated NADPH oxidase is associated with the phagocyte membranes. Recent studies have indicated how the oxidase is distributed in the membrane, and on which membranes it is located. Immunoelectron microscopy showed that cytosolic oxidase components were grouped together with Rac on the inner face of neutrophil plasma membranes in 3- to 10-nm clusters34 (Fig2). In a study of exceptional interest, Kobayashi et al35 used cytochemical staining to show that O2− production in phorbol-stimulated neutrophils initially took place in small alkaline phosphatase-containing cytoplasmic vesicles (Fig3). These vesicles then fused together, forming larger vesicles that then merged with the plasma membrane. After a time, O2−-producing vesicles containing a marker of extracellular fluid appeared within the neutrophils. These results strongly imply that in resting cells, the membrane-associated oxidase components are located exclusively in intracellular organelles (secretory vesicles as shown here, and specific granules as demonstrated earlier36), and that the active oxidase in the plasma membrane is delivered there by membrane fusion events. They further imply that vesicles whose membranes contain the active oxidase cycle between the interior of the cell and the plasma membrane.
Localization of the oxidase to the neutrophil plasma membrane.34 Normal neutrophils were activated and unroofed, and the cytoplasmic face of the plasma membrane was labeled by the immunogold technique with anti-p67PHOX (large beads) and anti-gp91PHOX (small beads). The oxidase assemblies are gathered together in small clusters. Similar results were obtained with anti-p67PHOX/anti-p47PHOX and anti-p67PHOX/anti-Rac antibody pairs. (Reprinted with permission.34)
Localization of the oxidase to the neutrophil plasma membrane.34 Normal neutrophils were activated and unroofed, and the cytoplasmic face of the plasma membrane was labeled by the immunogold technique with anti-p67PHOX (large beads) and anti-gp91PHOX (small beads). The oxidase assemblies are gathered together in small clusters. Similar results were obtained with anti-p67PHOX/anti-p47PHOX and anti-p67PHOX/anti-Rac antibody pairs. (Reprinted with permission.34)
Sites of O2− production in neutrophils as a function of time after activation.35 (Top) O2− production starts in secretory vesicles (clumped precipitate; single arrow). The membranes of these vesicles are distinct from the plasma membrane, because none of the O2−-forming vesicles contain the ferritin (punctate precipitate, double arrow) that was added to the extracellular medium during the incubation and then was internalized by pinocytosis. (Center) The O2−-forming secretory vesicles later fuse with pinocytotic vesicles to form secondary vesicles. The oxidase in the membranes of these secondary vesicles continue to deliver O2− into the vesicle lumina. (Bottom) O2−-forming secretory vesicles also fuse with the plasma membrane itself, leading to the secretion of O2− into the extracellular environment. (Reprinted with permission from Kobayashi T, Robinson JM, Seguchi H: Identification of intracellular sites of superoxide production in stimulated neutrophils. J Cell Sci 111:81, 1998, published by The Company of Biologists, Ltd, Cambridge, UK.35)
Sites of O2− production in neutrophils as a function of time after activation.35 (Top) O2− production starts in secretory vesicles (clumped precipitate; single arrow). The membranes of these vesicles are distinct from the plasma membrane, because none of the O2−-forming vesicles contain the ferritin (punctate precipitate, double arrow) that was added to the extracellular medium during the incubation and then was internalized by pinocytosis. (Center) The O2−-forming secretory vesicles later fuse with pinocytotic vesicles to form secondary vesicles. The oxidase in the membranes of these secondary vesicles continue to deliver O2− into the vesicle lumina. (Bottom) O2−-forming secretory vesicles also fuse with the plasma membrane itself, leading to the secretion of O2− into the extracellular environment. (Reprinted with permission from Kobayashi T, Robinson JM, Seguchi H: Identification of intracellular sites of superoxide production in stimulated neutrophils. J Cell Sci 111:81, 1998, published by The Company of Biologists, Ltd, Cambridge, UK.35)
Inhibitors
Sulfhydryl groups are important for the function of the leukocyte NADPH oxidase. Recently, naturally occurring sulfhydryl blockers have been found to inhibit the oxidase. The aldehyde 4-hydroxynonenal inhibited the oxidase with an I50 of 19 μmol/L.37Inhibition was reversed with dithiothreitol, suggesting that the blockade of -SH groups is responsible for the action of the aldehyde. Inhibition by 4-hydroxynonenal is of physiological significance because the aldehyde is a major product of lipid peroxidation, and therefore will be present in tissues undergoing oxidative attack.
Nitric oxide (NO·) also inhibits the oxidase, but it works by preventing the assembly of the oxidase during activation.38 It has no effect on the fully active oxidase, and does not interact with either the flavin or the hemes on cytochrome b558. Nitrosothiols (RSNO) also inhibit oxidase activation, preventing translocation of the cytosolic oxidase components p47PHOX and p67PHOX through an action on the membrane.39 The effect of nitrosothiols can be reversed by mercaptoethanol. It is likely that both NO· and RSNO act by combining with cysteine-SH groups to form nitrosothiols on components of the oxidase.
It has recently been reported that PR-39, an antimicrobial peptide from neutrophils, is able to inhibit the NADPH oxidase by binding to the SH3 domains of p47PHOX, thereby blocking the interaction between p47PHOX and p22PHOX that normally takes place during oxidase activation.40 This 39–amino acid peptide is very unusual in that it contains 10 arginines, 19 prolines, and no acidic residues. A role for this peptide in the regulation of oxidase activity has been proposed, but evidence supporting this proposal is scanty. It is true that exposure of neutrophils to PR-39 inhibits O2− production in response to phorbol myristate acetate, but nonspecific toxicity of this peptide was not ruled out.
The Oxidase as a Battery
The phagocyte secretes O2− as the anion, leaving behind the proton produced in the O2−-forming reaction. In accord with this finding are observations showing that the leukocyte NADPH oxidase is an electrogenic enzyme, and that its activation is associated with the opening of a channel through which the protons left behind can leave the cell before they shut down the enzyme.41-44 In a recent publication, the existence of an inward current associated with the activation of the oxidase has been directly measured by patch clamping,45 confirming earlier work by O.T.G. Jones and associates.41 In the discussion of their report, these investigators postulate that the electric current per se may be directly related to the function of the oxidase—ie, microbial killing. They further state that “the importance [of O2−] in microbial killing is unclear.” This statement is rather surprising in view of the very large body of evidence showing that this O2− is the precursor of the powerful oxidants the phagocytes use as microbicidal agents.
Activation of the oxidase.
During oxidase activation, the cytosolic oxidase subunits are transferred to the membrane, where they bind to the membrane-associated oxidase components (cytochrome b558 and possibly Rap1A) to assemble the active enzyme. Translocation is preceded by the phosphorylation of certain oxidase-related proteins, some identified and some not. Low-molecular-weight guanine nucleotide binding proteins (GNBP) are also involved in oxidase activation, but the nature of their participation is unknown.
Protein-Protein Interactions Among the Oxidase Subunits
Examination of interactions between the various subunits of the oxidase is a very active field of investigation, and a remarkable number of studies on this subject have appeared in the published literature in the last few years.2,30,31,33,46-62 These studies have shown that in both the resting and active oxidases, the various subunits are associated with each other in the form of well-defined complexes. Resting cytosol contains an ∼250-kD complex of undetermined stoichiometry comprising the three oxidase subunits p47PHOX, p67PHOX, and p40PHOX. Activation of the oxidase brings this cytosolic complex to the membrane, where it associates with cytochrome b558, a membrane-bound dimer containing gp91PHOX and p22PHOX that in turn is probably interacting with Rap1A. A very large amount of effort has been expended in mapping the protein-protein interactions responsible for the formation of these complexes. An earlier review63 listed those protein-protein interactions that had been reported as of 1996. Since then, many more pairs of interactions have been mapped in whole or in part.
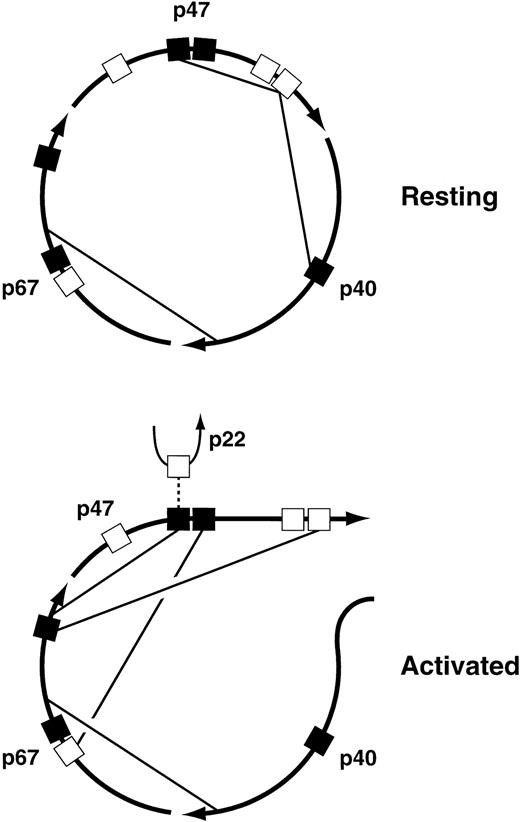
The major interactions among the cytosolic subunits that have been mapped to date are illustrated in Fig 4. The pairwise interactions themselves are well established, but the proposed allocation of the various interactions to the resting versus the activated state is strictly hypothetical, although it is based on evidence that rearrangements of the cytosolic complex take place during oxidase activation64 65 and on the identification of interactions that are required for translocation of the complex to the membrane. Of particular interest are the interactions between the C-terminal proline-rich SH3 binding domain of p47PHOX and the SH3 domains of both p47PHOX and p40PHOX in the resting complex. The interaction of p47PHOX with itself is thought to be responsible for the inability of resting p47PHOX to bind to cytochrome b558, the membrane-associated electron-carrying component of the oxidase. During activation, however, the C-terminal proline-rich domain of p47PHOX is occupied by the C-terminal SH3 domain of p67PHOX, an interaction that is required for translocation. This presumably means that this proline-rich domain separates from the p47PHOX SH3 domain that it occupies in the resting state, and perhaps from the SH3 domain of p40PHOX as well. Another interaction required for translocation is the one between the C-terminal SH3 domain of p47PHOX and the N-terminal proline-rich domain of p67PHOX; in the diagram this is shown only in the activated complex, but it could be present in the resting complex as well. Finally, the N-terminal SH3 domain of p47PHOX, freed from its interaction with the distal p47PHOX proline-rich domain, binds to the C-terminal portion of p67PHOX. But in the active oxidase, this p47PHOX SH3 domain has to interact with p22PHOX. Maybe oxidase activation is a multi-step process, one step consisting of the rearrangements shown in the figure, and a subsequent step involving an exchange at the N-terminal SH3 domain of p47PHOX in which the C-terminal portion of p67PHOX is swapped for the proline-rich domain of p22PHOX. The possibility that oxidase activation is a multi-step process is also implied by experiments discussed below in the section on protein phosphorylation. These experiments suggest that oxidase activation can be dissected into three successive events: partial phosphorylation of p47PHOX, translocation of p47PHOX and the other cytosolic oxidase components to the membrane, and then final phosphoryation of p47PHOX coupled to the acquisition of catalytic activity by the enzyme. I must emphasize, however, that the foregoing discussion of the changes in protein-protein interactions undergone by the cytosolic complex during oxidase activation, though consistent with current findings, is pure speculation.
Proposed protein-protein interactions among the cytosolic components of the leukocyte NADPH oxidase in the resting and activated states. For an explanation of the figure, see text. (▪), SH3 domains; (□), proline-rich SH3 binding domains.
Proposed protein-protein interactions among the cytosolic components of the leukocyte NADPH oxidase in the resting and activated states. For an explanation of the figure, see text. (▪), SH3 domains; (□), proline-rich SH3 binding domains.
Although interactions among the cytosolic subunits, and between the cytosolic subunits and p22PHOX, are reasonably well defined, interacting domains between gp91PHOX and the remainder of the oxidase subunits are uncertain. Phage display libraries have identified certain p47PHOX peptides that recognize gp91PHOX,66 and the examination of hydrophobic domains67 has identified certain peptides in gp91PHOX that could be important in oxidase activity. At relatively high concentrations, these peptides inhibit oxidase activity in the sodium dodecyl sulfate (SDS)-dependent cell-free oxidase activating system and in electropermeabilized neutrophils. However, peptide inhibition studies can be deceptive. Illustrating this is work carried out on a peptide from the C-terminus of gp91PHOX. For a number of years, an interaction involving seven residues near the C-terminus of gp91PHOX (RGVHFIF) was thought to be of considerable functional significance because a peptide with this sequence was found to be a potent inhibitor of oxidase activity in an SDS-dependent cell-free oxidase activating system. Recently, however, it was shown that mutations in this sequence had no effect on oxidase activity in a gp91PHOX-deficient leukemia line; O2− production by cells expressing these mutants was no different than O2− production in cells expressing wild-type gp91PHOX.68 This means either that this sequence is of no functional significance, or that its function can be replaced by some other element in the gp91PHOXprotein.
Nevertheless, this region of gp91PHOX does appear to bind to p47PHOX, and the structure of a complex between p47PHOX and the 17-mer gp91PHOX 551-568 (the C-terminus of gp91PHOX, lacking only K569) was studied by two-dimensional nuclear magnetic resonance spectroscopy (2-D NMR).56 This study showed that the stretch of peptide from S555 to F564 could be clearly defined, showing an extended bend with immobilized side chains (except for H561). This is a novel approach to the study of interactions among the oxidase components.
Phosphorylation
Phosphorylation is an essential element in the activation of the NADPH oxidase. Phosphorylation of p47PHOX during oxidase activation has been recognized for many years. More recently, the phosphorylation of p67PHOX and p40PHOX has been shown, and the phosphorylation of a component in the membrane has been implicated in the activation process.
The protein whose phosphorylation has been studied most thoroughly is p47PHOX. During oxidase activation it is extensively phosphorylated, with 8 to 9 serines in the C-terminal quarter of the molecule acquiring phosphates. Of these target serines, S379 is the only one whose conversion to alanine as a sole mutation results in a major loss of oxidase activity.69Phosphorylation of S379 is necessary for both the translocation of p47PHOX and the activation of the oxidase. It has recently been found, however, that the mutation of both of a pair of target serines to alanine can inactivate p47PHOX. Serines S303 and S304 are known to be phosphorylated during oxidase activation. The mutation of this serine pair to alanines greatly decreases oxidase activity, though the phosphoryation of other serines and the translocation of the mutant p47PHOX to the membrane is unaffected. Activity is normal, however, if these serines are replaced by glutamates, or, surprisingly, by lysines.70The activity of p47PHOX S303K,S304K mutant raises the possibility that p47PHOX may in part be linked to the rest of the active oxidase by a cation bridge. Interestingly, the mutation to alanines of the MAP kinase target pair S345/S348 had no effect on the activity of the oxidase.69 71
A subsequent study showed another pair of serines, S359 and S370, whose phosphorylation was necessary for oxidase activation.72 The mutation of both these serines to alanines results not only in the loss of oxidase activity, but also in a complete failure of phosphorylation of p47PHOX, both recombinant and in whole cells. Replacement of those serines by glutamate or aspartate allows phosphorylation and translocation to take place, but oxidase activity is still greatly reduced. In contrast to the results obtained with S303K,S304K, the replacement of S359 and S370 with lysines led to the total loss of activity of the oxidase; p47PHOX-deficient cells expressing p47PHOX S359K,S370K showed no more O2− production than the untransfected p47PHOX-deficient cells. The conclusion drawn from these studies was that during oxidase activation, S359 and/or S370 has to be phosphorylated first; S379 then acquires a phosphate, allowing the cytosolic complex to translocate to cytochrome b558; and finally, S303 and/or S304 are phosphorylated, endowing the oxidase with full catalytic activity.
The phosphorylation of p47PHOX is regulated by protein kinase A. It had been known for some time that oxidase activation is diminished in neutrophils containing elevated levels of cyclic adenosine monophosphate (cAMP) or cAMP analogs. It has now been shown that the phosphorylation of p47PHOX in response to N-formylmethionylleucyl phenylalanine (fMLP) is inhibited by cAMP agonists, and that this effect is prevented by an inhibitor of protein kinase A. In contrast, cAMP agonists had no effect on p47PHOXphosphorylation induced by phorbol myristate acetate, an activator of protein kinase C.73
Recently, both p40PHOX and p67PHOX were found to take up phosphate when neutrophils are stimulated.74,75 It was shown that p40PHOX is phosphorylated in the resting cell, takes up additional phosphate when the NADPH oxidase is activated, and loses the additional phosphate when the oxidase is deactivated. Both p40PHOX and p67PHOX acquire phosphate when the cells are activated with either fMLP or phorbol; the latter suggests that protein kinase C participates in the phosphorylation of both oxidase components. On the basis of in vitro experiments, however, it was claimed that the phosphorylation of p40PHOX and p47PHOX involve distinct signal transduction pathways. In regard to the phosphorylation of p67PHOX, an earlier report76 claimed that two 67K proteins are phosphorylated in activated neutrophils, but that neither one is p67PHOX; their results, however, do not rule out the possibility that a small amount of p67PHOX is phosphorylated during oxidase activation, a qualification that becomes important in the light of observations showing that only ∼5% of the p67PHOX translocates to the membrane during oxidase activation.77,78 Inhibition of MAP kinase kinase by PD098059 prevents oxidase activation by either opsonized zymosan or phorbol myristate acetate,79 while inhibition of p38 by SB203580 prevents oxidase activation by fMLP.80 MAP kinase kinase activates ERK, a member of the MAP kinase family, and p38, another member of the MAP kinase family. To the extent that these inhibitors are truly specific (always an open question with any inhibitor), these results imply that both ERK and p38 participate in the activation of the NADPH oxidase. However, the nature of their participation is unknown.
The recent development of cell-free systems in which the NADPH oxidase is activated by kinases81 82 offers an opportunity to study the signal transduction pathway(s) responsible for oxidase activation in a system that is at least partly defined. In one of these systems, oxidase activation requires both adenosine triphosphate (ATP) and phosphatidic acid, the latter an anionic detergent that is able to activate the oxidase by itself, although ATP doubles O2− production in that system. A phosphatidic acid–dependent protein kinase is postulated to account for those findings. The other system uses no detergents, but requires the phosphorylation of both p47PHOX and a membrane component to activate the NADPH oxidase. The identity of the phosphorylated membrane component is currently under investigation.
With kinase near, is phosphatase remote? Not likely, but studies of phosphatases in relation to the leukocyte NADPH oxidase are lagging. Three recent studies, all using whole neutrophils and inhibitors, have implied that phosphatases are involved in the deactivation of activated NADPH oxidase. Two studies showed an enhancement of oxidase activity and O2− production in fMLP-activated neutrophils treated with okadaic acid83 or calyculin A,84 both of which are inhibitors of protein phosphatases 1 and 2A. The release of p47PHOX from the cytoskeleton of activated neutrophils in response to protein kinase inhibitors was prevented by both okadaic acid and calyculin A.85 This interesting study suggests that the activity of the oxidase is determined by the prevailing steady-state rates of protein phosphorylation and dephosphorylation, with increases in phosphorylation activating and increases in dephosphorylation deactivating the enzyme, lending further support to inferences that could be drawn from a large number of earlier studies on protein phosphorylation and oxidase activity.
Small Guanine Nucleotide Binding Proteins
Two low-molecular-weight guanine nucleotide binding proteins (G proteins) have been implicated in the function of the leukocyte NADPH oxidase: Rac2 (in mouse macrophages, Rac1) and Rap1A. Rac2, found chiefly in the cytosol of resting neutrophils, is a member of the Rho family of G proteins, a family known principally for its function in regulating the cytoskeleton. Rap1A, located in the membranes of resting neutrophils, is in the Ras family. Like Ras, it regulates cell proliferation, acting as an antagonist of Ras-dependent transformation.86
Most of the recent work on low-molecular-weight G proteins and the NADPH oxidase has been performed on Rac2, probably because as a soluble protein, Rac2 is much easier to deal with experimentally than Rap1A. These studies focused on the protein-protein interactions that developed between Rac2 and other NADPH oxidase components when the oxidase was activated in a cell-free system using anionic detergents. Incubation of neutrophil membranes with p67PHOX-deficient cytosol under activating conditions had been shown to cause p47PHOX to be transferred to the membrane, but the reciprocal experiment with p47PHOX-deficient cytosol did not lead to the translocation of p67PHOX.87 In a similar experiment but with recombinant proteins instead of whole cytosols, the same laboratory showed that Rac2 also failed to translocate.61 These findings suggest that during oxidase activation, the translocation of p47PHOX precedes the translocation of both p67PHOX and Rac2. Using chimeras between Rac1, which strongly activates the leukocyte NADPH oxidase, and CDC42hs, a Rho-family G protein that barely activates the oxidase, it was shown that residues 27 and 33 were particularly important for oxidase activation.88 Both Rac1 and Rac2 were shown to bind to p67PHOX but not to p47PHOX. Both the effector region (residues 26-45) and the insert region (residues 125-145) of Rac1 were important for oxidase activation by this G protein, but binding to p67PHOX was only affected by mutations in the effector region.2
Rap1A was first implicated in the function of the leukocyte NADPH oxidase when it was found to copurify with cytochrome b558. Functional evidence for the participation of Rap1A in oxidase activation was not developed until much later, when it was shown using a transfected Epstein-Barr virus (EBV)-transformed B-lymphocyte system that both Rap1AS17N and Rap1AQ63E, which are locked in the GDP-bound and GTP-bound conformations respectively, inhibited phorbol-induced production of O2−, although the wild-type protein had no effect.89 The observation that O2− production was inhibited by both “locked” conformations of Rap1A raises the possibility that the GTP-bound form carries the oxidase from “state 1” to “state 2” with concomitant hydrolysis of the GTP, while the GDP-bound form brings the oxidase back to “state 1” with the concomitant exchange of GDP for GTP. Nothing is known about the nature of these hypothetical states. Another ingredient in the mixture is the finding that Rap1A can be phosphorylated by protein kinase A, and that the phosphorylated form binds somewhat more weakly to cytochrome b558 than the unphosphorylated form.90 Protein kinase A is known to suppress the activity of the NADPH oxidase, and the foregoing observations suggest that the kinase-regulated interaction between cytochrome b558 and Rap1A may be of functional significance. On the other hand, the observations concerning the phosphorylation of Rap1A were made in an in vitro system; there is no evidence to date that Rap1A is phosphorylated in whole cells under any circumstances.
A curious observation is the recent demonstration that suppressing p120Ras-GAP biosynthesis by an antisense oligonucleotide led to enhanced O2− production in EBV-transformed B lymphocytes.91 Because p120Ras-GAP binds to both Ras and Rap1A, the observed effect could be a result of its interaction with one or both of these G proteins. Another effect of p120Ras-GAP, however, is to inhibit phorbol-induced cellular events.92 This seems to be the likeliest explanation for the effect of the p120Ras-GAPantisense oligonucleotide, because the increase in O2− production was seen in phorbol-stimulated cells but not fMLP-stimulated cells.
The Cytoskeleton, Integrins, and Tyrosine Kinases
The role of the cytoskeleton in the function of the leukocyte NADPH oxidase has been recognized for some time. The two major pieces of evidence supporting this idea are: (1) the finding that in activated neutrophils, all the O2− producing activity and portions of all the oxidase components are found in the cortical cytoskeleton77 93; and (2) the demonstration that when neutrophils adhere to a surface, a process that involves integrins and is associated with far-reaching changes in the cytoskeleton, the time course of O2− production becomes greatly altered compared with the time course of O2−production in suspended cells.
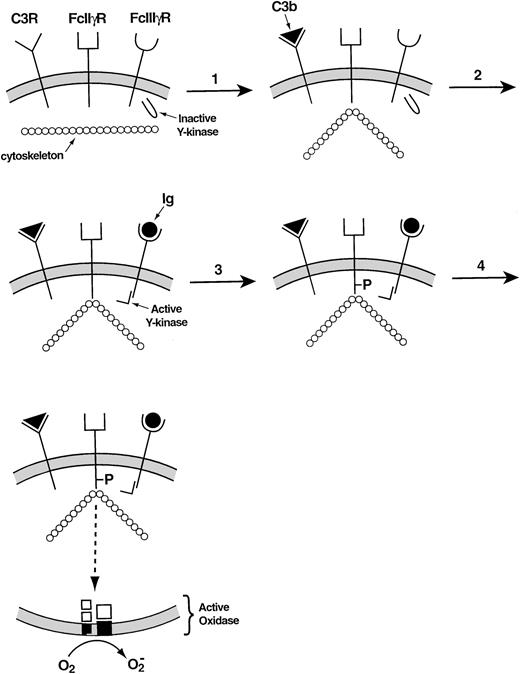
Recent studies have provided more details regarding the incompletely understood relationship between the cytoskeleton and the leukocyte NADPH oxidase. In adherent neutrophils, stimulation with tumor necrosis factor causes β2 integrins as well as the cytosolic oxidase components to move slowly from the Triton-soluble to the Triton-insoluble compartment, the latter generally regarded as representing the neutrophil cytoskeleton.94O2− is produced during this event, its time course resembling that of the migration of proteins to the cytoskeleton. A complicated series of steps involving a β2 integrin explains how antibodies and complement cooperate in the activation of the leukocyte NADPH oxidase.95 When the activated complement protein iC3b associates with the β2 integrin that serves as its receptor, the Ig receptor FcγII§ becomes attached to the cortical cytoskeleton. The occupation of the FcγIII receptor then allows the tyrosine phosphorylation of the cytoskeletally associated FcγII receptor to take place, triggering the signal transduction pathway that leads to the activation of the leukocyte NADPH oxidase (Fig 5).
Mechanism of collaboration of the C3 receptor (a β2 integrin) and the FcγIII receptor in the activation of the leukocyte NADPH oxidase.95 In the resting membrane, the C3, FcIIγ, and FcIIIγ receptors are empty. (1) Occupancy of the C3 receptor causes the FcIIγ receptor to bind to the cytoskeleton. (2) Occupancy of the FcIIIγ receptor activates a tyrosine kinase. (3) The activated tyrosine kinase phosphorylates the cytoplasmic portion of the FcIIγ receptor, which was rendered susceptible to phosphorylation through its interaction with the cytoskeleton. (4) The phosphorylated FcIIγ receptor activates the oxidase via a multistep signal transduction pathway.
Mechanism of collaboration of the C3 receptor (a β2 integrin) and the FcγIII receptor in the activation of the leukocyte NADPH oxidase.95 In the resting membrane, the C3, FcIIγ, and FcIIIγ receptors are empty. (1) Occupancy of the C3 receptor causes the FcIIγ receptor to bind to the cytoskeleton. (2) Occupancy of the FcIIIγ receptor activates a tyrosine kinase. (3) The activated tyrosine kinase phosphorylates the cytoplasmic portion of the FcIIγ receptor, which was rendered susceptible to phosphorylation through its interaction with the cytoskeleton. (4) The phosphorylated FcIIγ receptor activates the oxidase via a multistep signal transduction pathway.
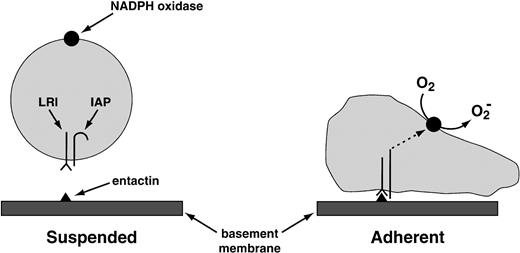
The same laboratory that worked out antibody-complement cooperation has also identified the “leukocyte response integrin (LRI).”96 This protein, which recognizes the basement membrane protein entactin, acts in association with another membrane protein, the “integrin-associated protein.” This combination of proteins activates the leukocyte NADPH oxidase when one of them—the LRI—interacts with entactin, or with the peptide KGAGDV (Fig 6). O2−production activated by the LRI/integrin-associated protein (IAP) combination occurs in neutrophils deficient in CD18, the common subunit of the β2 integrins, ruling out the participation of these more traditional leukocyte integrins in the response of the neutrophil to the LRI/IAP system.
Provisional mechanism for the activation of the NADPH oxidase by the LRI. The LRI and the IAP are thought to form a complex in the leukocyte plasma membrane. When leukocytes are in suspension, the signal transduction pathway between this complex and the NADPH oxidase is blocked. The blockade is lifted by the following combination of events: (1) the binding of LRI to entactin, a basement membrane protein; (2) adhesion of the phagocyte to a surface (in suspended cells, NADPH oxidase is not activated by the binding of LRI to entactin); and (3) possibly a conformational change in IAP. These events somehow activate protein kinase C, which then activates the oxidase. Tyrosine phosphorylation does not participate in oxidase activation by LRI/IAP.96
Provisional mechanism for the activation of the NADPH oxidase by the LRI. The LRI and the IAP are thought to form a complex in the leukocyte plasma membrane. When leukocytes are in suspension, the signal transduction pathway between this complex and the NADPH oxidase is blocked. The blockade is lifted by the following combination of events: (1) the binding of LRI to entactin, a basement membrane protein; (2) adhesion of the phagocyte to a surface (in suspended cells, NADPH oxidase is not activated by the binding of LRI to entactin); and (3) possibly a conformational change in IAP. These events somehow activate protein kinase C, which then activates the oxidase. Tyrosine phosphorylation does not participate in oxidase activation by LRI/IAP.96
Associations between particular cytoskeletal proteins and the NADPH oxidase have also been described. The human counterpart ofcoronin, a protein important for motility inDictyostelium, assocoates with p40PHOX and accumulates around phagocytic vesicles.97Cofilinloses its phosphate and moves to ruffled membranes when neutrophils are stimulated with fMLP. Oxidase components also migrate to the ruffled membranes under those conditions, and O2−production is stimulated with kinetics that resemble the kinetics of cofilin dephosphorylation, implying the possibility of some connection between cofilin and the oxidase.98 Stimulation of the oxidase in a detergent-activated cell-free system is augmented by G actin.99
Finally, some very interesting studies relating neutrophil tyrosine kinases to the activation of the NADPH oxidase have recently appeared from Berton’s laboratory.94,100 101 In their initial studies, this group showed that tumor necrosis factor, which activates the NADPH oxidase in adherent cells but not in suspended cells, caused the Src family tyrosine kinase p58fgr to move from the cytosol to the cytoskeleton. Neutrophils from knockout mice deficient in both p58fgr and p59/61hck, another tyrosine kinase in the Src family, were unable to produce O2− in the course of their attachment to surfaces coated with collagen or fibronectin, though O2− production in response to immune complexes and phorbol myristate acetate was normal. These results indicate that the trigger responsible for the adhesion-dependent activation of fgr and hck, and their subsequent actions on the oxidase, was β2 integrin. Conversely, reactive oxidizing species were found to be required for the activation of the tyrosine kinases p58fgr and p53/56lyn that takes place when neutrophils adhere to surfaces, a result which indicates that at least to some extent, the activation of these kinases is a bootstrap operation with autocatalytic features.
CGD.
CGD is an inherited immune deficiency in which phagocytes from affected patients are unable to manufacture O2−. All cases so far have been found to result from a deficiency of one of 4 oxidase-specific proteins: p47PHOX, p67PHOX, p22PHOX, or gp91PHOX. Virtually all the recent work on the oxidase in CGD has been directed toward developing a genetic cure of the disease.
B lymphocytes and hematopoietic progenitors from patients with various forms of CGD have been cured by transfection with vectors expressing the oxidase component missing from the patients’ cells. Various retroviral vectors have been used to correct CGD in cells from patients with the X-linked form of the disease, who are missing gp91PHOX. In the earliest study, the respiratory burst was restored in 15% of transfected human myeloid cells, with the active cells producing O2− at about 60% of the normal rate.102 Using a different vector, the same group transfected a CGD myeloid line as well as marrow cells from a knockout mouse with gp91PHOXdeficiency.103 Results with the new vector were greatly superior to results obtained previously, with oxidase activity being largely to completely restored in both human and mouse cells. CD34+ cells from a p67PHOX-deficient patient were cultured after transfection with a replication-defective amphotropic retrovirus that expressed p67PHOX. O2− production was restored in neutrophils arising from the transfected cells.104
Two groups of investigators have cured CGD in vivo by genetic methods, but only in mice. Knockout mice lacking gp91PHOXwere transplanted with their own marrow cells after transfection with a murine stem cell virus vector that expressed the missing protein.105 O2− was expressed by neutrophils from these transplanted mice, though at levels lower than seen in wild-type mice. Despite the fact that neutrophil O2− production was only partly corrected in the transplanted mice, these mice withstood a challenge withAspergillus fumigatus that produced pneumonia in all the untransplanted CGD mice. Similar results were obtained in p47PHOX knockouts that were transplanted with stem cells transfected with retroviral vectors that expressed p47PHOX,106 except that in these mice the challenge was with Burkholderia cepacia, a typical pathogen of patients with CGD, though normally its activities are confined to onions.
NADPH OXIDASE IN NONPHAGOCYTES
Like all other biological macromolecules and macromolecular assemblies, the leukocyte NADPH oxidase was created in the course of evolution. In considering its possible origin, it was hard to believe that an enzyme this lethal could have arisen de novo. With this in mind, it was speculated that the oxidase might have arisen through the mutation of a more ancient NADPH oxidase of much lower activity whose tissue distribution was much more widespread than that of the lethal leukocyte oxidase and whose principal function was to provide oxidants for signaling purposes.107 Recent findings have confirmed this speculation, showing clearly that a low-activity NADPH oxidase is present in a variety of nonphagocytic cells, most of which are derived from the embryonic mesoderm, and that this oxidase is a source of second messengers.
An NADPH oxidase similar to the one found in phagocytes has been reported to occur in all three layers of the aorta. O2− was generated in endothelial cell sonicates to which NADPH was added. In addition, mRNA for the four specific oxidase subunits and protein for the two cytosolic subunits were found, but no heme protein was detected by spectroscopy.108 Human aortic smooth-muscle cells produced O2− in response to platelet-derived growth factor (PDGF). This O2− production was inhibitable by diphenylene iodonium, suggesting that a phagocyte-like NADPH oxidase was responsible for its production. In these cells, the activation of NF-κB by PDGF was dependent on O2−, but its activation by interleukin-1β was not.109 In fibroblasts from aortic adventitia, O2− production occurred constitutively.110 Its rate of production was increased by angiotensin II110,111 but not by norepinephrine.111 It was postulated that the O2− generated by the aorta was functioning as a blood pressure regulator by consuming nitric oxide, a well-known hypotensive agent with which it reacts at a diffusion-limited rate.110 111
In joint tissues, O2− production has been detected in synoviocytes (both type A and type B)106 and in chondrocytes.112 In synoviocytes, O2− production is induced by phorbol myristate acetate, suggesting that it is generated by an NADPH oxidase. In chondrocytes, O2− production is elicited by a calcium ionophore, but not by phorbol myristate acetate. In these cells O2− production is inhibited by diphenylene iodonium, and mRNA for p22PHOX, p40PHOX, and p47PHOX (or their homologs) was detected by reverse transcriptase-polymerase chain reaction, relatively strong evidence that O2−from chondrocytes is produced by an NADPH oxidase like that in phagocytes.
There is evidence that NADPH oxidases serve as components of oxygen sensors in various tissues. Erythropoietin production in some hepatoma lines appears to be regulated by O2−, and p22PHOX, the α-subunit of cytochrome b558, has been detected immunologically in renal peritubular fibroblasts and in Ito cells of the liver, both of which may be sources of erythropoietin.113 In the lung, pulmonary neuroepithelial bodies have been proposed as airway oxygen sensors.114 The cells of these organs contain mRNAs corresponding to a subunit of a H2O2-regulated potassium channel as well as messages for p22PHOXand gp91PHOX, the two membrane-associated oxidase subunits, or their homologs. H2O2 production by the neuroepithelial body cells was constitutive, but was stimulated by phorbol myristate acetate and inhibited by diphenylene iodonium. (Inhibition of O2− or H2O2 production by diphenylene iodonium is generally regarded as presumptive evidence for an NADPH oxidase.) Furthermore, the K+ current in these cells increased when the cells were exposed to H2O2. These results suggest that the cells contain an NADPH oxidase whose output is a function of the ambient oxygen tension and whose dismuted product (ie, H2O2) regulates the flow of current through the K+ channel, the latter representing the signal by which the oxygen tension is communicated to the rest of the organism.
Plants contain an NADPH oxidase that they use for host defense. Cells from suspension cultures of Arabidopsis thaliana produced O2− when activated with phorbol myristate acetate. Harpin, a bacterial elicitor protein,115had the same effect, eliciting the production of O2− from the cultured cells. TheArabidopsis cells were found to contain proteins that were recognized by antibodies against human p47PHOX and p67PHOX, and membranes from the plant produced O2− when incubated with human neutrophil cytosol under activating conditions.
Finally, a b-type cytochrome homologous to human gp91PHOX was discovered in yeast.116This protein is an iron reductase that contains a low-potential heme and sequences homologous to flavin-binding sequences in other proteins. Binding of the heme to the apoprotein requires the presence of four essential histidine residues, because the conversion of any of these essential histidines to alanine resulted in a protein with no heme spectrum.117 This protein, the product of the yeast FRE1 gene, participates in iron uptake by the yeast.
The turnover number is the number of molecules of product made each second by one molecule of enzyme.
Cytochrome b−245 is the name uniquely given to cytochrome b558 by investigators from a particular laboratory.
The FcγII and FcγIII are Ig receptors that are expressed on the surfaces of phagocytes. Occupation of the FcγII receptor stimulates the oxidase, but occupation of the FcγIII receptor alone inhibits oxidase activation.
Secretory vesicles are small intracellular vesicles that fuse rapidly with plasma membrane to discharge their contents to the exterior (or to the lumen of a phagosome) in response to appropriate stimuli.
Supported in part by US Public Health Service Grants No. R01 AI-24227, R01 AI-28479, R21 AI-41697, RR-00833, and the Stein Endowment Fund.
REFERENCES
Author notes
Address reprint requests to Bernard M. Babior, MD, PhD, The Scripps Research Institute, Division of Biochemistry-NX7, 10550 N Torrey Pines Rd, La Jolla, CA 92037.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal