Abstract
The natural course and the clinical significance of hepatitis G virus (HGV) infection were investigated in 106 pediatric patients who received chemotherapy for hematological malignancy or underwent bone marrow transplantation (BMT) using HGV-RNA and antibodies to the HGV-E2 protein (anti-E2). HGV markers were detected in 21 patients (19.8%; HGV-RNA in 19 and anti-E2 in 2). Longitudinal analysis of these HGV-infected patients showed that 1 had anti-E2 before the initial blood transfusion, 14 had persistent viremia, and 6 became clear of circulating HGV-RNA after completion of therapy, although 5 of the 6 HGV-cleared patients never developed anti-E2. Reactivation of HGV infection during chemotherapy was observed in two anti-E2–positive, HGV-RNA–negative patients; the reappearance of the same HGV strain was confirmed by phylogenetic analysis. Among BMT survivors without other known causes of liver dysfunction, HGV-RNA–positive patients had a higher peak serum alanine amino transferase (ALT) value than negative patients. Contrary to previous reports, immunosuppressed patients can apparently recover from HGV infection without detectable anti-E2 and some patients who supposedly recovered from HGV infection can nonetheless suffer exacerbation when subsequently immunosuppressed.
PATIENTS WITH HEMATOLOGIC malignancies and bone marrow transplant (BMT) recipients are at high risk for acquiring blood-borne viruses because of a large number of blood transfusions.1-3 Furthermore, a severe immunodeficiency in these patients, caused by the underlying diseases and by intensive chemotherapy or immunosuppressive therapy, often affects the course of the viral infection.4 5
Two new blood-borne viruses were recently described, and the viral isolates were named GB virus C6 and hepatitis G virus (HGV).7 Sequence analyses of these viruses showed that they are possibly different genotypes of the same virus8 and belong to the Flaviviridae family, with a similar genomic organization to hepatitis C virus (HCV).9 We refer to this virus simply as HGV throughout this report. HGV is universally prevalent, and the viral RNA has been detected in 1.5% to 2.0% of volunteer blood donors in the United States7,10 and also in Japan.11 An increased prevalence of viremia has been reported for persons with parenteral exposure: more than 40% of leukemia patients and BMT recipients.3 12 Although HGV has been associated with transfusion-related or other types of hepatitis, its clinical relevance and the long-term course of infection remain largely unresolved.
The recent availability of serologic tests for the detection of antibodies to the recombinant HGV second envelope protein (anti-E2) permits us to clarify new aspects of HGV infection;13,14 longitudinal analysis of patients with posttransfusion hepatitis showed that the development of anti-E2 was associated with loss of HGV viremia, indicating that serum anti-E2 serves as a marker of recovery from HGV infection.15Studies detecting both HGV-RNA and anti-E2 should therefore bring progress in our understanding of the natural course and clinical significance of HGV infection.
In this report, we analyze HGV-RNA and anti-E2 sequentially in pediatric BMT recipients and hematological malignancies to make the following clear: first, the natural course and long-term clinical outcome of HGV infection in these patients, and second, whether this virus may contribute to liver dysfunction in survivors who have undergone BMT and chemotherapy.
MATERIALS AND METHODS
Patients.
A total of 106 consecutive patients who were treated at two pediatric hematological departments in Ibaraki prefecture in Japan were included in the cross-sectional study to assess the prevalence of HGV infection. They received BMT (42 patients) or were treated with conventional combined chemotherapy for hematological malignancy (64 patients). There were 54 boys and 52 girls. Mean age at diagnosis was 5.7 years (range, 6 months to 15 years). Anti-HCV antibody was detected in 14 patients. Those who had an increased serum alanine amino transferase (ALT) value for longer than 3 months were also tested with HCV-RNA, and 11 were positive for HCV-RNA. None was positive for hepatitis B virus surface (HBs) antigen or human immunodeficiency virus (HIV) antibody. The study population included 57 cases of acute lymphoblastic leukemia, 17 of acute myeloid leukemia, 11 of non-Hodgkin’s lymphoma, 6 of severe aplastic anemia, 5 of neuroblastoma, 3 of other solid tumors, 3 of chronic myeloid leukemia, 2 of Kostman’s hereditary neutropenia, and 1 of severe combined immunodeficiency. Seventeen of these patients were treated with related allogeneic BMT, 16 with unrelated allogeneic BMT, and 9 with autologous BMT.
In the longitudinal study to analyze the natural course of HGV infection, serial serum samples from patients with positive HGV-RNA and/or anti-E2 antibody in the cross-sectional study were retrospectively collected from frozen samples stored between 1987 and 1997. These stored samples included sera taken from each patient before treatment, at least twice while on therapy and about once yearly during the follow-up period. Samples for detection of anti-E2 were obtained at least 6 months apart from receiving transfusion or Ig preparations to avoid detection of passively transferred antibodies. The blood obtained was centrifuged within 4 hours, a part of the serum was examined for liver function, and another part was frozen and stored at −80°C until HGV testing. Patients gave consent for samples to be taken for investigational use.
The ALT value was measured at least twice a week during inpatient stay and on every hospital visit. Mean ALT values in individual patients were calculated by averaging the mean of ALT values measured during each month so as to exclude the influence of frequent measurement of ALT in those with abnormal liver function tests. To analyze the participation of HGV infection in liver dysfunction, we reviewed the medical records to determine the ALT value and other factors such as ferritin, transfused red blood cell units, and episodes of acute and chronic graft-versus-host disease (GVHD) and veno-occlusive disease (VOD). Donor exposures were counted using the total number of individual units of red blood cells, platelets, and fresh frozen plasma. Patients who had any hepatopathic factor, such as a positive hepatitis virus marker, including HCV antibodies, HCV-RNA, or chronic GVHD, were excluded from the analysis of liver function studies.
Detection of HGV-RNA.
Methods that detected HGV-RNA were reported elsewhere.16Briefly, RNA extracted from 125 μL of serum was reverse transcribed and served for nested polymerase chain reaction (PCR) with two sets of primers corresponding to 5′-noncoding (5NC) and helicase (NS3) regions of HGV. Primers specific for the 5NC region have been described by Orito et al17 and those for NS3 by Simons et al.6 The second-round PCR products were run on 3% SYNERGEL (Diversified Biotech, Boston, MA) and examined for size. The presence of HGV RNA in the tested sample was defined by the positive finding of PCR products of either the 5NC or NS3 region. The correspondence of the results by primer sets of 5NC and of NS3 was 98% of all samples.
Phylogenetic analysis.
The E2 region containing 312 bp was amplified from cDNA with primers, as described by Kao et al.18 Both first and second PCR included 35 cycles consisting of denaturation for 30 seconds at 94.0°C, annealing for 60 seconds at 53.0°C and 55.0°C for the first and second cycle, respectively, extension for 90 seconds at 72°C, and final extension for 7 minutes at 72°C. Three of the 50 μL of the first-round PCR product served as the template in the second-round PCR. PCR products were confirmed for size with SYNERGEL. PCR products of 5NC, NS3, and E2 regions were directly sequenced using cycle sequence protocol, with reagents supplied with the Taq Dye Terminator Cycle Sequencing Kit (ABI, Foster City, CA) and automated sequencer (ABI PRISM 377 DNA Sequencer; ABI). Phylogenetic analysis of the E2 region was performed by the neighbor-joining method,19 which is as efficient as other available methods, or more, for constructing the correct phylogenetic tree.20
Measurement of anti-E2 antibodies to HGV.
Anti-E2 antibody was measured with an immunoassay kit (Enzymun-Test Anti-HGenv; Boehringer Mannheim, Mannheim, Germany) developed by Tacke et al.13 The principle of assay is the enzyme-linked immunosorbent assay (ELISA)/3-step sandwich assay using Streptavidin technology. Briefly, recombinant HGV envelope protein from CHO cells binding to biotinylated monoclonal anti-E2 antibodies and a serum sample were incubated in a Streptavidin-coated ELISA plate and, after washing, again incubated with peroxidase-labeled antihuman-Fcγ antibodies. After adding a substrate of ABTS [2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonate)] and H2O2 and development of color, an absorbance of 422 nm was measured. Positive results were assured by the confirmatory test according to the instruction manual. That is, each serum sample was measured in parallel with either lysates of HGV-E2 region-transfected and untransfected CHO cells, and sera were defined as positive for anti-E2 if the absorbance of anti-E2/anti-CHO ratio was greater than 1.5.
Statistics.
Both the χ2 test and the Fisher exact test were used for qualitative variables, and the Student’s t-test was used for quantitative variables. A P value less than .05 was considered as being significant. All statistical analysis was performed using StatView for Macintosh (Abacus Concepts, Inc, Berkeley, CA).
RESULTS
Cross-sectional study in 1993.
To assess the prevalence of HGV infection, serum samples collected in 1993 were tested for HGV-RNA and anti-E2. Among 106 patients, HGV-RNA and anti-E2 were detected in 19 (17.9%) and 2 patients (1.9%), respectively. The two HGV markers did not coexist in any patient. At the time of sample collection, 74 patients were still under treatment with immunosuppressive agents or antileukemic drugs and the other 32 had completed treatment. The prevalence of HGV-RNA and anti-E2 according to the stage of treatment is indicated in Table 1. There was no significant difference in HGV positivity rate between those undergoing chemotherapy or immunosuppressive therapy and those having completed therapy. In total, the frequency of HGV infection in our cohort, calculated as the positivity rate of HGV-RNA and anti-E2, was 21 of 106 (19.8%).
Results of HGV Markers According to Treatment Stages in Cross-Sectional Study
| . | Patients Undergoing Treatment (n = 74) . | Patients Having Completed Therapy (n = 32) . |
|---|---|---|
| HGV-RNA positive | 16/74 (21.6%) | 3/32 (9.4%) |
| Anti-E2 positive | 1/74 (1.3%) | 1/32 (3.1%) |
| . | Patients Undergoing Treatment (n = 74) . | Patients Having Completed Therapy (n = 32) . |
|---|---|---|
| HGV-RNA positive | 16/74 (21.6%) | 3/32 (9.4%) |
| Anti-E2 positive | 1/74 (1.3%) | 1/32 (3.1%) |
Abbreviation: anti-E2, anti-E2 antibody.
Longitudinal study of HGV markers.
To clarify the natural course of HGV infection in hematological malignancy, the 21 patients who showed either HGV-RNA or anti-E2 positivity in cross-sectional study were precisely examined by analysis using serially collected sera. The results are shown in Table 2. Among the 21 patients with HGV infection, 6 (patients no. 1 through 6) had become clear of HGV-RNA during their off-therapy period. None became HGV-RNA–negative under chemotherapy or immunosuppressive therapy. Of the 6 who became clear of HGV-RNA, only 1 patient (patient no. 1) developed anti-E2 concomitantly with clearance of HGV-RNA; however, the anti-E2 had disappeared after 3 years and he presented no HGV marker thereafter. The other 9 survivors (patients no. 7 through 15) remained HGV-RNA–positive at the last sample collection. The HGV-RNA viremia persisted over a follow-up period of 3 to 10 years. Five patients (patients no. 16 through 20) had died with HGV viremia while undergoing chemotherapy. One other patient (patient no. 21) had been positive for anti-E2 before initial blood transfusion. The long-term outcome of HGV infection in the survivors was as follows: HGV viremia was cleared in 3 of 6 (50%) of BMT recipients, 3 of 9 (33%) of children treated with conventional chemotherapy, and 6 of 15 (40%) of all survivors. There was no significant difference in the clearance rate according to the treatment modality.
Longitudinal Study of 21 HGV-Infected Patients
| Patient No. . | Sex/Age at Diagnosis . | Outcome of HGV Infection . | Duration of Viremia (first to last detection date [yr]) . | Duration of Treatment . | Pattern of Development of Anti-E2 . | Modality of Treatment . | Diseases . |
|---|---|---|---|---|---|---|---|
| 1 | F/15 | Clear with anti-E2 | 1989-92 (3) | 1989-90 | − → + → − | CX | NHL |
| 2 | M/8 | Clear without anti-E2 | 1992-95 (3) | 1991-92 | − | CX | NHL |
| 3 | M/4 | Clear without anti-E2 | 1993-96 (3) | 1993-95 | − | rBMT | ALL |
| 4 | F/4 | Clear without anti-E2 | 1993-95 (2) | 1993-94 | − | CX | ALL |
| 5 | F/4 | Clear without anti-E2 | 1993-95 (2) | 1992-93 | − | rBMT | NB |
| 6 | M/13 | Clear without anti-E2 | 1992-94 (2) | 1989-93 | − | rBMT | ALL |
| 7 | F/3 | Persistence of viremia | Before 1987-97 (>10) | 1982-83 | − | CX | ALL |
| 8 | M/2 | Persistence of viremia | 1988-97 (>9) | 1988-90 | − | CX | ALL |
| 9 | F/2 | Persistence of viremia | 1991-97 (>6) | 1990-92 | − | CX | NHL |
| 10 | M/2 | Persistence of viremia | 1991-97 (>6) | 1987-97 | − | CX | ALL |
| 11 | M/3 | Persistence of viremia | 1992-97 (>5) | 1990-92 | − | rBMT | SAA |
| 12 | M/14 | Persistence of viremia | 1993-97 (>4) | 1992-97 | − → + → − | uBMT | ALL |
| 13 | M/8 | Persistence of viremia | 1993-97 (>4) | 1992-97 | + → − | CX | ALL |
| 14 | F/11 | Persistence of viremia | 1993-97 (>3) | 1993-96 | − | uBMT | AML |
| 15 | F/6 | Persistence of viremia | 1993-96 (>3) | 1993-94 | − | CX | ALL |
| 16 | F/4 | Died with positive HGV-RNA | Before 1987-97 (>10) | 1984-97 | − | aBMT | ALL |
| 17 | M/6 | Died with positive HGV-RNA | Before 1987-93 (>6) | 1985-93 | − | CX | ALL |
| 18 | F/3 | Died with positive HGV-RNA | Before 1987-93 (>6) | 1986-93 | − | CX | ALL |
| 19 | M/8 | Died with positive HGV-RNA | 1991-94 (3) | 1990-94 | − | uBMT | AML |
| 20 | F/11 | Died with positive HGV-RNA | 1993-94 (1) | 1992-94 | − | aBMT | ALL |
| 21 | M/11 | Acquired anti-E2 before transfusion | — (0) | 1993-95 | + | CX | ALL |
| Patient No. . | Sex/Age at Diagnosis . | Outcome of HGV Infection . | Duration of Viremia (first to last detection date [yr]) . | Duration of Treatment . | Pattern of Development of Anti-E2 . | Modality of Treatment . | Diseases . |
|---|---|---|---|---|---|---|---|
| 1 | F/15 | Clear with anti-E2 | 1989-92 (3) | 1989-90 | − → + → − | CX | NHL |
| 2 | M/8 | Clear without anti-E2 | 1992-95 (3) | 1991-92 | − | CX | NHL |
| 3 | M/4 | Clear without anti-E2 | 1993-96 (3) | 1993-95 | − | rBMT | ALL |
| 4 | F/4 | Clear without anti-E2 | 1993-95 (2) | 1993-94 | − | CX | ALL |
| 5 | F/4 | Clear without anti-E2 | 1993-95 (2) | 1992-93 | − | rBMT | NB |
| 6 | M/13 | Clear without anti-E2 | 1992-94 (2) | 1989-93 | − | rBMT | ALL |
| 7 | F/3 | Persistence of viremia | Before 1987-97 (>10) | 1982-83 | − | CX | ALL |
| 8 | M/2 | Persistence of viremia | 1988-97 (>9) | 1988-90 | − | CX | ALL |
| 9 | F/2 | Persistence of viremia | 1991-97 (>6) | 1990-92 | − | CX | NHL |
| 10 | M/2 | Persistence of viremia | 1991-97 (>6) | 1987-97 | − | CX | ALL |
| 11 | M/3 | Persistence of viremia | 1992-97 (>5) | 1990-92 | − | rBMT | SAA |
| 12 | M/14 | Persistence of viremia | 1993-97 (>4) | 1992-97 | − → + → − | uBMT | ALL |
| 13 | M/8 | Persistence of viremia | 1993-97 (>4) | 1992-97 | + → − | CX | ALL |
| 14 | F/11 | Persistence of viremia | 1993-97 (>3) | 1993-96 | − | uBMT | AML |
| 15 | F/6 | Persistence of viremia | 1993-96 (>3) | 1993-94 | − | CX | ALL |
| 16 | F/4 | Died with positive HGV-RNA | Before 1987-97 (>10) | 1984-97 | − | aBMT | ALL |
| 17 | M/6 | Died with positive HGV-RNA | Before 1987-93 (>6) | 1985-93 | − | CX | ALL |
| 18 | F/3 | Died with positive HGV-RNA | Before 1987-93 (>6) | 1986-93 | − | CX | ALL |
| 19 | M/8 | Died with positive HGV-RNA | 1991-94 (3) | 1990-94 | − | uBMT | AML |
| 20 | F/11 | Died with positive HGV-RNA | 1993-94 (1) | 1992-94 | − | aBMT | ALL |
| 21 | M/11 | Acquired anti-E2 before transfusion | — (0) | 1993-95 | + | CX | ALL |
Abbreviations: CX, chemotherapy; rBMT, related allogeneic bone marrow transplantation; uBMT, unrelated allogeneic bone marrow transplantation; aBMT, autologous bone marrow transplantation.
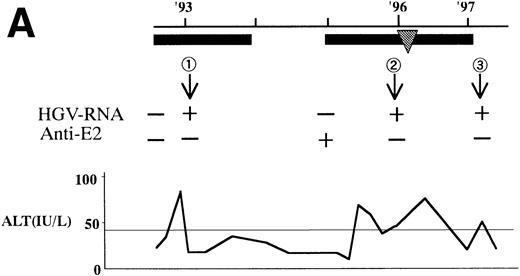
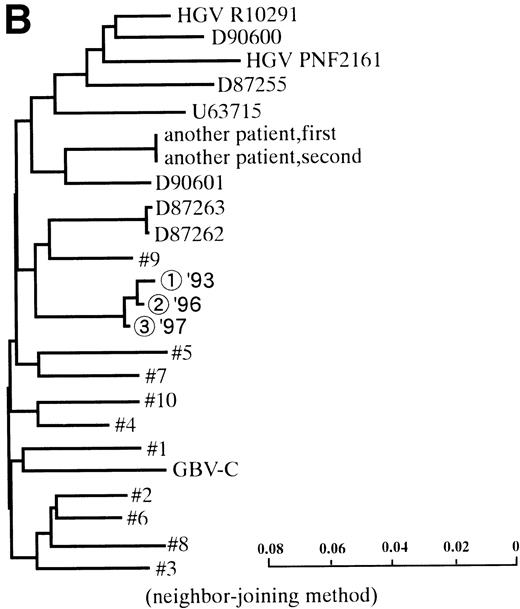
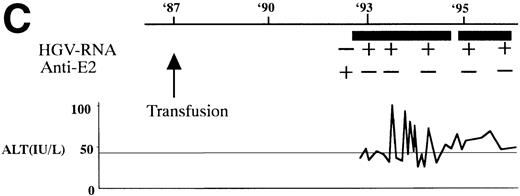
In 2 patients who were persistently HGV-RNA–positive in 1997, retrospective evaluation disclosed relapsing of HGV viremia during execution of chemotherapy for acute lymphoblastic leukemia (ALL). Patient no. 12 was infected with HGV during the first remission induction therapy. During the off-therapy period, which lasted for 1 year, while the immunocompetence seemed to be restored, HGV-RNA became undetectable simultaneously with the development of anti-E2. However, during the second remission induction against relapsing ALL, anti-E2 had disappeared concurrently with the reappearance of HGV-RNA (Fig 1A). To investigate whether this HGV-RNA was due to reactivation of the former strain or reinfection by other strains, phylogenetic analysis of HGV sequences from different isolates was performed (Fig 1B). In each of the 5NC, NS3 (data not shown), and E2 regions, the reappearing HGV consistently showed the nearest homology with the formerly infecting isolate. Patient no. 13 (Fig 1C) had been transfused with red blood cells at the time of operation for spontaneous occlusion of the circle of Willis (Moyamoya disease),21 5 years before the development of ALL. He had been anti-E2–positive and HGV-RNA–negative at the start of remission induction therapy for ALL, before the initial transfusion of blood products. After chemotherapy was initiated, anti-E2 disappeared and, concurrently, HGV-RNA became positive.
(A) HGV markers and serum ALT levels of a patient (patient no. 12) with relapse of HGV viremia during chemotherapy. The patient was 14 years old at development of ALL. The horizontal line and solid bar mean years from diagnosis and periods under immunosuppressive therapy or chemotherapy, respectively. The inverted triangle indicates the timing of BMT. In the clinical course of ALT, the normal range for ALT is indicated as a horizontal line. Phylogenetic analysis was performed with the strains obtained at the timing of figures in the circle. (B) Phylogenetic analysis of different HGV isolates from the patient with reactivation of HGV infection. Figures in the circles show the strains from patient no. 12. Homology of nucleotide sequence of 312-bp E2 cDNA between reappearing and former isolates from patient no. 12 were 98% to 99%, although the homology among the other sequences was 79.8% to 88.3%. In the phylogenetic tree, HGV R10291 and PNF2164 are in the same E2 regions of the original HGV strain, and GBV-C is also in the original. We sequenced two strains derived from another patient (patient no. 15), and the strain first appearing showed just the same nucleotide sequence as the other that was obtained 2 years later. D90600, 90601, 87262, 87263, and U63715 are the accession numbers of previously reported HGV or GBV-C sequences in GenBank. Numbers 1 through 10 are strains derived from Taiwanese patients reported by Kao et al.18 (C) HGV markers and serum ALT levels of another patient (patient no. 15) with reactivation of HGV viremia during chemotherapy. Clinical courses are represented by similar notations as in (A).
(A) HGV markers and serum ALT levels of a patient (patient no. 12) with relapse of HGV viremia during chemotherapy. The patient was 14 years old at development of ALL. The horizontal line and solid bar mean years from diagnosis and periods under immunosuppressive therapy or chemotherapy, respectively. The inverted triangle indicates the timing of BMT. In the clinical course of ALT, the normal range for ALT is indicated as a horizontal line. Phylogenetic analysis was performed with the strains obtained at the timing of figures in the circle. (B) Phylogenetic analysis of different HGV isolates from the patient with reactivation of HGV infection. Figures in the circles show the strains from patient no. 12. Homology of nucleotide sequence of 312-bp E2 cDNA between reappearing and former isolates from patient no. 12 were 98% to 99%, although the homology among the other sequences was 79.8% to 88.3%. In the phylogenetic tree, HGV R10291 and PNF2164 are in the same E2 regions of the original HGV strain, and GBV-C is also in the original. We sequenced two strains derived from another patient (patient no. 15), and the strain first appearing showed just the same nucleotide sequence as the other that was obtained 2 years later. D90600, 90601, 87262, 87263, and U63715 are the accession numbers of previously reported HGV or GBV-C sequences in GenBank. Numbers 1 through 10 are strains derived from Taiwanese patients reported by Kao et al.18 (C) HGV markers and serum ALT levels of another patient (patient no. 15) with reactivation of HGV viremia during chemotherapy. Clinical courses are represented by similar notations as in (A).
Liver dysfunction in HGV infection.
There seemed to be many causes of liver damage in the subjects studied. To determine the possible role of HGV in liver dysfunction, patients without any known cause of hepatotoxicity, including HCV-antibodies, HCV-RNA, HBs-antigen, HBV-DNA, antinuclear antibodies, anti-smooth muscle antibody, acute and chronic GVHD, VOD, and iron overload, were analyzed. To exclude drug influence, we examined the liver function only after completion of chemotherapy or immunosuppressive therapy. Among this subgroup, liver function was compared between the HGV-infected and uninfected patients in each treatment modality (Table 3). In the BMT recipients, HGV-infected subjects showed higher maximum ALT values than HGV-uninfected (P = .0064), although there was no such difference in the patients who received conventional chemotherapy alone. The clinical characteristics of these BMT recipients were evaluated according to their HGV status (Table 4), but there was no significant difference in respect of BMT modalities, conditioning regimen, donor exposure, or ferritin within 1 year after completion of immunosuppressive therapy. Transfused red blood cell units of HGV-infected BMT recipients were more than those of the uninfected, but the serum ferritin was elevated equally in both groups. Among these HGV-infected BMT recipients, 3 (patients no. 3, 5, and 6) later became cleared of HGV viremia. All three had transient peak ALT levels (77 to 91 IU/L) only during the viremic period. The maximum ALT values during the viremic period in these 3 patients were higher than those after clearance of HGV-RNA (P = .0366).
Liver Function in 31 Evaluable Patients According to HGV Status and Treatment Modalities After Completion of Drug Therapy
| . | HGV-RNA . | |||
|---|---|---|---|---|
| Positive . | Negative . | |||
| BMT (n = 6) . | Chemotherapy (n = 4) . | BMT (n = 10) . | Chemotherapy (n = 11) . | |
| Maximum ALT, IU/L (mean ± SD) | 88.0 ± 19.63-150,3-151 | 16.3 ± 0.4 | 37.2 ± 4.23-152 | 18.4 ± 5.0 |
| Abnormal/total | 6/6 | 0/4 | 4/10 | 0/11 |
| % Patients with abnormal levels | 100 | 0 | 40 | 0 |
| Mean ALT (mean ± SD) | 44.9 ± 15.6 | 10.7 ± 0.8 | 20.9 ± 2.33-153 | 11.1 ± 2.9 |
| . | HGV-RNA . | |||
|---|---|---|---|---|
| Positive . | Negative . | |||
| BMT (n = 6) . | Chemotherapy (n = 4) . | BMT (n = 10) . | Chemotherapy (n = 11) . | |
| Maximum ALT, IU/L (mean ± SD) | 88.0 ± 19.63-150,3-151 | 16.3 ± 0.4 | 37.2 ± 4.23-152 | 18.4 ± 5.0 |
| Abnormal/total | 6/6 | 0/4 | 4/10 | 0/11 |
| % Patients with abnormal levels | 100 | 0 | 40 | 0 |
| Mean ALT (mean ± SD) | 44.9 ± 15.6 | 10.7 ± 0.8 | 20.9 ± 2.33-153 | 11.1 ± 2.9 |
All of the patients in the table were negative for other hepatopathic factors, including HCV-RNA, HBs antigen, autoimmune antibodies, and drug use. The values of mean ALT represent the average of the mean ALT values of each patient measured during each month of the hospital course.
Abbreviation: ALT, alanine aminotransferase, normal value <40 IU/L.
P = .0064 HGV-infected versus uninfected.
P = .0189 BMT versus chemotherapy.
P = .0003 BMT versus chemotherapy.
P = .0005 BMT versus chemotherapy.
ALT Levels After Completion of Immunosuppressive Therapy Accompanying BMT With and Without HGV
| Patient No. . | BMT . | Conditioning Regimen . | Donor Exposure (person) . | Transfused RBC (U) . | Ferritin (ng/dL) . | During HGV Viremic Period . | |
|---|---|---|---|---|---|---|---|
| Maximum ALT (IU/L) . | Mean ALT (IU/L) . | ||||||
| HGV-infected population (n = 6) | |||||||
| 3 | Related | TBI + L-PAM + VP | 309 | 86 | 1,100 | 91 | 29.8 ± 6.1 |
| 5 | Related | TBI + L-PAM + BU | 26 | 20 | 620 | 82 | 38.8 ± 58.1 |
| 6 | Related | TBI + L-PAM + ATG | 55 | 23 | 390 | 77 | 23.5 ± 16.8 |
| 11 | Related | ALG + CY | 46 | 15 | 1,440 | 179 | 118.5 ± 39.2 |
| 12 | Unrelated | TBI + L-PAM + VP | 126 | 39 | 280 | 55 | 27.3 ± 13.6 |
| 14 | Unrelated | TBI + CY | 31 | 8 | >500 | 44 | 17.4 ± 8.6 |
| Mean ± SD | 98.8 ± 109.1 | 31.8 ± 28.54-150 | 1,242 ± 1,188 | 88.0 ± 19.64-151 | 44.9 ± 15.6 | ||
| HGV uninfected population (n = 10) | |||||||
| 22 | Autologous | CP + VP + L-PAM | 34 | 12 | — | 27 | 12.1 ± 6.3 |
| 23 | Unrelated | TBI + CY + ATG | 16 | 10 | — | 30 | 18.7 ± 4.5 |
| 24 | Related | BU + BP + CY | 77 | 12 | 2,500 | 29 | 15.6 ± 2.2 |
| 25 | Related | TBI + CY | 15 | 5 | 270 | 65 | 18.4 ± 10.6 |
| 26 | Related | TBI + L-PAM | 67 | 17 | 650 | 28 | 15.4 ± 3.8 |
| 27 | Related | TBI + L-PAM | 16 | 8 | — | 21 | 15.6 ± 2.8 |
| 28 | Related | BU + VP | 56 | 25 | 3,000 | 46 | 26.9 ± 9.3 |
| 29 | Related | TBI + VP + L-PAM | 15 | 3 | 380 | 34 | 23.8 ± 5.8 |
| 30 | Related | BU + L-PAM | 32 | 12 | — | 46 | 35.3 ± 10.4 |
| 31 | Related | TBI + CY + HDAC + ATG | 18 | 0 | 650 | 46 | 27.5 ± 10.0 |
| Mean ± SD | 34.6 ± 23.7 | 10.4 ± 7.2 | 722 ± 452 | 37.2 ± 4.2 | 20.9 ± 2.3 | ||
| Patient No. . | BMT . | Conditioning Regimen . | Donor Exposure (person) . | Transfused RBC (U) . | Ferritin (ng/dL) . | During HGV Viremic Period . | |
|---|---|---|---|---|---|---|---|
| Maximum ALT (IU/L) . | Mean ALT (IU/L) . | ||||||
| HGV-infected population (n = 6) | |||||||
| 3 | Related | TBI + L-PAM + VP | 309 | 86 | 1,100 | 91 | 29.8 ± 6.1 |
| 5 | Related | TBI + L-PAM + BU | 26 | 20 | 620 | 82 | 38.8 ± 58.1 |
| 6 | Related | TBI + L-PAM + ATG | 55 | 23 | 390 | 77 | 23.5 ± 16.8 |
| 11 | Related | ALG + CY | 46 | 15 | 1,440 | 179 | 118.5 ± 39.2 |
| 12 | Unrelated | TBI + L-PAM + VP | 126 | 39 | 280 | 55 | 27.3 ± 13.6 |
| 14 | Unrelated | TBI + CY | 31 | 8 | >500 | 44 | 17.4 ± 8.6 |
| Mean ± SD | 98.8 ± 109.1 | 31.8 ± 28.54-150 | 1,242 ± 1,188 | 88.0 ± 19.64-151 | 44.9 ± 15.6 | ||
| HGV uninfected population (n = 10) | |||||||
| 22 | Autologous | CP + VP + L-PAM | 34 | 12 | — | 27 | 12.1 ± 6.3 |
| 23 | Unrelated | TBI + CY + ATG | 16 | 10 | — | 30 | 18.7 ± 4.5 |
| 24 | Related | BU + BP + CY | 77 | 12 | 2,500 | 29 | 15.6 ± 2.2 |
| 25 | Related | TBI + CY | 15 | 5 | 270 | 65 | 18.4 ± 10.6 |
| 26 | Related | TBI + L-PAM | 67 | 17 | 650 | 28 | 15.4 ± 3.8 |
| 27 | Related | TBI + L-PAM | 16 | 8 | — | 21 | 15.6 ± 2.8 |
| 28 | Related | BU + VP | 56 | 25 | 3,000 | 46 | 26.9 ± 9.3 |
| 29 | Related | TBI + VP + L-PAM | 15 | 3 | 380 | 34 | 23.8 ± 5.8 |
| 30 | Related | BU + L-PAM | 32 | 12 | — | 46 | 35.3 ± 10.4 |
| 31 | Related | TBI + CY + HDAC + ATG | 18 | 0 | 650 | 46 | 27.5 ± 10.0 |
| Mean ± SD | 34.6 ± 23.7 | 10.4 ± 7.2 | 722 ± 452 | 37.2 ± 4.2 | 20.9 ± 2.3 | ||
The values of mean ALT represent the average of the mean ALT values of each patient measured during each month of the hospital course.
Abbreviations: TBI, total body irradiation; L-PAM, melphalan; BU, busulfan; VP, etoposide; ALG, antilymphocyte globulin; ATG, antithymocyte globulin; CY, cyclophosphamide; HDAC, high-dose cytosine arabinoside.
P = .0366 HGV-infected versus uninfected.
P = .0064 HGV-infected versus uninfected.
Among the 9 survivors with persistent HGV infection, only 1 (patient no. 11) suffered from chronic liver dysfunction, with ALT values at 100 to 180 IU/L, for more than 5 years after cessation of drug therapy. He did not have any autoantibodies, HCV antibodies, or HCV-RNA. Anti-HBs and anti-HBc antibody were positive, but HBs antigen was negative and HBV-DNA was never detected by nested PCR in either serum or liver tissue. No marker of other hepatitis virus, EB virus, cytomegalovirus, or herpesvirus indicated any other etiologic factor for the liver dysfunction.
DISCUSSION
The natural course of HGV infection is poorly understood.15This is the first study investigating the natural history and clinical outcome of HGV infection in patients with hematological malignancy and BMT recipients. Our findings clearly show that these patients are frequently exposed to HGV due to multiple blood transfusions and that they suffer from severe immunosuppression, which has a significant influence on the clearance of HGV viremia and the production of anti-E2 immune response.
The prevalence of HGV-RNA in these pediatric patients with hematological malignancy or BMT recipients was 17.9%. This percentage is lower than that reported in a group of adult patients (30% to 48%).3,12 The discrepancy may be related to the age, therapeutic stage, or geographic area of the study populations. The study of serial samples showed that all the patients with HGV viremia became positive for HGV-RNA after the beginning of the therapy, indicating that the HGV was transmitted almost solely by blood transfusion. It is noteworthy that the prevalence of anti-E2 was much lower than that of HGV-RNA in this cohort. In contrast, all previous reports, including our own data,16 from various immunocompetent subjects showed that the prevalence of anti-E2 was higher than or at least equal to that of HGV-RNA. This difference implies an immunodeficiency status in our study population that induces the persistence of HGV viremia and suppression of the anti-E2 immune response. It is theoretically possible that the pediatric age of the study population may play some role in lowering the prevalence of anti-E2, because in HBV infection, infants hardly acquire any anti-HBs antibody.22 Up to now, there has been no report concerning anti-E2 production in children, but we observed that 4 of 6 immunocompetent children who were infected with HGV by transfusion developed a persistent anti-E2 response (unpublished data). Further study will clarify this point.
The results of the longitudinal study of HGV markers in this cohort, which demonstrated the natural course of HGV infection in the immunodeficient status, differ in several points from those in immunocompetent subjects, which have been previously reported. In studies of posttransfusion hepatitis and intravenous drug users, more than half of the acute HGV infections were transient and HGV-RNA cleared spontaneously within a rather short period.23,24Among the 19 cases of acute HGV infection in this study, none had eliminated HGV viremia in the immunosuppression status under chemotherapy or BMT, indicating that this immunosuppression interfered with HGV clearance. This may account for the higher prevalence of HGV-RNA in BMT and renal transplant recipients.25 26
Anti-E2 has been interpreted as the marker of clearance of HGV infection, because the antibody appears after or just before the clearance of HGV-RNA.13 In previous reports, almost all of the patients who became clear of HGV-RNA developed anti-E2.13-15 In contrast, among the 6 patients with self-limiting HGV infections in our series, 5 became clear of HGV viremia without any anti-E2 immune response. These findings evidently show that the immune response to anti-E2 does not play an essential role in the recovery from HGV viremia. We previously reported hemophiliacs who became clear of HGV-RNA several years before the appearance of anti-E2. Tacke et al15 also reported one case who became HGV-RNA–negative before any anti-E2 response and another who had coexistent anti-E2 and HGV-RNA for at least 75 weeks and suggested that the cytotoxic T cell may play an important role in the elimination of HGV. This plausible hypothesis is further supported by the present study.
In addition, anti-E2 has been used as a marker of recovery from HGV infection in calculating the HGV-exposure rate in epidemiological studies. Anti-E2 was detected in 3% to 14% of volunteer blood donors,13,15,27 41% to 85.2% of drug users,13,14,27 and 32% of hemophiliacs.16 In this series, the first study of immunocompromised patients, about half of the survivors of BMT and hematological malignancy were clear of HGV-RNA; however, anti-E2 was prevalent in only 3% or less in the cross-sectional and longitudinal studies. The lower prevalence of antiviral antibodies holds true also for HBV and HCV infections under the same immunosuppressed condition. Among patients seronegative for HBV and HCV after treatment of acute leukemia, 10.5% were revealed to be HBV antigen positive by histology.28 Only 70% of HCV-infected pediatric patients with acute lymphoblastic leukemia were positive for the HCV antibody in various serological assays, and the remaining 30% were seronegative despite positive HCV-RNA.29 These findings imply that the use of serological assay of anti-E2 is of limited value and that determination of HGV-RNA by PCR should be recommended in these patients.
Reactivation of HGV after anti-E2 immune response has never before been observed. It is important that these 2 patients became anti-E2–negative and HGV-RNA–positive simultaneously. These observations suggest the following two possibilities: ongoing replication of HGV at undetectable levels, or latent HGV, may occur after loss of detectable HGV viremia in concurrence with the development of anti-E2 response; and there may be neutralizing or protective activity in at least some anti-E2 antibodies, which usually suppress large-scale replication of HGV. In some cases of impaired immunocompetence, the loss of these antibodies may induce the reactivation of HGV. Future studies will clarify the mechanisms of persistent infection with HGV, a unique member of theFlaviviridae family.
Liver dysfunction frequently develops in patients with hematologic malignancy. Especially in BMT recipients, liver disease sometimes has a major impact on the prognosis. Besides the hepatotoxic effects of drugs, viral hepatitis (HBV or HCV), chronic GVHD, and iron overload are possible causes of such liver damage in long-term survivors.30 The pathogen remained undefined in 30% of hepatitis in survivors of pediatric malignancies.28 To clarify the influence of HGV infection alone on liver damage, we compared ALT levels, hepatitis markers, conditioning regimen, dose of transfusion, and serum ferritin between HGV-viremic and uninfected patients during off-therapy periods, according to the treatment modality. HGV infection seemed likely to have some relation to liver dysfunction in long-term survivors of BMT recipients, because, after exclusion of other known kinds of viral hepatitis and chronic GVHD, maximum ALT values of HGV-infected patients were statistically higher than those of uninfected patients, whereas mean values of ALT showed no difference. This finding would imply that the effect of HGV on ALT is transient and self-limiting, whereas the viral infection persists in almost all cases even after abnormal liver function is resolved. We also found a patient who was infected solely with HGV and presented persistent liver dysfunction over 5 years after therapy withdrawal. The clinical significance of the HGV infection remains unclear. Recent studies have suggested that transfusion-associated HGV infection rarely results in liver disease,31 although there are some reports indicating the pathogenic role of acute HGV infection in posttransfusion liver dysfunction.13,14 Up to now, HGV-related hepatotoxicity has been discussed in terms of viral factors such as specific viral strains in fulminant hepatitis32,33; however, our study implies that alteration of the host’s immunocompetence may influence the hepatotoxicity of HGV. A parallel can be drawn with HCV infection in immunodeficient patients; rapidly progressive HCV infection sometimes occurs in HIV-infected hemophiliacs34,35 and in congenital hypogammaglobulinemia.36 37 Further studies of large numbers of HGV-infected patients combined with pathological studies will be needed to establish the clinical significance of HGV infection in an immunosuppressed population.
Supported in part by a grant from the Mother and Child Health Foundation (No.10-6) and the Ministry of Health and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ryo Sumazaki, MD, Department of Pediatrics, Institute of Clinical Medicine, University of Tsukuba, Tsukuba, Ibaraki, 305 Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal