Abstract
We show a dramatic downregulation of the stem cell factor (SCF) receptor in different hematopoietic cell lines by murine stroma. Growth of the human erythroid/macrophage progenitor cell line TF-1 is dependent on granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-3 (IL-3). However, TF-1 cells clone and proliferate equally well on stroma. Independent stroma-dependent TF-1 clones (TF-1S) were generated on MS-5 stroma. Growth of TF-1S and TF-1 cells on stroma still requires interaction between c-kit (SCF receptor) and its ligand SCF, because antibodies against c-kit inhibit growth to less than 2%. Surprisingly, c-kit receptor expression (RNA and protein) was downregulated by 2 to 3 orders of magnitude in TF-1S and TF-1 cells grown on stroma. This stroma-dependent regulation of the kit receptor in TF-1 was also observed on exposure to kit ligand-negative stroma, thus indicating the need for heterologous receptor ligand interaction. Removal of stroma induced upregulation by 2 to 4 orders of magnitude. Downregulation and upregulation of c-kit expression could also be shown for the megakaryocytic progenitor cell line M-07e and was comparable to that of TF-1, indicating that stroma-dependent regulation of c-kit is a general mechanism. Downregulation may be an economic way to compensate for the increased sensitivity of the c-kit/ligand interaction on stroma. The stroma-dependent c-kit regulation most likely occurs at the transcriptional level, because mechanisms, such as splicing, attenuation, differential promoter usage, or mRNA stability, could be excluded.
THE c-kit GENE ENCODES a tyrosine kinase receptor of critical importance for dermal, gonadal, and hematopoietic stem cell development.1,2 The c-kit protein functions as a receptor for two different forms of stem cell factor (SCF), a soluble (sSCF) and a membrane bound ligand (mSCF). Both act in a qualitatively different manner.3,4Secretion of sSCF or presentation of mSCF by stroma cells suggests that the hematopoietic microenvironment regulates hematopoiesis by close cell-to-cell contact.5,6 In its soluble form, SCF induces short-term proliferation either alone or in synergism with other growth factors. Thus, primitive murine Sca-1+ hematopoietic progenitor cells respond better to a combination of hematopoietic factors if SCF is included in the cocktail.7 Stroma cells expressing mSCF, as opposed to those releasing sSCF, maintain human progenitors for several weeks.8 The different functions of SCF can be explained by mechanisms either involving the SCF ligand itself or its receptor.
To test the expression and function of molecules expected to play a key role in cell-cell interactions, we established systems for long-term growth of murine (ELM, Myl-D7)9,10 and human hematopoietic progenitor cells using coculture with a murine SCF expressing stroma cell line (MS-5). By inclusion of stroma, our cell culture systems combine the advantages of cell lines (eg, long-term proliferation and relatively stable phenotype) with an in vivo comparable situation of so far undefined cell-cell interactions. The human TF-1 cells used in this study were originally described to be strictly dependent on granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-3 (IL-3).11 We observed that MS-5 stroma cells can alternatively stimulate long-term growth of TF-1 cells (manuscript in preparation and this report). Several stroma adapted TF-1S lines (TF-1S #MB1-#MB8) were established by continued passage of cloned TF-1 on MS-5 stroma cells. After 3 months, neither GM-CSF nor IL-3 could maintain the long-term growth of the TF-1S clones (W. Ostertag, unpublished results). Thus, the TF-1S clones were considered to be stroma-dependent.
We used TF-1 and the M-07e megakaryoblastic cell line12 to establish the general relevance of the c-kit regulation. M-07e was either grown in suspension with the soluble growth factors GM-CSF or IL-3 or by coculture with stroma cells to test the c-kit receptor expression. We observed a stroma-dependent c-kit receptor modulation of up to 3 orders of magnitude within 90 days.
Because regulation of c-kit could be the result of different mechanisms, we performed expression analysis on several levels. The influence of stroma cells on the structure of the chromatin flanking the c-kit promoter as well as the influence of stroma on differential promoter usage, the transcript level, and alternative splicing was examined. Posttranscriptional controls may also affect the level of c-kit receptor, because low-level posttranscriptional downregulation of the related CSF-1R has been shown to occur by activation of other receptors in early murine multipotent progenitor cells13and in a special subset of late progenitor cells.14 Our data show that downregulation of the c-kit mRNA level does not result from stroma cell-mediated reduction of the mRNA stability. Therefore, c-kit expression is most likely regulated on the transcriptional level.
MATERIALS AND METHODS
Cell lines.
TF-1 cells show an early erythro-myeloid progenitor phenotype and depend for growth on either GM-CSF or IL-3.11 TF-1 cells were maintained in RPMI1640 medium supplemented with 10% fetal calf serum (FCS; GIBCO BRL, Grand Island, NY), 2 mmol/L glutamine, and 5 × 102 U/mL of recombinant human GM-CSF (rhGM-CSF) in tissue culture flasks. Strictly stroma-dependent TF-1 cell lines (TF-1S) were isolated by cloning (end point dilution) of TF-1 on lethally irradiated murine MS-515 (17,600 rad) or UNC Sl−/Sl−16 (3,900 rad) stroma cells and sequential transfer of the clones to new feeder cells every 1 to 2 weeks for several weeks. The medium for these stroma-dependent clones was the same as described above, except that GM-CSF was not used. For studies of the c-kit receptor expression, TF-1S cells were washed twice with medium and were subsequently transferred to uncoated petri dishes in the presence of RPMI medium with 10% FCS, 2 mmol/L glutamine, and 5 × 102 U/mL rhGM-CSF.
The megakaryoblastic cell line M-07e was cultured in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO BRL) supplemented with 10% FCS, 2 mmol/L glutamine, and 2 ng/mL rhIL-3 (Pharma Biotechnologie, Hannover, Germany).
The murine MS-5 stroma cells15 do not secrete GM-CSF.10 They were maintained in α-MEM supplemented with 20% horse serum (HS; BioWhittaker UK Ltd, Berkshire, UK) and 2 mmol/L glutamine. The stroma cell feeder was prepared by growing cells up to 40% confluency in tissue culture flasks and then 17,600 rad lethal irradiation. UNC Sl−/Sl−cells16 were cultured in RPMI/10% FCS/2 mmol/L glutamine.
Inhibition of growth by monoclonal c-kit antibody.
The monoclonal antibody (MoAb) YB5.B8 (anti–c-kit) has already been described.17 For analyzing proliferation of TF-1 on stroma, MS-5 cells (103 cells/well of microtiter plates) were plated in α-MEM, 20% HS, and 2 mmol/L glutamine and allowed to grow overnight. MS-5 cells were irradiated (17,600 rad). The medium was removed. One hundred TF-1 cells per well were seeded in prewarmed complete medium (RPMI/10% FCS/2 mmol/L glutamine) on MS-5 feeder. After incubation of the cells for 24 hours at 37°C, fresh medium containing the monoclonal c-kit antibody, as described in Fig 1, was added. TF-1 cells were counted after 5 days and the numbers were compared with a control without c-kit antibody (100% proliferation). TF-1 grown in suspension with GM-CSF was used as a negative control.
Flow cytometric analysis of c-kit+ cells.
Phycoerythrin (PE)-conjugated MoAb (MoAb YB5.B8) specific for c-kit was obtained from Dianova (Hamburg, Germany). PE-conjugated isotype-matched Ab (Simultest control; Becton Dickinson, San Jose, CA) was used as a negative control. Washed cells (106) were resuspended in 30 μL staining buffer (phosphate-buffered saline [PBS]/1% FCS) and incubated with appropriate amounts of PE-conjugated MoAb for detection of c-kit protein. Labeling was performed for 30 minutes at 4°C for anti–c-kit and control antibody. Labeled cells were washed three times with PBS/1% bovine serum albumin (BSA). Cells were analyzed with a FACScan (Becton Dickinson). Laser excitation was at 488 nm for PE.
RNA extraction and analysis.
RNA isolation and Northern analysis were performed as described.18 DNA fragments for hybridization probes were obtained from plasmid pUC 19/hckit,19 containing a full-length cDNA for c-kit, or were synthesized by reverse transcriptase-dependent polymerase chain reaction (RT-PCR), cloned into a PCR cloning vector (pCR; Invitrogen, NV Leek, The Netherlands), and checked for specificity by sequencing.
Specific RNA transcripts were amplified by the RT-PCR. RNA (1 μg) was reverse transcribed into cDNA by using avian myeloblastosis virus reverse transcriptase and primed by a mixture of oligo (dT)12-18 and random primer (hexanucleotide).
Specific DNA fragments were subsequently amplified with Taq polymerase (GIBCO BRL, Life Technologies GmbH, Karlsruhe, Germany) in a thermocycler (Perkin-Elmer, Langen, Germany), as described by Saiki et al.20 The amplified product was separated by agarose gel electrophoresis and detected in the presence of ethidium bromide under UV light. To confirm specificity and to detect low quantities, DNA was transferred to a nylon membrane and hybridized to specific probes. For semiquantitative RT-PCR, various cycle numbers and different concentrations of cDNA were tested to ensure a linear range of amplification. The number of amplification cycles, if not indicated elsewere, was as follows: for c-kit, 20; for GM-CSFRβ, 20; and for actin, 15. Primers used for PCR were as follows: c-kit: forward primer KE3 (CTTGTTGACCGCTCCTTG) (nt 376-393) and reverse primer KE7 (GCTATGGCAGCATTGACG) (nt 1215-1232) or alternatively forward primer 3′UTS66 (TGGACCACTGCATGAGCTTT) (nt 3521-3540) and reverse primer 3′UTS67 (TCCTGTGGGAGCCATGCAGT) (nt 3928-3947)19; GM-CSFRβ: forward primer 1 (CTGGTCCCTCTGGCCCAGGC) (nt 2115-2134) and reverse primer 2 (GACCTCCCAAGGGGGCAG) (nt 2853-2870)21; c-myc: forward primer 1 (TACTGCGACGAGGAGGAGAAC) (nt 70-90) and reverse primer 2 (GCTGTGGCCTCCAGCAGAAG) (nt 845-864)22; actin: forward primer 1 (CGCCGCGCTCGTCGTCGACA) (nt 56-75) and reverse primer 2 (GTCACGCACGATTTCCCGCT) (nt 655-674).23 Quantitative determination was performed by either densitometric scanning of the autoradiographs or scanning of phosphorimages using a phosphorimager (Fuji Photo Film Co Ltd, Tokyo, Japan) and TINA (version 2.0; Raytest, Straubenhardt, Germany) software for evaluation.
To determine the start site of c-kit transcription, for both TF-1 and stroma-dependent clones, rapid amplification of cDNA ends (RACE)-PCR was performed (GIBCO BRL). Length and specificity of the resulting PCR products were checked by size separation, blotting, and hybridization to a probe containing the first and the second c-kit exons. For cloning, the amplification was performed and the products were visualized after separation by agarose gel electrophoresis with ethidium bromide and UV light. The 5′ ends of c-kit cDNA were extracted from the gel using an NA-45 DNA binding membrane (Schleicher & Schuell, Dassel, Germany). Subsequently, the cDNAs were subcloned and sequenced.
Mapping of DNaseI hypersensitive sites (HSS).
Isolation of nuclei and DNaseI digestion was performed as described.18 Genomic DNA was then purified, restricted withEcoRI, and transferred to Biodyne B membranes (Pall, Dreilich, Germany) after separation by gel electrophoresis. Genomic c-kit DNA fragments used as probes for indirect end labeling are indicated in the figure legends.
RESULTS
The SCF/c-kit interaction is required for the proliferation of TF-1 cells on stroma.
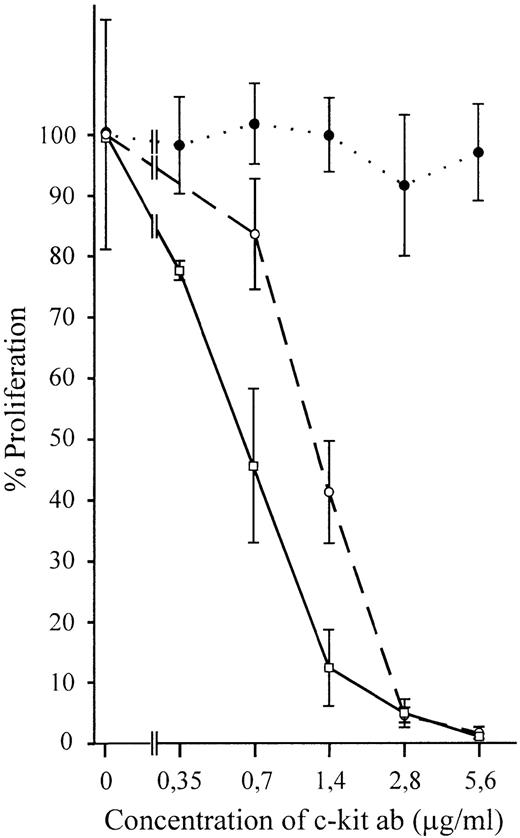
The importance of SCF/c-kit interaction in supporting hematopoiesis suggested that SCF expressed by MS-5 cells may be responsible for the sustained proliferation of TF-1 cells on these cells. GM-CSF was excluded to be a mediator of MS-5 stroma-induced growth, because the cells do not express this factor.10 Furthermore, murine GM-CSF is unable to activate the human receptor. To test the function of SCF for TF-1 proliferation, anti–c-kit antibody (YB5.B8) was added at different concentrations to TF-1 cells growing on MS-5 stroma cells. A dramatic decrease in proliferation was observed (Fig 1). Within 5 days, viable cells were reduced to 2% or less, as compared with controls without specific antibody. Growth of the parental TF-1 cells in suspension with hGM-CSF was not reduced by addition of the anti–c-kit antibody (specificity control).
Both growth of wild-type TF-1 and stroma-dependent TF-1S clones on stroma but not growth of suspended cells in absence of stroma can be inhibited by the monoclonal human antibody YB5.B8. (•) Parental TF-1 grown in suspension with GM-CSF (control); (○) parental TF-1 cells grown on stroma; (□) stroma-dependent clones TF-1S #MB5, #MB6, and #MB8. Only one line is drawn for the stroma-dependent clones, because the inhibition was almost identical. Means of three (TF-1 in suspension with GM-CSF), three (TF-1 on stroma), and two (TF-1S #5, #6, and #8 each) independent experiments are shown.
Both growth of wild-type TF-1 and stroma-dependent TF-1S clones on stroma but not growth of suspended cells in absence of stroma can be inhibited by the monoclonal human antibody YB5.B8. (•) Parental TF-1 grown in suspension with GM-CSF (control); (○) parental TF-1 cells grown on stroma; (□) stroma-dependent clones TF-1S #MB5, #MB6, and #MB8. Only one line is drawn for the stroma-dependent clones, because the inhibition was almost identical. Means of three (TF-1 in suspension with GM-CSF), three (TF-1 on stroma), and two (TF-1S #5, #6, and #8 each) independent experiments are shown.
Not only proliferation of the parental TF-1 cells, but also that of all three stroma-dependent TF-1S clones examined, was inhibited. Thus, the mSCF/c-kit interaction is essential for the stroma-induced proliferation of both the parental TF-1 cell line and the stroma-dependent TF-1S clones. It obviously replaces the requirement of suspended TF-1 for GM-CSF/IL-3.
Stroma cell interaction induces downregulation of c-kit mRNA.
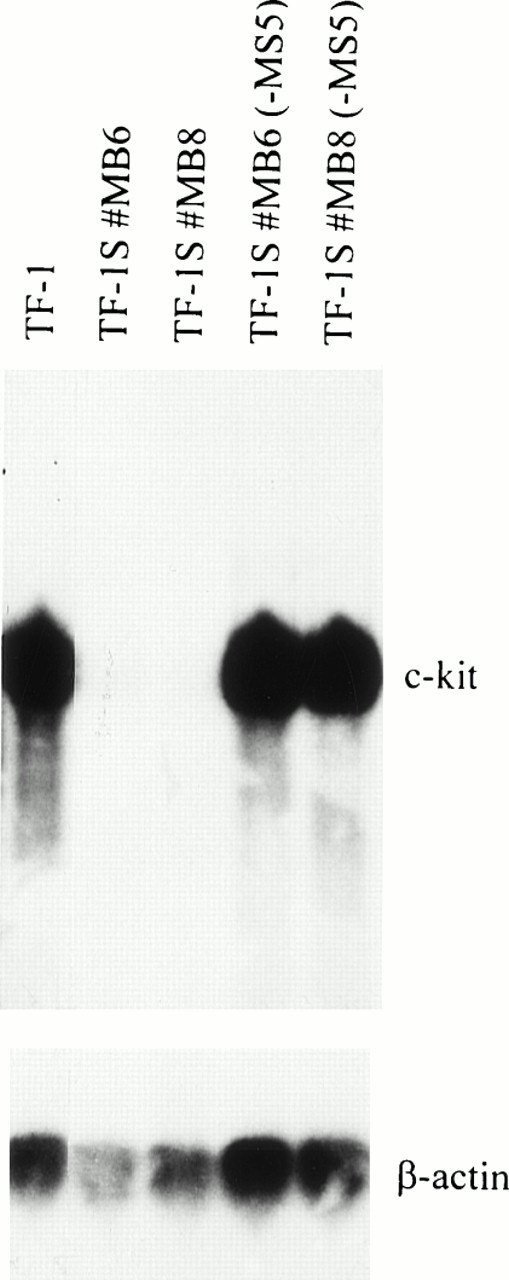
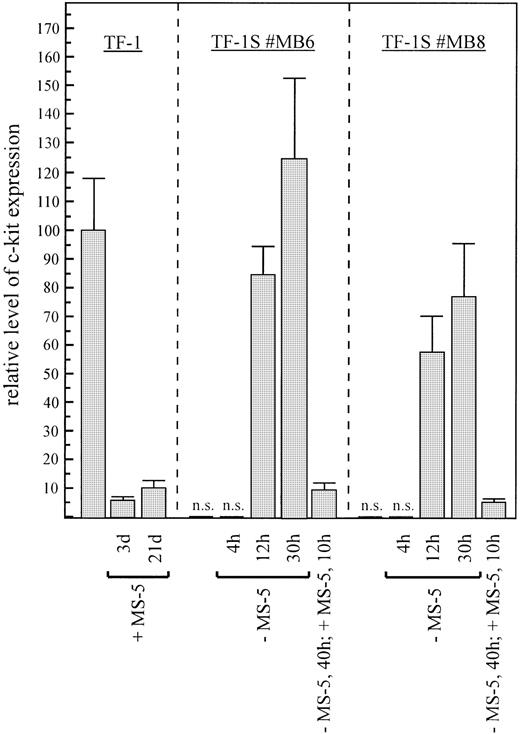
To investigate if c-kit is expressed in TF-1 grown on stroma cells (here MS-5), as predicted by our antibody blocking experiments, we analyzed the steady-state level of c-kit RNA by Northern analysis and semiquantitative RT-PCR. Surprisingly, the transcription levels of stroma-dependent TF-1S cells were 2 to 3 orders of magnitude lower, as compared with the parental TF-1 cells (Fig2). The levels of β-actin used as a control were only slightly reduced, in accordance with the decrease in cell growth.
High levels of c-kit RNA are expressed in parental TF-1 cells in absence of stroma and low levels in stroma-dependent TF-1S clones. To determine c-kit expression, RNA (15 μg) from cell line TF-1 and stroma-dependent clones (TF-1S #MB6 and TF-1S #MB8) was subjected to Northern analysis. Absence of MS-5 stroma cells for 1 day is indicated by “−MS5” (lanes 4 and 5).
High levels of c-kit RNA are expressed in parental TF-1 cells in absence of stroma and low levels in stroma-dependent TF-1S clones. To determine c-kit expression, RNA (15 μg) from cell line TF-1 and stroma-dependent clones (TF-1S #MB6 and TF-1S #MB8) was subjected to Northern analysis. Absence of MS-5 stroma cells for 1 day is indicated by “−MS5” (lanes 4 and 5).
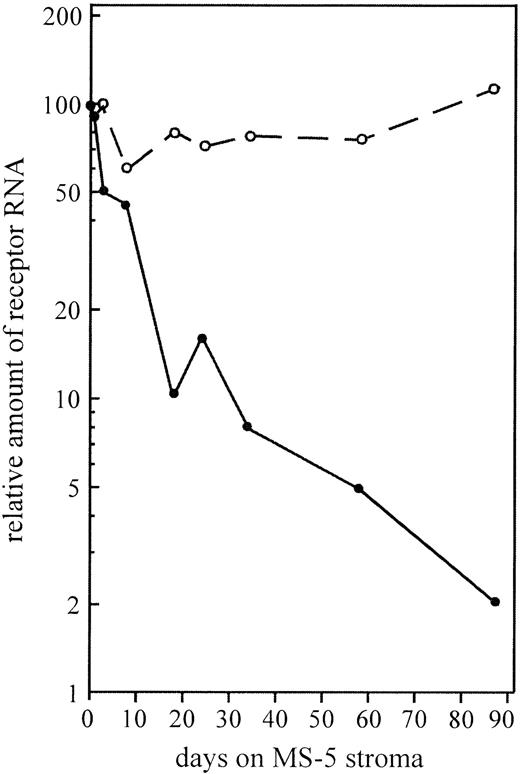
Downregulation of c-kit RNA was also observed in the parental TF-1 cells when transferred to stroma in the absence of GM-CSF (Fig 3). The 2 orders of magnitude reduction was a steady and slow process requiring as much as 12 weeks. In contrast, the level of the GM-CSF receptor β-chain remained fairly constant.
The level of c-kit mRNA (•) but not that of the GM-CSF receptor β-chain mRNA (○) is downregulated by stroma interaction. Semiquantitative PCR was performed from TF-1 grown for different times on MS-5 stroma. Actin was used as an internal standard for the quantification. The relation of receptor RNAs to β-actin RNA in TF-1 cells grown in suspension were set to 100.
The level of c-kit mRNA (•) but not that of the GM-CSF receptor β-chain mRNA (○) is downregulated by stroma interaction. Semiquantitative PCR was performed from TF-1 grown for different times on MS-5 stroma. Actin was used as an internal standard for the quantification. The relation of receptor RNAs to β-actin RNA in TF-1 cells grown in suspension were set to 100.
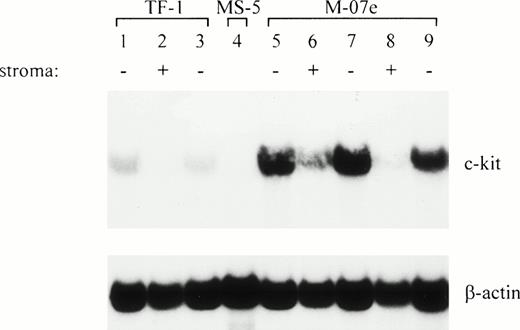
To show that downregulation is a general property not only of TF-1 cells, we also used a different hematopoietic cell line, M-07e,12 with a megakaryoblast phenotype. M-07e, normally grown with IL-3 or GM-CSF in suspension, can alternatively be maintained on stroma. M-07e cells were transferred to MS-5 and analyzed for expression of c-kit. The c-kit transcript level was also downregulated in M-07e in coculture with stroma (Fig 4). Both M-07e and TF-1 cells (control) showed a fivefold reduction of the c-kit mRNA level within 2 weeks, as opposed to the c-kit transcript levels of the corresponding cells grown in suspension with soluble factor.
The level of c-kit transcripts is similarly downregulated in M-07e cells upon interaction with stroma cells. The level is upregulated when the stroma is removed. RNA (12 μg/lane) was subjected to Northern analysis. M-07e cells grown in suspension with rhIL-3 or alternatively on stroma for 1 and 2 weeks were analyzed for c-kit expression. In addition, an aliquot of the M-07e cells was removed from the stroma (at days 7 and 14, respectively) and was grown for 1 day in suspension with IL-3 added. In parallel, TF-1 was used as a control for downregulation and upregulation (lanes 1 through 3). Lane 1, TF-1; lane 2, TF-1, 2 weeks on MS-5; lane 3, TF-1, 2 weeks on MS-5, 1 day in suspension (+GM-CSF); lane 4, MS-5; lane 5, M-07e; lane 6, M-07e, 1 week on MS-5; lane 7, M-07e, 1 week on MS-5, 1 day in suspension (+IL-3); lane 8, M-07e, 2 weeks on MS-5; lane 9, M-07e, 2 weeks on MS-5, 1 day in suspension (+IL-3).
The level of c-kit transcripts is similarly downregulated in M-07e cells upon interaction with stroma cells. The level is upregulated when the stroma is removed. RNA (12 μg/lane) was subjected to Northern analysis. M-07e cells grown in suspension with rhIL-3 or alternatively on stroma for 1 and 2 weeks were analyzed for c-kit expression. In addition, an aliquot of the M-07e cells was removed from the stroma (at days 7 and 14, respectively) and was grown for 1 day in suspension with IL-3 added. In parallel, TF-1 was used as a control for downregulation and upregulation (lanes 1 through 3). Lane 1, TF-1; lane 2, TF-1, 2 weeks on MS-5; lane 3, TF-1, 2 weeks on MS-5, 1 day in suspension (+GM-CSF); lane 4, MS-5; lane 5, M-07e; lane 6, M-07e, 1 week on MS-5; lane 7, M-07e, 1 week on MS-5, 1 day in suspension (+IL-3); lane 8, M-07e, 2 weeks on MS-5; lane 9, M-07e, 2 weeks on MS-5, 1 day in suspension (+IL-3).
Downregulation of the c-kit receptor is reversible.
To check if downregulation of the c-kit mRNA level is a reversible process, we removed TF-1S stroma-dependent cells from the stroma for 1 day and performed Northern analysis and semiquantitative RT-PCR. Surprisingly, the amount of c-kit mRNA increased by 3 to 4 orders of magnitude (Fig 2).
The complete upregulation required 1 to 3 days and varied between clones. The levels of c-kit mRNA were higher than that of control TF-1 cells. The study could not be extended for more than 3 days because of the reduced viability of TF-1S without stroma. Stroma-dependent TF-1S cells, maintained in suspension with GM-CSF, showed the same degree of upregulation as cells grown in suspension without GM-CSF (data not shown). GM-CSF was added to the medium in most experiments, because the cells looked healthier in medium containing the factor. Nevertheless and surprisingly, the cells died within 1 week due to loss of responsiveness to GM-CSF.
Upregulation of c-kit mRNA could be shown for both the parental TF-1 cells and the megakaryoblast M-07e cells (Fig 4). The c-kit mRNA was upregulated by both cell lines upon growth in suspension for 1 day.
To determine if the degree of downregulation is inherent to the state of the cell, we decided to test whether downregulation occurs at the same rate in TF-1S cells as compared with wildtype TF-1 cells. As described above, c-kit mRNA was quickly upregulated by 3 orders of magnitude in TF-1S cells when transferred to suspension culture with GM-CSF (Table 1). After 2 days, the cells were reexposed to stroma. The c-kit RNA levels in the TF-1S clones decreased again, but downregulation was now a fast process. The magnitude and rate of downregulation was dependent on the time of cocultivation with stroma cells.
Downregulation and Upregulation of the c-kit mRNA Levels Are Immediate Consequences of Stroma Cell Interaction
| Clones . | Relative Level of c-kit mRNA Expression . | ||
|---|---|---|---|
| Continuous Growth on . | Removal of . | Reexposure to Stroma . | |
| TF-1S #MB6 | 1 ± 0.2* | 7,980 ± 1,860* | 9 ± 2* |
| TF-1S #MB8 | 1 ± 0.3† | 360 ± 100† | 4 ± 1† |
| Clones . | Relative Level of c-kit mRNA Expression . | ||
|---|---|---|---|
| Continuous Growth on . | Removal of . | Reexposure to Stroma . | |
| TF-1S #MB6 | 1 ± 0.2* | 7,980 ± 1,860* | 9 ± 2* |
| TF-1S #MB8 | 1 ± 0.3† | 360 ± 100† | 4 ± 1† |
The mRNA levels of the TF-1S clones each were set to 1. The c-kit mRNA level of #MB8 is 2.7-fold higher than the c-kit mRNA level of #MB6. Removal of and reexposure to stroma was performed for a period of 2 days each.
n = 4.
n = 3.
Downregulation of c-kit expression is not caused by presentation of the SCF ligand on stroma cells.
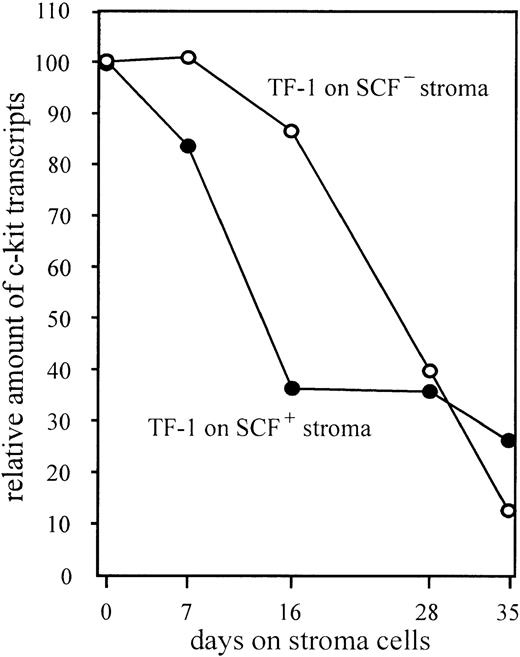
Downregulation of the c-kit mRNA level could either be mediated by SCF or by an alternative ligand presented by the stroma. We analyzed TF-1 cells grown on SCF-deficient (UNC Sl−/Sl−) stroma to directly prove the relevance of SCF for downregulation of c-kit. SCF-deficient stroma cells induced similar levels of downregulation of the c-kit receptor as stroma-presenting SCF (Fig 5). These results clearly show that downregulation of c-kit is caused by stroma, but not by the homologous ligand.
Downregulation of c-kit expression in absence of c-kit activation by use of SCF-deficient (UNC Sl−/Sl−) stroma cells. Stroma feeder was prepared by seeding 5 × 104 stroma cells per well into 6-well tissue culture plates (Nunclon). TF-1 cells grown in GM-CSF–containing medium were washed three times in RPMI medium and subsequently transferred to irradiated (17,600 rad) stroma. Usually every 7 days the TF-1 cells were sequentially transferred to fresh prepared stroma. An aliquot of cells was analyzed for expression of c-kit as described in Materials and Methods. Aliquots of each transfer were confirmed to be negative for factor-independent mutant cells by recloning in medium with and without GM-CSF. Cultures that contained mutant cells were excluded.
Downregulation of c-kit expression in absence of c-kit activation by use of SCF-deficient (UNC Sl−/Sl−) stroma cells. Stroma feeder was prepared by seeding 5 × 104 stroma cells per well into 6-well tissue culture plates (Nunclon). TF-1 cells grown in GM-CSF–containing medium were washed three times in RPMI medium and subsequently transferred to irradiated (17,600 rad) stroma. Usually every 7 days the TF-1 cells were sequentially transferred to fresh prepared stroma. An aliquot of cells was analyzed for expression of c-kit as described in Materials and Methods. Aliquots of each transfer were confirmed to be negative for factor-independent mutant cells by recloning in medium with and without GM-CSF. Cultures that contained mutant cells were excluded.
Downregulation of mRNA results in reduction of the c-kit receptor protein.
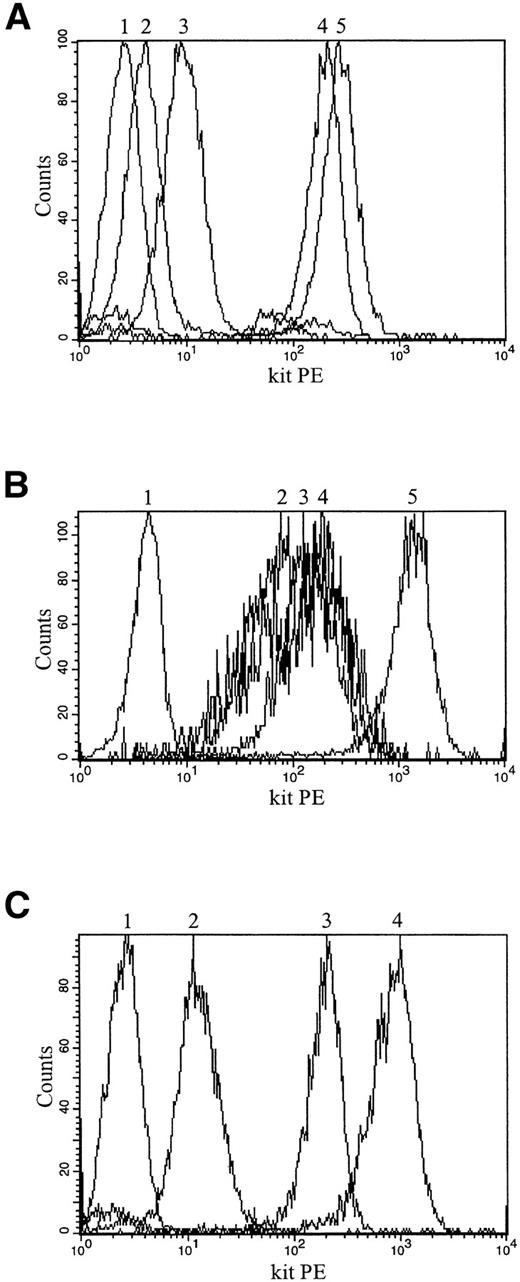
Control mechanisms acting at the posttranscriptional or translational level might overcome the stroma-induced reduction of c-kit RNA. To determine if the amount of c-kit protein correlates with the different RNA levels, we quantitated the receptor by antibody binding and FACS analysis (Fig 6). Compared with the parental TF-1 cells, the amount of c-kit protein was reduced by more than 2 orders of magnitude in both stroma-dependent TF-1S clones that were tested (Figs 6 and 7A).
c-kit receptor protein is also downregulated by stroma cells, indicating that translational mechanisms are not involved in downregulation and upregulation. Quantification of c-kit protein expression by binding of monoclonal anti–c-kit antibody (YB5.B8) and FACS analysis. Mean fluorescence was compared with the mean fluorescence of TF-1 cells grown in suspension with GM-CSF, which was set to 100%. Unspecific binding as measured by the mouse IgG standard control antibody was gated out. The fluorescence of the stroma-dependent clones was in the range of the control value without c-kit antibody and thus the values were considered not significant (n.s.). Results of at least three independent experiments are shown.
c-kit receptor protein is also downregulated by stroma cells, indicating that translational mechanisms are not involved in downregulation and upregulation. Quantification of c-kit protein expression by binding of monoclonal anti–c-kit antibody (YB5.B8) and FACS analysis. Mean fluorescence was compared with the mean fluorescence of TF-1 cells grown in suspension with GM-CSF, which was set to 100%. Unspecific binding as measured by the mouse IgG standard control antibody was gated out. The fluorescence of the stroma-dependent clones was in the range of the control value without c-kit antibody and thus the values were considered not significant (n.s.). Results of at least three independent experiments are shown.
Upregulation and downregulation of c-kit protein expression in TF-1S, parental TF-1, and M-07e cells is shown by anti–c-kit antibody binding (YB5.B8) and FACS analysis (histograms). (A) Upregulation and downregulation of c-kit protein expression in stroma-dependent TF-1S #MB6 clone. (1) TF-1S #MB6 labeled with mouse IgG isotype control antibody; (2) TF-1S #MB6 on MS-5; (3) TF-1S #MB6 maintained for 40 hours in suspension with GM-CSF and then rexposed for 10 hours to stroma; (4) TF-1 grown in suspension with GM-CSF; (5) TF-1S #MB6 maintained for 48 hours in suspension with GM-CSF. (B) Downregulation of c-kit protein expression in M-07e on exposure to stroma cells. (1) M-07e cells labeled with mouse IgG isotype control antibody; (2) M-07e, 21 days on MS-5; (3) M-07e, 14 days on MS-5; (4) M-07e, 7 days on MS-5; (5) M-07e grown in suspension with IL-3. (C) Downregulation and upregulation of c-kit protein expression in the parental TF-1 cells. (1) TF-1 labeled with mouse IgG isotype control antibody; (2) TF-1 on MS-5; (3) TF-1 grown in suspension with GM-CSF; (4) TF-1, 14 days on stroma and then cultured for 48 hours in suspension with GM-CSF.
Upregulation and downregulation of c-kit protein expression in TF-1S, parental TF-1, and M-07e cells is shown by anti–c-kit antibody binding (YB5.B8) and FACS analysis (histograms). (A) Upregulation and downregulation of c-kit protein expression in stroma-dependent TF-1S #MB6 clone. (1) TF-1S #MB6 labeled with mouse IgG isotype control antibody; (2) TF-1S #MB6 on MS-5; (3) TF-1S #MB6 maintained for 40 hours in suspension with GM-CSF and then rexposed for 10 hours to stroma; (4) TF-1 grown in suspension with GM-CSF; (5) TF-1S #MB6 maintained for 48 hours in suspension with GM-CSF. (B) Downregulation of c-kit protein expression in M-07e on exposure to stroma cells. (1) M-07e cells labeled with mouse IgG isotype control antibody; (2) M-07e, 21 days on MS-5; (3) M-07e, 14 days on MS-5; (4) M-07e, 7 days on MS-5; (5) M-07e grown in suspension with IL-3. (C) Downregulation and upregulation of c-kit protein expression in the parental TF-1 cells. (1) TF-1 labeled with mouse IgG isotype control antibody; (2) TF-1 on MS-5; (3) TF-1 grown in suspension with GM-CSF; (4) TF-1, 14 days on stroma and then cultured for 48 hours in suspension with GM-CSF.
When the MO7 or parental TF-1 cells were plated on stroma, a striking decrease in c-kit expression was observed within 2 weeks (Fig 7B and C). Consistent with the mRNA levels, removal from stroma cells induced a correlated upregulation of the c-kit protein (data shown for TF-1; Fig 7C). The degree of upregulation of the c-kit receptor measured by RNA and protein levels was comparable and indicated that differential translational controls, if at all, were not of primary importance. Because the regulation of the c-kit protein is similar to the regulation of the c-kit mRNA, we conclude that the c-kit expression of TF-1 cells is primarily controlled at the level of c-kit RNA.
Downregulation of c-kit mRNA is not due to reduced mRNA stability.
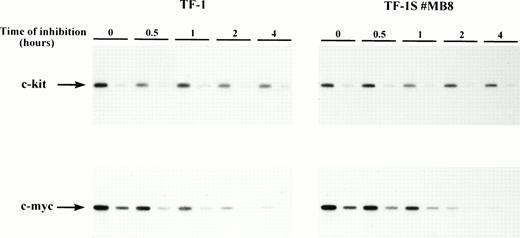
Stroma cells might induce a drastic decrease of the c-kit mRNA stability. The result would also be a decrease in the steady-state c-kit mRNA level, indistinguishable from a reduction of transcription. We thus measured the mRNA stability of the c-kit transcript in TF-1 cells by Actinomycin D inhibition of RNA synthesis and semiquantitative RT-PCR. The half-life of c-kit is quite long (>10 hours) in both the parental TF-1 and stroma-grown TF-1S #MB8 cells (Fig 8). Northern blot analysis of the c-kit mRNA of TF-1 cells grown in suspension confirmed these results (data not shown). The level of c-myc mRNA, which was used as a control for inhibition, rapidly decreased with time (half-life of ∼30 minutes). Therefore, we conclude that the stroma cell interaction does not affect the stability of the c-kit transcript.
The half-life of the c-kit transcript is not reduced by the stroma cell interaction. The reduction of the c-kit mRNA level was monitored after actinomycin D inhibition (5.0 μg/mL) of RNA synthesis in TF-1 cells. Only data of the first 4 hours of inhibition were evaluated, because the cells tended to clump under prolonged action of actinomycin D. The decrease of c-kit mRNA was measured by semiquantitative RT-PCR. One-fifth dilutions of the RNA were reverse transcribed and amplified to check linearity of amplification. These amplification products correspond to the second lane at each time point. Amplification of c-kit–specific transcripts in TF-1 cells and c-myc–specific transcripts was performed for 18 cycles. Amplification of c-kit specific transcripts was performed for 22 cycles in case of the TF-1S #MB8 cells.
The half-life of the c-kit transcript is not reduced by the stroma cell interaction. The reduction of the c-kit mRNA level was monitored after actinomycin D inhibition (5.0 μg/mL) of RNA synthesis in TF-1 cells. Only data of the first 4 hours of inhibition were evaluated, because the cells tended to clump under prolonged action of actinomycin D. The decrease of c-kit mRNA was measured by semiquantitative RT-PCR. One-fifth dilutions of the RNA were reverse transcribed and amplified to check linearity of amplification. These amplification products correspond to the second lane at each time point. Amplification of c-kit–specific transcripts in TF-1 cells and c-myc–specific transcripts was performed for 18 cycles. Amplification of c-kit specific transcripts was performed for 22 cycles in case of the TF-1S #MB8 cells.
Start of transcription and length of the c-kit transcript do not differ in TF-1 and stroma cell-dependent TF-1S clones.
We could show that the expression of c-kit is reduced as a consequence of stroma cell interaction and that binding of SCF to the SCF receptor on TF-1 cells is necessary for proliferation on MS-5 cells. Expression of an alternative spliced variant with an increased affinity for binding SCF (mSCF) might explain some of our puzzling results. To elucidate this possibility, we examined the size of the c-kit transcripts of TF-1 and stroma-dependent clones by extensive RT-PCR using a broad spectrum of c-kit–specific primers. No differences in c-kit transcript lengths were detectable (data not shown).
We then analyzed the start site of c-kit transcription by RACE-PCR and RT-PCR. RACE products of TF-1 cells, stroma cell-dependent TF-1S clones, and clones induced to high c-kit expression (by removal of stroma cells) had the same mobility in agarose gel electrophoresis. Sequencing of the cloned fragments showed that all identified start sites are clustered within a 17-bp sequence, which maps 56 bp 5′ to the published initiation codon of translation (Fig 9). In agreement with the positions of the identified start sites, we could not detect any RT-PCR product using 5′ primers located further upstream (P56 and P57). Thus, primer P58, which maps well with the transcription start, is the most upstream primer yielding an amplification. Results of the RACE-PCR and the RT-PCR thus clearly show that c-kit, regardless of growth conditions, has multiple starts of transcription located between position −72 and −56.
The start sites of the c-kit transcription are the same in cells downregulated (+ stroma) or upregulated (− stroma) for c-kit expression. Start sites of c-kit transcription as defined by RACE-PCR. Start sites mapped at position −72 (TF-1S #MB6), −57 (TF-1S#MB6), and −56 (TF-1 and TF-1S#MB6). No differences were found between our sequences and the sequence published by Giebel et al.24 5′ primers that were positive (+) or negative (−) in giving a signal from RT-PCR are indicated in the sequence.
The start sites of the c-kit transcription are the same in cells downregulated (+ stroma) or upregulated (− stroma) for c-kit expression. Start sites of c-kit transcription as defined by RACE-PCR. Start sites mapped at position −72 (TF-1S #MB6), −57 (TF-1S#MB6), and −56 (TF-1 and TF-1S#MB6). No differences were found between our sequences and the sequence published by Giebel et al.24 5′ primers that were positive (+) or negative (−) in giving a signal from RT-PCR are indicated in the sequence.
Heterogeneous initiation of transcription of the c-kit gene is not unexpected, because sequences upstream of this site contain more than 80% guanine/cytosine residues and lack a typical TATA or CAAT motif. Therefore, the c-kit promoter appears to be similar to that of other receptors or housekeeping genes with long GC stretches and multiple binding motifs for the transcription factor SP1.
The results of our transcription analysis thus indicate that neither stroma-specific c-kit variants of TF-1 are expressed nor initiation of transcription is altered after stroma-dependent downregulation.
The chromatin proximal to the c-kit promoter is not altered by stroma interaction.
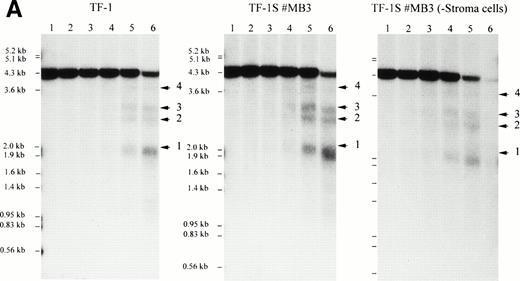
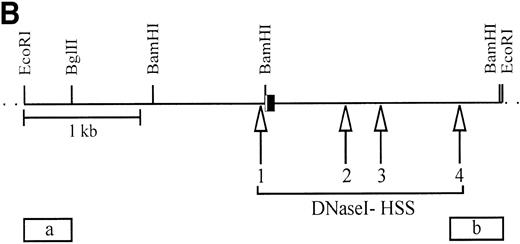
To analyze if specific control elements of the c-kit promoter of TF-1 cells reduce the activity of the gene due to stroma cell interaction, we mapped DNaseI hypersensitive sites (HSS). This method is commonly used to identify control domains of transcription with an open chromatin structure. HSS have not been previously mapped for the c-kit gene. HSS in the c-kit gene and 5′ flanking sequences in TF-1 cells grown in suspension with GM-CSF or alternatively on stroma (TF-1S) were compared. Two probes were used that hybridized to a 4.2-kbEcoRI genomic fragment. We could detect four HSS in TF-1 and stroma-grown clones (Fig 10A). The first site, HSS1, was seen as a 2.1-kb degradation band of the 4.2-kbEcoRI fragment using probe (a) for end labeling. Interestingly HSS1 maps to the c-kit promoter region used in hematopoietic cells identified here (see below; Fig 10B). Three other sites (HSS2, HSS3, and HSS4) are located at the 5′ end of intron 1. Whereas HSS2 and HSS3 had about the same intensity as HSS1, HSS4 was quite weak and not always detectable. The same pattern of HSS was seen independently using different culture conditions for TF1 cells: all stroma cell-dependent TF-1S clones (Fig 10A, data for #MB6 and #MB8 are not shown) showed the same DNA conformation as TF-1 grown without stroma. Thus, stroma cell interaction does not alter the chromatin structure of the c-kit promoter.
The DNaseI hypersensitive sites (HSS) in TF-1, independent of downregulation or upregulation of the c-kit receptor, are identical. (A) Mapping of HSS (1-4) (arrowheads) in different TF-1 cell lines. All major and minor degradation bands of the 4.2-kbEcoRI fragment are indicated and numbered according to the map of (B). HSS1 directly maps within the c-kit promoter region. HSS2, HSS3, and HSS4 are located in the 5′ region of the first intron. DNaseI-treated genomic DNA (10 μg) was digested with EcoRI and probed with probe (a). DNaseI concentrations were as follows: lane 1, no DNaseI; lane 2, 0.8 μg/mL; lane 3, 1.2 μg/mL; lane 4, 1.8 μg/mL; lane 5, 2.7 μg/mL; lane 6, 4.0 μg/mL. (B) The hypersensitive sites of the c-kit promoter region of TF-1 cells (TF-1; TF-1S #MB3, #MB6, #MB8; and TF-1S #MBs removed from stroma for 1 day). HSS4 is a minor site and was not always found. Exon 1 is boxed, and the coding region is indicated by shading. Probes used for mapping HSS (a and b) are indicated.
The DNaseI hypersensitive sites (HSS) in TF-1, independent of downregulation or upregulation of the c-kit receptor, are identical. (A) Mapping of HSS (1-4) (arrowheads) in different TF-1 cell lines. All major and minor degradation bands of the 4.2-kbEcoRI fragment are indicated and numbered according to the map of (B). HSS1 directly maps within the c-kit promoter region. HSS2, HSS3, and HSS4 are located in the 5′ region of the first intron. DNaseI-treated genomic DNA (10 μg) was digested with EcoRI and probed with probe (a). DNaseI concentrations were as follows: lane 1, no DNaseI; lane 2, 0.8 μg/mL; lane 3, 1.2 μg/mL; lane 4, 1.8 μg/mL; lane 5, 2.7 μg/mL; lane 6, 4.0 μg/mL. (B) The hypersensitive sites of the c-kit promoter region of TF-1 cells (TF-1; TF-1S #MB3, #MB6, #MB8; and TF-1S #MBs removed from stroma for 1 day). HSS4 is a minor site and was not always found. Exon 1 is boxed, and the coding region is indicated by shading. Probes used for mapping HSS (a and b) are indicated.
DISCUSSION
Hematopoiesis requires two interacting cell systems: hematopoietic cells and supporting stroma cells. The necessity of interaction with mesenchymal stroma cells has been shown to be essential for long-term culture systems.25,26 The molecular mechanisms that govern these interactions are poorly understood. To obtain in vitro systems to study interaction of hematopoietic cells with stroma, we have successfully isolated strictly stroma-dependent clones of the human TF-1 and murine ELM myeloid progenitor cell lines9,27 and more recently of other even earlier hematopoietic cells, such as Myl-D7.10 With the exception of ELM-D, showing a high frequency of reversion to stroma cell independence, the other stroma-dependent cells provide an excellent tool to investigate the molecular mechanisms of cell attachment and stroma cell-induced long-term proliferation, as well as the means to pin down the regulation of molecules required for this interaction.
The growth of the human parental TF-1 on mouse stroma and of all stroma-dependent TF-1S subclones could be inhibited by monoclonal anti–c-kit antibody. Surprisingly, previous studies have shown that the presence of soluble SCF in absence of stroma cells had only a limited effect as an inducer of short-term but not long-term proliferation (manuscript in preparation). However, inhibition of TF-1 cell proliferation on SCF positive (Sl+/Sl+) MS-5 stroma cells indicates that the YB5.B8 antibody used here17 is sufficient not only to block the short-term proliferation stimulus of sSCF, but also of additional SCF-dependent signals that provide long-term proliferation. These signals most likely depend on presentation of the membrane-inserted SCF (mSCF; manuscript in preparation).
Growth of all stroma-interacting myeloid precursor cells tested (TF-1 and ELM-D)9 27 could always be inhibited by using neutralizing anti–c-kit antibody. Thus, the c-kit receptor may have a general function in hematopoietic/stroma cell interaction in different species. However, we could directly demonstrate the existence of an alternative, yet to be identified growth-promoting activity by interaction of TF-1 with SCF− stroma cells. Cloning and long-term growth of TF-1 cells on SCF− stroma cells demonstrate that other molecules can substitute for the function of the c-kit ligand.
Despite the SCF/c-kit requirement for proliferation in our stroma-dependent system (Fig 1), we detected a massive downregulation of the c-kit mRNA of TF-1S cells cocultured with stroma (Fig 2). Downregulation of c-kit mRNA could be shown for both the parental TF-1 and the megakaryoblast M-07e cells, suggesting that the mechanism of SCF receptor modulation is shared in cells of different hematopoietic lineages (Fig 4).
Downregulation of c-kit as a consequence of hematopoietic cell/stroma interaction occurred at both the RNA and protein levels. In TF-1 cells grown on stroma, both the c-kit mRNA and the protein expression were coordinately reduced by several orders of magnitude as compared with TF-1 cells grown with GM-CSF in suspension. We therefore conclude that translational controls have no significant overall effect on c-kit downregulation in our system.
Alternative splicing of the c-kit mRNA, resulting in expression of a variant with a decreased half-life, might explain the reduced levels of c-kit. The c-kit RNA was examined for possible splice variants induced by stroma-dependent growth, because alternative splicing has been shown for other members of the tyrosine kinase family and found to generate functionally different isoforms.28-30 For the c-kit receptor, a minor splice variant (kitA) has been described.31-33 However, no evidence for a specific c-kit splice product in TF-1 or TF-1S cells was found.
Moreover, we analyzed the stability of c-kit transcripts in TF-1 cells grown in suspension and in stroma-dependent TF-1S #MB8 cells. Our results demonstrate that downregulation is not due to an altered half-life of the c-kit mRNA. Taken together, the reduction of c-kit mRNA is most probably controlled by the transcription machinery.
Several mechanisms that could account for a reduced level of transcription were therefore analyzed. Using RACE-PCR, we identified the initiation site of c-kit transcription and by this we can further exclude that different promoters are activated in TF-1 cells grown with or without stroma. All identified transcription start sites, interestingly enough, mapped to a region that has been identified as the melanocyte-specific c-kit promoter.24 Thus, cell type and stroma cell-specific c-kit expression is not regulated by differential promoter usage, as has been shown for the c-kit–related M-CSFR gene.34 Additionally, the downregulation of the c-kit gene is not the result of a stroma cell-induced transcriptional stop in elongation, because RT-PCR for the 5′ end of the c-kit transcript was as efficient as for the 3′ end (data not included due to negative results).
We showed that the DNaseI hypersensitive sites of the c-kit receptor gene of cells grown with and without stroma are identical. Thus, the reduction of the transcriptional activity of the c-kit gene as a consequence of direct chromatin alterations can be excluded. This result moreover shows that the chromatin structure of the c-kit promoter is open and therefore not fixed to an inactive state when transcriptional activity is reduced by 3 orders of magnitude on stroma coculture. Indeed, upregulation of the c-kit expression after stroma cell removal and subsequent downregulation was a fast process and often showed an overshoot (4 orders of magnitude).
Our result of the chromatin structure of the c-kit gene further indicates that the sequences adjacent to the transcriptional start site might not be sufficient to permit a cell-specific transcription level. As expected, differences in the activities of promoter-reporter-gene constructs transfected in cell lines with high or low c-kit expression were quite low (C. Heberlein, unpublished results).35,36 Results reported by others support the notion that far upstream DNA control elements are needed for high expression of the c-kit gene. In the murine system, a locus control region has been identified that is more than 50 kb upstream of the gene.37 We cannot exclude that changes in the chromatin structure have occurred in this region. Alternatively, transcription is regulated either by enhancement of the basal promoter by cis-acting elements located at far distance to the initiation start of transcription or by the binding of transcription factors to the promoter region with no significant (obvious) effect on the chromatin structure.
Summarizing our data, we demonstrate that the c-kit receptor expression is downregulated in myeloid progenitor cells due to stroma cell interaction by mainly transcriptional control mechanisms. The downregulation appears to be a consequence of an insufficient usage of transcriptional activators during stroma cell interaction or alternatively by the presence of inhibitors.
ACKNOWLEDGMENT
The authors thank Dr R.A. Spritz providing plasmid pUC KIT 27-1R4.2.
Supported by the Deutsche Forschungsgemeinschaft (Os 31) and Amgen. The Heinrich-Pette-Institut is financially supported by Freie und Hansestadt Hamburg and the Bundesministerium für Gesundheit.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Christoph Heberlein, PhD, Heinrich-Pette-Institut für experimentelle Virologie und Immunologie an der Universität Hamburg, Martinistr. 52, 20251 Hamburg, Germany.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal