Abstract
Colony-stimulating factors (CSFs) promote the proliferation, differentiation, commitment, and survival of myeloid progenitors, whereas cyclic AMP (cAMP)-mediated signals frequently induce their growth arrest and apoptosis. The ERK/mitogen-activated protein kinase (MAPK) pathway is a target for both CSFs and cAMP. We investigated how costimulation by cAMP and colony-stimulating factor-1 (CSF-1) or interleukin-3 (IL-3) modulates MAPK in the myeloid progenitor cell line, 32D. cAMP dramatically increased ERK activity in the presence of CSF-1 or IL-3. IL-3 also synergized with cAMP to activate ERK in another myeloid cell line, FDC-P1. The increase in ERK activity was transmitted to a downstream target, p90rsk. cAMP treatment of 32D cells transfected with oncogenic Ras was found to recapitulate the superactivation of ERK seen with cAMP and CSF-1 or IL-3. ERK activation in the presence of cAMP did not appear to involve any of the Raf isoforms and was blocked by expression of dominant-negative MEK1 or treatment with a MEK inhibitor, PD98059. Although cAMP had an overall inhibitory effect on CSF-1–mediated proliferation and survival, the inhibition was markedly increased if ERK activation was blocked by PD98059. These findings suggest that upregulation of the ERK pathway is one mechanism induced by CSF-1 and IL-3 to protect myeloid progenitors from the growth-suppressive and apoptosis-inducing effects of cAMP elevations.
HEMATOPOIESIS, the formation and functional activation of blood cells, is controlled by dynamic and precisely coordinated events, many of which are still poorly understood. Central to this regulatory process in mammals are the hematopoietic colony-stimulating factors (CSFs), critical mediators of cellular proliferation, differentiation commitment, survival, and activation of mature cell functions. Cells of the myeloid lineage have been most clearly shown to be those affected by CSFs.1 The earliest cells committed to differentiate along this lineage are the granulocyte-macrophage (myeloid) progenitors. Myeloid progenitors and their progeny can respond to several CSFs1; the relative importance of each factor may vary depending on the differentiation status of the cell and on the availability of the factor from the microenvironment. The macrophage CSF, CSF-1, is a factor specifically responsible for maintenance of monocyte/macrophage populations. Its receptor, CSF-1R, is a member of the tyrosine kinase family of growth factor receptors.2,3 CSF-1R is expressed on the majority of murine bone marrow cells with blast morphology (myeloid progenitors); its expression then becomes more restricted but not exclusively to those progeny further committed to differentiate along the monocyte/macrophage series.4 That CSF-1 is an important growth factor for these cells is illustrated by the finding of a macrophage deficiency in the op/op mouse lacking functional CSF-1,5 with the deficiency being severe in certain macrophage populations, including that in the blood. op/op mice also show a significant reduction in hematopoietic stem cells and progenitors,5 indicating that CSF-1 acts on early precursor cells as well as on the more mature monocytes and macrophages. In agreement with the notion that CSF-1 can act on early precursors, CSF-1 is known to cooperate with interleukin-1 (IL-1) to dramatically stimulate proliferation of multipotent progenitor cells more primitive than those that normally respond to CSF-1.6
Cyclic AMP (cAMP) is another important modulator of myeloid cell proliferation. cAMP is produced when specific serpentine receptors coupled to adenylate cyclase are activated. Some examples of such receptors expressed on myeloid cells are β-adrenergic receptors7 and receptors for prostaglandin E2(PGE2)8 and the chemokine, macrophage inflammatory protein-1α (MIP-1α).9 The bone marrow is densely innervated by adrenergic fibers,10whereas PGE2 and MIP-1α are both secreted by monocytes/macrophages.11,12 Although PGE2 and MIP-1α binding can induce other mediators as well, their effects on growth can be mimicked in some cells by directly increasing intracellular cAMP levels or by activating the cAMP-dependent protein kinase, protein kinase A (PKA).9,13 cAMP inhibits CSF-1–induced proliferation of functionally differentiated macrophages,14 15 but the effect of cAMP and CSF-1 costimulation in myeloid progenitors is not known.
Maintenance of hematopoietic homeostasis requires the ability to respond dynamically to a wide range of environmental stresses, such as infection and trauma, as well as normal growth and development. Progenitor cells must be able to appropriately integrate signals, both positive and negative, from multiple sources. The ability of cAMP to modulate growth factor-stimulated proliferation/differentiation is traced in some cell types to its effects on the extracellular signal regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) module. This module consists of three kinases that act sequentially: an MAPKKK (Raf isoforms), an MAPKK (MEK1/2), and an MAPK (ERK1/2).16ERKs phosphorylate cytosolic proteins, eg, p90rsk17 and MAPKAP,18 and also translocate to the nucleus to phosphorylate transcriptional factors, eg, TCF/Elk.19 cAMP binds to and activates PKA, which then phosphorylates Raf-1, leading to a reduction of the latter’s affinity for Ras-GTP and inhibition of its enzymatic activity.20 In diverse cell types such as NIH 3T3 cells,21 arterial smooth muscle cells,22 and cortical astrocytes,23 inhibition of Raf-1 by PKA leads to an inhibition of growth factor-stimulated ERK activity that can correlate with growth suppression. On the other hand, cAMP acting through the Rap1/B-Raf pathway activates ERK and induces differentiation of PC12 cells.24-27
Despite their manifest importance, many questions remain open on the signaling mechanisms used by CSF-1 in myeloid progenitors. In part, this reflects the difficulty of obtaining from the bone marrow, sufficient numbers of pure populations of CSF-1R–bearing cells at the same stage of precursor development, a prerequisite for biochemical studies. To overcome this problem, our studies of CSF-1–dependent signaling events in myeloid precursors have used the 32Dcl23 cell line transfected with the murine CSF-1R.28-30 32Dcl23 is a nonleukemic murine myeloid progenitor cell line that self-renews in the presence of IL-3. They lack endogenous CSF-1R, but, when transduced with an exogenous CSF-1R, will use CSF-1 as a growth and survival factor.28 Support for a proliferative rather than a differentiative role of CSF-1R in myeloid progenitors comes from studies in which infection of blast cells harvested from 5-flurouracil–treated mice with a CSF-1R retrovirus enhanced their proliferation and not their differentiation capacity.31 A survival role for CSF-1R is implicated by the observation thatop/op mice engineered to express the bcl-2 transgene in myeloid progenitors showed marked restoration of monocytopoiesis in the bone marrow.32 32D/CSF-1R cells therefore represent a relevant in vitro model to study the proliferative and survival function of CSF-1 in myeloid progenitors and have the additional advantage of being a homogeneous cell population compared with bone marrow blast cells. The purpose of the present study was to examine in a myeloid cell line transduced with the murine CSF-1 receptor how cAMP and myeloid growth factor costimulation affects activation of ERK, a common target for both agents. Because serpentine receptors frequently activate multiple G-proteins that couple to different intracellular signaling pathways, we have used pharmacological agents to directly assess the role of the cAMP second messenger system. cAMP synergized with CSF-1 (acting on a tyrosine kinase receptor) or IL-3 (acting on a cytokine receptor) to greatly increase ERK activity. Despite the marked upregulation of ERK activity, cAMP still antagonized growth factor-dependent mitogenesis and cell survival. However, blockage of ERK activation in 32D cells accelerated the growth inhibition and apoptosis induced by cAMP. These results indicate that the ability of growth factors to cooperate with cAMP and enhance ERK activation protected 32D cells from the antiproliferative and proapoptotic effects mediated by cAMP. The implications of these findings in hematopoietic homeostasis are discussed.
MATERIALS AND METHODS
Antibodies and reagents.
Polyclonal antibodies against ERK2 (sc-154), Raf-1 (sc-133), B-Raf (sc-166 and competing peptide), A-Raf (sc-408 and competing peptide), and p90rsk (RSK1, sc-231) were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies were from the following sources: anti–Raf-1, MEK1, MEK2, ERK1, ERK2, ERK3, pan ERK, PY20 antiphosphotyrosine antibody (Transduction Laboratories, Lexington, KY), ERK1/2 (Zymed, San Francisco, CA), Y13-258 and Ab-4 anti-Ras antibodies (Oncogene Research, Cambridge, MA), and antihemagglutinin antibody (HA.11, BAbCo, Richmond, CA). Secondary antibodies were from GIBCO BRL (Gaithersburg, MD) or Zymed. Recombinant human CSF-1 was a gift from Genetics Institute (Cambridge, MA), recombinant murine IL-3 was from Becton Dickinson (Bedford, MA), protein A and protein G sepharose were from Zymed, S6 peptide (RRRLSSLRA) was from Upstate Biotechnology (Lake Placid, NY), myelin basic protein (MBP) and cell culture media were from GIBCO BRL, PD98059 was from Calbiochem (La Jolla, CA), and all other reagents were from Sigma (St Louis, MO).
Plasmids and plasmid construction.
The murine CSF-1R cDNA described previously28 was cloned into the mammalian expression vector pCEN/MPSV to generate plasmid pCEN/MSPV-CSF-1R. The parental pCEN vector from John Majors (Washington University Medical School, St Louis, MO) was modified to pCEN/MPSV, which contains the enhancer/promoter sequences from the myeloproliferative sarcoma virus so as to extend host range.29 The 61LRas fragment was released from pZIP-RasH61L (a gift of Channing Der, University of North Carolina, Chapel Hill, NC) and inserted into the pcDNAIneo expression vector (Invitrogen, Carlsbad, CA). pCMV5-MEK1(S218A, S222A) was from Kun-Liang Guan (University of Michigan, Ann Arbor, MI). To avoid potential interference from the Epstein-Barr virus sequences present in pCEP4-HA–tagged ERK2 (a gift of Melanie Cobb, University of Texas, Southwestern, Dallas, TX), they were deleted from HA-ERK2/pCEP4 to generate HA-ERK2/pCEP4Δ. Bacterial expression plasmids encoding His-tagged wild-type MAPK, kinase-dead (KD) MAPK, and KD-MEK were from Gary Johnson (National Jewish Center for Immunology and Respiratory Medicine, Denver, CO) and His-tagged proteins were purified as described.33
Cell culture and treatments.
FDC-P134 and 32Dcl2335 are murine nonleukemic myeloid precursor cell lines dependent on IL-3 for growth and survival. FDC-P1 can also use granulocyte-macrophage growth factor (granulocyte-macrophage colony-stimulating factor [GM-CSF]) instead of IL-3. The FDC-P1 cell line was obtained from Larry Rohrschneider (Fred Hutchinson Cancer Center, Seattle, WA) and maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 5% WEHI-CM (conditioned medium) as a source of murine IL-3; the maintenance of 32D cells has been described previously.29 The rat pheochromocytoma cell line, PC12,36 was obtained from the American Type Culture Collection (Manassas, VA) and maintained in F-12K medium supplemented with 15% horse serum and 2.5% FBS. For kinase assays, cycling cells were rinsed twice in Hanks’ Buffered Salt Solution (HBSS) before starving for 2 hours in serum-free medium whose components were as described.37 Cells were then treated as indicated in the figure legends. In cases in which stock solutions of test agents (forskolin, 3-isobutyl-1–methylxanthine [IBMX], PD98059) were in dimethyl sulfoxide (DMSO), an equal volume of DMSO was added to control cells. Optimal doses of CSF-1 (1 to 10 nmol/L) and IL-3 (50-100 U/mL) were used in all experiments.
Transfections.
To establish stable cell lines expressing CSF-1R, 32D cells were electroporated with 20 μg of pCEN/MPSV-CSF-1R plasmid using a Gene pulser (Bio-Rad, Hercules, CA). Forty-eight hours later, transfected cells were placed in complete medium containing 1 mg/mL of G418. Drug-resistant mass populations were selected over 1 week, and individual clones were isolated by limiting dilution and screened for surface expression of CSF-1R by binding to 125I-CSF-1 as described previously.28 Two clones, WT8 and WT10, were selected for further study. Efficiency of transient transfections was determined using 5 to 10 μg of a pcDNA1Neo construct expressing β-galactosidase. Cells (1 × 107) were electroporated at 280 V, 960 μF. Similar to transfection efficiencies reported for other hematopoietic cells,38 39 we found that 20% to 22% of viable cells stained positive with X-gal 24 hours after transfection. Higher transfection efficiencies could be achieved with larger amounts of DNA but at the expense of increased cell death. Accordingly, a tagged ERK2 construct (HA-ERK) was used as a reporter in transient cotransfections. The total transfected DNA was kept constant with pcDNA1Neo. Twenty-four hours after electroporation, dead cells were removed by spinning through a Ficoll cushion (Organon Teknika, Durham, NC), rinsed in HBSS, and placed in serum-free medium. Cells were treated as described above.
Immunoprecipitation and kinase assays.
After treatment, cells were rinsed in HBSS before lysis in ice-cold buffer containing 20 mmol/L Tris, pH 7.5, 2 mmol/L EDTA, 50 mmol/L NaCl, 10 mmol/L sodium pyrophosphate, 50 mmol/L NaF, 1% (vol/vol) Triton X-100, 0.5% (wt/vol) sodium deoxycholate, 50 mmol/L β-glycerophosphate, and 10% (vol/vol) glycerol containing 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 μg/mL pepstatin A, 1 mmol/L Na3VO4, 1 mmol/L benzamidine, 1 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L dithiothreitol (DTT). Insoluble material was pelleted by centrifugation and the protein content of the lysate determined by the Bio-Rad protein assay. For ERK and MEK assays, lysates were incubated with the appropriate antibody and protein A sepharose for 4 hours. In cotransfection experiments with HA-ERK2, HA-ERK2 expression was first quantitated by Western blotting and the amount of lysate used for each condition adjusted to contain equivalent levels of HA-ERK2. ERK or HA immunoprecipitates were washed twice with buffer A (10 mmol/L Tris, pH 7.4, 1% Nonidet P-4, 0.5% deoxycholate, 0.1 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 0.2 mmol/L Na3VO4), twice with buffer B (10 mmol/L Tris pH 7.4, 0.1 % Nonidet P-40, 1 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 0.2 mmol/L Na3VO4), once with buffer C (50 mmol/L Tris, pH 7.4, 0.15 mol/L NaCl), and once with kinase buffer (20 mmol/L HEPES, pH 7.4, 10 mmol/L MgCl2, 1 mmol/L MnCl2, 1 mmol/L EGTA, 0.03% Brij35, 2 mmol/L DTT, 0.2 mmol/L Na3VO4). Immunoprecipitates were resuspended in kinase buffer containing 10 μmol/L ATP, 5 μCi [32P]γATP (3,000 Ci/mmole; NEN, Boston, MA), 20 μg/mL aprotinin, 5.35 μmol/L protein kinase A inhibitor peptide (PKI), and 0.25 mg/mL MBP and incubated at 30°C for 15 minutes. The reaction was terminated by boiling in Laemmli sample buffer and the products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For MEK assays, immunoprecipitates were washed twice in lysis buffer, twice in lithium chloride buffer (0.1 mol/L Tris, pH 7.4, 0.5 mol/L LiCl), and once in kinase buffer (25 mmol/L HEPES, pH 7.4, 25 mmol/L β-glycerophosphate, pH 7.5, 10 mmol/L MgCl2, 1 mmol/L MnCl2, 0.5 mmol/L EGTA, 0.03% Brij35, 1 mmol/L DTT, 0.2 mmol/L Na3VO4) before resuspending in kinase buffer containing 200 to 400 ng recombinant KD-MAPK as substrate and other components as described above. The reaction was continued for 15 minutes at 30°C and processed as described above. ERK and MEK assay conditions were chosen such that substrate phosphorylation was in the linear range with respect to lysate amount and incubation time. For MEK-coupled assays, immunoprecipitates were first incubated for 30 minutes at 30°C in kinase buffer containing 100 μmol/L ATP, 20 μg/mL aprotinin, and 150 ng of recombinant wild-type MAPK followed by the addition of 5 μCi [32P]γATP and 0.25 mg/mL MBP and further incubation for 15 minutes. For Raf assays, 1 mg of cell lysate was immunoprecipitated with 2 μg of antibody and the immunoprecipitates were washed and kinase activity was measured as described for MEK, except that 400 ng of recombinant KD-MEK was used as substrate. When a competing peptide was used to test the specificity of A-Raf or B-Raf immunoprecipitations, the antibody and peptide were allowed to incubate for 30 minutes before the addition of lysates.
Gels were Coomassie Blue-stained to locate substrate bands, dried, and subjected to autoradiography. To quantitate substrate phosphorylation, the appropriate bands were either cut out and Cerenkov-counted or quantitated using the PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
For p90rsk assays, immunoprecipitates were washed twice in lysis buffer, once in Tris-buffered saline, and twice in kinase buffer. Activity of p90rsk was determined by measuring32P incorporation into S6 peptide in a reaction mixture containing kinase buffer (25 mmol/L HEPES, pH 7.4, 25 mmol/L β-glycerophosphate, 1 mmol/L EGTA, 0.5 mmol/L DTT, 10 mmol/L MgCl2), 250 μmol/L S6 peptide, 6 μmol/L PKI, 50 μmol/L ATP, and 6 μCi [32P]γATP. Incubation was for 15 minutes at 30°C, and the reaction supernatant was spotted onto P81 paper, rinsed four times in 0.5% phosphoric acid, dried in acetone, and counted.
Western blotting.
After boiling in Laemmli sample buffer, cell lysates (20 μg) or immunoprecipitates were fractionated by SDS-PAGE and transferred to Immobilon-P (Millipore, Bedford, MA) membranes. Membranes were immunoblotted with primary antibodies at the recommended dilutions, incubated with horseradish peroxidase-conjugated secondary antibodies, and developed by enhanced chemiluminescence (Amersham, Arlington Heights, IL). In some cases, autoradiography images were scanned with an Epson ES-1000C scanner and transparency module (Epson, Torrance, CA) using Adobe Photoshop version 3 software (Mountain View, CA). Their intensities were quantitated using NIH Image 1.6 software (provided by the Research Services Branch, NIMH, National Institutes of Health, Bethesda, MD) without further manipulation of the images.
Fluorescence-activated cell sorting (FACS) analysis.
To examine Ras expression in transiently transfected cells, 32D/CSF-1R cells were harvested 24 hours after electroporation, fixed in 0.5% formaldehyde, and permeabilized with methanol before staining with 20 μg/mL of Y13-258 anti-Ras antibody and a phycoerythrin-conjugated secondary antibody. FACS analysis was performed by the Washington University Pathology Department. As a negative control, cells were stained with an isotype-controlled antibody, anti-CD4.
Cell cycle analysis, proliferation, and apoptosis assays.
Proliferation was determined either by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay or by cell counts. For the MTS assay, 2 × 104cells were aliquoted per well of a 96-well dish in a total volume of 100 μL RPMI with 10% FBS and various test reagents. Each condition was performed in duplicate. After 48 hours, MTS and phenazine methosulfate (PMS) were added according to the manufacturer’s protocol (Promega CellTiter 96 Aqueous kit; Promega, Madison, WI). Cells were returned to the incubator for another 2 to 3 hours. The tetrazolium is reduced by metabolically active cells into formazan products, which are detected at 490 nm in a plate reader. For cell counts, cells were seeded at 5 × 104/mL in the various test media and cell numbers were determined daily in duplicate by counting with a hemacytometer. Viability was assessed by Trypan Blue dye exclusion. Apoptosis was detected using the method of Kinoshita et al.40 Cells were seeded at a density of 2 × 105/mL in 8 mL of various test media. Twenty-four hours later, they were harvested and lysed in 600 μL of lysis buffer (10 mmol/L Tris, pH 7.5, 10 mmol/L EDTA, 0.2% Triton X-100). Nuclei and other cellular debris were removed by centrifugation and the supernatant subjected to three rounds of extraction with phenol:chloroform (1:1). Low molecular weight chromosomal DNA was precipitated and dissolved in 10 mmol/L Tris, pH 8, 1 mmol/L EDTA, and RNA digested by incubation with 50 μg/mL RNAase A for 3 hours at 37°C. An equal volume of each sample was then loaded onto 1.5% agarose gels and DNA laddering was visualized by ethidium bromide staining.
RESULTS
cAMP inhibits CSF-1 and IL-3–stimulated growth of a myeloid progenitor cell line.
A cell line, 32Dcl23, which is differentiated to the myeloid progenitor stage but before the onset of CSF-1R expression, was transfected by electroporation with a mammalian expression vector encoding the murine CSF-1R cDNA. Two clones, WT8 and WT10, were selected for further study. By Scatchard analysis, WT8 and WT10 expressed 4.5 × 104 and 1 × 104 receptors/cell, respectively. For each clone, maximal proliferative response as determined by cell counting was induced by 1 nmol/L CSF-1 in the presence of serum, comparable to that elicited by 10% WEHI-CM, a source of murine IL-3. Data presented in the figures of this report were derived from WT8, but results have been confirmed with WT10 in most instances. Other investigators have reported that transduction of CSF-1R into a 32D subclone, 32Dcl3 (G), permits partial monocytic differentiation in the presence of CSF-1.41,42 Our clones (at least 3 have been analyzed in detail) have been monitored for 7 days in culture for signs of adhesion, morphological changes, and the acquisition of monocytic markers such as Mac-1 and Fcγ in response to CSF-1 (A.W.L., unpublished observations), and none has been observed. The basis for the difference is likely to be due to differences in lineage commitment, because 32Dcl3 (G) is already committed to differentiate along the myelomonocytic lineage and undergoes granulocyte colony-stimulating factor (G-CSF)–dependent terminal differentiation, whereas 32Dcl23 does not.43
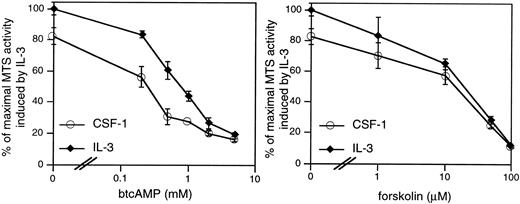
cAMP elevations are frequently but not universally linked to growth suppression. We first determined if the 32D/CSF-1R cell line is appropriate for investigations of the growth-inhibitory effects of cAMP. CSF-1– or IL-3–stimulated mitogenesis of 32D/CSF-1R cells was examined in the presence of varying concentrations of two agents known to activate PKA, which is the primary effector of cAMP in eukaryotic cells. They are forskolin, which directly stimulates adenylate cyclase to produce cAMP, and dibutyrl cAMP (btcAMP), a cell-permeable cAMP analog. Mitogenesis was assayed by the colorimetric MTS assay and the results are shown for WT8 in Fig 1. Very similar results were obtained for WT10. Both btcAMP and forskolin exerted a dose-dependent inhibition of CSF-1– and IL-3–dependent mitogenesis, with IL-3 being somewhat more resistant to the antiproliferative effects of btcAMP (ED50 is 0.35 mmol/L for CSF-1 and 0.8 mmol/L for IL-3).
cAMP elevations inhibit CSF-1– and IL-3–stimulated mitogenesis in 32D/CSF-1R myeloid cells. Exponentially growing cells were seeded into RPMI, 10% FBS with either 5 nmol/L CSF-1 or 100 U/mL of murine recombinant IL-3 and the indicated doses of btcAMP (left panel) or forskolin (right panel). Forty-eight hours later, mitogenesis was determined by the colorimetric assay as described in Materials and Methods. Results are shown as a percentage of the absorbance at 490 nm obtained for cells growing in IL-3 and represent the mean ± SE of duplicate samples.
cAMP elevations inhibit CSF-1– and IL-3–stimulated mitogenesis in 32D/CSF-1R myeloid cells. Exponentially growing cells were seeded into RPMI, 10% FBS with either 5 nmol/L CSF-1 or 100 U/mL of murine recombinant IL-3 and the indicated doses of btcAMP (left panel) or forskolin (right panel). Forty-eight hours later, mitogenesis was determined by the colorimetric assay as described in Materials and Methods. Results are shown as a percentage of the absorbance at 490 nm obtained for cells growing in IL-3 and represent the mean ± SE of duplicate samples.
cAMP and CSF-1 costimulation synergistically activate ERK.
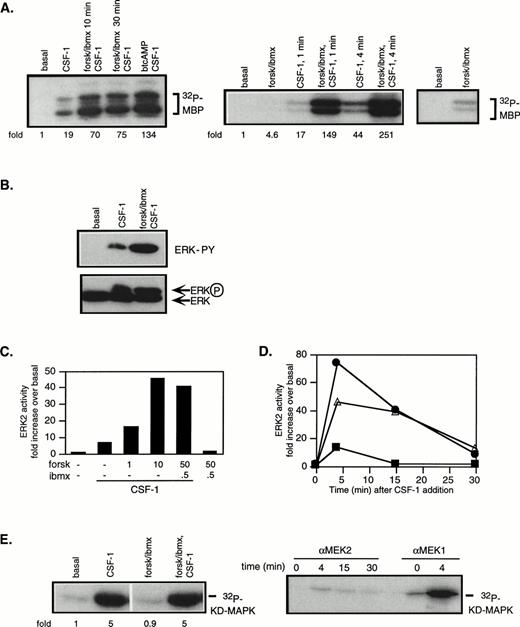
cAMP is known to inhibit growth factor-stimulation of the Ras/MAPK pathway in a variety of cell types. We investigated if cAMP’s growth inhibitory effects in 32D/CSF-1R cells could be a consequence of inhibition of CSF-1–stimulated ERK activity. Western blotting with different anti-ERK antibodies indicated p42ERK2 to be the predominant ERK species and MEK1 and MEK2 antibodies both recognized 46-kD proteins (data not shown). To measure ERK and MEK activities, cells were starved in serum-free medium for 2 hours before CSF-1 addition. CSF-1 stimulated a robust increase in the activities of both kinases (Fig 2A and E): on average, ERK and MEK1 activities were increased by 15.4- ± 3.1-fold (SE; n = 16) and 4.7- ± 0.8-fold (SE; n = 13), respectively, over untreated cells after 4 minutes of CSF-1 stimulation.
cAMP cooperates with CSF-1 to synergistically activate ERK/MAPK. 32D/CSF-1R cells were starved in serum-free media for 2 hours. Where indicated, 50 μmol/L forskolin (forsk) and 0.5 mmol/L IBMX or 1 mmol/L btcAMP were added during the last 20 minutes of the incubation or as indicated and then stimulated with or without CSF-1 for 4 minutes or as indicated at 37°C. Immune complex kinase assays were performed on lysates normalized for protein content. Fold refers to increase in substrate phosphorylation relative to that in untreated cells. (A) ERK2 was immunoprecipitated and activity measured in an in vitro kinase assay with MBP as a substrate. The left panel shows that similar results were obtained with either a 10- or 30-minute pretreatment period with forskolin/IBMX and with btcAMP. The right panel shows that forskolin/IBMX pretreatment increased ERK activity at both 1 and 4 minutes after CSF-1 addition. The rightmost panel is a longer exposure to compare basal ERK activity with that in the presence of forskolin/IBMX only. The magnitude of the increase induced by forskolin/IBMX varies between experiments (see Fig 3). (B) Immunoprecipitated ERK2 was analyzed for tyrosine phosphorylation by Western blotting with an antiphosphotyrosine antibody (top). The blot was then stripped and reprobed with anti-ERK2 monoclonal antibody (bottom). (C) Cells were pretreated with varying doses of forskolin or IBMX as indicated, followed by CSF-1 stimulation. ERK immune complex kinase assays were performed as described above. (D) Cells were stimulated with CSF-1 alone (▪), pretreated with forskolin/IBMX for 20 minutes and then stimulated with CSF-1 (•), or treated with CSF-1 and forskolin/IBMX simultaneously (▵). Aliquots were taken at the indicated time points, and lysates were prepared and assayed for ERK2 activity. (E) MEK1 was immunoprecipitated and activity measured with recombinant KD MAPK protein as substrate (left panel). MEK1 or MEK2 was immunoprecipitated from the same preparation of lysates and assayed for kinase activity (right panel). Parallel MEK1 and MEK2 immune complex kinase assays were repeated twice with similar results.
cAMP cooperates with CSF-1 to synergistically activate ERK/MAPK. 32D/CSF-1R cells were starved in serum-free media for 2 hours. Where indicated, 50 μmol/L forskolin (forsk) and 0.5 mmol/L IBMX or 1 mmol/L btcAMP were added during the last 20 minutes of the incubation or as indicated and then stimulated with or without CSF-1 for 4 minutes or as indicated at 37°C. Immune complex kinase assays were performed on lysates normalized for protein content. Fold refers to increase in substrate phosphorylation relative to that in untreated cells. (A) ERK2 was immunoprecipitated and activity measured in an in vitro kinase assay with MBP as a substrate. The left panel shows that similar results were obtained with either a 10- or 30-minute pretreatment period with forskolin/IBMX and with btcAMP. The right panel shows that forskolin/IBMX pretreatment increased ERK activity at both 1 and 4 minutes after CSF-1 addition. The rightmost panel is a longer exposure to compare basal ERK activity with that in the presence of forskolin/IBMX only. The magnitude of the increase induced by forskolin/IBMX varies between experiments (see Fig 3). (B) Immunoprecipitated ERK2 was analyzed for tyrosine phosphorylation by Western blotting with an antiphosphotyrosine antibody (top). The blot was then stripped and reprobed with anti-ERK2 monoclonal antibody (bottom). (C) Cells were pretreated with varying doses of forskolin or IBMX as indicated, followed by CSF-1 stimulation. ERK immune complex kinase assays were performed as described above. (D) Cells were stimulated with CSF-1 alone (▪), pretreated with forskolin/IBMX for 20 minutes and then stimulated with CSF-1 (•), or treated with CSF-1 and forskolin/IBMX simultaneously (▵). Aliquots were taken at the indicated time points, and lysates were prepared and assayed for ERK2 activity. (E) MEK1 was immunoprecipitated and activity measured with recombinant KD MAPK protein as substrate (left panel). MEK1 or MEK2 was immunoprecipitated from the same preparation of lysates and assayed for kinase activity (right panel). Parallel MEK1 and MEK2 immune complex kinase assays were repeated twice with similar results.
To examine the effect of cAMP, PKA was activated with either btcAMP or forskolin in combination with IBMX, a cyclic nucleotide phosphodiesterase inhibitor. Cells were pretreated with PKA-activating agents for 10 or 30 minutes before CSF-1 stimulation. In the absence of CSF-1, cAMP had a modest effect on ERK activity, increasing it by 4.6-fold above basal in the experiment shown (Fig 2A, right panel), although the magnitude of increase varies between experiments. On average, forskolin/IBMX alone increased ERK activity by 2.6- ± 0.5-fold (n = 10, P < .01 using the Student’s two-sidedt-test; Fig 3). In the presence of CSF-1, cAMP markedly enhanced ERK activation by an additional 5.6- ± 0.5-fold (n = 11, P < .001) and 4.6- ± 1.1-fold (n = 4, P < .05) for forskolin/IBMX and btcAMP, respectively (Figs 2A and 3). To eliminate the possibility that the increased MBP phosphotransferase activity present in ERK immunoprecipitates was a result of coprecipitating PKA, all ERK assays included PKI, a PKA inhibitor. ERK activation requires the phosphorylation of a threonine and tyrosine. ERK immunoprecipitates were blotted with an antiphosphotyrosine antibody, and the results (Fig 2B, top panel) confirm that the increase in ERK kinase activity in the presence of cAMP and CSF-1 was accompanied by enhanced ERK tyrosine phosphorylation. Gel mobility shifts of ERK have frequently been used as evidence for kinase activation. In agreement, Fig 2B (bottom panel) shows an increase in the amount of a slower migrating species when ERK immunoprecipitates were probed with a monoclonal ERK antibody. We also determined the dose-dependence of ERK activity on forskolin and found that the magnitude of synergistic activation reached a maximum with 10 μmol/L forskolin and remained constant between 10 and 50 μmol/L forskolin (Fig 2C). A dose of 50 μmol/L forskolin with 0.5 mmol/L IBMX was used in all subsequent experiments, unless otherwise stated.
Plot summarizing data from multiple experiments. ERK2 and MEK1 activities were measured as described in Fig 2 and the fold activation was calculated by normalizing to activities measured in untreated controls (− CSF-1) or to activities in samples treated with CSF-1 only (+ CSF-1) using the formula 1+ ([with cAMP] − [no cAMP])/([no cAMP] − [untreated]). Data shown represent the means ± SE, with the number of experiments (n) indicated. Statistically significant differences between untreated and cAMP-treated pairs are denoted by asterisks: *P < .05; **P < .001.
Plot summarizing data from multiple experiments. ERK2 and MEK1 activities were measured as described in Fig 2 and the fold activation was calculated by normalizing to activities measured in untreated controls (− CSF-1) or to activities in samples treated with CSF-1 only (+ CSF-1) using the formula 1+ ([with cAMP] − [no cAMP])/([no cAMP] − [untreated]). Data shown represent the means ± SE, with the number of experiments (n) indicated. Statistically significant differences between untreated and cAMP-treated pairs are denoted by asterisks: *P < .05; **P < .001.
cAMP is growth-inhibitory for CCL39 fibroblasts, and this was reported to correlate with inhibition of mitogen-stimulated ERK activation when measured at a fixed time point. However, it was subsequently demonstrated that cAMP pretreatment delayed ERK activation by 5 to 10 minutes, but did not affect the magnitude of the response.44 To determine if the observed synergism between cAMP and CSF-1 reflects a shift in activation kinetics rather than true synergism, we compared the time course of ERK activation in response to (1) CSF-1 addition alone, (2) a 20-minute pretreatment with forskolin/IBMX followed by CSF-1, and (3) the addition of CSF-1 and forskolin/IBMX simultaneously. Figure 2D shows that CSF-1–stimulated ERK activity was transient and was back to unstimulated levels after 15 minutes. Both cAMP treatment regimens resulted in a synergistic activation of ERK: at 4 minutes after stimulation, ERK activity was increased by approximately 70-fold if cells were pretreated and by approximately 45-fold if CSF-1 and forskolin/IBMX were added together. At the subsequent time points, ERK activity was elevated to a similar degree for both cAMP treatment regimens: 40-fold above basal at 15 minutes and 10-fold above basal at 30 minutes. We also determined the time course of PKA activation (assayed by Kemptide phosphorylation, data not shown) and found that the extent of synergistic ERK activation in response to cAMP and CSF-1 costimulation tracked closely the magnitude of PKA activation. These data demonstrate that maximal cooperation between CSF-1 and cAMP occurs when there is maximal overlap in the intensity of the individual signals. A 20-minute pretreament period with cAMP-elevating agents was adopted in all subsequent experiments.
CSF-1 activates MEK1 and not MEK2 and neither is affected by cAMP treatment.
MEKs are the predominant ERK kinases in mammalian cells. To determine if the enhanced ERK stimulation can be explained by corresponding changes in MEK activity, MEK1 was immunoprecipitated and its activity measured against a recombinant KD MAPK as substrate. Despite the much increased ERK activity in the presence of cAMP and CSF-1 compared with CSF-1 alone, cAMP elevation was found to have a minimal effect on CSF-1–induced MEK activity (Fig 2E). Because small changes in MEK activity may not be detected by phosphorylation of KD-MAPK, a coupled MEK assay was performed in which immunoprecipitated MEK1 was allowed to phosphorylate recombinant wild-type MAPK and activation of MAPK was determined by MBP phosphorylation. Even this more sensitive assay did not detect a significant increase in MEK1 activity in the presence of cAMP elevation (data not shown). We also determined ERK2 and MEK1 activities in the absence and presence of forskolin/IBMX pretreatment at different CSF-1 concentrations (0, 0.01, 0.1, and 1 nmol/L) and at different times after CSF-1 addition (0, 1, 4, 10, 15, and 30 minutes). A similar dissociation between ERK2 and MEK1 activities was observed in response to cAMP stimulation under all conditions tested (data not shown). In addition to MEK1, MEK2 is also known to activate ERK1/2. To determine if MEK2 may be mediating the increase in ERK activation, lysates from 32D/CSF-1R cells treated with CSF-1 for various times were immunoprecipitated with a MEK2 antibody in common usage45and assayed for activity against KD-MAPK (Fig 2E). Maximal MEK2 activity was estimated to be less than 1% that of MEK1 measured simultaneously. Effective immunoprecipitation of MEK2 was verified by blotting the immunoprecipitates with MEK2 antibodies (not shown). Thus, MEK2 did not contribute significantly towards ERK activation by CSF-1 in the absence (Fig 2E) or presence (not shown) of cAMP treatment. Figure 3 summarizes the results from multiple experiments, illustrating the synergistic activation of ERK but not of MEK1 by cAMP and CSF-1.
cAMP cooperates with IL-3 to synergistically activate ERK in 32D and FDC-P1 cells.
We next determined if the observed cooperative activation of ERK between cAMP and CSF-1 is unique to CSF-1 or if cAMP has a similar effect on ERK stimulation by other mitogens in these cells. 32D cells are IL-3–dependent for proliferation and survival. Figure 4A shows that IL-3–activated ERK2 and pretreatment with forskolin/IBMX further increased ERK activation by more than eightfold. As with CSF-1, the increase in ERK activity in the presence of cAMP was not reflected by detectable increases in MEK1 activity (the small increase in MEK1 activity depicted in Fig 4A was not consistently observed between experiments). MEK2 was also not activated in response to IL3 (data not shown). IL-3 and cAMP effects were further investigated in a second IL-3–dependent, nonleukemic, myeloid progenitor cell line, FDC-P1, and synergistic activation was also observed (Fig 4A). These data demonstrate that cAMP cooperates with two different classes of growth factor receptors, a tyrosine kinase receptor (CSF-1R) and a cytokine receptor (IL-3R), to activate the ERK pathway in murine myeloid progenitors.
cAMP synergizes with IL-3 to activate ERK2 in 32D/CSF-1R and FDC-P1 cells. Cells were treated as described in the legend to Fig2, except that they were stimulated with murine rIL-3. (A) Cells were pretreated or not with forskolin/IBMX and stimulated with IL-3 for 15 minutes. ERK (top) and MEK1 (bottom) assays were performed as described in Fig 2. (B) Time dependence of ERK2 stimulation by CSF-1 (top panel) or IL-3 (bottom panel) in the presence (•) or absence (▪) of forskolin (10 μmol/L) and IBMX (0.1 mmol/L) pretreatment. Cells were starved in medium containing 10% FBS but no IL-3 for 18 hours and CSF-1 or IL-3 was added. Aliquots were taken at the indicated time points (0, 5 minutes, 30 minutes, 1 hour, 3 hours, 5 hours, 7 hours, 9 hours, 12 hours, and 22 hours) and processed for ERK activity. The experiments shown in (A) and (B) have been repeated twice with very similar results.
cAMP synergizes with IL-3 to activate ERK2 in 32D/CSF-1R and FDC-P1 cells. Cells were treated as described in the legend to Fig2, except that they were stimulated with murine rIL-3. (A) Cells were pretreated or not with forskolin/IBMX and stimulated with IL-3 for 15 minutes. ERK (top) and MEK1 (bottom) assays were performed as described in Fig 2. (B) Time dependence of ERK2 stimulation by CSF-1 (top panel) or IL-3 (bottom panel) in the presence (•) or absence (▪) of forskolin (10 μmol/L) and IBMX (0.1 mmol/L) pretreatment. Cells were starved in medium containing 10% FBS but no IL-3 for 18 hours and CSF-1 or IL-3 was added. Aliquots were taken at the indicated time points (0, 5 minutes, 30 minutes, 1 hour, 3 hours, 5 hours, 7 hours, 9 hours, 12 hours, and 22 hours) and processed for ERK activity. The experiments shown in (A) and (B) have been repeated twice with very similar results.
ERK stimulation by IL-3 in the presence or absence of forskolin/IBMX was compared with that observed for CSF-1 and monitored over a 22-hour time period (Fig 4B). Because 32D/CSF-1R cells cannot survive for prolonged periods with CSF-1 as the sole growth factor without serum (A.W.L., unpublished observations), they were starved for 24 hours in medium containing 10% FBS but no IL-3 or CSF-1. This resulted in a higher basal level of ERK activity and a somewhat lower fold induction compared with that seen with serum-free medium. In the absence of forskolin/IBMX, the peak of IL-3–stimulated ERK activity was slightly delayed (maximum at 15 minutes) compared with that induced by CSF-1 (maximum at 5 minutes). cAMP did not alter the kinetics of ERK activation during the 22 hours of monitoring; rather, the primary effect was on the magnitude of the response, as was seen with cells starved in serum-free medium (Fig 2D). Because the kinetics of cAMP-mediated activation was very similar for both CSF-1 and IL-3, pathways common to both growth factors are likely to being modulated by cAMP.
Upregulation of ERK activity by cAMP and CSF-1 is reflected in a synergistic increase in p90rsk activity.
We sought to determine if the synergism between cAMP and CSF-1 was transmitted downstream of ERK. MEK1-ERK is both necessary and sufficient for activation of all three isoforms of the ribosomal S6 kinase, p90rsk46; furthermore, there is evidence supporting a direct role for ERK in phosphorylating and activating p90rsk.46 Cell lysates were immunoprecipitated with an anti-RSK1 antibody and kinase activity towards S6 peptide was determined (Fig 5). CSF-1 stimulated a 20-fold increase in RSK1 activity that was further increased by almost twofold in the presence of forskolin/IBMX. Because the targets for p90rsk are transcriptional factors, these data suggest that the synergistic activation of ERK by cAMP and CSF-1 is likely to have a physiological role.
cAMP upregulates CSF-1–stimulated p90rskactivity. 32D/CSF-1R cells were treated as described in the legend to Fig 2. Lysates were immunoprecipitated with anti-p90rsk and the immunoprecipitates were assayed for kinase activity towards S6 peptide. Data represent the means ± SE from two independent experiments.
cAMP upregulates CSF-1–stimulated p90rskactivity. 32D/CSF-1R cells were treated as described in the legend to Fig 2. Lysates were immunoprecipitated with anti-p90rsk and the immunoprecipitates were assayed for kinase activity towards S6 peptide. Data represent the means ± SE from two independent experiments.
Oncogenic Ras cooperates with cAMP to activate ERK in a Raf-1–independent and MEK1-dependent manner.
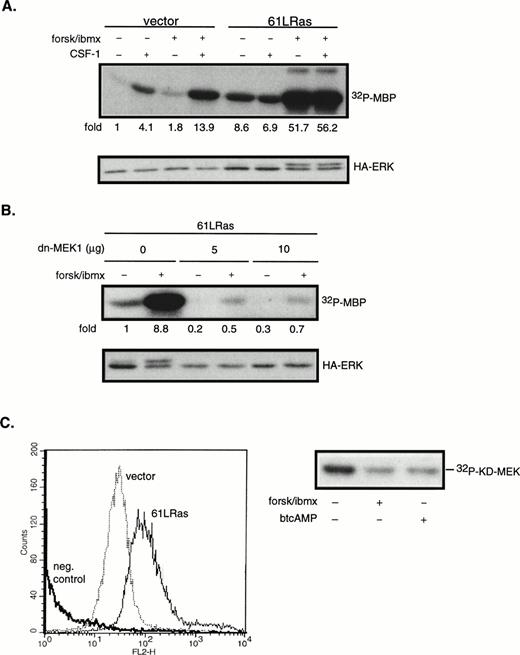
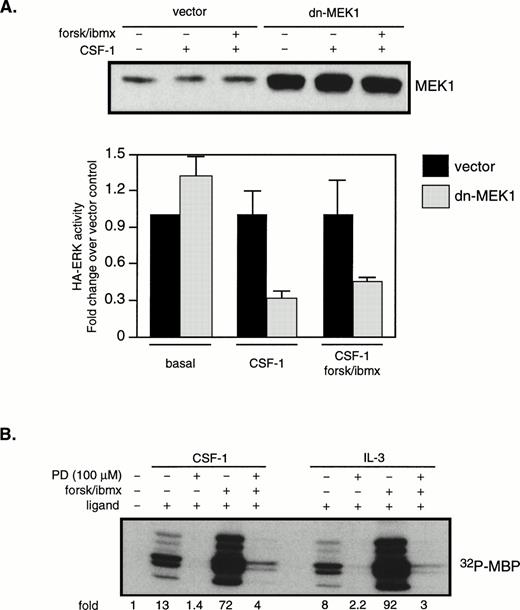
The next series of experiments were designed to determine the steps in the CSF-1–induced ERK activation cascade necessary for mediating cAMP’s synergistic effects. ERK can be activated by both Ras-dependent and independent pathways. To determine if Ras is involved in the synergistic activation of ERK by cAMP in 32D/CSF-1R cells, oncogenic Ras (61L) or its empty vector control was cotransfected with a hemagglutinin (HA)-tagged ERK2 (HA-ERK2). HA-ERK2 serves as a reporter to monitor 61LRas’s effect on ERK activity in transfected cells. Cells were processed 24 hours after transfection. In this and subsequent experiments using HA-ERK2, Western blotting with anti-HA antibody was first performed to assess expression levels for the different transfections. Lysates containing equivalent amounts of HA-ERK2 were then used in immune complex kinase assays. Results of a representative experiment are shown in Fig6A. Based on five independent transfections, it was observed that the steady-state expression of 61LRas resulted in a 10.1- ± 2.6-fold increase in basal HA-ERK2 activity compared with vector control. HA-ERK activity was not further increased by CSF-1, which suggests that, under normal conditions, Ras-GTP may be limiting for CSF-1–mediated ERK activation. In contrast to the lack of an effect by CSF-1, forskolin/IBMX treatment synergized strongly with 61LRas to further activate ERK. The average induction by forskolin/IBMX from four separate experiments was 4.7- ± 1.8-fold (P < .04) over that obtained in its absence, similar in magnitude to that observed for the cooperation between CSF-1 and cAMP (Fig 3). To determine if the activation of HA-ERK2 by cAMP and 61LRas was MEK1-dependent, we made use of a dn-MEK1 expression construct, in which the serines required for its activation (S218, S222) have been replaced by alanines. Figure6B shows that dn-MEK1 inhibited 61LRas-stimulated HA-ERK2 activity by 70% to 80% and eliminated most of the increase in activity induced by cAMP. To investigate if Raf-1 is involved in the cooperation between 61LRas and cAMP, 32D/CSF-1R cells were transfected with fourfold more 61LRas DNA (20 μg) than normally used in cotransfection experiments, because an epitope-tagged Raf-1 construct was not available. FACS analysis showed that a quantitative shift in mean Ras fluorescence was achieved for the transfectants (Fig 6C), indicating that the majority of the cells expressed similar levels of 61LRas. It was found that 61LRas expression increased basal Raf-1 activity by 2.7-fold compared with vector control (not shown), and this activity was significantly inhibited by cAMP (Fig 6C). Thus, the data indicate that cAMP can synergize with oncogenic Ras to markedly increase ERK activity via an MEK1-dependent mechanism but that Raf-1 did not appear to be involved.
cAMP synergizes with oncogenic Ras to stimulate ERK in an MEK1-dependent manner. 32D/CSF-1R cells were cotransfected with vector (7 μg) and HA-ERK2 (2 μg) or with 61LRas (6 μg), HA-ERK2 (1 μg), and vector (2 μg). Twenty-four hours later, surviving cells were starved and pretreated or not with forskolin/IBMX before stimulation with CSF-1 for 4 minutes. (A) Transfected ERK2 from lysates normalized to contain equivalent levels of HA-ERK2 was immunoprecipitated with anti-HA antibody and kinase activity towards MBP was determined (top). Anti-HA Western blot of transfected HA-ERK2 is shown (bottom). The results are representative of four independent sets of transfections. (B) Cells were cotransfected with 61LRas (3 μg), HA-ERK2 (1 μg), and the indicated amounts of dn-MEK1, with the total DNA content kept constant with empty vector. HA-ERK2 kinase activity (top) and Western blot (bottom) are shown. (C) FACS analysis shows Ras expression level in cells 24 hours after transfection with either vector (20 μg) or 6LRas (20 μg). Cells were permeabilized and stained with anti-Ras antibody (Y13-258). As a negative control, Ras-transfected cells were stained with an irrelevant primary antibody (anti-CD4). Abscissa: log relative fluorescence intensity; ordinate: relative cell number. Raf-1 kinase activity in cells transfected with 61LRas, in the absence or presence of cAMP-elevating agents, is shown on the right.
cAMP synergizes with oncogenic Ras to stimulate ERK in an MEK1-dependent manner. 32D/CSF-1R cells were cotransfected with vector (7 μg) and HA-ERK2 (2 μg) or with 61LRas (6 μg), HA-ERK2 (1 μg), and vector (2 μg). Twenty-four hours later, surviving cells were starved and pretreated or not with forskolin/IBMX before stimulation with CSF-1 for 4 minutes. (A) Transfected ERK2 from lysates normalized to contain equivalent levels of HA-ERK2 was immunoprecipitated with anti-HA antibody and kinase activity towards MBP was determined (top). Anti-HA Western blot of transfected HA-ERK2 is shown (bottom). The results are representative of four independent sets of transfections. (B) Cells were cotransfected with 61LRas (3 μg), HA-ERK2 (1 μg), and the indicated amounts of dn-MEK1, with the total DNA content kept constant with empty vector. HA-ERK2 kinase activity (top) and Western blot (bottom) are shown. (C) FACS analysis shows Ras expression level in cells 24 hours after transfection with either vector (20 μg) or 6LRas (20 μg). Cells were permeabilized and stained with anti-Ras antibody (Y13-258). As a negative control, Ras-transfected cells were stained with an irrelevant primary antibody (anti-CD4). Abscissa: log relative fluorescence intensity; ordinate: relative cell number. Raf-1 kinase activity in cells transfected with 61LRas, in the absence or presence of cAMP-elevating agents, is shown on the right.
To determine if HA-ERK2 might be activated by autocrine factors secreted by cells expressing oncogenic Ras, a mixing experiment was performed. Two pools of cells, transfected independently with 61LRas or HA-ERK2, were washed 2 hours after transfection and cocultured in fresh media for the remainder of the experiment. Under these conditions, HA-ERK2 activity of HA-ERK–transfected cells mixed with cells transfected with 61LRas was 3.2-fold higher than when mixed with cells transfected with the vector-control, compared with a 8.2-fold increase in cells cotransfected with 61LRas and HA-ERK2. This indicates that there was some secretion of autocrine factors but that 61LRas also directly activated HA-ERK2.
None of the Raf isoforms is responsible for cAMP-dependent ERK superactivation.
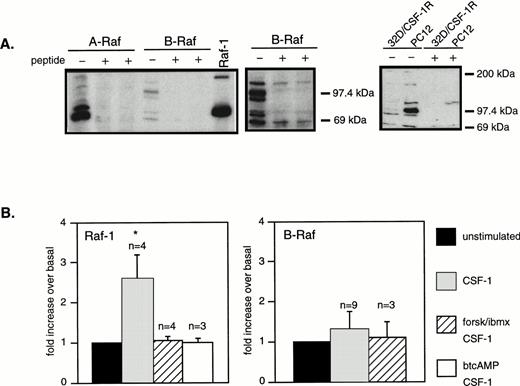
Because Raf-1 did not appear to be activated in the presence of cAMP, we sought to determine if MEK was activated by other Raf family members. Expression of the three Raf members in 32D/CSF-1R cells was determined by Western blotting (Fig 7A). Raf-1 and A-Raf were easily detected. Multiple bands (90 to 95 kD and 65 to 70 kD) were observed on the B-Raf blot but appeared to be specific as they were competed off by a blocking peptide. Also, B-Raf is present at significantly lower levels in 32D cells, in comparison to PC12, a cell line in which cAMP activates MEK/ERK by the Ras/B-Raf route.27 We next determined the activity of the three Rafs in response to CSF-1 ± cAMP by means of immune complex kinase assays using as substrate recombinant KD MEK1. CSF-1 stimulated a twofold to threefold increase in Raf-1 activity that was completely inhibited by pretreatment with forskolin/IBMX or btcAMP (Fig 7B), similar to what was observed for 61LRas (Fig 6C). Although B-Raf was present at low levels, it exhibited very high basal kinase activity; however, no increase in B-Raf activity could be detected in response to CSF-1 or IL-3, alone or in combination with cAMP-elevating agents (Fig7B). The kinase activity detected was confirmed to be due to B-Raf, because preincubation of the B-Raf antibody with its blocking peptide completely prevented the phosphorylation of KD-MEK by B-Raf immunoprecipitates (data not shown). Also, A-Raf kinase activity towards KD-MEK could not be detected, basally or in response to treatments, despite abundant amounts of A-Raf protein in the immunoprecipitates (data not shown). These results indicate that Raf-1 but not A-Raf or B-Raf was activated by CSF-1 and IL-3 (not shown) in 32D/CSF-1R cells and none of the Raf isoforms appeared to be responsible for ERK activation in the presence of cAMP and CSF-1.
CSF-1 activates Raf-1 but not B-Raf or A-Raf and none is activated in the presence of cAMP and CSF-1. (A) Western blot analysis of the three Raf isoforms in 32D/CSF-1R cells. Where indicated, blocking peptide (1 and 3 μg/mL) was preincubated with the appropriate antibody. The middle panel is a longer exposure of the B-Raf segment of the blot shown on the left. Equal amounts of lysates (50 μg) from 32D/CSF-1R and PC12 cells were also Western blotted for B-Raf, showing significantly higher levels of expression in PC12 cells (right panel). “+” refers to a parallel blot that was incubated with anti–B-Raf and its blocking peptide (1 μg/mL). (B) 32D/CSF-1R cells were treated as described in Fig 2 and Raf-1 or B-Raf was immunoprecipitated. Kinase activity was measured using a recombinant KD-MEK1 as substrate. Shown is a plot summarizing data (means ± SE) from multiple (n) experiments. Only CSF-1–stimulated Raf-1 activity is significant (*) compared with basal (P < .05).
CSF-1 activates Raf-1 but not B-Raf or A-Raf and none is activated in the presence of cAMP and CSF-1. (A) Western blot analysis of the three Raf isoforms in 32D/CSF-1R cells. Where indicated, blocking peptide (1 and 3 μg/mL) was preincubated with the appropriate antibody. The middle panel is a longer exposure of the B-Raf segment of the blot shown on the left. Equal amounts of lysates (50 μg) from 32D/CSF-1R and PC12 cells were also Western blotted for B-Raf, showing significantly higher levels of expression in PC12 cells (right panel). “+” refers to a parallel blot that was incubated with anti–B-Raf and its blocking peptide (1 μg/mL). (B) 32D/CSF-1R cells were treated as described in Fig 2 and Raf-1 or B-Raf was immunoprecipitated. Kinase activity was measured using a recombinant KD-MEK1 as substrate. Shown is a plot summarizing data (means ± SE) from multiple (n) experiments. Only CSF-1–stimulated Raf-1 activity is significant (*) compared with basal (P < .05).
The role of MEK1 in mediating ERK2 activation by CSF-1 and cAMP.
Figures 2 and 3 show that CSF-1 and cAMP activated ERK disproportionately compared with MEK1, but Fig 6 indicates that MEK1 lies on the major pathway leading to synergistic ERK activation by cAMP and oncogenic Ras. We therefore examined the role of MEK1 in CSF-1–stimulated ERK activation. Cells were cotransfected with HA-ERK and dn-MEK1 or a vector control. Western blotting with a MEK1 antibody indicated that, 24 hours after transfection, overall MEK1 levels were increased by 10-fold in cells transfected with dn-MEK1 compared with vector-transfected cells (Fig 8A, top). The results from four independent transfections are shown in Fig 8A (bottom). It is seen that dn-MEK1 significantly blocked activation of HA-ERK in cells stimulated with CSF-1 alone or in combination with cAMP. A concern with dn-MEK1 is that it may be exerting its inhibitory function by binding to an upstream activator shared by MEK and another MAPKK, resulting in the unknown MAPKK rather than MEK being uncoupled from the activation signal. To further confirm the critical role of MEK, we used the well-characterized and widely used synthetic inhibitor, PD98059, reported to be specific for MEK1/2.47PD98059 was added to the culture medium 1 hour and 40 minutes before the addition of forskolin/IBMX, which was followed 20 minutes later by CSF-1 (or IL-3) stimulation for 4 (or 10) minutes. ERK activities were measured and shown in Fig 8B. They demonstrate that 100 μmol/L PD98059 inhibited the majority of the ERK activity in response to ligand alone or in combination with forskolin/IBMX. A similar extent of ERK inhibition was also obtained with 50 μmol/L of PD98059. Taken together, our results suggest that the synergistic activation of ERK by CSF-1 (or IL-3) and cAMP depends on MEK1 activation.
MEK activity is required for the synergistic activation of ERK by CSF-1 and cAMP. (A) 32D/CSF-1R cells were cotransfected with HA-ERK2 (2 μg) and either vector (10 μg) or dn-MEK1 (10 μg). Cells were processed 24 hours later as described in Fig 5. Western blot of total MEK1 (endogenous plus transfected) is shown in the top panel. MBP kinase activity in HA-ERK2 immunoprecipitates was determined and the results from four independent cotransfections with HA-ERK2 and dn-MEK1 or vector control are shown in the bottom panel (means ± SE). To emphasize the effect of dn-MEK1, HA-ERK activities measured in the presence of dn-MEK1 are normalized to those from vector-control cells for each condition. (B) 32D/CSF-1R cells were starved and treated with 100 μmol/L PD98059 for 1 hour and 40 minutes before the addition of forskolin/IBMX. This was followed 20 minutes later by CSF-1 or IL-3. Endogenous ERK2 was immunoprecipitated and MBP kinase activity determined. The experiment has been repeated with 50 μmol/L PD98059 with very similar results.
MEK activity is required for the synergistic activation of ERK by CSF-1 and cAMP. (A) 32D/CSF-1R cells were cotransfected with HA-ERK2 (2 μg) and either vector (10 μg) or dn-MEK1 (10 μg). Cells were processed 24 hours later as described in Fig 5. Western blot of total MEK1 (endogenous plus transfected) is shown in the top panel. MBP kinase activity in HA-ERK2 immunoprecipitates was determined and the results from four independent cotransfections with HA-ERK2 and dn-MEK1 or vector control are shown in the bottom panel (means ± SE). To emphasize the effect of dn-MEK1, HA-ERK activities measured in the presence of dn-MEK1 are normalized to those from vector-control cells for each condition. (B) 32D/CSF-1R cells were starved and treated with 100 μmol/L PD98059 for 1 hour and 40 minutes before the addition of forskolin/IBMX. This was followed 20 minutes later by CSF-1 or IL-3. Endogenous ERK2 was immunoprecipitated and MBP kinase activity determined. The experiment has been repeated with 50 μmol/L PD98059 with very similar results.
ERK activation opposes cAMP’s growth inhibitory and apoptosis-promoting effects.
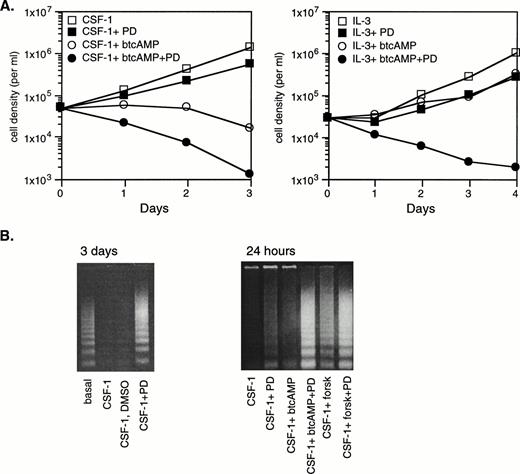
Because cAMP is growth-inhibitory (Fig 1) yet synergizes with growth factors to activate the ERK pathway (Fig 3), we wish to assess whether the synergistic activation of ERK plays a role, if at all, in cellular proliferation. To do this, we made use of PD98059, which was demonstrated in Fig 8 to abolish most of ERK activation in response to cAMP and CSF-1 or IL-3. No direct toxic effects have been reported for PD98059. Exponentially growing cells were thoroughly washed before seeding into fresh media containing 10% FBS. They were treated with 50 μmol/L PD98059 for 2 hours before the addition of btcAMP, and this was followed 20 minutes later by CSF-1 or IL-3. Cells were counted daily and representative results are shown in Fig 9A. Based on three independent experiments, PD98059 was found to reduce both CSF-1– and IL-3–dependent growth by 60% to 70% (P < .02) after 3 days of culture, indicating that ERK activity is required for optimal growth. btcAMP addition to CSF-1–stimulated cells caused growth cessation and cell death, and this was markedly enhanced by the inclusion of PD98059. IL-3–treated cells still proliferated in the presence of btcAMP, although cell numbers after 4 days of culture were only 30% of those growing without btcAMP. The addition of PD98059 to cells cultured with IL-3 and btcAMP had a dramatic, greater-than-additive effect, as evidenced by rapid cell death (Fig9A). PD98059 similarly affected cells treated with forskolin in the presence of CSF-1 or IL-3 (data not shown). The ability of IL-3 to support proliferation in the presence of cAMP, albeit at a significantly reduced level, indicates that IL-3 may activate pathways important for proliferation that are not inhibited by cAMP, or that the strength of the IL-3 signal exceeds the threshold necessary for cell cycle progression. In both CSF-1– and IL-3–stimulated growth, ERK activity is necessary for maintaining the residual proliferation observed in the presence of cAMP, because inhibition of the MEK-ERK pathway resulted in growth arrest and eventual cell death.
ERK activation by CSF-1 and cAMP opposes cAMP’s growth-inhibitory and apoptosis-promoting effects. (A) Exponentially growing 32D/CSF-1R cells were thoroughly washed and then seeded into medium without CSF-1 or IL-3 in the presence or absence of 50 μmol/L PD98059 (PD) and allowed to incubate for 1 hour and 40 minutes. btcAMP (1 mmol/L) was added, followed by 5 nmol/L CSF-1 (left) or 100 U/mL of rIL-3 (right) 20 minutes later. DMSO was present in the same amounts in all samples. Cell counts were performed in duplicate daily. Duplicate cell counts showed less than a 5% error in most cases. (B) Cells were treated as described in (A) using either 1 mmol/L btcAMP or 30 μmol/L forskolin. Cytoplasmic DNA was isolated from a fixed number of starting cells as described in Materials and Methods and analyzed by agarose gel electrophoresis and ethidium bromide staining after 3 days (left) or 24 hours (right). Relative numbers of viable cells remaining after 24 hours under the different conditions for the experiment on the right are as follows: CSF-1 (100%), CSF-1+PD (67%), CSF-1+btcAMP (46%), CSF-1+btcAMP+PD (35%), CSF-1+forskolin (67%), and CSF-1+forskolin+PD (43%).
ERK activation by CSF-1 and cAMP opposes cAMP’s growth-inhibitory and apoptosis-promoting effects. (A) Exponentially growing 32D/CSF-1R cells were thoroughly washed and then seeded into medium without CSF-1 or IL-3 in the presence or absence of 50 μmol/L PD98059 (PD) and allowed to incubate for 1 hour and 40 minutes. btcAMP (1 mmol/L) was added, followed by 5 nmol/L CSF-1 (left) or 100 U/mL of rIL-3 (right) 20 minutes later. DMSO was present in the same amounts in all samples. Cell counts were performed in duplicate daily. Duplicate cell counts showed less than a 5% error in most cases. (B) Cells were treated as described in (A) using either 1 mmol/L btcAMP or 30 μmol/L forskolin. Cytoplasmic DNA was isolated from a fixed number of starting cells as described in Materials and Methods and analyzed by agarose gel electrophoresis and ethidium bromide staining after 3 days (left) or 24 hours (right). Relative numbers of viable cells remaining after 24 hours under the different conditions for the experiment on the right are as follows: CSF-1 (100%), CSF-1+PD (67%), CSF-1+btcAMP (46%), CSF-1+btcAMP+PD (35%), CSF-1+forskolin (67%), and CSF-1+forskolin+PD (43%).
The role of ERK in mediating survival was next assessed by DNA fragmentation analysis. Exponentially growing cells (1.6 × 106) were thoroughly rinsed and seeded into media containing CSF-1 and various combinations of PD98059 and forskolin or btcAMP as described above. Cells were harvested 24 hours later and low molecular weight, soluble DNA was extracted and analyzed for DNA laddering as described in Materials and Methods. The results demonstrate that DNA laddering was already evident in cells that have been growing in the presence of PD98059 for 1 day (Fig 9B, right panel), suggesting that ERK activity is required for optimal protection against apoptosis by CSF-1. By 3 days, the intensity of laddering was comparable to that observed in cells that have been completely deprived of CSF-1 and IL-3 for the same period (Fig 9B, left panel). Furthermore, PD98059 markedly enhanced the amount of apoptosis observed in cells treated with either btcAMP or forskolin, showing that the synergistic activation of ERK protects cells from cAMP-induced apoptotic effects. Thus, it appears that CSF-1 and cAMP activated a growth- and survival-promoting signal mediated by ERK and that cAMP also activated other opposing, growth-inhibitory, and apoptosis-promoting signals in myeloid progenitors.
DISCUSSION
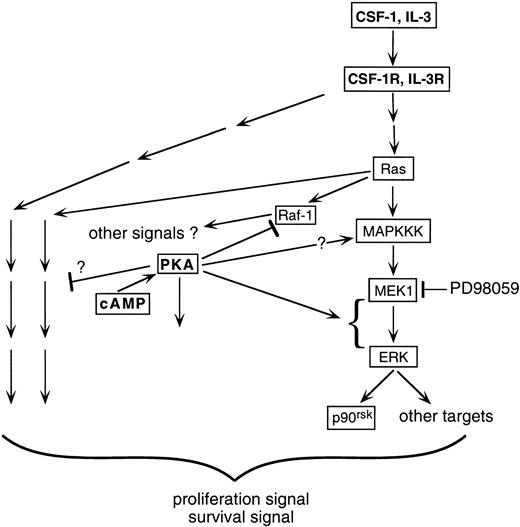
ERK/MAPK and cAMP are ubiquitous and critical cellular signaling components. A wide range of cell-type–dependent and receptor-type–dependent interactions between them have been observed.21-27 The present study focuses on the effect of cross-talk between cAMP and growth factors on the ERK pathway in myeloid progenitors. The key findings are as follows. (1) cAMP synergized with CSF-1 or IL-3 to markedly upregulate ERK activity. This synergism was transmitted downstream to at least one ERK substrate, p90rsk. (2) The cooperative activation of ERK by cAMP and growth factors was recapitulated by cAMP treatment of 32D cells transfected with oncogenic Ras. (3) The synergistic activation of ERK by cAMP and CSF-1 or IL-3 did not involve any of the Raf isoforms. (4) Both expression of dn-MEK1 and treatment with PD98059 prevented the observed synergistic activation, indicating that MEK1 activity was required for the cooperation between cAMP and growth factors; however, there is a dissociation between the levels of MEK1 and ERK2 activities, suggesting that cAMP also targets other factors that modulate ERK activity. (5) Finally, an intact ERK response was required for optimal CSF-1– and IL-3–dependent cell proliferation and survival, and its inhibition markedly accelerated the growth inhibition and apoptosis induced by cAMP. This is the first report describing cooperation in myeloid progenitors between cAMP and growth factors such as CSF-1 and IL-3 to activate the ERK pathway. This cooperation occurs at the level of ERK and is Ras- and MEK-dependent but independent of the Rafs. The upregulated ERK activity is important for protecting hematopoietic cells from the antiproliferative and proapoptotic effects of cAMP. A schematic summarizing our findings is shown in Fig 10.
Schematic illustrating how growth factors such as CSF-1 and IL-3 may cooperate with cAMP/PKA to activate the ERK/p90rsk pathway in myeloid progenitors. CSF-1 stimulates the activities of both Raf-1 and ERK in 32D cells. In the presence of cAMP, Raf-1 activation is prevented yet ERK activity is enhanced. The simplest explanation is that CSF-1 uses an alternate (not Raf-1) cAMP-insensitive kinase as the MAPKKK. Activation of MAPKKK is likely to be Ras-dependent, because expression of oncogenic Ras together with elevations in cAMP levels can recapitulate the synergistic stimulation of ERK by CSF-1 and cAMP. Whether cAMP can itself activate MAPKKK is not clear (see text). Instead, the dominant stimulatory effect of cAMP appears to be at a step after MEK1 activation, because MEK1 activity itself is not enhanced in the presence of cAMP but is required for the synergistic action of cAMP. Inhibition of the MEK-ERK pathway results in a significant but incomplete suppression of cell proliferation, indicating that growth factors such as CSF-1 and IL-3 activate other pathways required for optimal proliferation and survival in myeloid progenitor cell lines. These pathways could be mediated by both Ras-independent (depicted by pathways emanating from the receptor) and -dependent (depicted by pathways emanating from Ras) mechanisms and both are potentially targets for cAMP action. cAMP and PKA can also presumably influence pathways in the cell not directly activated by CSF-1 or IL-3 (depicted by an arrow emanating from PKA not targeted at a receptor-activated pathway).
Schematic illustrating how growth factors such as CSF-1 and IL-3 may cooperate with cAMP/PKA to activate the ERK/p90rsk pathway in myeloid progenitors. CSF-1 stimulates the activities of both Raf-1 and ERK in 32D cells. In the presence of cAMP, Raf-1 activation is prevented yet ERK activity is enhanced. The simplest explanation is that CSF-1 uses an alternate (not Raf-1) cAMP-insensitive kinase as the MAPKKK. Activation of MAPKKK is likely to be Ras-dependent, because expression of oncogenic Ras together with elevations in cAMP levels can recapitulate the synergistic stimulation of ERK by CSF-1 and cAMP. Whether cAMP can itself activate MAPKKK is not clear (see text). Instead, the dominant stimulatory effect of cAMP appears to be at a step after MEK1 activation, because MEK1 activity itself is not enhanced in the presence of cAMP but is required for the synergistic action of cAMP. Inhibition of the MEK-ERK pathway results in a significant but incomplete suppression of cell proliferation, indicating that growth factors such as CSF-1 and IL-3 activate other pathways required for optimal proliferation and survival in myeloid progenitor cell lines. These pathways could be mediated by both Ras-independent (depicted by pathways emanating from the receptor) and -dependent (depicted by pathways emanating from Ras) mechanisms and both are potentially targets for cAMP action. cAMP and PKA can also presumably influence pathways in the cell not directly activated by CSF-1 or IL-3 (depicted by an arrow emanating from PKA not targeted at a receptor-activated pathway).
Our observation of a cooperative activation of the ERK pathway by cAMP and growth factors is not an artifact of the engineered cell line used, because very similar effects were found for both CSF-1 and IL-3, with the latter acting on endogenous IL-3 receptors. IL-3 also cooperated with cAMP to activate ERK in a second myeloid progenitor cell line, FDC-P1. The receptors for CSF-1 and IL-3 are different: CSF-1R is a receptor tyrosine kinase, whereas IL-3R is a heterodimeric cytokine receptor that is not a tyrosine kinase but is phosphorylated by the cytoplasmic JAK family of tyrosine kinases. Coupling to the Ras/MAPK pathway by both receptor systems is thought to proceed through similar links, namely SHC tyrosine phosphorylation and association with Grb2/Sos.48 Therefore, it is not surprising to find that cAMP modulated CSF-1 and IL-3 stimulation of the ERK pathway in similar ways.
The synergistic activation of ERK induced by costimulation with cAMP and growth factors was recapitulated by cAMP treatment of cells expressing oncogenic Ras. This suggests that cross-talk between cAMP and CSF-1 or IL-3 is likely to be occurring at the level of Ras or downstream of Ras in the Ras/MAPK pathway. In data not shown, PKA activation had no effect on CSF-1–stimulated elements upstream of Ras, including receptor tyrosine phosphorylation, SHC tyrosine phosphorylation, and SHC/Grb2 association. Raf-1 was activated in response to CSF-1 in the absence but not in the presence of cAMP-elevating agents, in agreement with the well-documented inhibition of Raf-1 by PKA.20 Because inhibition of Raf-1 by cAMP is associated with an increase in ERK activity stimulated by CSF-1 and IL-3, the simplest explanation is that there is an MAPKKK in myeloid progenitors whose activity is not inhibited by cAMP. Other reports have also described the possible existence of Raf-1–independent mechanisms for activating ERK in hematopoietic cells: in macrophages, dominant-negative Ras expression inhibited CSF-1–stimulated MEK and ERK activities but had no effect on Raf-1 activation,49 and in a myeloid leukemic cell line, G-CSF–stimulated ERK activation is not inhibited by cAMP.50 However, ERK insensitivity to cAMP is not universal in hematopoietic cells, because cAMP inhibited ERK activation by GM-CSF and steel factor in a megakaryocytic leukemic cell line.51 One question is whether the MAPKKK implicated by our findings is also stimulated by cAMP. Figure 3 shows that, on average, forskolin/IBMX increases basal ERK activity by 2.6-fold. Because it is possible that the increase in ERK activity is due to cooperation of cAMP with residual activated Ras not eliminated by our starvation conditions, we cannot conclude, at this time, that cAMP can stimulate MAPKKK activity.
What is the possible identity of the cAMP-insensitive MAPKKK? In some PC12 sublines, cAMP activates the small G protein Rap1, which in turn activates the 95-kD form of B-Raf to stimulate ERK.27 32D cells express several forms of B-Raf, including a 90- to 95-kD species, but the overall expression level is low compared with PC12 cells (Fig 7A). This may account for our inability to detect B-Raf activation in response to cAMP. In addition to the Rafs, other molecules have been described as MAPKKKs. MEKK1 and MEKK3 apparently can activate MEK-ERK in transient overexpression studies.52 MEKK1 is expressed in 32D cells as determined by Western blotting, but we did not find evidence for its activation by CSF-1 (A.W.L., unpublished observations); the expression pattern of MEKK3 in myeloid cells is not known. Work is in progress to assess candidate signaling molecules capable of mediating the cooperative activation of ERK by cAMP and CSF-1.
The experiments with PD98059 and dn-MEK1 (Fig 8) provide strong evidence for a MEK1 requirement in the synergistic activation of ERK by cAMP and CSF-1. Yet there is not a corresponding synergistic activation of MEK1 (Fig 3), suggesting that cAMP is modulating other factors that affect either the ability of ERK to be activated by MEK1 or the inactivation of ERK subsequent to its activation by MEK1. A similar dissociation between MEK and ERK activation has been reported by Samuels et al53; in contrast to the findings of the present study, these investigators reported activation of MEK but not ERK in Rat1 fibroblasts upon Raf-1 stimulation. The dissociation was attributed to phosphatases acting on ERK. In support of the possibility that ERK activity in 32D cells is regulated by phosphatases and that cAMP may act through phosphatases to influence ERK activation, we have observed that inhibition of serine/threonine phosphatases such as PP2A by okadaic acid resulted in a marked (11.4-fold) increase in ERK activity in response to CSF-1 over that observed in the presence of CSF-1 alone. In comparison, MEK1 activity was only increased by 68% under the same conditions. Another possible mechanism is for cAMP to be affecting the mutual accessibility of MEK and ERK. In yeast, Ste5 serves as a scaffolding protein that holds Ste11 (MAPKKK), Ste7 (MAPKK), and Fus3/Kss1 (MAPK) together in a productive kinase cascade.54 An Ste5 analog has not been definitively identified in mammalian cells, but an attractive model would be for cAMP to modify this scaffolding protein in a way to enhance the ability of ERK to be phosphorylated by MEK. Further work will be needed to distinguish between these possibilities.
To the best of our knowledge, this study also shows for the first time a role for ERK activation in CSF-1–mediated survival and proliferation of myeloid cells. We have obtained similar findings for IL-3 that are generally in agreement with those of Perkins et al,55 who expressed a dn-MEK1 in BAF3 cells to examine ERK function in IL-3–mediated proliferation. These investigators reported that inhibition of ERK activation increased the IL-3 concentration necessary for optimal DNA synthesis and survival, although the effect was abrogated at high concentrations of IL-3. In contrast, we saw a significant reduction (by 60% to 70%) in cell numbers in the presence of PD98059 at saturating amounts of IL-3. A possible explanation is that dn-MEK1 is not as effective as PD98059 in inhibiting ERK activation. The decrease in CSF-1–dependent cell growth when ERK activity was inhibited could be a consequence not only of reduced cell cycle transit, but also of decreased survival as well, because 32D cells apoptose to some extent in the presence of PD98059. Because blocking the ERK pathway by PD98059 did not completely eliminate proliferation, other CSF-1– and IL-3–stimulated pathways must also contribute (Fig 10).
cAMP elevation had a net growth-inhibitory effect (Fig 1), so it was unexpected to find that CSF-1– and IL-3–induced ERK2 activity was increased by fivefold and ninefold, respectively, by costimulation with cAMP. These results suggest that cAMP is exerting inhibitory influences that ERK activation cannot override or the supranormal levels of ERK activity may itself be instrumental in mediating growth suppression. The latter possibility has been invoked to explain the correlation observed between IgM cross-linking–induced ERK activation and apoptosis in B cells.56 To distinguish between these possibilities, we used PD98059 to inhibit ERK activation. We found that synergistic activation of ERK by cAMP and CSF-1 or IL-3 contributed towards protection of cells from cAMP-induced apoptosis and growth inhibition. Thus, in myeloid progenitor cell lines such as 32D and FDC-P1, it appears that growth factors can use the ERK pathway to counter inhibitory effects produced by increases in intracellular cAMP. How might increased ERK activity modulate cAMP’s inhibitory actions? Cyclin D1 induction has been linked to ERK activation in a number of studies57,58 and may be a target for cAMP’s inhibitory action.59 One can speculate that a consequence of the synergistic activation of ERK by cAMP and growth factors is to increase G1 cyclin levels to partially overcome the negative effects of cAMP. cAMP also has targets not involving the ERK pathway: cAMP was found to increase the levels of the cell cycle inhibitor, p27kip1, in macrophages60 and to antagonize growth factor-dependent activation of p70s6k, the major S6 kinase in mammalian cells whose activation depends on phosphatidylinositol 3-kinase.61 We have previously demonstrated that CSF-1 activates phosphtaidylinositol 3-kinase in 32D/CSF-1R cells29 and is likely to represent one of the pathways required for optimal proliferation and survival (Fig 10). Lastly, it should be noted that Raf-1 can have functions independent of its role in the ERK pathway. In v-abl–transformed fibroblasts, inhibition of Raf-1 by cAMP did not inhibit ERK activation but instead eliminated override mechanisms that would otherwise have suppressed c-myc–induced apoptosis.62
The findings presented here lead to a number of additional questions. In addition to CSF-1R and IL-3R, do other growth factor receptors expressed on myeloid progenitors, eg, c-kit, GM-CSF receptor, and IL-6 receptor, also cooperate with cAMP to stimulate ERK and oppose cAMP’s growth-suppressive effects? If the interactions with cAMP differ between different myeloid growth factors as a consequence, for example, of growth factors coupling to different MAPKKKs, then expansion or depletion of subsets of myeloid progenitors could be achieved through the amount of cAMP produced and its selective interactions with the repertoire of growth factors and their receptors expressed on the cell surface. Differentiation along hematopoietic lineages may be influenced by intracellular cAMP levels and PKA activation as well: during the differentiation of monocytes to macrophages, both adenylate cyclase-specific activity and PKA protein levels were reported to increase dramatically.63 The impact on the ERK/MAPK pathway is not known. Elucidating the complex signals generated by elevated cAMP levels in response to various extracellular stimuli and the interactions between cAMP and growth factor-mediated pathways in different cell lineages may be key to our understanding of hematopoietic homeostasis.
ACKNOWLEDGMENT
The author thanks Cathy Diec and Min Li for technical assistance; Drs Melanie Cobb, Channing Der, K.-L. Guan, Gary Johnson, and John Majors for plasmids; and the Genetics Institute for recombinant human CSF-1. I also thank Drs Jeffrey Pessin and David States for critical reading of the manuscript and their suggestions.
Supported by an American Cancer Society Institutional Grant No. (IN-36-34), by a grant-in-aid from the American Heart Association Missouri Affiliate, and by the National Institutes of Health (Grant No. R01 DK48929).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
Author notes
Address reprint requests to Angel Wai-mun Lee, MD, PhD, Department of Biochemistry and Molecular Biophysics, Washington University Medical School, Box 8231, 660 S Euclid Ave, St Louis, MO 63110; e-mail:lee@biochem.wustl.edu.

![Fig. 3. Plot summarizing data from multiple experiments. ERK2 and MEK1 activities were measured as described in Fig 2 and the fold activation was calculated by normalizing to activities measured in untreated controls (− CSF-1) or to activities in samples treated with CSF-1 only (+ CSF-1) using the formula 1+ ([with cAMP] − [no cAMP])/([no cAMP] − [untreated]). Data shown represent the means ± SE, with the number of experiments (n) indicated. Statistically significant differences between untreated and cAMP-treated pairs are denoted by asterisks: *P < .05; **P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.537/4/m_blod40230003x.jpeg?Expires=1769084726&Signature=hOsuYgHZWJzhPpDXtu4jlgH8lquygli0FZnCbCkUO1txrrg-Csuzmr3hgU24ycl1Dti2vvCAGfVPpQZYQTWZjWG4~NGw4TLrsAQ2uQ1LLDVY0tn2f4HjqNLYXkEohHP5AnXarISzYDau2z01Pj37PZ-VIhWSaTvAsrf9GjaF1g4kqsmh~eBuZXuGDw5USsuNdvM2pAKba-Pa-cNDEYIHVszbSpVwZdZXlzzUtC6hLeG-pdtNSLPIiDGvVe3sZiIgF7qWbGnf0pD3kEA~0LTOLuuSA~KqBI2K5paMI5pzPOC0YY0HqDiTXr5Wrw2YOmr2Qfck-MesWfx0SHsUp0TjLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal